Exploring Endogenous Processes in Water Supply Systems: Insights from Statistical Methods and δ18O Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Water Sampling and Analysis
2.3. Data Analysis
Hypothesis
2.4. Water Retention Time (Water Age)
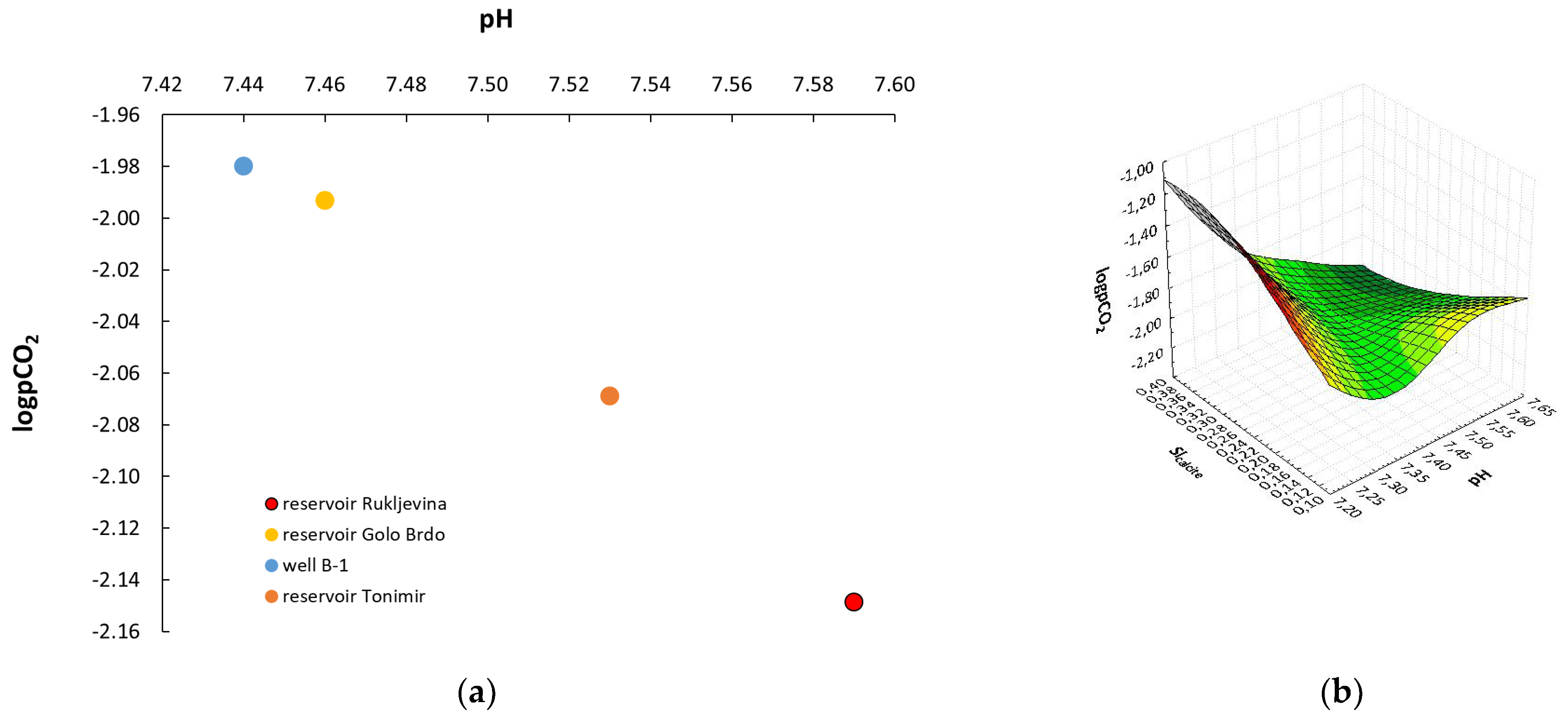
3. Results and Discussion
3.1. Hydrochemical Characteristics of Sampled Water
3.2. Water Retention Time in the Tonimir Reservoir
3.3. Findings of Statistical Data Processing
4. Conclusions
- The application of statistical data processing indicated the significance of certain changes within the WSS. However, the results of statistical processing should not be taken easily, because they can lead to wrong conclusions.
- Electrical conductivity decreases with increasing distance from the well due to the precipitation of calcium carbonate.
- In the example of the Tonimir reservoir, the water retention time is not long enough that it could deteriorate the water quality in the system.
- pH change is occurring. However, it will never reach MCL, which is pH 9.5, due to the dynamic status of WSSs—short water retention time.
- Stable isotopes have proven to be useful for calculating water retention time in the system, and they can be a useful complement to the management of WSSs.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast) (Text with EEA Relevance). Available online: http://data.europa.eu/eli/dir/2020/2184/oj (accessed on 7 November 2023).
- OG 30/2023, (Zakon o Vodi za Ljudsku Potrošnju Narodne Novine 30 2023). Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2023_03_30_509.html (accessed on 18 March 2024).
- Chu, S.; Xian, L.; Lai, C.; Yang, W.; Wang, J.; Long, M.; Ouyang, J.; Liao, D.; Zeng, S. Migration and risks of potentially toxic elements from sewage sludge applied to acid forest soil. J. For. Res. 2023, 34, 2011–2026. [Google Scholar] [CrossRef]
- Prest, E.I.; Hammes, F.; Van Loosdrecht, M.C.; Vrouwenvelder, J.S. Biological stability of drinking water: Controlling factors, methods, and challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.; Osborn, A.M.; Boxall, J.B. Biofilm structures (EPS and bacterial communities) in drinking water distribution systems are conditioned by hydraulics and influence discolouration. Sci. Total Environ. 2017, 593–594, 571–580. [Google Scholar] [CrossRef]
- Ainsworth, R. Save Piped Water: Managing Microbial Water Quality in Piped Distribution Systems; WHO: Geneva, Switzerland; IWA: London, UK, 2004. [Google Scholar]
- Liu, J.; Chen, H.; Huang, Q.; Lou, L.; Hu, B.; Endalkachew, S.D.; Mallikarjuna, N.; Shan, Y.; Zhou, X. Characteristics of pipe-scale in the pipes of an urban drinking water distribution system in eastern China. Water Sci. Technol. Water Supply 2015, 16, 715–726. [Google Scholar] [CrossRef]
- Boulay, N.; Edwards, M. Role of temperature, chlorine, and organic matter in copper corrosion by-product release in soft water. Water Res. 2001, 35, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Lopez-Roldan, R.; Cortina, J.-L. Presence of metals in drinking water distribution networks due to pipe material leaching: A review. Toxicol. Environ. Chem. 2013, 95, 870–889. [Google Scholar] [CrossRef]
- OG, 64/2023 Ordinance on (Pravilnik o Parametrima Sukladnosti, Metodama Analiza i Monitorintima Vode Namijenjene za Ljudsku Potrošnju, Narodne Novine 64 2023). Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2023_06_64_1057.html (accessed on 18 March 2024).
- OG, 88/2023 Ordinance on (Pravilnik o Sanitarno-Tehničkim i Higijenskim te Drugim Uvjetima Koje Moraju Ispunjavati Građevine za Vodoopskrbu i Poslovanje u Njima, Narodne Novine 88 2023). Available online: https://www.zakon.hr/cms.htm?id=57670 (accessed on 18 March 2024).
- Nemčić-Jurec, J.; Ruk, D.; Oreščanin, V.; Kovač, I.; Ujević Bošnjak, M.; Kinsela, A.S. Groundwater contamination in public water supply wells: Risk assessment, evaluation of trends and impact of rainfall on groundwater quality. Appl. Water Sci. 2022, 12, 172. [Google Scholar] [CrossRef]
- Ruk, D.; Nemčić-Jurec, J.; Oreščanin, V.; Kovač, I.; Horvat, I.; Ivaniš, B. Dimensional (temporal/spatial) and origin (natural/anthropogenic) characterisation of groundwater quality parameters in alluvial aquifer case study: Ivanščak catchment, Koprivnica, Croatia. Int. J. Hydrol. Sci. Technol. 2022, 13, 403–423. [Google Scholar] [CrossRef]
- Mohammed, A.; Samara, F.; Alzaatreh, A.; Knuteson, S.L. Statistical Analysis for Water Quality Assessment: A Case Study of Al Wasit Nature Reserve. Water 2022, 14, 3121. [Google Scholar] [CrossRef]
- Maiolo, M.; Pantusa, D. Multivariate Analysis of Water Quality Data for Drinking Water Supply Systems. Water 2021, 13, 1766. [Google Scholar] [CrossRef]
- de Wet, R.F.; West, A.G.; Harris, C. Seasonal variation in tap water δ2H and δ18O isotopes reveals two tap water worlds. Sci. Rep. 2020, 10, 13544. [Google Scholar] [CrossRef] [PubMed]
- Leslie, D.; Welch, K.; Lyons, W.B. Domestic Water Supply Dynamics Using Stable Isotopes δ18O, δD, and d-Excess. J. Water Resour. Prot. 2014, 6, 1517–1532. [Google Scholar] [CrossRef]
- Nagode, K.; Kanduč, T.; Bračič Železnik, B.; Jamnik, B.; Vreča, P. Multi-Isotope Characterization of Water in the Water Supply System of the City of Ljubljana, Slovenia. Water 2022, 14, 2064. [Google Scholar] [CrossRef]
- Sánchez-Murillo, R.; Esquivel-Hernández, G.; Birkel, C.; Ortega, L.; Sánchez-Guerrero, M.; Rojas-Jiménez, L.D.; Vargas-Víquez, J.; Castro-Chacón, L. From Mountains to Cities: A Novel Isotope Hydrological Assessment of a Tropical Water Distribution System. Isot. Environ. Health Stud. 2020, 56, 606–623. [Google Scholar] [CrossRef] [PubMed]
- Marković, T.; Karlović, I.; Perčec Tadić, M.; Larva, O. Application of Stable Water Isotopes to Improve Conceptual Model of Alluvial Aquifer in the Varaždin Area. Water 2020, 12, 379. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency: Effects of Water Age on Distribution System Water Quality. 2002. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/2007_05_18_disinfection_tcr_whitepaper_tcr_waterdistribution.pdf (accessed on 15 November 2023).
- Monteiro, L.; Algarvio, R.; Covas, D. Enhanced Water Age Performance Assessment in Distribution Networks. Water 2021, 13, 2574. [Google Scholar] [CrossRef]
- Smith, C.; Grayman, W.; Friedman, M. Evaluating Retention Time to Manage Distribution System Water Quality, Proposal for RFP2769; AwwaRF and KIWA: Rijswijk, The Netherlands, 2001. [Google Scholar]
- Waples, J.T.; Bordewyk, J.K.; Knesting, K.M.; Orlandini, K.A. Using naturally occurring radionuclides to determine drinking water age in a community water system. Environ. Sci. Technol. 2015, 49, 9850–9857. [Google Scholar] [CrossRef] [PubMed]
- Kralik, M. How to Estimate Mean Residence Times of Groundwater. Procedia Earth Planet. Sci. 2015, 13, 301–306. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. In U.S. Geological Survey Techniques and Methods, Book 6; U.S. Geological Survey: Road Asheville, NC, USA, 2013; Chapter A43; p. 497. [Google Scholar]
- Scribbr, Student’s t Table (Free Download)|Guide & Examples. Available online: https://www.scribbr.com/statistics/students-t-table/ (accessed on 22 January 2024).
- James, C.N.; Copeland, R.C.; Lytle, D.A. Relationships between oxidation-reduction potential, oxidant, and pH in drinking water. In Proceedings of the AWWA Water Quality Technology Conference, San Antonio, TX, USA, 14–18 November 2004. [Google Scholar]




| Year | 2021 | 2022 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | n | pH | Cl− (mg/L) | NO3− (mg/L) | EC (µS/cm) | n | pH | Cl− (mg/L) | NO3− (mg/L) | EC (µS/cm) | |
| B-1 | min | 6.68 | 17 | 13.55 | 584 | 7.19 | 19.45 | 16.6 | 570 | ||
| max | 8.06 | 29.3 | 30.7 | 681 | 7.92 | 29.3 | 22.0 | 663 | |||
| mean | 50 | 7.37 | 23.2 | 26.5 | 631 | 52 | 7.46 | 23.2 | 19.8 | 600 | |
| GB | min | 6.82 | 17.0 | 22.0 | 596 | 7.09 | 15.6 | 18.3 | 582 | ||
| max | 7.74 | 31.0 | 33.0 | 640 | 7.86 | 24.9 | 22.5 | 622 | |||
| mean | 51 | 7.42 | 23.2 | 27.9 | 628 | 50 | 7.52 | 22.2 | 20.5 | 603 | |
| R | min | 6.84 | 16.3 | 22.6 | 471 | 7.13 | 18.4 | 17.4 | 544 | ||
| max | 7.84 | 30.7 | 33.6 | 643 | 8.04 | 25.2 | 22.5 | 619 | |||
| mean | 51 | 7.43 | 22.9 | 27.8 | 625 | 49 | 7.65 | 22.4 | 20.5 | 601 | |
| T | min | 7.02 | 15.6 | 22.1 | 604 | 7.10 | 17.9 | 17.1 | 577 | ||
| max | 7.73 | 29.3 | 34.9 | 650 | 7.79 | 25.8 | 22.4 | 660 | |||
| mean | 52 | 7.41 | 23.1 | 27.4 | 629 | 50 | 7.47 | 22.6 | 20.2 | 603 | |
| Location | Date | EC (µS/cm) | T (oC) | pH | DO (mg/L) | ORP (mV) | HCO3− (mg/L) | Cl− (mg/L) | SO42− (mg/L) | NO3− (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Na+ (mg/L) | K+ (mg/L) | δ18O (‰) | δ2H (‰) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B-1 | 26 July 2022 | 661 | 14.6 | 7.25 | 0.9 | 184 | 342 | 22.2 | 31.5 | 18.3 | 96.5 | 17.8 | 15 | 4.5 | −9.85 | −69.2 |
| T | 26 July 2022 | 660 | 14.2 | 7.3 | 0.8 | 139 | 307 | 22.4 | 31.5 | 18.4 | 96.8 | 17.8 | 14.9 | 4.5 | −9.98 | −69.3 |
| B-1 | 30 November 2022 | 680 | 13 | 7.44 | 1.5 | 160 | 340 | 22.8 | 32.4 | 21.7 | 92.9 | 17.4 | 14.5 | 4.2 | −9.8 | −68.6 |
| T | 29 November 2022 | 674 | 12.6 | 7.52 | 4.4 | 162 | 348 | 22.3 | 32.4 | 21.4 | 93.9 | 17.8 | 14.5 | 4.3 | −9.81 | −69.3 |
| B-1 | 1 February 2023 | 670 | 12.7 | 7.46 | 2.1 | 130 | 375 | 21.4 | 31.8 | 19.7 | 100 | 18.7 | 15 | 4.3 | −9.73 | −68.7 |
| T | 2 February 2023 | 603 | 9.7 | 7.53 | 3.2 | 280 | 372 | 22.9 | 31.7 | 20.3 | 100.6 | 18.6 | 15.2 | 4.7 | −9.72 | −68.7 |
| R | 2 February 2023 | 578 | 5.7 | 7.59 | 8.9 | 240 | 371 | 21.8 | 31.8 | 20.5 | 100.2 | 18.6 | 15 | 4.3 | −9.72 | −68.7 |
| GB | 2 February 2023 | 586 | 6.7 | 7.44 | 7.6 | 273 | 371 | 22.4 | 31.4 | 20.1 | 100.2 | 18.6 | 15 | 4.3 | −9.76 | −68.8 |
| (a) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YEAR 2021 | pH | Cl− (mg/L) | NO3− (mg/L) | EC (µS/cm) | ||||||||||||
| Statistics | B-1 | T | GB | R | B-1 | T | GB | R | B-1 | T | GB | R | B-1 | T | GB | R |
| Mean () | 7.37 | 7.41 | 7.42 | 7.43 | 23.2 | 23.1 | 23.2 | 22.9 | 26.5 | 27.4 | 27.9 | 27.8 | 631 | 629 | 628 | 625 |
| Standard Deviation (σ) | 0.266 | 0.160 | 0.191 | 0.205 | 2.25 | 2.37 | 2.32 | 2.56 | 2.90 | 2.47 | 2.45 | 2.5 | 18.8 | 11.1 | 9.6 | 23.7 |
| Sample Variance (σ2) | 0.071 | 0.026 | 0.036 | 0.042 | 5.04 | 5.63 | 5.38 | 6.57 | 8.42 | 6.08 | 6.00 | 6.3 | 352.6 | 123.1 | 92.0 | 561.2 |
| s2 | 0.071 | 0.026 | 0.036 | 0.042 | 5.04 | 5.63 | 5.38 | 6.57 | 8.42 | 6.08 | 5.84 | 6.3 | 352.6 | 123.1 | 92.0 | 561.2 |
| Minimum | 6.68 | 7.02 | 6.82 | 6.84 | 17.00 | 15.6 | 17.0 | 16.3 | 13.6 | 22.1 | 22.0 | 22.6 | 584 | 604 | 596 | 471 |
| Maximum | 8.06 | 7.73 | 7.74 | 7.84 | 29.25 | 29.3 | 31.0 | 30.7 | 30.7 | 34.9 | 33.0 | 33.6 | 681 | 650 | 640 | 643 |
| n | 50 | 52 | 51 | 51 | 50 | 52 | 51 | 51 | 50 | 51 | 51 | 50 | 50 | 52 | 51 | 51 |
| sd2 | 0.00187 | 0.00211 | 0.00223 | 0.209 | 0.206 | 0.230 | 0.287 | 0.282 | 0.293 | 9.24 | 8.75 | 18.14 | ||||
| t | −0.868 | −0.992 | −1.260 | 0.125 | −0.033 | 0.533 | −1.640 | −2.674 | −2.350 | 0.612 | 0.887 | 1.362 | ||||
| tα | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | ||||
| Hypothesis | H0 | H0 | H0 | H0 | H0 | H0 | H0 | H1 | H1 | H0 | H0 | H0 | ||||
| (b) | ||||||||||||||||
| YEAR 2022 | pH | Cl− (mg/L) | NO3− (mg/L) | EC (µS/cm) | ||||||||||||
| Statistics | B-1 | T | GB | R | B-1 | T | GB | R | B-1 | T | GB | R | B-1 | T | GB | R |
| Mean () | 7.46 | 7.47 | 7.52 | 7.65 | 23.2 | 22.6 | 22.2 | 22.4 | 19.8 | 20.2 | 20.5 | 20.5 | 600 | 603 | 603 | 601 |
| Standard Deviation (σ) | 0.166 | 0.137 | 0.144 | 0.220 | 1.81 | 1.49 | 1.84 | 1.47 | 1.28 | 1.37 | 1.26 | 1.24 | 14.2 | 14.6 | 10.8 | 12.5 |
| Sample Variance (σ2) | 0.028 | 0.019 | 0.021 | 0.048 | 3.28 | 2.23 | 3.38 | 2.17 | 1.65 | 1.88 | 1.59 | 1.53 | 202.9 | 213.2 | 117.5 | 156.6 |
| s2 | 0.028 | 0.019 | 0.021 | 0.048 | 3.28 | 2.23 | 3.38 | 2.17 | 1.65 | 1.88 | 1.59 | 1.53 | 202.9 | 213.2 | 117.5 | 156.6 |
| Minimum | 7.19 | 7.1 | 7.09 | 7.13 | 19.5 | 17.9 | 15.6 | 18.4 | 16.6 | 17.1 | 18.3 | 17.4 | 570 | 577 | 582 | 544 |
| Maximum | 7.92 | 7.79 | 7.86 | 8.04 | 29.3 | 25.8 | 24.9 | 25.2 | 22.0 | 22.4 | 22.5 | 22.5 | 663 | 660 | 622 | 619 |
| n | 52 | 50 | 50 | 49 | 52 | 50 | 50 | 49 | 52 | 50 | 50 | 49 | 52 | 50 | 50 | 49 |
| sd2 | 0.00091 | 0.00095 | 0.00149 | 0.108 | 0.131 | 0.109 | 0.0691 | 0.0636 | 0.0630 | 8.16 | 6.32 | 7.15 | ||||
| t | −0.617 | −2.076 | −5.106 | 1.714 | 2.671 | 2.288 | −1.467 | −2.859 | −2.693 | −1.070 | −0.953 | −0.268 | ||||
| tα | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | 1.984 | ||||
| Hypothesis | H0 | H1 | H1 | H0 | H1 | H1 | H0 | H1 | H1 | H0 | H0 | H0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novotni-Horčička, N.; Marković, T.; Kovač, I.; Karlović, I. Exploring Endogenous Processes in Water Supply Systems: Insights from Statistical Methods and δ18O Analysis. Water 2024, 16, 1425. https://doi.org/10.3390/w16101425
Novotni-Horčička N, Marković T, Kovač I, Karlović I. Exploring Endogenous Processes in Water Supply Systems: Insights from Statistical Methods and δ18O Analysis. Water. 2024; 16(10):1425. https://doi.org/10.3390/w16101425
Chicago/Turabian StyleNovotni-Horčička, Nikolina, Tamara Marković, Ivan Kovač, and Igor Karlović. 2024. "Exploring Endogenous Processes in Water Supply Systems: Insights from Statistical Methods and δ18O Analysis" Water 16, no. 10: 1425. https://doi.org/10.3390/w16101425
APA StyleNovotni-Horčička, N., Marković, T., Kovač, I., & Karlović, I. (2024). Exploring Endogenous Processes in Water Supply Systems: Insights from Statistical Methods and δ18O Analysis. Water, 16(10), 1425. https://doi.org/10.3390/w16101425








