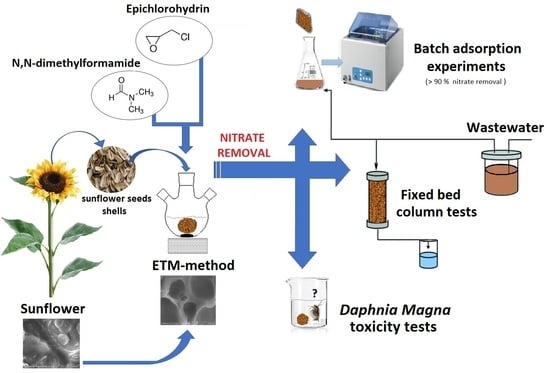
Utilization of Modified Sunflower Seed as Novel Adsorbent for Nitrates Removal from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
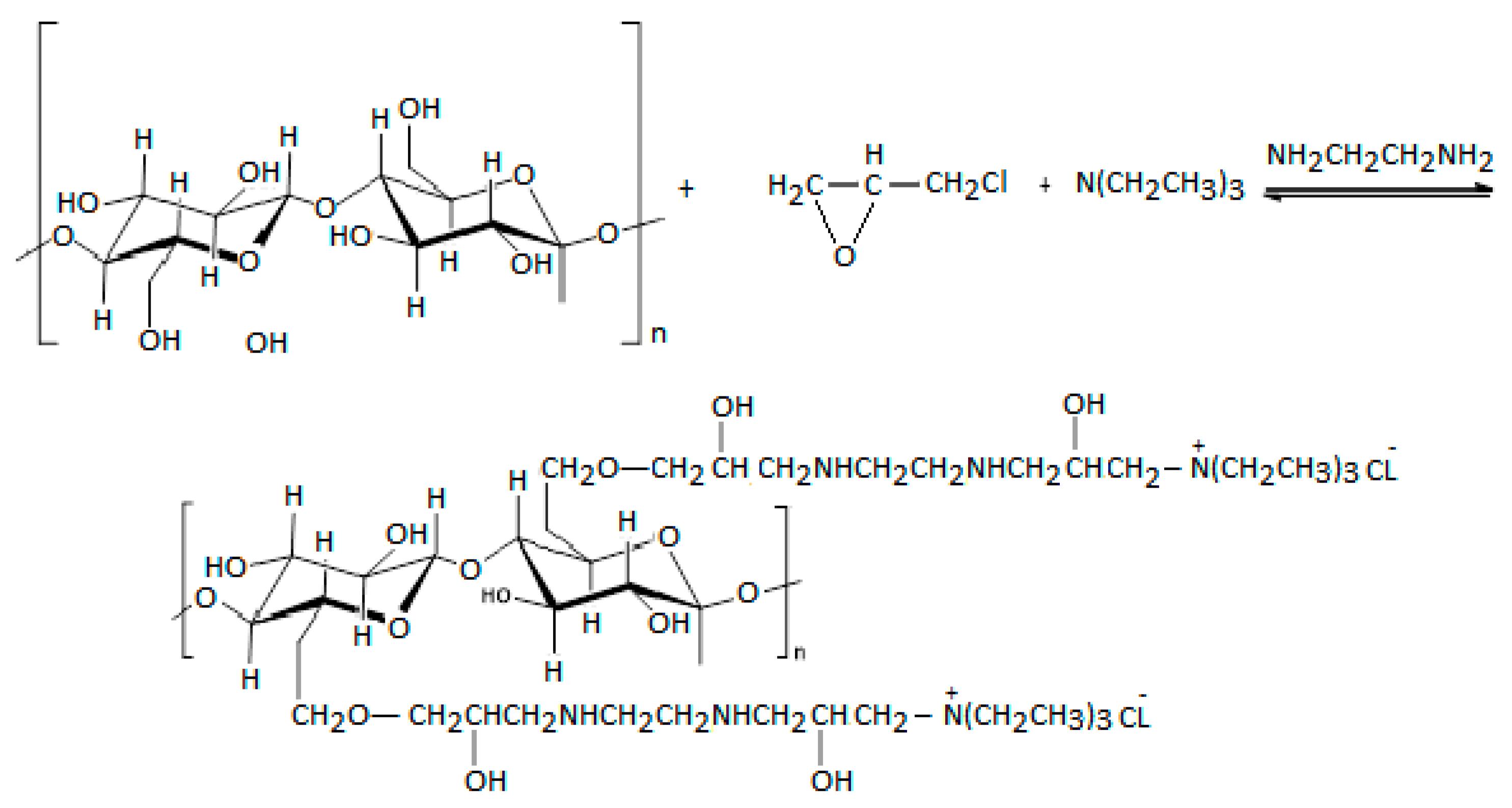
2.2. Adsorbent Preparation
2.3. Structural Characterization of Raw and Modified Sunflower Seed Shells
2.4. Batch Adsorption Experiments
2.5. Fixed-Bed Column Regeneration Test
2.6. Adsorption Equilibrium Modeling
2.6.1. Langmuir Isotherm Model
2.6.2. Freundlich Isotherm Model
2.7. Adsorption Kinetic Modeling and Mechanism
2.8. Determination of Acute Toxicity Using Daphnia Magna
3. Results and Discussion
3.1. Structural Characterization of Raw and Modified Sunflower Seed Shells
3.2. Batch Adsorption Experiments
3.2.1. Effect of MSS Concentration on Nitrate Removal
3.2.2. Effect of Initial N-NO3 Concentrations on Nitrate Removal by MSS
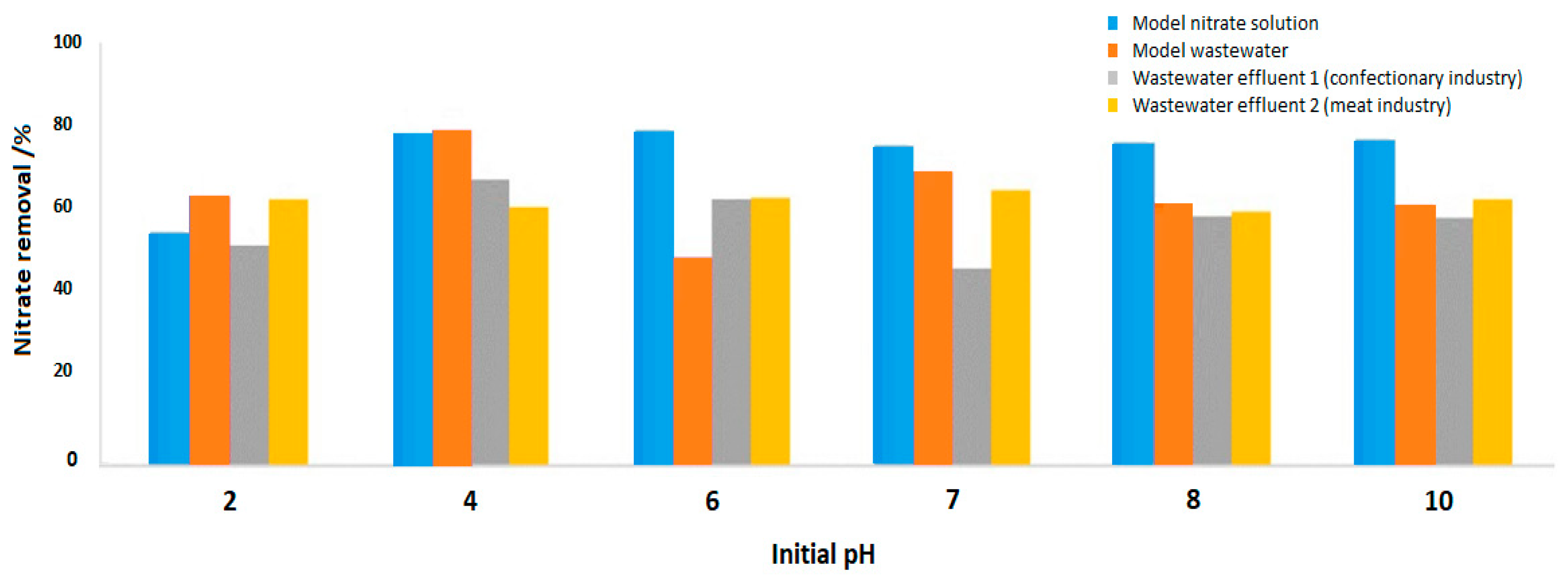
3.2.3. Effect of pH on Nitrate Removal by MSS
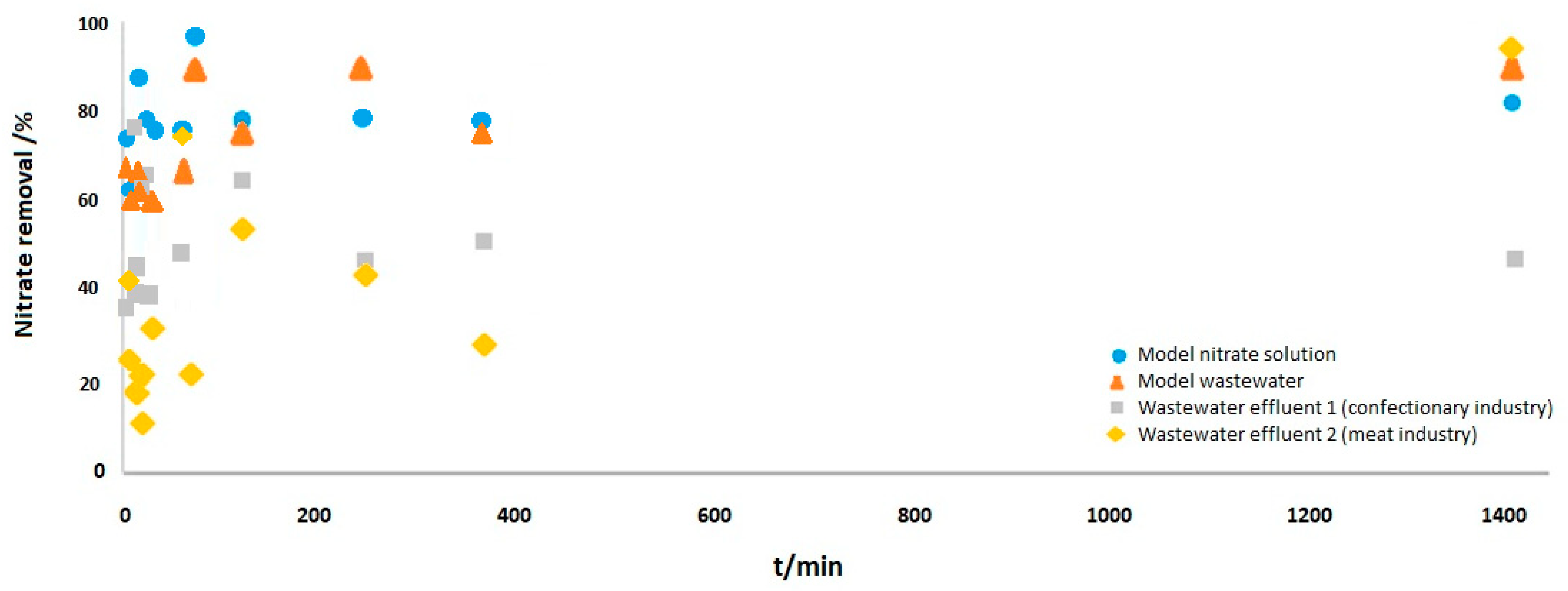
3.2.4. Effect of Contact Time on Nitrate Removal by MSS
3.3. Adsorption Equilibrium Modeling
3.4. Adsorption Kinetic Modeling and Mechanism
3.5. Breakthrough and Desorption Studied
3.6. Determination of Acute Toxicity of MSS Samples by Daphnia Magna Toxicity Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Olgun, A.; Atar, N.; Wang, S. Batch and column studies of phosphate and nitrate adsorption on waste solids containing boron impurity. Chem. Eng. J. 2013, 222, 108–119. [Google Scholar] [CrossRef]
- WHO. Nitrate and Nitrite in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality. Available online: https://www.who.int/ (accessed on 17 October 2021).
- Xu, X.; Gao, B.Y.; Yue, Q.Y.; Zhong, Q.Q.; Zhan, X. Preparation, characterization of wheat residue based anion exchangers and its utilization for the phosphate removal from aqueous solution. Carbohydr. Polym. 2010, 82, 1212–1218. [Google Scholar] [CrossRef]
- Mohamad Nor, N.; Lau, L.C.; Lee, K.T.; Mohamed, A.R. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—A review. J. Environ. Chem. Eng. 2013, 1, 658–666. [Google Scholar] [CrossRef]
- Bhatnagar, A. Conventional and Non-Conventional Adsorbents for Removal of Pollutants from Water—A review Removal of Anionic Dyes from Water Using Citrus Limonum (Lemon) Peel: Equilibrium Studies and Kinetic Modeling View project. Available online: https://www.researchgate.net/publication/267822355 (accessed on 17 October 2021).
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Al-Maswari, B.M.; Abdullah Alqadami, A.; Alshehri, S.M. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem. Eng. J. 2017, 330, 1351–1360. [Google Scholar] [CrossRef]
- Stjepanović, M.; Velić, N.; Habuda- Stanić, M. Modified Hazelnut Shells as a Novel Adsorbent for the Removal of Nitrate from Wastewater. Water 2022, 14, 816. [Google Scholar] [CrossRef]
- Yao, J.; Wang, Z.; Liu, M.; Bai, B.; Zhang, C. Nitrate-Nitrogen Adsorption Characteristics and Mechanisms of Various Garden Waste Biochars. Materials 2023, 16, 5726. [Google Scholar] [CrossRef]
- Stjepanović, M.; Velić, N.; Lončarić, A.; Gašo-Sokač, D.; Bušić, V.; Habuda-Stanić, M. Adsorptive removal of nitrate from wastewater using modified lignocellulosic waste material. J. Mol. Liq. 2019, 285, 535–544. [Google Scholar] [CrossRef]
- Kuang, P.; Cui, Y.; Zhang, Z.; Ma, K.; Zhang, W.; Zhao, K.; Zhang, X. Increasing Surface Functionalities of FeCl3-Modified Reed Waste Biochar for Enhanced Nitrate Adsorption Property. Processes 2023, 11, 1740. [Google Scholar] [CrossRef]
- Angosto, J.M.; Obón, J.M.; Roca, M.J.; Alacid, M.; Fernández-López, J.A. A Promising, Highly Effective Nitrate Sorbent Derived from Solid Olive Mill Residues. Agronomy 2023, 13, 1325. [Google Scholar] [CrossRef]
- Stjepanović, M.; Velić, N.; Habuda-Stanić, M. Modified Grape Seeds: A Promising Alternative for Nitrate Removal from Water. Materials 2021, 14, 4791. [Google Scholar] [CrossRef] [PubMed]
- Villabona-Ortíz, A.; Ortega-Toro, R.; Tejada-Tovar, C. Selective and Competitive Adsorption of Anions in Solution on Porous Adsorbent from Zea mays Steams: Kinetic and Equilibrium Study. Water 2022, 14, 2906. [Google Scholar] [CrossRef]
- Geng, N.; Ren, B.; Xu, B.; Li, D.; Xia, Y.; Xu, C.; Hua, E. Bamboo Chopstick Biochar Electrodes and Enhanced Nitrate Removal from Groundwater. Processes 2022, 10, 1740. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, X.H.; Nguyen, T.P.; Tran, T.H.; Cuong, D.X.; Van, N.T.; Le, H.N.; Van, H.T.; Nguyen, L.H. Lychee peels-derived biochar-supported CaFe2O4 magnetic nanocomposite as an excellent adsorbent for effective removal of nitrate and phosphate from wastewater. J. Environ. Chem. Eng. 2023, 11, 110991. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Zhang, P.; Shen, J.; Wang, S.; Li, Y. Development of iron-based biochar for enhancing nitrate adsorption: Effects of specific surface area, electrostatic force, and functional groups. Sci. Total Environ. 2023, 856, 159037. [Google Scholar] [CrossRef]
- Chanda, R.; Islam, S.; Biswas, B.K. N and P removal from wastewater using rice husk ash-derived silica-based Fe-ZSM-5 zeolite. Clean. Eng. Technol. 2023, 16, 100675. [Google Scholar] [CrossRef]
- Sousa, F.W.; Oliveira, A.G.; Ribeiro, J.P.; Rosa, M.F.; Keukeleire, D.; Nascimento, R.F. Green coconut shells applied as adsorbent for removal of toxic metal ions using fixed-bed column technology. J. Environ. Manag. 2010, 91, 1634–1640. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W. Nitrate Reduction by Zerovalent Iron: Effects of Formate, Oxalate, Citrate, Chloride, Sulfate, Borate, and Phosphate. Environ. Sci. Technol. 2004, 38, 2715–2720. [Google Scholar] [CrossRef]
- Orlando, U.S.; Baes, A.U.; Nishijima, W.; Okada, M. A new procedure to produce lignocellulosic anion exchangers from agricultural waste materials. Bioresour. Technol. 2002, 83, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Heath, E.; Kompare, B. Removal of pharmaceutical residues in a pilot wastewater treatment plant. Anal. Bioanal. Chem. 2007, 387, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Ighalo, J.O.; Omoarukhe, F.O.; Ojukwu, V.E.; Iwuozor, K.O.; Igwegbe, C.A. Cost of adsorbent preparation and usage in wastewater treatment: A review. Clean. Chem. Eng. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Saleh, M.E.; El-Refaey, A.A.; Mahmoud, A.H. Effectiveness of sunflower seed husk biochar for removing copper ions from wastewater: A comparative study. Soil Water Res. 2016, 11, 53–63. [Google Scholar] [CrossRef]
- ISO 6060: 1989; Water Quality—Determination of the Chemical Oxygen Demand. International Organization for Standardization: Geneva, Switzerland, 1989.
- ISO 5663: 2001; Water Quality—Determination of Kjeldahl Nitrogen—Method after Mineralization with Selenium. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 7890-3: 1988; Water Quality—Determination of Nitrate—Part 3: Spectrometric Method Using Sulfosalicylic Acid. International Organization for Standardization: Geneva, Switzerland, 1988.
- HRN EN 26777:1998; Water quality—Determination of nitrite—Molecular absorption spectrometric method (ISO 6777:1984; EN 26777:1993). Croatian Institute for Standards: Zagreb, Croatia, 1998.
- ISO 6878:2004; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 10523:1998; Water Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 1998.
- Keränen, A.; Leiviskä, T.; Gao, B.Y.; Hormi, O.; Tanskanen, J. Preparation of novel anion exchangers from pine sawdust and bark, spruce bark, birch bark and peat for the removal of nitrate. Chem. Eng. Sci. 2013, 98, 59–68. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die Adsorption in Losungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Stjepanović, M.; Velić, N.; Galić, A.; Kosović, I.; Jakovljević, T.; Habuda-Stanić, M. From waste to biosorbent: Removal of congo red from water by waste wood biomass. Water 2021, 13, 279. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Zhen, Y.; Ning, Z.; Shaopeng, Z.; Yayi, D.; Xuntong, Z.; Jiachun, S.; Weiben, Y.; Yuping, W.; Jianqiang, C. A pH- and Temperature-Responsive Magnetic Composite Adsorbent for Targeted Removal of Nonylphenol. ACS Appl. Mater. Interfaces 2015, 7, 24446–24457. [Google Scholar] [CrossRef]
- Xu, X.; Gao, B.; Yue, Q.; Li, Q.; Wang, Y. Nitrate adsorption by multiple biomaterial based resins: Application of pilot-scale and lab-scale products. Chem. Eng. J. 2013, 234, 397–405. [Google Scholar] [CrossRef]
- ISO 6341:2012 (E); Water Quality—Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. International Organization for Standardization: Geneva, Switzerland, 2012.
- Katal, R.; Baei, M.S.; Rahmati, H.T.; Esfandian, H. Kinetic, isotherm and thermodynamic study of nitrate adsorption from aqueous solution using modified rice husk. J. Ind. Eng. Chem. 2012, 18, 295–302. [Google Scholar] [CrossRef]
- El Ouardi, M.; Qourzal, S.; Alahiane, S.; Assabbane, A.; Douch, J. Effective Removal of Nitrates Ions from Aqueous Solution Using New Clay as Potential Low-Cost Adsorbent. J. Encapsulation Adsorpt. Sci. 2015, 5, 178–190. [Google Scholar] [CrossRef]
- Rezaei Kalantary, R.; Dehghanifard, E.; Mohseni-Bandpi, A.; Rezaei, L.; Esrafili, A.; Kakavandi, B.; Azari, A. Nitrate adsorption by synthetic activated carbon magnetic nanoparticles: Kinetics, isotherms and thermodynamic studies. Desalin Water Treat. 2016, 57, 16445–16455. [Google Scholar] [CrossRef]
- Mehdinejadiani, B.; Amininasab, S.M.; Manhooei, L. Enhanced adsorption of nitrate from water by modified wheat straw: Equilibrium, kinetic and thermodynamic studies. Water Sci. Technol. 2019, 79, 302–313. [Google Scholar] [CrossRef]
- Divband Hafshejani, L.; Hooshmand, A.; Naseri, A.A.; Mohammadi, A.S.; Abbasi, F.; Bhatnagar, A. Removal of nitrate from aqueous solution by modified sugarcane bagasse biochar. Ecol. Eng. 2016, 95, 101–111. [Google Scholar] [CrossRef]
- Mehrabinia, P.; Ghanbari-Adivi, E. Examining nitrate surface absorption method from polluted water using activated carbon of agricultural wastes. Model. Earth Syst. Environ. 2021, 8, 1553–1561. [Google Scholar] [CrossRef]
- Chu, K.H. Removal of copper from aqueous solution by chitosan in prawn shell: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2002, 14, 77–95. [Google Scholar] [CrossRef]
- Karachalios, A.; Wazne, M. Nitrate removal from water by quaternized pine bark using choline-based ionic liquid analogue. J. Chem. Technol. Biotechnol. 2013, 88, 664–671. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Heydaripour, J. Onion membrane: An efficient adsorbent for decoloring of wastewater. J. Environ. Health Sci. Eng. 2015, 13, 1. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sangeetha, D. Application of coconut coir pith for the removal of sulfate and other anions from water. Desalination 2008, 219, 1–3. [Google Scholar] [CrossRef]
- John, Y.; David, V.E.; Mmereki, D.A. Comparative Study on Removal of Hazardous Anions from Water by Adsorption: A Review. Int. J. Chem. Eng. 2018, 2018, 3975948. [Google Scholar] [CrossRef]
- Banu, H.T.; Meenakshi, S. Synthesis of a novel quaternized form of melamine–formaldehyde resin for the removal of nitrate from water. J. Water Process Eng. 2017, 16, 81–89. [Google Scholar] [CrossRef]
- Mondal, N.K.; Ghosh, P.; Sen, K.; Mondal, A.; Debnath, P. Efficacy of onion peel towards removal of nitrate from aqueous solution and field samples. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100222. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Operations, 3rd ed; McGraw-Hill: New York, NY, USA, 1981. [Google Scholar]
- Naushad, M.; Ahamad, T.; Sharma, G.; Al-Muhtaseb, A.H.; Albadarin, A.B.; Alam, M.M.; ALOthman, Z.A.; Alshehri, S.M.; Ghfar, A.A. Synthesis and characterization of a new starch/SnO2 nanocomposite for efficient adsorption of toxic Hg2+ metal ion. Chem. Eng. J. 2016, 300, 306–316. [Google Scholar] [CrossRef]
- Kalaruban, M.; Loganathan, P.; Shim, W.G.; Kandasamy, J.; Ngo, H.H.; Vigneswaran, S. Enhanced removal of nitrate from water using amine-grafted agricultural wastes. Sci. Total Environ. 2016, 565, 503–510. [Google Scholar] [CrossRef]





| Adsorbent | Nitrate Adsorption Capacity | Reference |
|---|---|---|
| Modified hazelnut shells | 25.79 mg g−1 | [10] |
| Franco biochar | 1.339 mg g−1 | [11] |
| Modified brewers’ spent grain | 22.65 mg g−1 | [12] |
| Modified reed straw | 272.024 mg g−1 | [13] |
| Modified olive mill residues | 110 mg g−1 | [14] |
| Modified grape seeds | 25.626 mg g−1 | [15] |
| Modified corn stalks | 23.59 mg g−1 | [16] |
| Modified bambo chopstick | 16.39 mg g−1 | [17] |
| Modified lychee peels | 60.3 mg g−1 | [18] |
| Modified corn-cob | 9.35 mg g−1 | [19] |
| Modified rice husk ash | 30.86 mg g−1 | [20] |
| Parameters | Model Wastewater * | Confectionery Industry Wastewater | Meat Industry Wastewater |
|---|---|---|---|
| COD (mgO2 L−1) | 785 | 14,488 | 1200 |
| Ntotal (mg L−1) | 330 | 83 | 48 |
| N-NH4 (mg L−1) | 25 | 35 | 8 |
| N-NO3 (mg L−1) | 2.45 | 50 | 65 |
| N-NO2 (mg L−1) | <0.002 | <0.002 | 0.45 |
| P-PO4 (mg L−1) | 27.51 | 16 | 42 |
| pH | 7.48 | 5.7 | 9.4 |
| Colour | yellowish | yellow-brown | gray-brown |
| Element | Atomic No. | SS | MSS | ||||
|---|---|---|---|---|---|---|---|
| Weight (%) | Atomic (%) | Abs. Error (1 sigma) | Weight (%) | Atomic (%) | Abs. Error (1 Sigma) | ||
| C | 6 | 68.55 | 76.22 | 7.49 | 32.97 | 67.52 | 5.59 |
| N | 7 | 4.27 | 4.07 | 0.86 | 4.16 | 7.30 | 1.52 |
| O | 8 | 22.80 | 19.03 | 2.86 | 5.38 | 8.28 | 1.31 |
| Cl | 17 | 0.04 | 0.01 | 0.01 | 19.38 | 13.45 | 0.69 |
| Cu | 29 | 0.59 | 0.12 | 0.08 | 0.69 | 0.27 | 0.14 |
| Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|
| T/°C | qm (mg g−1) | KL (L mg−1) | R2 | RL | n | KF (mg g−1)(mg L−1)1/n | R2 | |
| Model nitrate solution | 25 | 13.351 | 0.017 | 0.3262 | 1.499 | 2.234 | 1.111 | 0.7387 |
| 35 | 9.737 | 0.030 | 0.4304 | 1.879 | 2.364 | 1.036 | 0.8028 | |
| 45 | 12.063 | 0.028 | 0.4797 | 1.807 | 2.294 | 1.079 | 0.8255 | |
| Model wastewater | 25 | 11.779 | 0.029 | 0.6070 | 1.849 | 1.749 | 1.433 | 0.8781 |
| 35 | 16.611 | 0.032 | 0.9379 | 1.938 | 1.484 | 1.031 | 0.8903 | |
| 45 | 11.261 | 0.046 | 0.7051 | 2.348 | 2.061 | 1.078 | 0.7903 | |
| Real wastewater from the confectionary industry | 25 | 18.282 | 0.019 | 0.7539 | 1.744 | 1.504 | 1.623 | 0.9597 |
| 35 | 31.348 | 0.007 | 0.6499 | 1.274 | 1.184 | 3.505 | 0.9781 | |
| 45 | 14.684 | 0.025 | 0.6527 | 1.979 | 1.877 | 1.160 | 0.9577 | |
| Real wastewater from the meat industry | 25 | 7.189 | 0.006 | 0.2680 | 1.231 | 0.754 | 42.052 | 0.8397 |
| 35 | 7.117 | 0.006 | 0.2299 | 1.231 | 0.753 | 39.591 | 0.8003 | |
| 45 | 12.870 | 0.006 | 0.1625 | 1.231 | 0.771 | 21.546 | 0.8249 | |
| T/°C | Pseudo-First Order | Pseudo-Second Order | |||||
|---|---|---|---|---|---|---|---|
| qe cal (mg g−1) | kL (min −1) | R2 | qe cal (mg g−1) | kL (g mg−1)(min−1) | R2 | ||
| Model nitrate solution | 25 | 0.998 | 0.716 | 0.3337 | 2.851 | 0.569 | 0.999 |
| 35 | 0.999 | 0.380 | 0.0923 | 2.721 | 1.716 | 0.999 | |
| 45 | 0.996 | 0.979 | 0.5836 | 2.758 | 0.389 | 0.999 | |
| Model wastewater | 25 | 0.996 | 0.014 | 0.3390 | 2.929 | 0.057 | 0.987 |
| 35 | 0.999 | 0.265 | 0.1256 | 2.630 | 0.471 | 0.992 | |
| 45 | 1.019 | 1.034 | 0.2373 | 2.765 | 0.165 | 0.995 | |
| Real wastewater from the confectionary industry | 25 | 0.999 | 0.282 | 0.0545 | 1.714 | 0.329 | 0.981 |
| 35 | 1.001 | 0.139 | 0.0493 | 1.570 | 0.120 | 0.978 | |
| 45 | 1.002 | 0.038 | 0.0884 | 1.316 | 0.070 | 0.970 | |
| Real wastewater from the meat industry | 25 | 0.999 | 1.037 | 0.0493 | 1.639 | 0.186 | 0.860 |
| 35 | 0.999 | 1.071 | 0.0061 | 1.177 | 0.328 | 0.945 | |
| 45 | 0.988 | 0.572 | 0.5943 | 1.093 | 0.204 | 0.890 | |
| T/°C | ki1 (mg g−1min0.5) | c1 (mg g−1) | R12 | ki2 (mg g−1min0.5) | c2 (mg g−1) | R22 | |
|---|---|---|---|---|---|---|---|
| Model nitrate solution | 25 | 0.104 | 2.361 | 0.3005 | 0.009 | 2.702 | 0.7737 |
| 35 | 0.117 | 2.196 | 0.6484 | 0.004 | 2.655 | 0.0253 | |
| 45 | 0.106 | 2.135 | 0.8639 | 0.007 | 2.702 | 0.0721 | |
| Model wastewater | 25 | 0.038 | 2.229 | 0.5151 | 0.061 | 1.777 | 0.7312 |
| 35 | 0.002 | 2.642 | 0.0014 | 0.004 | 2.555 | 0.0189 | |
| 45 | 0.009 | 2.587 | 0.0291 | 0.054 | 1.681 | 0.7399 | |
| Real wastewater from the confectionary industry | 25 | 0.047 | 1.475 | 0.1964 | 0.039 | 2.378 | 0.2901 |
| 35 | 0.040 | 1.961 | 0.0007 | 0.046 | 2.374 | 0.3259 | |
| 45 | 0.006 | 1.897 | 0.0009 | 0.084 | 2.799 | 0.5957 | |
| Real wastewater from the meat industry | 25 | 0.083 | 1.052 | 0.5431 | 0.064 | 3.089 | 0.0895 |
| 35 | 0.082 | 0.709 | 0.4763 | 0.073 | 2.612 | 0.0641 | |
| 45 | 0.006 | 1.135 | 0.0100 | 0.026 | 1.843 | 0.0235 | |
| Adsorption Capacitiy (mg g−1) | ||
|---|---|---|
| Batch experiments | Fixed-bed column | |
| Model nitrate solution | 13.351 | 43.38 |
| Model wastewater | 9.737 | 24.59 |
| Real wastewater from the confectionary industry | 12.063 | - |
| Real wastewater from the meat industry | 11.779 | 33 |
| Nitrate Saturated MSS | Solution | Immobilization | |
|---|---|---|---|
| % | 24 h | 48 h | |
| MS | 0.5 | 20 | 30 |
| 1 | 25 | 35 | |
| 2 | 25 | 45 | |
| CW | 0.5 | 0 | 20 |
| 1 | 10 | 30 | |
| 2 | 20 | 40 | |
| MW | 0.5 | 5 | 10 |
| 1 | 10 | 20 | |
| 2 | 20 | 40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristek Janković, A.; Habuda-Stanić, M.; Dong, H.; Tutić, A.; Romić, Ž.; Ergović Ravančić, M.; Landeka Dragičević, T.; Šiljeg, M. Utilization of Modified Sunflower Seed as Novel Adsorbent for Nitrates Removal from Wastewater. Water 2024, 16, 73. https://doi.org/10.3390/w16010073
Kristek Janković A, Habuda-Stanić M, Dong H, Tutić A, Romić Ž, Ergović Ravančić M, Landeka Dragičević T, Šiljeg M. Utilization of Modified Sunflower Seed as Novel Adsorbent for Nitrates Removal from Wastewater. Water. 2024; 16(1):73. https://doi.org/10.3390/w16010073
Chicago/Turabian StyleKristek Janković, Antonija, Mirna Habuda-Stanić, Huiyu Dong, Ana Tutić, Željka Romić, Maja Ergović Ravančić, Tibela Landeka Dragičević, and Mario Šiljeg. 2024. "Utilization of Modified Sunflower Seed as Novel Adsorbent for Nitrates Removal from Wastewater" Water 16, no. 1: 73. https://doi.org/10.3390/w16010073