The Potential of AOP Pretreatment in the Biodegradation of PS and PVC Microplastics by Candida parapsilosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Preparation and Characterization of Microplastics
2.3. AOP Treatments
Analysis of Influential Parameters and Process Optimization
2.4. Biodegradation Experiments
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eyerer, P.; Gettwert, V. Properties of Plastics in Structural Components. In Polymers—Opportunities and Risks I; Eyerer, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 47–165. [Google Scholar] [CrossRef]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in environment: Global concern, challenges, and controlling measures. Int. J. Environ. Sci. Technol. 2023, 20, 4673–4694. [Google Scholar] [CrossRef] [PubMed]
- Amobonye, A.; Bhagwat, P.; Raveendran, S.; Singh, S.; Pillai, S. Environmental Impacts of Microplastics and Nanoplastics: A Current Overview. Front. Microbiol. 2021, 12, 768297. [Google Scholar] [CrossRef] [PubMed]
- The 17 Goals. Available online: https://sdgs.un.org/goals (accessed on 26 April 2024).
- Plastic Soup Foundation. Available online: https://www.plasticsoupfoundation.org/en/plastic-problem/sustainable-development/individual-sdgs/ (accessed on 26 April 2024).
- Alqahtani, S.; Alqahtani, S.; Saquib, Q.; Mohiddin, F. Toxicological impact of microplastics and nanoplastics on humans: Understanding the mechanistic aspect of the interaction. Front. Toxicol. 2023, 5, 1193386. [Google Scholar] [CrossRef]
- Zhao, H.; Hong, X.; Chai, J.; Wan, B.; Zhao, K.; Han, C.; Zhang, W.; Huan, H. Interaction between Microplastics and Pathogens in Subsurface System: What We Know So Far. Water 2024, 16, 499. [Google Scholar] [CrossRef]
- Maddela, N.R.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M. Additives of plastics: Entry into the environment and potential risks to human and ecological health. J. Environ. Manag. 2023, 348, 119364. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Mo, A.; Jiang, J.; Liang, Y.; Cao, X.; He, D. Removal of microplastics in water: Technology progress and green strategies. Green Anal. Chem. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Ceretta, M.B.; Nercessian, D.; Wolski, E.A. Current Trends on Role of Biological Treatment in Integrated Treatment Technologies of Textile Wastewater. Front. Microbiol. 2021, 12, 651025. [Google Scholar] [CrossRef]
- Okal, E.J.; Heng, G.; Magige, E.A.; Khan, S.; Wu, S.; Ge, Z.; Zhang, T.; Mortimer, P.E.; Xu, J. Insights into the mechanisms involved in the fungal degradation of plastics. Ecotoxicol. Environ. Saf. 2023, 262, 115202. [Google Scholar] [CrossRef]
- Miri, S.; Saini, R.; Davoodi, S.M.; Pulicharla, R.; Brar, S.K.; Magdouli, S. Biodegradation of microplastics: Better late than never. Chemosphere 2022, 286, 131670. [Google Scholar] [CrossRef]
- Lokesh, P.; Shobika, R.; Omer, S.; Reddy, M.; Saravanan, P.; Rajeshkannan, R.; Saravanan, V.; Venkatkumar, S. Bioremediation of plastics by the help of microbial tool: A way for control of plastic pollution. Sustain. Chem. Environ. 2023, 3, 100027. [Google Scholar] [CrossRef]
- Cai, Z.; Li, M.; Zhu, Z.; Wang, X.; Huang, Y.; Li, T.; Gong, H.; Yan, M. Biological Degradation of Plastics and Microplastics: A Recent Perspective on Associated Mechanisms and Influencing Factors. Microorganisms 2023, 11, 1661. [Google Scholar] [CrossRef]
- Ghosh, S.; Qureshi, A.; Purohit, H.J. Microbial Degradation of Plastics: Biofilms and Degradation Pathways. In Contaminants in Agriculture and Environment: Health Risks and Remediation; Kumar, V., Kumar, R., Singh, J., Kumar, P., Eds.; Agro Environ Media: Haridwar, India, 2019; pp. 184–199. [Google Scholar] [CrossRef]
- Hanpanich, O.; Wongkongkatep, P.; Pongtharangkul, T.; Wongkongkatep, J. Turning hydrophilic bacteria into biorenewable hydrophobic material with potential antimicrobial activity via interaction with chitosan. Bioresour. Technol. 2017, 230, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Bayry, J.; Aimanianda, V.; Guijarro, J.I.; Sunde, M.; Latgé, J.-P. Hydrophobins—Unique Fungal Proteins. PLoS Pathog. 2012, 8, e1002700. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Wang, J. Microplastic degradation methods and corresponding degradation mechanism: Research status and future perspectives. J. Hazard. Mater. 2021, 418, 126377. [Google Scholar] [CrossRef] [PubMed]
- Hamd, W.; Daher, E.A.; Tofa, T.S.; Dutta, J. Recent Advances in Photocatalytic Removal of Microplastics: Mechanisms, Kinetic Degradation, and Reactor Design. Front. Mar. Sci. 2022, 9, 885614. [Google Scholar] [CrossRef]
- Mousset, E.; Loh, W.H.; Lim, W.S.; Jarry, L.; Wang, Z.; Lefebvre, O. Cost comparison of advanced oxidation processes for wastewater treatment using accumulated oxygen-equivalent criteria. Water Res. 2021, 200, 117234. [Google Scholar] [CrossRef]
- Rodríguez, M. Fenton and UV-Vis Based Advanced Oxidation Processes in Wastewater Treatment: Degradation, Mineralization and Biodegradability Enhancement. Ph.D. Thesis, University of Barcelona, Faculty of Chemistry, Barcelona, Spain, 2003. [Google Scholar]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merényi, G.; Neta, P.; Ruscic, B.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1139–1150. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Zoschke, K.; Dietrich, N.; Börnick, H.; Worch, E. UV-based advanced oxidation processes for the treatment of odour compounds: Efficiency and by-product formation. Water Res. 2012, 46, 5365–5373. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F. Hydrogen peroxide photolysis with different UV light sources including a new UV-LED light source. New Front. Chem. 2014, 23, 99–110. [Google Scholar]
- Aye, T.T.; Low, T.Y.; Sze, S.K. Nanosecond laser-induced photochemical oxidation method for protein surface mapping with mass spectrometry. Anal Chem. 2005, 77, 5814–5822. [Google Scholar] [CrossRef] [PubMed]
- Urbina-Suarez, N.A.; López-Barrera, G.L.; García-Martínez, J.B.; Barajas-Solano, A.F.; Machuca-Martínez, F.; Zuorro, A. Enhanced UV/H2O2 System for the Oxidation of Organic Contaminants and Ammonia Transformation from Tannery Effluents. Processes 2023, 11, 3091. [Google Scholar] [CrossRef]
- Račytė, J.; Rimeika, M. Factors influencing efficiency of UV/H2O2 advanced oxidation process. In 2007: Proceedings from Kalmar ECO-TECH’07: Technologies for Waste and Wastewater Treatment, Energy from Wast, Remediation of Contaminated Sited, Emissions Related to Climate, Kalmar, Sweden, 26–28 November 2007; Kaczala, F., Hogland, W., Marques, M., Vinrot, E., Eds.; Linnaeus University: Växjö, Sweden, 2007; pp. 757–769. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, N.; Deng, Y.; Chu, W.; Rong, W.; Zhou, S. Factors affecting ultraviolet irradiation/hydrogen peroxide (UV/H2O2) degradation of mixed N-nitrosamines in water. J. Hazard. Mater. 2012, 43, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alvarez, B.; Villegas-Guzman, P.; Peñuela, G.A.; Torres-Palma, R.A. Degradation of a Toxic Mixture of the Pesticides Carbofuran and Iprodione by UV/H2O2: Evaluation of Parameters and Implications of the Degradation Pathways on the Synergistic Effects. Water Air Soil Pollut. 2016, 227, 215. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.; Rodríguez-Chueca, J. Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A Review Study on Sulfate-Radical-Based Advanced Oxidation Processes for Domestic/Industrial Wastewater Treatment: Degradation, Efficiency, and Mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef]
- Moreno-Andrés, J.; Rueda-Márquez, J.J.; Homola, T.; Vielma, J.; Moríñigo, M.Á.; Mikola, A.; Sillanpää, M.; Acevedo-Merino, A.; Nebot, E.; Levchuk, I. A comparison of photolytic, photochemical and photocatalytic processes for disinfection of recirculation aquaculture systems (RAS) streams. Water Res. 2020, 181, 115928. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Weniger, A.-K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Yan, X.; Yue, Y.; Li, W.; Luo, W.; Wang, Y.; Sun, J.; Li, Y.; Liu, M.; Fan, M. H2O2 concentration influenced the photoaging mechanism and kinetics of polystyrene microplastic under UV irradiation: Direct and indirect photolysis. J. Clean. Prod. 2022, 380, 135046. [Google Scholar] [CrossRef]
- Hankett, J.M.; Welle, A.; Lahann, J.; Chen, Z. Evaluating UV/H2O2 Exposure as a DEHP Degradation Treatment for Plasticized PVC. J. Appl. Polym. Sci. 2014, 131, 40649. [Google Scholar] [CrossRef]
- Hankett, J.M.; Collin, W.R.; Chen, Z. Molecular Structural Changes of Plasticized PVC after UV Light Exposure. J. Phys. Chem. B 2013, 117, 16336–16344. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, H.; Wang, H.; Wang, C. Separation of hazardous polyvinyl chloride from waste plastics by flotation assisted with surface modification of ammonium persulfate: Process and mechanism. J. Hazard. Mater. 2020, 389, 121918. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A Comprehensive Insight into Fungal Enzymes: Structure, Classification, and Their Role in Mankind’s Challenges. J. Fungi 2021, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Gangola, S.; Joshi, S.; Kumar, S.; Pandey, S.C. Chapter 10—Comparative Analysis of Fungal and Bacterial Enzymes in Biodegradation of Xenobiotic Compounds. In Smart Bioremediation Technologies; Bhatt, P., Ed.; Academic Press: Chennai, India, 2019; pp. 169–189. [Google Scholar] [CrossRef]
- Harding, M.W.; Marques, L.L.R.; Howard, R.J.; Olson, M.E. Can filamentous fungi form biofilms? Trends Microbiol. 2009, 17, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Cvetnić, M.; Ocelić Bulatović, V.; Mandić, V.; Bafti, A.; Ukić, Š.; Kučić Grgić, D.; Bolanča, T. Bacteria and Yeasts Isolated from the Environment in Biodegradation of PS and PVC Microplastics: Screening and Treatment Optimization. Environments 2023, 10, 207. [Google Scholar] [CrossRef]
- Hock, O.G.; Lum, H.W.; De Qin, D.; Kee, W.K.; Shing, W.L. The growth and laccase activity of edible mushrooms involved in plastics degradation. Curr. Top. Toxicol. 2019, 15, 57–62. [Google Scholar]
- Atiq, N. Biodegradability of Synthetic Plastics Polystyrene and Styrofoam by Fungal Isolates. Ph.D. Thesis, Quaid-i-Azam University, Islamabad, Pakistan, 2011. [Google Scholar]
- Chaudhary, A.K.; Vijayakumar, R.P. Studies on biological degradation of polystyrene by pure fungal cultures. Environ. Dev. Sustain. 2020, 22, 4495–4508. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Chaitanya, K.; Vijayakumar, R.P. Synergistic effect of UV and chemical treatment on biological degradation of Polystyrene by Cephalosporium strain NCIM 1251. Arch. Microbiol. 2021, 203, 2183–2191. [Google Scholar] [CrossRef]
- Motta, O.; Proto, A.; De Carlo, F.; De Caro, F.; Santoro, E.; Brunetti, L.; Capunzo, M. Utilization of chemically oxidized polystyrene as co-substrate by filamentous fungi. Int. J. Hyg. Environ. Health 2009, 212, 61–66. [Google Scholar] [CrossRef]
- Galgali, P. Fungal degradation of carbohydrate-linked polystyrenes. Carbohydr. Polym. 2004, 55, 393–399. [Google Scholar] [CrossRef]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef]
- Ekanayaka, A.H.; Tibpromma, S.; Dai, D.; Xu, R.; Suwannarach, N.; Stephenson, S.L.; Dao, C.; Karunarathna, S.C. A Review of the Fungi That Degrade Plastic. J. Fungi 2022, 8, 772. [Google Scholar] [CrossRef]
- Sakhalkar, S.; Mishra, R. Screening and Identification of Soil Fungi with Potential of Plastic Degrading Ability. Indian J. Appl. Res. 2013, 3, 62–64. [Google Scholar] [CrossRef]
- Malachová, K.; Novotný, Č.; Adamus, G.; Lotti, N.; Rybková, Z.; Soccio, M.; Šlosarčíková, P.; Verney, V.; Fava, F. Ability of Trichoderma hamatum Isolated from Plastics-Polluted Environments to Attack Petroleum-Based, Synthetic Polymer Films. Processes 2020, 8, 467. [Google Scholar] [CrossRef]
- Sumathi, T.; Viswanath, B.; Sri Lakshmi, A.; SaiGopal, D.V.R. Production of Laccase by Cochliobolus sp. Isolated from Plastic Dumped Soils and Their Ability to Degrade Low Molecular Weight PVC. Biochem. Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kirbaş, Z.; Keskin, N.; Güner, A. Biodegradation of polyvinylchloride (PVC) by white rot fungi. Bull. Environ. Contam. Toxicol. 1999, 63, 335–342. [Google Scholar] [CrossRef]
- Ali, M.I.; Ahmed, S.; Robson, G.; Javed, I.; Ali, N.; Atiq, N.; Hameed, A. Isolation and molecular characterization of polyvinyl chloride (PVC) plastic degrading fungal isolates. J. Basic Microbiol. 2013, 54, 18–27. [Google Scholar] [CrossRef]
- Webb, J.S.; Nixon, M.; Eastwood, I.M.; Greenhalgh, M.; Robson, G.D.; Handley, P.S. Fungal Colonization and Biodeterioration of Plasticized Polyvinyl Chloride. Appl. Environ. Microbiol. 2000, 66, 3194–3200. [Google Scholar] [CrossRef]
- Gómez-Méndez, L.D.; Moreno-Bayona, D.A.; Poutou-Piñales, R.A.; Salcedo-Reyes, J.C.; Pedroza-Rodríguez, A.M.; Vargas, A.; Bogoya, J.M. Biodeterioration of plasma pretreated LDPE sheets by Pleurotus ostreatus. PLoS ONE 2018, 13, e0203786. [Google Scholar] [CrossRef]
- Scally, L.; Gulan, M.; Weigang, L.; Cullen, P.J.; Milosavljevic, V. Significance of a Non-Thermal Plasma Treatment on LDPE Biodegradation with Pseudomonas aeruginosa. Materials 2018, 11, 1925. [Google Scholar] [CrossRef]
- Jeyakumar, D.; Chirsteen, J.; Mukesh, D. Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour. Technol. 2013, 148, 78–85. [Google Scholar] [CrossRef]
- Tribedi, P.; Dey, S. Pre-oxidation of low-density polyethylene (LDPE) by ultraviolet light (UV) promotes enhanced degradation of LDPE in soil. Environ. Monit. Assess. 2017, 189, 624. [Google Scholar] [CrossRef]
- Fang, J.; Xuan, Y.; Li, Q. Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci. China Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Amar, Z.H.; Chabira, S.F.; Sebaa, M.; Ahmed, B. Structural changes undergone during thermal aging and/or processing of unstabilized, dry-blend and rigid PVC, investigated by FTIR-ATR and curve fitting. Ann. Chim. Sci. Mater. 2019, 43, 59–68. [Google Scholar] [CrossRef]
- Gong, Y.; Ding, P.; Xu, M.-J.; Zhang, C.-M.; Xing, K.; Qin, S. Biodegradation of phenol by a halotolerant versatile yeast Candida tropicalis SDP-1 in wastewater and soil under high salinity conditions. J. Environ. Manag. 2021, 289, 112525. [Google Scholar] [CrossRef] [PubMed]
- Black, J.G. Microbiology: Principles and Explorations, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 975. [Google Scholar]
- Liu, X. Organic Chemistry I; Kwantlen Polytechnic University: Surrey, BC, Canada, 2021; p. 198. [Google Scholar]
- Herrera-Ordonez, J. The role of sulfate radicals and pH in the decomposition of persulfate in aqueous medium: A step towards prediction. Chem. Eng. J. Adv. 2022, 11, 100331. [Google Scholar] [CrossRef]
- Honarmandrad, Z.; Sun, X.; Wang, Z.; Naushad, M.; Boczkaj, G. Activated persulfate and peroxymonosulfate based advanced oxidation processes (AOPs) for antibiotics degradation—A review. Water Resour. Ind. 2023, 29, 100194. [Google Scholar] [CrossRef]
- Mahbobi, M.; Tiemann, T.K. Chapter 8. Regression Basics. In Introductory Business Statistics with Interactive Spreadsheets—1st Canadian Edition, 1st ed.; Mahbobi, M., Tiemann, T.K., Eds.; Pressbooks: Montreal, QC, Canada; Available online: https://opentextbc.ca/introductorybusinessstatistics/chapter/regression-basics-2/ (accessed on 28 February 2024).
- Frost, J. Statistics By Jim. T-Distribution Table of Critical Values. Available online: https://statisticsbyjim.com/hypothesis-testing/t-distribution-table/ (accessed on 28 February 2024).
- Trofa, D.; Gácser, A.; Nosanchuk, J.D. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 2008, 21, 606–625. [Google Scholar] [CrossRef]
- Branco, J.; Miranda, I.M.; Rodrigues, A.G. Candida parapsilosis Virulence and Antifungal Resistance Mechanisms: A Comprehensive Review of Key Determinants. J. Fungi 2023, 9, 80. [Google Scholar] [CrossRef]
- Tóth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vágvölgyi, C.; Gácser, A. Candida parapsilosis: From Genes to the Bedside. Clin. Microbiol. Rev. 2019, 32, e00111-18. [Google Scholar] [CrossRef]
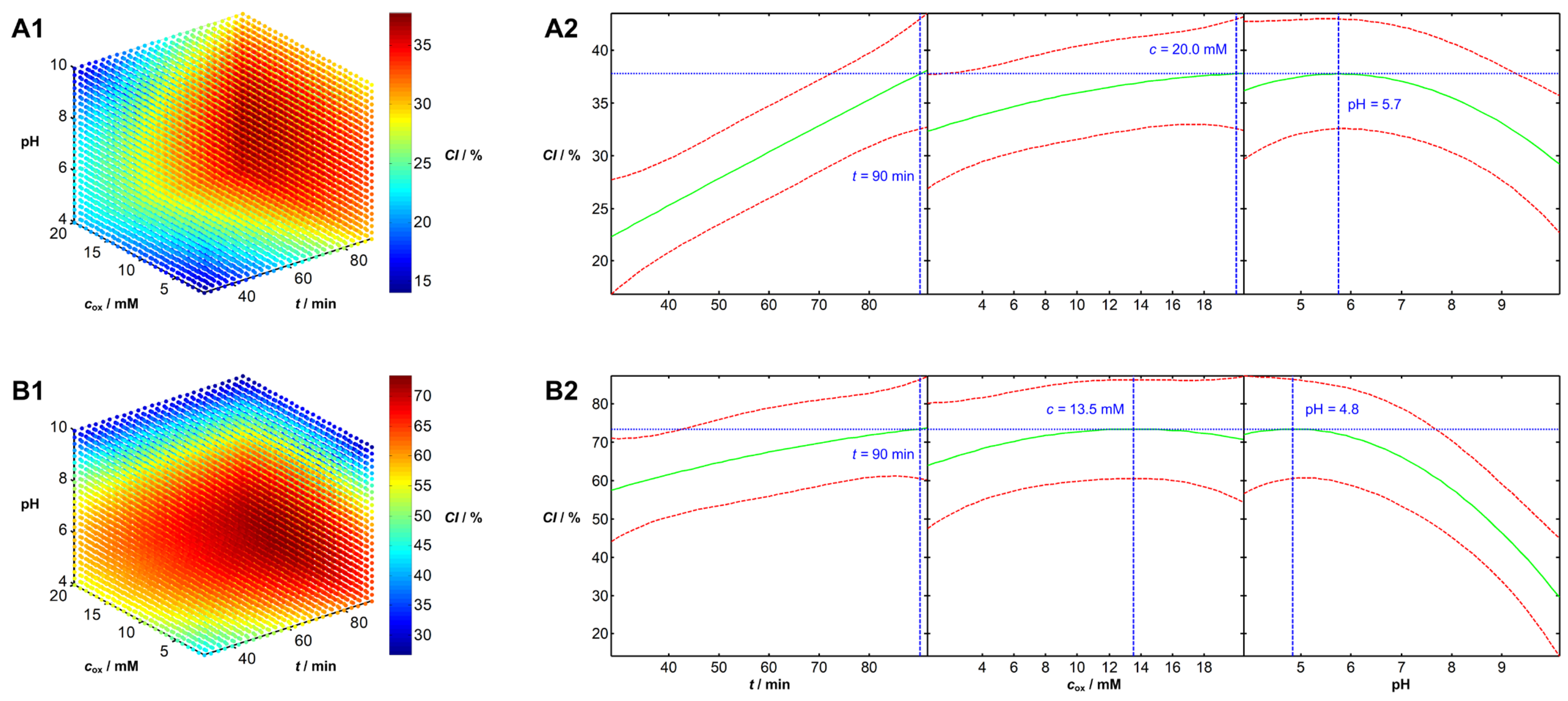
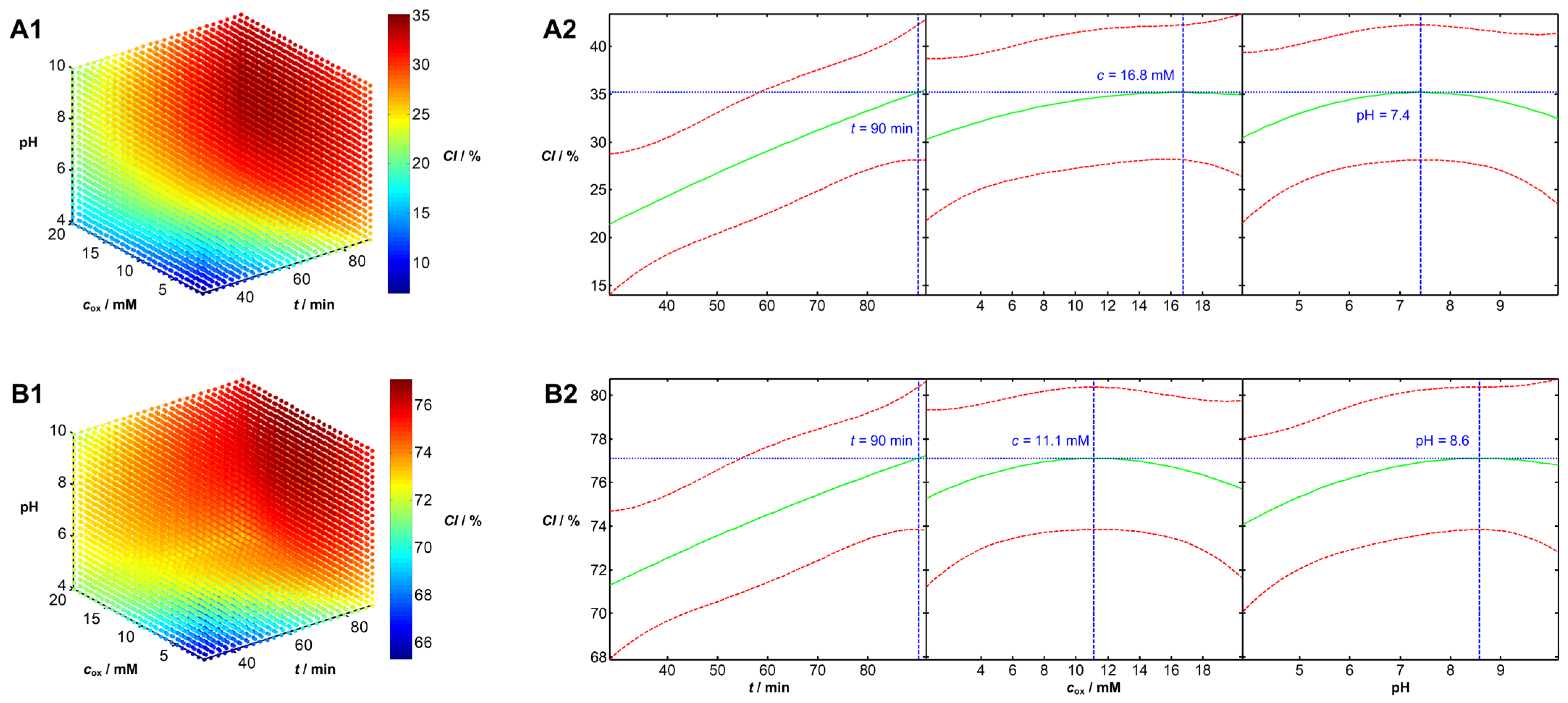
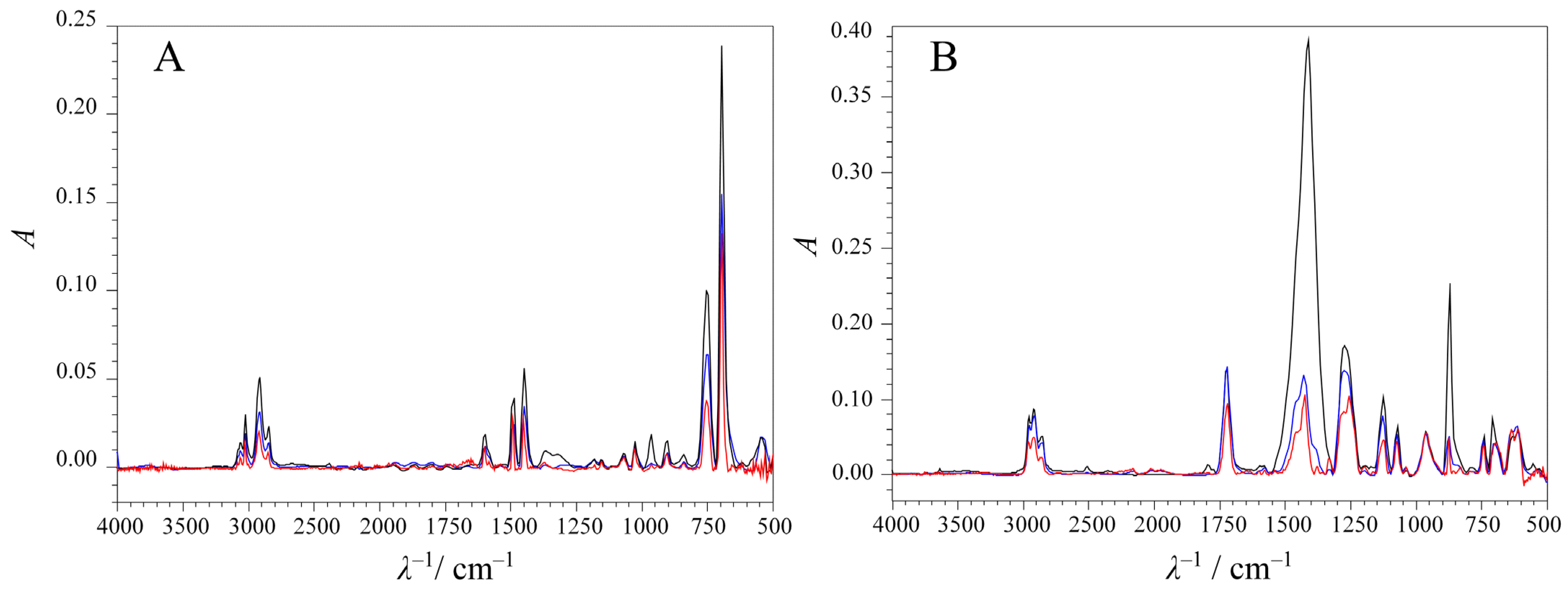
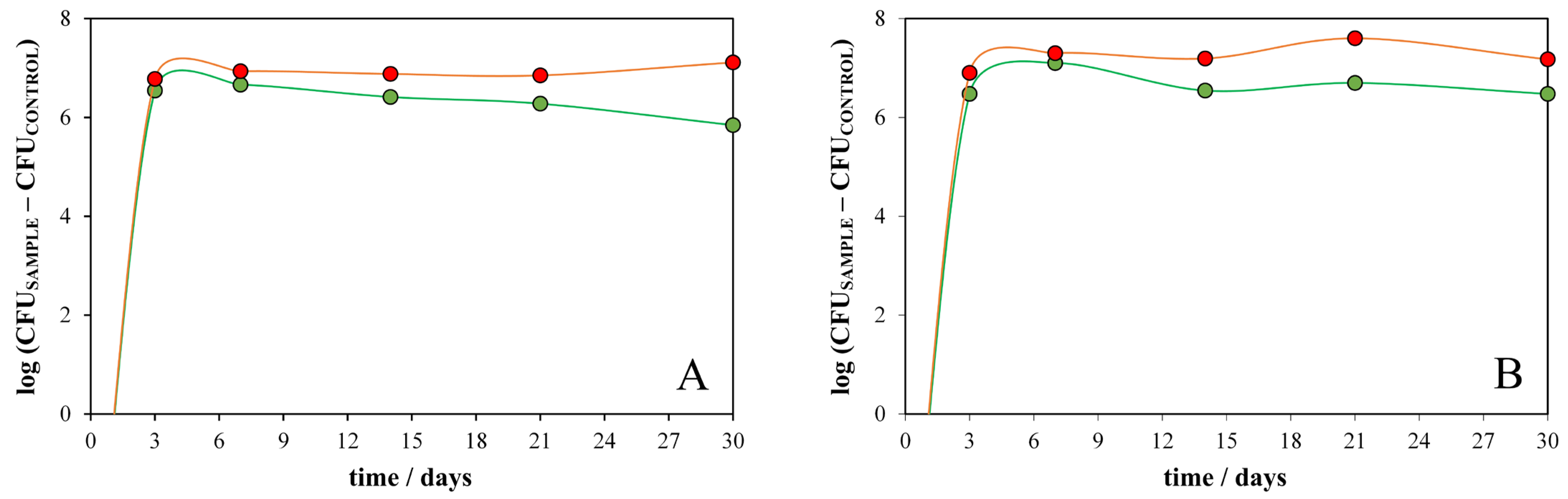
| No. | t/min | pH | c/mM | No. | t/min | pH | c/mM | No. | t/min | pH | c/mM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 4.0 | 1.0 | 10 | 60 | 4.0 | 1.0 | 19 | 90 | 4.0 | 1.0 |
| 2 | 30 | 4.0 | 10.5 | 11 | 60 | 4.0 | 10.5 | 20 | 90 | 4.0 | 10.5 |
| 3 | 30 | 4.0 | 20.0 | 12 | 60 | 4.0 | 20.0 | 21 | 90 | 4.0 | 20.0 |
| 4 | 30 | 7.0 | 1.0 | 13 | 60 | 7.0 | 1.0 | 22 | 90 | 7.0 | 1.0 |
| 5 | 30 | 7.0 | 10.5 | 14 | 60 | 7.0 | 10.5 | 23 | 90 | 7.0 | 10.5 |
| 6 | 30 | 7.0 | 20.0 | 15 | 60 | 7.0 | 20.0 | 24 | 90 | 7.0 | 20.0 |
| 7 | 30 | 10.0 | 1.0 | 16 | 60 | 10.0 | 1.0 | 25 | 90 | 10.0 | 1.0 |
| 8 | 30 | 10.0 | 10.5 | 17 | 60 | 10.0 | 10.5 | 26 | 90 | 10.0 | 10.5 |
| 9 | 30 | 10.0 | 20.0 | 18 | 60 | 10.0 | 20.0 | 27 | 90 | 10.0 | 20.0 |
| PS | PVC | |||||||
|---|---|---|---|---|---|---|---|---|
| AOP | 694.4 cm−1 | 748.4 cm−1 | 1450.5 cm−1 | 871.8 cm−1 | 1280.8 cm−1 | 1411.9 cm−1 | ||
| UV-C/H2O2 | 694.4 cm−1 | 1.0000 | 871.8 cm−1 | 1.0000 | ||||
| 748.4 cm−1 | 0.9201 | 1.0000 | 1280.8 cm−1 | −0.0794 | 1.0000 | |||
| 1405.5 cm−1 | 0.8352 | 0.9410 | 1.0000 | 1411.9 cm−1 | 0.9860 | −0.0723 | 1.0000 | |
| UV-C/S2O82− | 694.4 cm−1 | 1.0000 | 871.8 cm−1 | 1.0000 | ||||
| 748.43 cm−1 | 0.9672 | 1.0000 | 1280.8 cm−1 | 0.4849 | 1.0000 | |||
| 1405.5 cm−1 | 0.9252 | 0.9540 | 1.0000 | 1411.9 cm−1 | 0.9372 | 0.5454 | 1.0000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bule Možar, K.; Miloloža, M.; Martinjak, V.; Ujević Bošnjak, M.; Markić, M.; Bolanča, T.; Cvetnić, M.; Kučić Grgić, D.; Ukić, Š. The Potential of AOP Pretreatment in the Biodegradation of PS and PVC Microplastics by Candida parapsilosis. Water 2024, 16, 1389. https://doi.org/10.3390/w16101389
Bule Možar K, Miloloža M, Martinjak V, Ujević Bošnjak M, Markić M, Bolanča T, Cvetnić M, Kučić Grgić D, Ukić Š. The Potential of AOP Pretreatment in the Biodegradation of PS and PVC Microplastics by Candida parapsilosis. Water. 2024; 16(10):1389. https://doi.org/10.3390/w16101389
Chicago/Turabian StyleBule Možar, Kristina, Martina Miloloža, Viktorija Martinjak, Magdalena Ujević Bošnjak, Marinko Markić, Tomislav Bolanča, Matija Cvetnić, Dajana Kučić Grgić, and Šime Ukić. 2024. "The Potential of AOP Pretreatment in the Biodegradation of PS and PVC Microplastics by Candida parapsilosis" Water 16, no. 10: 1389. https://doi.org/10.3390/w16101389
APA StyleBule Možar, K., Miloloža, M., Martinjak, V., Ujević Bošnjak, M., Markić, M., Bolanča, T., Cvetnić, M., Kučić Grgić, D., & Ukić, Š. (2024). The Potential of AOP Pretreatment in the Biodegradation of PS and PVC Microplastics by Candida parapsilosis. Water, 16(10), 1389. https://doi.org/10.3390/w16101389










