Abstract
This study evaluated the potential of a biocoagulant produced from prickly pear peel waste valorization and its use as a biocoagulant aid mixed with aluminum sulfate to remove turbidity in domestic wastewater. A central composite design (CCD) and a simplex lattice design (SLD) of two components (biocoagulant and aluminum sulfate) were developed to determine the optimal doses and pH of the biocoagulant and optimal mixing proportions. Both designs optimized the coagulation process from an analysis of variance to fit the experimental data to mathematical models and an optimization analysis to obtain the highest percentage of turbidity removal. The results showed that a water pH of 4 and a biocoagulant dose of 100 mg/L are optimal conditions for a turbidity removal of 76.1%. The potential decreases to 51.7% when the wastewater pH is maintained at 7.8 and a dose of 250 mg/L is used. This efficiency could be increased to 58.2% by using a mixture with optimal proportions of 30% biocoagulant and 70% aluminum sulfate. The experimental data were fitted to two quadratic models, estimating model prediction errors of 0.42% and 2.34%, respectively. Therefore, these results support the valorization of prickly pear peel waste to produce a biocoagulant, which could be used in acid and alkaline wastewater or as a biocoagulant aid mixed with aluminum sulfate.
1. Introduction
Freshwater resources globally have been further stressed by climate change, severe droughts, population growth, demand increase, and inadequate management in recent decades, resulting in severe water shortages in many regions [1]. Worldwide, 2.4 billion people lived in water-stressed countries in 2020, and this value is expected to increase due to the causes mentioned [2]. One of the targets of Goal 6 of Sustainable Development: Cleaner Water and Sanitation is to improve water quality by reducing pollution, eliminating dumping, minimizing the release of hazardous chemicals and materials, halving the proportion of untreated wastewater, and substantially increasing recycling and safety reuse globally by 2030 [2]. However, the availability of safe water for the population will not be ensured increasingly due to water contamination problems and lack of wastewater treatment [1].
Water contamination refers to the accidental, intentional, or negligent intrusion of biological, chemical, or radioactive contaminants and the frequent disposal of untreated or partially treated wastewater into surface water bodies performed by industrial activities, agricultural production, and urban life [3,4]. Contamination intensifies water scarcity in a region by making water inadequate for different uses and reducing freshwater availability [5]. Furthermore, exposure to contaminated water affects human health and the environment [6]. Therefore, affordable wastewater treatment alternatives to avoid the deterioration of water quality and increase reclaimed water and safe reuse remain an opportunity area for reducing freshwater extraction.
Coagulation–flocculation is applied in conventional water treatment plants to remove turbidity from the raw water associated with colloidal contaminants. Aluminum salts are commonly used as coagulants during this process, generating a high amount of sludge that contains a considerable part of aluminum hydroxide precipitate, which cannot be reused, increasing the cost of disposing of this waste [3,7]. This situation becomes worse when a high coagulant dose above its optimal dose is used during the treatment, leaving traces of these compounds in the treated water and the sludge. The discharge or use of treated water with chemical coagulant traces or direct disposal of sludge into nearby water bodies or soil has contributed to ecotoxicological issues, causing damage to the environment and human health [3,8]. Therefore, research has been conducted on studying the potential of biocoagulants in coagulation–flocculation to avoid the possible toxicity for human health and biota caused by using chemical agents in wastewater treatment [9].
Biocoagulants are based on cellulose derivatives, alginate, chitosan, galactomannans, gelatin, glues, mucilage, starch, and polysaccharides derived from microorganisms, animals, or plants [10,11]. These materials are biodegradable, eco-friendly, and non-toxic, generating biodegradable sludge, which could be used in agricultural practices, landfills, or construction [10,12]. Some biocoagulants applied to remove turbidity from natural and synthetic water and wastewater have been reported with significant efficiencies, such as Uncaria tomentosa, 17.2% [13]; Aloe vera, 53.5% [14]; Quercus Branti, 63.5% [15]; Opuntia robusta, 68.7% [13]; Azadirachta indica, 73.0% [16]; Plantago ovata, 80.0% [17]; Dolichos lablab, 89.0% [18]; Moringa oleifera, 94.0% [18]; Cicer arietinum, 96.0% [18]; Jatropha curcas, 96.0% [19]; and banana pith, 98.5% [20]. Likewise, biocoagulants have been studied in dual systems as coagulant aids; they are mixed with chemical coagulants (ferric and aluminum salts) to improve their effectiveness of removing turbidity from water and reduce the amount of used chemical agents and generated sludge [9,15,21,22].
Research has been conducted on biocoagulants from plants and fruit waste (biomass) in recent decades since these materials are considered green coagulants for being biodegradable and safe in water treatment [23]. This work proposed producing a biocoagulant from prickly pear peel waste. The waste obtained as a by-product of the fruit (prickly pear) produced by Opuntia ficus-indica species is a rich source of mucilage, which is a hydrocolloid biopolymer that could have potential application in wastewater treatment as a biocoagulant. The mucilage shows surfactant properties like natural gums in precipitating particles and ions from aqueous solutions [24]. The prickly pear is a fruit with high nutritional value since it provides vitamins, minerals, proteins, and fiber with excellent properties for digestion. Therefore, prickly pear is also a powerful natural remedy for treating multiple stomach diseases. Mexico has 20 thousand producers of prickly pear, who harvest 48 thousand hectares and obtain around 352 thousand tons annually [25]. The fruit is generally consumed fresh. So, this practice generates high accumulations of prickly pear peel waste in the country, where only a minimal part is reused in animal feed production [26]. It is estimated that more than one hundred seventy-six thousand tons of prickly pear peel waste is generated per year [25], representing an opportunity to valorize this by-product in the production of biocoagulants.
Otálora et al. [24] studied the physicochemical and molecular characteristics of Opuntia ficus-indica fruit peel mucilage. These properties were favorable for application as a biocoagulant in water and wastewater treatment. However, a study of its application was not found. Therefore, this work aimed to evaluate the potential of a biocoagulant produced from prickly pear peel waste valorization and its use as a biocoagulant aid mixed with aluminum sulfate to remove turbidity in domestic wastewater. The optimal pH and doses of the biocoagulant and optimal mixing proportions were determined using a central composite design (CCD) and a simplex lattice design (SLD) of two components (biocoagulant and aluminum sulfate), respectively, optimizing the coagulation process to achieve the highest percentage of turbidity removal. This study presents an integrated solution to two problems: the first one is the valorization of a waste product (prickly pear peel), and the second one is the production of a biocoagulant that can be used in wastewater treatment, reducing the use of chemical coagulants.
2. Materials and Methods
2.1. Feedstock
This study produced a biocoagulant from prickly pear peel waste. The prickly pear peel waste was provided by merchants of a marketplace in Zacatecas (Zacatecas, Mexico) (latitude 22°37′3″ N and longitude 102°51′40″ W). The wastes of green peel and white prickly pear (fruit of Opuntia ficus-indica) were selected as samples. Only this type of peel was used to avoid some uncertainty caused by using varieties of prickly pear since more than nine varieties of this species are cultivated in Mexico [27]. Approximately 5 kg of prickly pear was collected and used to produce the biocoagulant.
Domestic wastewater was used as a case study for evaluating the potential of the biocoagulant produced from prickly pear peel waste. The samples were collected at the urban wastewater discharge point in Zacatecas (Zacatecas, Mexico), located at latitude 22°37′3″ N and longitude 102°51′40″ W. On two occasions, 15 L of wastewater was collected following the guidelines established by the Mexican standard NMX-AA-003-1980 [28]. The physicochemical characteristics of the wastewater samples are shown in Table 1. Each value shows the mean of three measures.

Table 1.
Wastewater sample physicochemical characteristics.
It can be noted that electrical conductivity, turbidity, and TSS are higher than the reference values established by water quality standards (Table 1). Therefore, it is necessary to remove these contaminants by evaluating the prickly pear peel waste biocoagulant.
Aluminum sulfate, reagent grade (Mca. Meyer, Mexico City, Mexico), was used as a conventional coagulant. Likewise, a commercial flocculant (anionic polymer) provided by a Mexican company was used for all the jar tests at a concentration of 1 mg/L.
2.2. Biocoagulant Preparation and Characterization
The biocoagulant was prepared from mucilage content in the prickly pear peel waste. The mucilage extraction was performed based on the technique proposed by Sáenz et al. [32] with some modifications. The prickly pear peel waste was washed and cleaned first with tap water and second with deionized water for 48 h at room temperature. Then, the prickly pear peel waste was crushed using a commercial blender with the previously used deionized water. Afterward, the mixture was heated at 50 °C for one hour in a magnetic stirrer with a hot plate. Later, it was cooled at room temperature. Then, the obtained suspension was centrifuged at 3500 rpm for 10 min using a J-40 SOLBAT centrifuge. The supernatant was decanted and recuperated in a graduated cylinder to measure its volume. Subsequently, absolute ethanol was added with a 1:3 (v/v) ratio, and the mixture was kept at rest for 24 h at 4 °C. Afterward, it was vacuum filtered to obtain the mucilage extract. Then, it was dried at room temperature for 24 h. Finally, it was crushed in a mortar to form a powder (biocoagulant). The biocoagulant was stored in glass bottles at 4 °C. A biocoagulant production yield of 0.25% w/w was estimated.
The biocoagulant characterization was carried out by Fourier transform infrared (FTIR) spectroscopy (FTIR spectrometer, Bruker, Billerica, MA, USA). The spectrum was collected in 32 scans at 4 cm−1 in the mid-IR range 4000–400 cm−1 with automatic signal gain and rationed against a background spectrum recorded from the clean empty cell at 25 °C. The spectral data were analyzed using the OPUS 3.0 data collection software program (Bruker, Billerica, MA, USA). This analysis was performed to identify functional groups and predict the coagulation mechanisms.
2.3. Experimental Designs
A response surface design was developed to determine the optimal pH and doses of the prickly pear peel waste biocoagulant for wastewater treatment. A central composite design (CCD) of two numeric factors (pH and doses) with five central points and one block was selected. The factor ranges in terms of alphas were 4 and 7, and 50 and 450, respectively. The percentage of turbidity removal was the response to be observed and analyzed. The design resulted in 13 trials, as is shown in Table 2.

Table 2.
Trial numbers obtained in the CCD.
Furthermore, a mixture experimental design was developed to evaluate the potential of prickly pear peel waste as a biocoagulant aid (in a mixture with aluminum sulfate) for wastewater treatment. A simplex lattice design (SLD) of two components (biocoagulant and aluminum sulfate) with three replicates and a cubic order model was selected. The component values were interpreted as proportions as a function of the used doses. The proportion levels ranged from 0 to 1 for each component, and the proportions’ sum was 1 for each trial. The percentage of turbidity removal was the response to be observed and analyzed. The design resulted in 10 trials, as shown in Table 3. The wastewater pH (Table 1) and doses of 250 mg/L of biocoagulant were used in the trials of the SLD. These values were the conditions obtained in the CCD when the wastewater pH was not modified.

Table 3.
Trial numbers obtained in the SLD.
2.4. Jar Tests
The domestic wastewater sample was mixed well at the beginning of the jar test. A volume of 500 mL of wastewater was used in each trial. The jar test was performed using a jar floc test instrument (Flocculator SW6, Bibby Stuart, Armfield, Clarksburg, NJ, USA) at ambient conditions. The adjustment of the pH value reported in Table 2 was performed by drip-adding sulfuric acid 1 M or sodium hydroxide 1 M as necessary. The jar test involved three steps: first, a rapid mixing at 150 rpm for 5 min with coagulant addition according to the experimental designs (Table 2 and Table 3); second, a low mixing at 50 rpm for 30 min with 0.5 mL of flocculant addition; third, a sedimentation for 60 min. After these three steps, a volume of liquor supernatant (~20 mL) was pulled at 5 cm from the sample surface for conducting the water physicochemical characterization (final conditions). Each trial was performed in duplicate, and the average value was reported.
2.5. Water Physicochemical Characterization
After the jar test, the wastewater samples were physiochemically characterized by analyzing temperature, electrical conductivity, pH, turbidity, and TSS. These parameters were measured using a mercury thermometer, a conductivity meter with RS-232 Cable (Eutech Instruments ThermoScientific, Singapore), a LAQUAact PH110 Potentiometer (Horiba Scientific, Kyoto, Japan), a TB200TM Portable Turbidimeter (Orbeco-Hellige, Inc., Sarasota, FL, USA), and the method established by the Mexican Standard NMX-AA-034-SCFI-2015 [33], respectively.
After the jar test, the turbidity was measured to calculate the percentage of turbidity removal, which was used as the response in the experimental designs (CCD and SLD). This value was calculated by Equation (1).
Xi and Xf were the initial and final turbidity values before and after the coagulation tests. These results were expressed as the mean of two measurements.
2.6. Statistical Analysis and Process Optimization
An analysis of variance (ANOVA) was performed considering the percentage of turbidity removal as the response in both experimental designs (CCD and SLD) and using Design-Expert® Version 12 Software (Trial version) (Stat-Ease, Inc., Minneapolis, MN, USA). A p-value was established for the significance of the model and the lack of fit. Therefore, the experimental data were adjusted to mathematical models based on the numeric factors considered in each design. Likewise, the model equations were used to perform an optimization analysis, obtaining the optimal conditions for achieving the highest percentage of turbidity removal within the studied range of the factors.
3. Results and Discussion
3.1. Biocoagulant Properties
The mucilage extracted from prickly pear peel waste was characterized by FTIR analysis since this compound is the base of the biocoagulant. Figure 1 shows the FTIR spectrum, identifying functional groups from previously reported FTIR conversion tables [34,35].
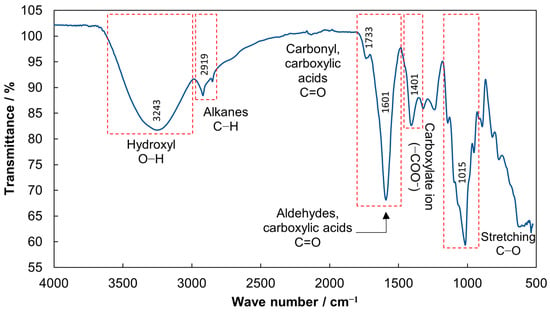
Figure 1.
FTIR spectrum of the biocoagulant produced from prickly pear peel waste.
The peak observed in the spectrum at 3243 cm−1 (Figure 1) is associated with the stretching vibration of the hydroxyl group bonds (O-H), including hydrogen bonds [34,36]. Otálora et al. [24] characterized mucilage extracted from the peel of the fruit of Opuntia ficus-indica. They attributed this band equally to the stretching of the hydroxyl groups (OH), where these groups adhered to the polysaccharide structure and galacturonic acid carboxylic terminals. Likewise, the peak observed at 2919 cm−1 (Figure 1) corresponds to the presence of the C-H bond. Bernardino et al. [37] characterized Opuntia mucilage and associated this peak with C-H bond stretching of the methyl ester of galacturonic acid present in it.
A weaker peak at 1733 cm−1 (Figure 1) is associated with the stretching vibration of the C=O bond, which indicates the presence of the carbonyl group [24,38,39,40]. This carbonyl indicates an ester form of carboxylic acid where carbonyl is esterified [41,42]. Choudhary et al. [43] attributed this peak to the C=O stretching of the methylated or protonated (non-ionized) form of the carboxyl group, indicating the presence of the methyl ester of galacturonic acid [44] since the galacturonic acid can be found with the free carboxyl group or with the carboxyl esterified with methanol. Therefore, galacturonic acid in the mucilage is the main factor in carrying out a coagulation process and reduces the turbidity in the wastewater, as was reported by Aguilera-Flores et al. [13].
The peak at 1601 cm−1 (Figure 1) indicates the presence of the stretching of the C=O bond. This peak is found in polysaccharides and is characteristic of a carboxyl group [45]. However, Gómez and Rupérez [41] and Zhao et al. [46] reported that this peak indicates the presence of uronic acid and represents stretches of carboxylate ions derived from galacturonic acid. Li et al. [47] mentioned that peaks near 1401 cm−1 indicate the presence of uronic acid in the polysaccharide molecule, in this case, mucilage. Choudhary et al. [43] and Zhao et al. [46] associated this peak with the ionization of carboxyl groups and the stretching vibrations of COO- (negatively charged carboxylate ion) of galacturonic acid. Likewise, the peak observed at 1234 cm−1 (Figure 1) corresponds to the stretching of the C-O bond, so this bond could be associated with esters and the carboxylic acid that are in the galacturonic acid present in the mucilage of the prickly pear peel.
Finally, the peak observed at 1015 cm−1 (Figure 1) is associated with the different groups of carbohydrates present in mucilage since the range between 1000 and 1200 cm−1 indicates the C-O stretching of galactose, rhamnose, and galacturonic acid [48,49]. Therefore, the uronic acid or galacturonic acid in the mucilage of prickly pear peel is the main component for use as a biocoagulant.
Some coagulation mechanism types are patch flocculation, sweep coagulation, bridging, and charge neutralization. The last two are the possible coagulation mechanisms for plant-based biocoagulants [50]. Galacturonic acid has been classified as an anionic coagulant and is considered non-ionic in the pH range of 6.5 to 8.5 or higher. The presence of this acid in the biocoagulant is responsible for the bridging mechanism in the coagulation process since, being predominantly in polymeric form (polygalacturonic acid), it presents a bridge that allows the particles to adsorb [51,52,53,54]. When galacturonic acid is found as a non-ionic polymer, it provides an H+ bridge to adsorb colloidal particles [55]. Therefore, the FTIR results support the assumption that bridging is the coagulation mechanism of the biocoagulant produced from prickly pear peel waste due to the presence of galacturonic acid.
3.2. Optimal pH and Doses of the Prickly Pear Peel Waste Biocoagulant
Table 4 reports the results of water physicochemical characterization after the coagulation treatment using the CCD shown in Table 2. The temperature was not included in Table 4 since the value of this parameter remained at 13 ± 0.50 °C.

Table 4.
Water physicochemical characterization after the coagulation treatment using the CCD to determine optimal pH and doses of the biocoagulant.
It can be observed in Table 4 that ΔpH showed an increasing trend in all the trials. This condition coincides with the results of Vaca et al. [56], who observed that an Opuntia-based biocoagulant increased the water pH after treatment in all cases with a maximum value of four-tenths. The experimental results obtained in this study showed this same maximum value. An advantage of biocoagulants is that they do not significantly alter the pH of the treated water [57]. Therefore, this parameter remains within the reference range (6.5–8.5) established by the World Health Organization [29] for the cases where the water pH was not modified.
The SST concentration decreased (ΔSST values) in all the trials after coagulation treatment (Table 4), and percentages of SST removal between 27.63% and 92.55% were obtained. The final SST concentrations are within the maximum reference value (140 mg/L) reported by the Ministry of the Environment and Natural Resources [31]. So, the prickly pear peel waste biocoagulant shows the capacity to improve the wastewater quality regarding this parameter.
It can be noted that ΔEC shows positive and negative values. This comportment could not be associated with a trend since there is no relationship between the doses, the pH, and the final value of the obtained conductivity. Medellín-Castillo et al. [23] also reported increments and decrements in the water’s electrical conductivity after using a devilfish-based biocoagulant mixed with aluminum and ferric sulfate. They associated this behavior with the dissolved ions of the wastewater and coagulants. Therefore, this parameter must be controlled before the discharge of treated water.
The percentages of turbidity removal were from 12.19% to 67.17% (Table 4). The biocoagulant can remove the turbidity in the wastewater in the pH range of 4.4–7.7. It can be noted that a biocoagulant dose of 250 mg/L is required to achieve a percentage of turbidity removal of 51.7% at wastewater pH (Trial 6). Therefore, 250 mg/L was used as doses in the trials of evaluating the potential of prickly pear peel waste as a biocoagulant aid mixed with aluminum sulfate (Section 3.4), maintaining the wastewater pH (7.69). Likewise, the results shown in Table 4 depended on the doses used in the jar tests. Therefore, both parameters were used as the factors in the experimental design. Table 5 shows the analysis of variance (ANOVA) performed for the CCD.

Table 5.
Analysis of variance (ANOVA) of the CCD to determine optimal pH and doses of the biocoagulant.
It can be observed in Table 5 that the model is statistically significant (p-value < 0.05), and the lack of fit is not statistically significant (p-value > 0.05). Therefore, the experimental data were adjusted to the quadratic model described by Equation (2).
The response (turbidity removal) is sensitive to the doses (main factor), pH × doses (interaction), and pH2 (quadratic effect). Figure 2 shows a 3D response surface and contour graph for the turbidity removal in domestic wastewater using a prickly pear peel waste biocoagulant.
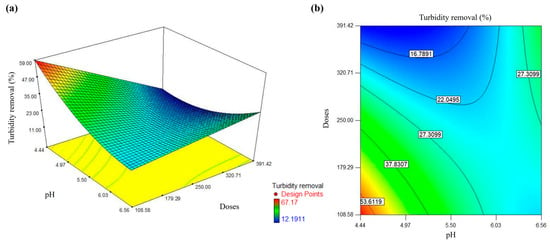
Figure 2.
Turbidity removal in domestic wastewater using a prickly pear peel waste biocoagulant: (a) 3D response surface graph, (b) interaction contour graph.
The red area in Figure 2 shows the conditions for the highest percentage of turbidity removal that could be obtained. These conditions correspond to pH 4.44–4.6 and doses 108–138 mg/L. Likewise, it can be noted that turbidity removal decreases when the pH and doses increase. Therefore, prickly pear peel waste biocoagulant is more effective in acid media. Nharingo et al. [58] reported that the predominant mechanisms of Opuntia-based biocoagulants are adsorption, charge neutralization, and stretching due to the anionic nature and deprotonated galacturonic acid macromolecule.
On the other hand, Yongabi [59] reported that a higher particle number with positive charges is obtained at acid pH in the wastewater, which is available to react with the negatively charged colloidal and suspended material. Marichamy et al. [60] indicated that wastewater is saturated with positive charge ions (H+) at acid pH, improving the electrostatic attraction between acid ions and contaminant ions. Hence, the electrostatic attraction force between the ions is increased at acid pH, and the biocoagulant can rapidly destabilize contaminants particles. Therefore, prickly pear peel waste biocoagulant has more potential in acid media (pH 4).
Although various factors influence the effectiveness of wastewater treatment by coagulation, the pH and the doses have been considered the most essential parameters in this process [61]. Therefore, an optimization analysis was performed using the quadratic model described in Equation (2) to achieve the maximum percentage of turbidity removal.
The optimal conditions obtained in the optimization analysis were a pH of 4 and doses of 100 mg/L to achieve a percentage of turbidity removal of 76.13%. A trial was performed in the laboratory under these optimal conditions and following the methodology described in Section 2.4, obtaining a percentage of turbidity removal of 76.45%. Therefore, an error of 0.42% was estimated for the model’s prediction, confirming that the optimal pH and doses of the prickly pear peel waste biocoagulant are 4 and 100 mg/L, respectively. These results will be discussed in Section 3.4.
3.3. Potential of Prickly Pear Peel Waste as a Biocoagulant Aid Mixed with Aluminum Sulfate
Table 6 reports the results of water physicochemical characterization after the coagulation treatment using the SLD shown in Table 3. The temperature is not included in Table 6 since the value of this parameter remained at 14 ± 0.50 °C.

Table 6.
Water physicochemical characterization after the coagulation treatment using the SLD to evaluate the potential of prickly pear peel waste as a biocoagulant aid (in a mixture with aluminum sulfate) for wastewater treatment.
It can be observed in Table 6 that ΔpH showed an increment when the biocoagulant was used in a proportion of 1 in the mixture. This condition coincides with the results obtained in the CCD (Section 3.2). On the other hand, as the proportion of aluminum sulfate increases, the pH tends to decrease, acidifying the water pH. The highest decrement was detected when aluminum sulfate was used in a proportion of 1 in the mixture (Trials 4, 8, and 10). This behavior was reported by Gandiwa et al. [62] since they indicated that the water pH decreases with aluminum sulfate-based coagulants. However, all the trials remained within the reference range (6.5–8.5) of the World Health Organization [29].
SST concentration decreased (ΔSST values) in all the trials after coagulation treatment (Table 6), and percentages of SST removal between 28.18% and 99.99% were obtained. Hence, the final SST concentrations were lower than the maximum reference value (140 mg/L) reported by the Ministry of the Environment and Natural Resources [31]. So, this water quality parameter is improved using the prickly pear peel waste biocoagulant alone or mixed with aluminum sulfate.
It can be observed that ΔEC increased in all the trials (Table 6). The highest increments occurred when aluminum sulfate was used in a proportion of 1 in the mixture (Trials 4, 8, and 10). This behavior is associated with coagulant dissociation, generating dissolved ions that increase the water EC [62]. Marobhe et al. [63] reported that Opuntia-based coagulants show low dissociation capacity in water regarding aluminum sulfate. Therefore, ΔEC was lower in the trials where prickly pear peel waste biocoagulant was used in a high proportion in the mixture (Table 6).
The percentages of turbidity removal were from 0% to 99.99% (Table 6). The highest removal percentage was achieved when the aluminum sulfate was used alone (Trials 8 and 10), and the removal percentage decreased as the biocoagulant proportion in the mixture increased. The reference value (<5 NTU) emitted by the World Health Organization [29] was exceeded in all the trials, except for the cases where aluminum sulfate was used with a proportion of 1 in the mixture (Trials 8 and 10). Instead, a percentage of turbidity removal was not observed when the biocoagulant was mixed with aluminum sulfate in Trials 2 and 5 (Table 6).
The turbidity value must be less than the quality parameter since high turbidity values may be indicative of organic particulates, which harbor microorganisms, increasing the possibility of waterborne diseases and posing health risks to effluent users. Likewise, high turbidity in water may increase treatment costs due to problems caused by the flocculation, disinfection, and filtration processes. If the wastewater is discharged into water bodies without a reduction in the turbidity levels, the water bodies become unpleasant for full-contact recreation [64].
Therefore, the percentages of turbidity removal (Table 6) were used as the responses in the SLD to determine the optimal conditions of the mixture between the biocoagulant and aluminum sulfate. Table 7 shows the analysis of variance (ANOVA) performed for the SLD to evaluate the potential of prickly pear peel waste as a biocoagulant aid (in a mixture with aluminum sulfate) for wastewater treatment.

Table 7.
Analysis of variance (ANOVA) of the SLD to evaluate the potential of prickly pear peel as a biocoagulant aid.
It can be observed in Table 7 that the model is statistically significant (p-value < 0.05), and the lack of fit is not statistically significant (p-value > 0.05). Therefore, the experimental data were adjusted to the quadratic model described by Equation (3).
where A is the biocoagulant and B is aluminum sulfate.
It can be observed in Equation (3) that the linear mixture (A and B), the interaction between both coagulants (A × B), and the interaction A × B × (A − B) were significant (Table 7). Therefore, turbidity removal depends on the mixture and the interaction of the coagulants. Figure 3 shows a two-component mix graph for the turbidity removal in domestic wastewater using prickly pear peel waste as a biocoagulant aid (in a mixture with aluminum sulfate) for wastewater treatment.
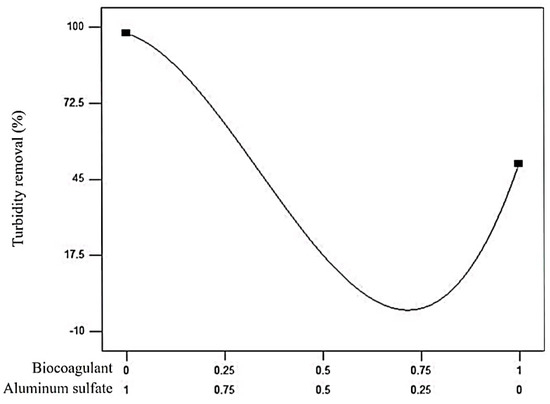
Figure 3.
Two-component mix graph for the turbidity removal in domestic wastewater using a prickly pear peel waste biocoagulant in a mixture with aluminum sulfate.
It can be noted in Figure 3 that turbidity removal is more sensitive to the aluminum sulfate effect than the biocoagulant. Its potential decreases as the biocoagulant proportion increases in the mixture. The turbidity removal could be improved using a prickly pear peel waste biocoagulant as a coagulant aid mixed with aluminum sulfate [21] since the turbidity removal is 1.22 times higher with a mixture’s proportion of 0.25 of the biocoagulant and 0.75 of aluminum sulfate than 1.00 of the biocoagulant. However, the prickly pear peel waste biocoagulant as a coagulant aid is not functional when used with a mixture proportion of between 0.67 and 0.75 since the turbidity removal decreases (Figure 3). Therefore, an optimization analysis was performed using the mathematical model shown in Equation (3) for achieving the highest percentage of turbidity removal under the following criteria: minimum and maximum amounts of aluminum sulfate and biocoagulant, respectively.
The optimization analysis revealed that a percentage of turbidity removal of 57.28% is achieved using a dose of 250 mg/L with a proportion of a mixture of 0.3 prickly pear peel waste biocoagulant and 0.7 aluminum sulfate at domestic wastewater pH (7.8). A trial was performed in the laboratory under these treatment-optimal conditions, and the same methodology was applied, obtaining a turbidity removal of 58.62%, showing an error of 2.34% between the experimentally observed removal and the model’s prediction.
3.4. Potential of Prickly Pear Peel Waste Biocoagulant
A comparison of the potential between different biocoagulants applied to the water and wastewater treatment with the results found in this study is shown in Table 8.

Table 8.
Comparison of the potential of biocoagulants in water and wastewater treatment.
Several biocoagulants have been used to treat urban, domestic, and industrial wastewater; drinking water; and synthetic turbid water. Biocoagulants produced from Cicer arietinum seeds, Moringa oleifera seeds, and tamarind seeds show high efficiencies of turbidity removal (>94%) in different water types in the pH range of 7.3 to 7.5 (Table 8). Likewise, banana pith and Jatropha curcas have a high potential (>96%) as biocoagulants but at water acid pH (4.0 and 3.0, respectively). The optimal pH of the prickly pear peel waste biocoagulant was 4.0, showing turbidity removal efficiency 1.3 times lower than the biocoagulants mentioned. However, this biocoagulant shows potential 4.4 times higher than other biocoagulants such as Oputnia and Uncaria tomentosa at a pH of 4.0 and 2.9 times at a pH of 7.8 (Table 8).
On the other hand, using prickly pear peel waste biocoagulant as a coagulant aid could be feasible since its application could represent an alternative for reducing the use of aluminum sulfate, mitigating the negative environmental impacts generated by this chemical coagulant, and minimizing treatment cost [21]. However, a decision on the operation conditions of the treatment must be made. Three scenarios could be evaluated: (1) using prickly pear peel waste biocoagulant with doses of 100 mg/L, modifying wastewater pH at 4.0 to achieve a turbidity removal of 76.1%; (2) using prickly pear peel waste biocoagulant with doses of 250 mg/L and maintaining wastewater pH (7.8) to achieve a turbidity removal of 51.7%; (3) using prickly pear peel waste biocoagulant as a coagulant aid (mixed with aluminum sulfate) with doses of 250 mg/L, a proportion of 30% biocoagulant and 70% chemical coagulant, and maintaining wastewater pH (7.8) to achieve a turbidity removal of 58.2%. A cost–benefit analysis must be performed to evaluate these three scenarios and make the best decision. Ratnayaka et al. [67] reported that high amounts of aluminum sulfate are more expensive than acidifying or alkalinizing the wastewater. Likewise, applying dual systems (a mixture of biocoagulants with chemical coagulants) could reduce the treatment cost associated with the chemical coagulant amount and sludge management [22]. In this sense, the best scenario would probably be (1), followed by (3) and (2).
The production cost of prickly pear peel waste biocoagulant was estimated under the experimental conditions of this study. The estimation included the cost of tap water, deionized water, and ethanol (with recovering) and the cost of energy used in heating, centrifugation, and filtration. The cost of feedstock (prickly pear peel waste) collection and transport was discarded. An approximate value of 390 USD/kg of biocoagulant was estimated. This value is 780 times higher than aluminum sulfate (0.5 USD/kg) [68]. However, cost analyses of process scaling could reduce its value since it has been reported that the price of plant-based biocoagulant industrial production is eight times lower than aluminum sulfate [69]. Therefore, prickly pear peel waste biocoagulant could represent a biodegradable and lower-cost alternative material in domestic wastewater treatment.
4. Conclusions
This study produced a biocoagulant from prickly pear peel waste valorization. The potential of the biocoagulant to remove turbidity in domestic wastewater was evaluated. A water pH of 4 and biocoagulant dose of 100 mg/L were determined from an experimental design as the optimal condition to achieve an efficiency of turbidity removal of 76.1%. Its potential decreases to 51.7%, maintaining the wastewater pH (7.8) and using 250 mg/L. Likewise, this efficiency could be increased to 58.2% using a mixture with optimal proportions of 30% biocoagulant and 70% aluminum sulfate. These experimental data were fitted to two quadratic models, and estimated model prediction errors were 0.42% and 2.34%, respectively. These results support the valorization of prickly pear peel waste to produce a biocoagulant that could be used in acid and alkaline wastewater or as a biocoagulant aid mixed with aluminum sulfate. Therefore, this prickly pear peel waste biocoagulant could represent a biodegradable and lower-cost alternative material in domestic wastewater treatment.
Author Contributions
Conceptualization, M.M.A.F. and O.E.R.M.; methodology, M.M.A.F., O.E.R.M., N.A.M.C. and O.S.M.; software, M.M.A.F. and R.V.B.; validation, M.M.A.F., N.A.M.C., O.S.M. and R.V.B.; formal analysis, M.M.A.F., N.A.M.C. and O.E.R.M.; investigation, M.M.A.F., O.E.R.M., N.A.M.C., V.Á.V., O.S.M., R.V.B. and C.E.C.M.; resources, M.M.A.F., V.Á.V. and N.A.M.C.; data curation, M.M.A.F., N.A.M.C. and R.V.B.; writing—original draft preparation, M.M.A.F.; writing—review and editing, M.M.A.F., O.E.R.M., N.A.M.C., V.Á.V., O.S.M., R.V.B. and C.E.C.M.; visualization, M.M.A.F.; supervision, M.M.A.F., V.Á.V., N.A.M.C. and O.S.M.; project administration, M.M.A.F.; funding acquisition, M.M.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto Politécnico Nacional, grant numbers SIP-20220024 and SIP-20240116, and by Consejo Zacatecano de Ciencia, Tecnología e Innovación.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Salehi, M. Global water shortage and potable water safety; Today’s concern and tomorrow’s crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef] [PubMed]
- United Nations. The Sustainable Development Goals Report 2023. Available online: https://unstats.un.org/sdgs/report/2023/The-Sustainable-Development-Goals-Report-2023.pdf (accessed on 24 December 2023).
- Suman, A.; Ahmad, T.; Ahmad, K. Dairy wastewater treatment using water treatment sludge as coagulant: A novel treatment approach. Environ. Dev. Sustain. 2018, 20, 1615–1625. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 80246. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Liu, M.; Ma, Z.; Fang, W.; Bi, J. Quality matters: Pollution exacerbates water scarcity and sectoral output risks in China. Water Res. 2022, 224, 119059. [Google Scholar] [CrossRef] [PubMed]
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Natural-based coagulants/flocculants as sustainable market-valued products for industrial wastewater treatment: A review of recent developments. RSC Adv. 2023, 13, 19335. [Google Scholar] [CrossRef] [PubMed]
- Bachand, P.A.M.; Bachand, S.M.; Lopus, S.E.; Heyvaert, A.; Werner, I. Treatment with chemical coagulants at different dosing levels changes ecotoxicity of stormwater from the Tahoe basin, California, USA. J. Environ. Sci. Health Part A 2010, 45, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Bin Omar, M.A.; Mohd Zin, N.S.B.; Mohd Salleh, S.N.A.B. A review on performance of chemical, natural and composite coagulant. Int. J. Eng. Technol. 2018, 7, 56–60. [Google Scholar]
- Koul, B.; Bhat, N.; Abubakar, M.; Mishra, M.; Arukha, A.P.; Yadav, D. Application of Natural Coagulants in Water Treatment: A Sustainable Alternative to Chemicals. Water 2022, 14, 3751. [Google Scholar] [CrossRef]
- Aragaw, T.; Bogale, F.M. Role of coagulation/flocculation as a pretreatment option to reduce colloidal/bio-colloidal fouling in tertiary filtration of textile wastewater: A review and future outlooks. Front. Environ. Sci. 2023, 11, 1142227. [Google Scholar] [CrossRef]
- Dos Santos, J.D.; Veit, M.T.; Juchen, P.T.; da Cunha Gonçalves, G.; Palacio, S.M.; Fagundes-Klen, M. Use of different coagulants for cassava processing wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 1821–1827. [Google Scholar] [CrossRef]
- Aguilera-Flores, M.M.; Valdivia-Cabral, G.I.; Medellín-Castillo, N.A.; Ávila-Vázquez, V.; Sánchez-Mata, O.; García-Torres, J. Study on the Effectiveness of Two Biopolymer Coagulants on Turbidity and Chemical Oxygen Demand Removal in Urban Wastewater. Polymers 2023, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Gisonni, C.; Crispino, G.; Pirozzi, F.; Pizzi, A. Use of Aloe vera as an Organic Coagulant for Improving Drinking Water Quality. Water 2021, 13, 2024. [Google Scholar] [CrossRef]
- Jamshidi, A.; Rezaei, S.; Hassani, G.; Firoozi, Z.; Ghaffari, H.R.; Sadeghi, H. Coagulating potential of Iranian oak (Quercus branti) extract as a natural coagulant in turbidity removal from water. J. Environ. Health Sci. Eng. 2020, 18, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandham, K.; Karri, R.R. Preparation and characterization of Azadirachta indica A. Juss. plant based natural coagulant for the application of urban sewage treatment: Modelling and cost assessment. Environ. Technol. Innov. 2021, 23, 101733. [Google Scholar] [CrossRef]
- Dhivya, S.; Ramesh, S.T.; Gandhimathi, R.; Nidheesh, P.V. Performance of natural coagulant extracted from Plantago ovata seed for the treatment of turbid water. Water Air Soil Pollut. 2017, 228, 423. [Google Scholar] [CrossRef]
- Asrafuzzaman, M.; Fakhruddin, A.N.M.; Hossain, M.A. Reduction of Turbidity of Water Using Locally Available Natural Coagulants. ISRN Microbiol. 2011, 2011, 632189. [Google Scholar] [CrossRef] [PubMed]
- Abidin, Z.Z.; Ismail, N.; Yunus, R.; Ahamad, I.S.; Idris, A. A preliminary study on Jatropha curcas as coagulant in wastewater treatment. Environ. Technol. 2011, 32, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kakoi, B.; Kaluli, J.W.; Ndiba, P.; Thiong’o, G. Banana pith as a natural coagulant for polluted river water. Ecol. Eng. 2016, 95, 699–705. [Google Scholar] [CrossRef]
- Mohd-Salleh, S.N.A.; Mohd-Zin, N.S.; Othman, N. A review of Wastewater Treatment using Natural Material and Its Potential as Aid and Composite Coagulant. Sains Malays. 2019, 48, 155–164. [Google Scholar] [CrossRef]
- Aguilera-Flores, M.M.; Medellín-Castillo, N.A.; Ávila-Vázquez, V.; González-García, R.; Cardona-Benavides, A.; Carranza-Álvarez, C. Evaluation of a biocoagulant from devilfish invasive species for the removal of contaminants in ceramic industry wastewater. Sci. Rep. 2022, 12, 9917. [Google Scholar] [CrossRef] [PubMed]
- Medellín-Castillo, N.A.; Aguilera-Flores, M.M.; Ávila-Vázquez, V.; González-García, R.; García-Torres, J. Evaluation of the Devilfish (Pterygoplichthys spp.) Natural Coagulant as a Treatment for the Removal of Turbidity in Fish Farm Wastewater. Water Air Soil. Pollut. 2022, 233, 167. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches, A.; Lara, C.; Cifuentes, R.; Gómez, A. Use of Opuntia ficus-indica fruit peel as a novel source of mucilage with coagulant physicochemical/molecular characteristics. Polymers 2022, 14, 3832. [Google Scholar] [CrossRef]
- Fideicomiso de Riesgo Compartido. La TUNA, una fruta muy mexicana. Available online: https://www.gob.mx/firco/articulos/la-tuna-una-fruta-muy-mexicana?idiom=es (accessed on 1 December 2023).
- Cerezal, P.; Duarte, G. Use of Skin in the Elaboration of Concentrated Products of Cactus Pear (Opuntia ficus-indica (L.) Miller). J. Prof. Assoc. Cactus Dev. 2005, 7, 61–83. [Google Scholar]
- Pimienta-Barrios, E. Prickly pear (Opuntia spp.): A valuable fruit crop for the semi-arid lands of Mexico. J. Arid. Environ. 1994, 28, 1–11. [Google Scholar] [CrossRef]
- Secretaría de Comercio y Fomento Industrial. Norma Mexicana NMX-AA-003-1980 Aguas Residuales—Muestreo. Available online: https://www.gob.mx/cms/uploads/attachment/file/166762/NMX-AA-003-1980.pdf (accessed on 1 December 2023).
- World Health Organization. Guidelines for Drinking-Water Quality. Available online: https://iris.who.int/bitstream/handle/10665/254637/9789241549950-eng.pdf?sequence=1 (accessed on 25 December 2023).
- United States Environmental Protection Agency. Water: Monitoring and Assessment, 5.9 Conductivity. Available online: https://archive.epa.gov/water/archive/web/html/vms59.html (accessed on 24 December 2023).
- Secretaría de Medio Ambiente y Recursos Naturales. Norma Oficial Mexicana NOM-001-SEMARNAT-2021, Que Establece Los Límites Permisibles de Contaminantes en Las Descargas de Aguas Residuales en Cuerpos Receptores Propiedad de la Nación. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5645374&fecha=11/03/2022 (accessed on 25 December 2023).
- Sáenz, C.; Berger, H.; Corrales-García, J.; Galletti, L.; García de Cortázar, V.; Higuera, I.; Mondragón, C.; Rodríguez-Félix, A.; Sepúlveda, E.; Varnero, M.T. FAO Agricultural Services Bulletin 162, Agroindustrial Use of Nopal. Available online: https://repositorio.uchile.cl/bitstream/handle/2250/120301/Utilizacion-agroindustrial-del-nopal.pdf?sequence=1 (accessed on 22 September 2023).
- Secretaría de Economía. Norma Mexicana NMX-AA-034-SCFI-2015 Análisis de Agua—Medición de Sólidos y Sales Disueltas en Aguas Naturales, Residuales y Residuales Tratadas—Método de Prueba. Available online: https://www.gob.mx/cms/uploads/attachment/file/166146/nmx-aa-034-scfi-2015.pdf (accessed on 22 September 2023).
- Skoog, D.A.; Holler, F.J.; Nieman, T.A. Principios de Análisis Instrumental, 6th ed.; Cengage Learning: Ciudad de Mexico, Mexico, 2008; pp. 461–463. [Google Scholar]
- Chang, R. Fisicoquímica, 3rd ed.; McGraw-Hill/Interamericana: Ciudad de Mexico, Mexico, 2008; pp. 724–725. [Google Scholar]
- Peláez, A.; Velázquez, I.; Herrera, A.; García, J. Textile dyes removal from aqueous solution using Opuntia ficus-indica fruit waste as adsorbent and its characterization. J. Environ. Manag. 2013, 130, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, A.; Montañez, J.; Conde, E.; Negrete, M.; Teniente, G.; Vargas, E.; Juárez, J.; Acosta, G.; González, L. Spectroscopic and structural analyses of Opuntia Robusta mucilage and its potential as an edible coating. Coatings 2018, 8, 466. [Google Scholar] [CrossRef]
- Gopi, D.; Kanimozhi, K.; Kavitha, L. Opuntia ficus-indica peel derived pectin mediated hydroxyapatite nanoparticles: Synthesis, spectral characterization, biological and antimicrobial activities. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2015, 141, 135–143. [Google Scholar] [CrossRef]
- Lodhi, M.S.; Shaheen, A.; Khan, M.T.; Shafiq, M.I.; Samra, Z.Q.; Wei, D.Q. A novel method of affinity purification and characterization of polygalacturonase of Aspergillus flavus by galacturonic acid engineered magnetic nanoparticle. Food Chem. 2022, 372, 131317. [Google Scholar] [CrossRef]
- Teng, H.; He, Z.; Li, X.; Shen, W.; Wang, J.; Zhao, D.; Sun, H.; Xu, X.; Li, C.; Zha, X. Chemical structure, antioxidant and anti-inflammatory activities of two novel pectin polysaccharides from purple passion fruit (Passiflora edulia Sims) peel. J. Mol. Struct. 2022, 1264, 133309. [Google Scholar] [CrossRef]
- Gómez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Méndez, D.A.; Martínez-Abad, A.; Martínez-Sanz, M.; López-Rubio, A.; Fabra, M.J. Tailoring structural, rheological and gelling properties of watermelon rind pectin by enzymatic treatments. Food Hydrocoll. 2023, 135, 108119. [Google Scholar] [CrossRef]
- Choudhary, M.; Ray, M.B.; Neogi, S. Evaluation of the potential application of cactus (Opuntia ficus-indica) as a bio-coagulant for pre-treatment of oil sands process-affected water. Sep. Purif. Technol. 2019, 209, 714–724. [Google Scholar] [CrossRef]
- Santos, J.D.; Espeleta, A.F.; Branco, A.; de Assis, S.A. Aqueous extraction of pectin from sisal waste. Carbohydr. Polym. 2013, 92, 1997–2001. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, K.; Zhang, X.; Li, L.; Zhang, H.; Zhou, L.; Liang, J.; Li, X. In vitro binding capacities, physicochemical properties and structural characteristics of polysaccharides fractionated from Passiflora edulis peel. Food Biosci. 2022, 50, 102016. [Google Scholar] [CrossRef]
- Zhao, X.; Qiao, L.; Wu, A.M. Effective extraction of Arabidopsis adherent seed mucilage by ultrasonic treatment. Sci. Rep. 2017, 7, 40672. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.M.; Wang, J.F.; Zha, X.Q.; Pan, L.H.; Zhang, H.L.; Luo, J.P. Structural characterization and immunomodulatory activity of a new polysaccharide from jellyfish. Carbohydr. Polym. 2017, 159, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Ghori, M.U.; Mohammad, M.A.; Rudrangi, S.R.; Fleming, L.T.; Merchant, H.A.; Smith, A.M.; Conway, B.R. Impact of purification on physicochemical, surface and functional properties of okra biopolymer. Food Hydrocoll. 2017, 71, 311–320. [Google Scholar] [CrossRef]
- Mirbahoush, S.M.; Chaibakhsh, N.; Moradi-Shoeili, Z. Highly efficient removal of surfactant from industrial effluents using flaxseed mucilage in coagulation/photo-Fenton oxidation process. Chemosphere 2019, 231, 51–59. [Google Scholar] [CrossRef]
- Kristianto, H. The potency of Indonesia native plants as natural coagulant: A mini review. Water Conserv. Sci. Eng. 2017, 2, 51–60. [Google Scholar] [CrossRef]
- Yin, C. Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem. 2010, 45, 1437–1444. [Google Scholar] [CrossRef]
- Choy, S.; Prasad, N.; Wu, Y.; Raghunandan, E.; Ramanan, N. Utilization of plant-based natural coagulants as future alternatives towards sustainable water clarification. J. Environ. Sci. 2014, 26, 2178–2189. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Unuabonah, E.I.; Amuda, O.S.; Kolawole, O.M. Mechanistic insight into the coagulation efficiency of polysaccharide-based coagulants. In Polysaccharides as a Green and Sustainable Resources for Water and Wastewater Treatment, 1st ed.; Navard, P., Antipolis, S., Eds.; Springer Nature: Cham, Switzerland, 2017; pp. 13–35. [Google Scholar]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public. Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Kursun, I. Determination of flocculation and adsorption-desorption characteristics of Na-feldspar concentrate in the presence of different polymers. Physicochem. Probl. Miner. Process 2010, 44, 127–142. [Google Scholar]
- Vaca, M.; López, R.; Flores, J.; Terres, H.; Lizardi, A.; Rojas, N. Aplicación del Nopal (Opuntia ficus indica) como coagulante primario para aguas residuales. Rev. AIDIS Ing. Cienc. Ambient. 2014, 7, 210–216. [Google Scholar]
- Villabona, A.; Paz, I.; Martínez, J. Caracterización de Opuntia ficus-indica para su uso como coagulante natural. Rev. Colomb. Biotecnol. 2013, 15, 137–144. [Google Scholar]
- Nharingo, T.; Zivurawa, M.T.; Guyo, U. Exploring the use of cactus Opuntia ficus-indica in the biocoagulation-flocculation of Pb (II) ions from wastewaters. Int. J. Environ. Sci. Technol. 2015, 12, 3791–3802. [Google Scholar] [CrossRef]
- Yongabi, K. Biocoagulants for Water and Wastewater Purification: A Review. Int. Rev. Chem. Eng. 2010, 2, 444–458. [Google Scholar]
- Marichamy, M.K.; Kumaraguru, A.; Jonna, N. Particle size distribution modeling and kinetic study for coagulation treatment of tannery industry wastewater at response surface optimized condition. J. Cleaner Prod. 2021, 297, 126657. [Google Scholar] [CrossRef]
- Altaher, H.; ElQada, E.; Omar, W. Pretreatment of Wastewater Streams from Petroleum/Petrochemical Industries Using Coagulation. Adv. Chem. Eng. Sci. 2011, 4, 245–251. [Google Scholar] [CrossRef]
- Gandiwa, B.I.; Moyo, L.B.; Ncube, S.; Mamvura, T.A.; Mguni, L.L.; Hlabangana, N. Optimisation of using a blend of plant based natural and synthetic coagulants for water treatment: (Moringa Oleifera-Cactus Opuntia-alum blend). S. Afr. J. Chem. Eng. 2020, 34, 158–164. [Google Scholar] [CrossRef]
- Marobhe, N.; Renman, G.; Jackson, M. Investigation on the performance of local plant materials for coagulation of turbid river water. J. Inst. Eng. Tanzan. 2007, 8, 50–62. [Google Scholar]
- Osode, A.N.; Okoh, A.I. Impact of discharged wastewater final effluent on the physicochemical qualities of a receiving watershed in a suburban community of the Eastern Cape Province. CLEAN Soil Air Water 2009, 37, 938–944. [Google Scholar] [CrossRef]
- Carpinteyro-Urban, S.; Vaca, M.; Torres, L.G. Can vegetal biopolymers work as coagulant-flocculant aids in the treatment of high-load cosmetic industrial wastewaters? Water Air Soil Pollut. 2012, 223, 4925–4936. [Google Scholar] [CrossRef]
- Ayangunna, R.R.; Giwa, S.O.; Giwa, A. Coagulation-flocculation treatment of industrial wastewater using tamarind seed powder. Int. J. Chemtech Res. 2016, 9, 771–780. [Google Scholar]
- Ratnayaka, D.D.; Brandt, M.J.; Johnson, K.M. Storage, clarification and chemical treatment. Water Supply 2009, 7, 267–314. [Google Scholar]
- Yonge, D.A. Comparison of Aluminum and Iron-Based Coagulants for Treatment of Surface Water in Sarasota County, Florida. Available online: https://stars.library.ucf.edu/etd/2435 (accessed on 6 January 2024).
- Ang, T.H.; Kiatkittipong, K.; Kiatkittipong, W.; Chua, S.C.; Lim, J.W.; Show, P.L.; Bashir, M.J.K.; Ho, Y.C. Insight on Extraction and Characterisation of Biopolymers as the Green Coagulants for Microalgae Harvesting. Water 2020, 12, 1388. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).