Seasonal and Size-Related Fish Microhabitat Use Upstream and Downstream from Small Hydropower Plants
Abstract
:1. Introduction
2. Materials and Methods
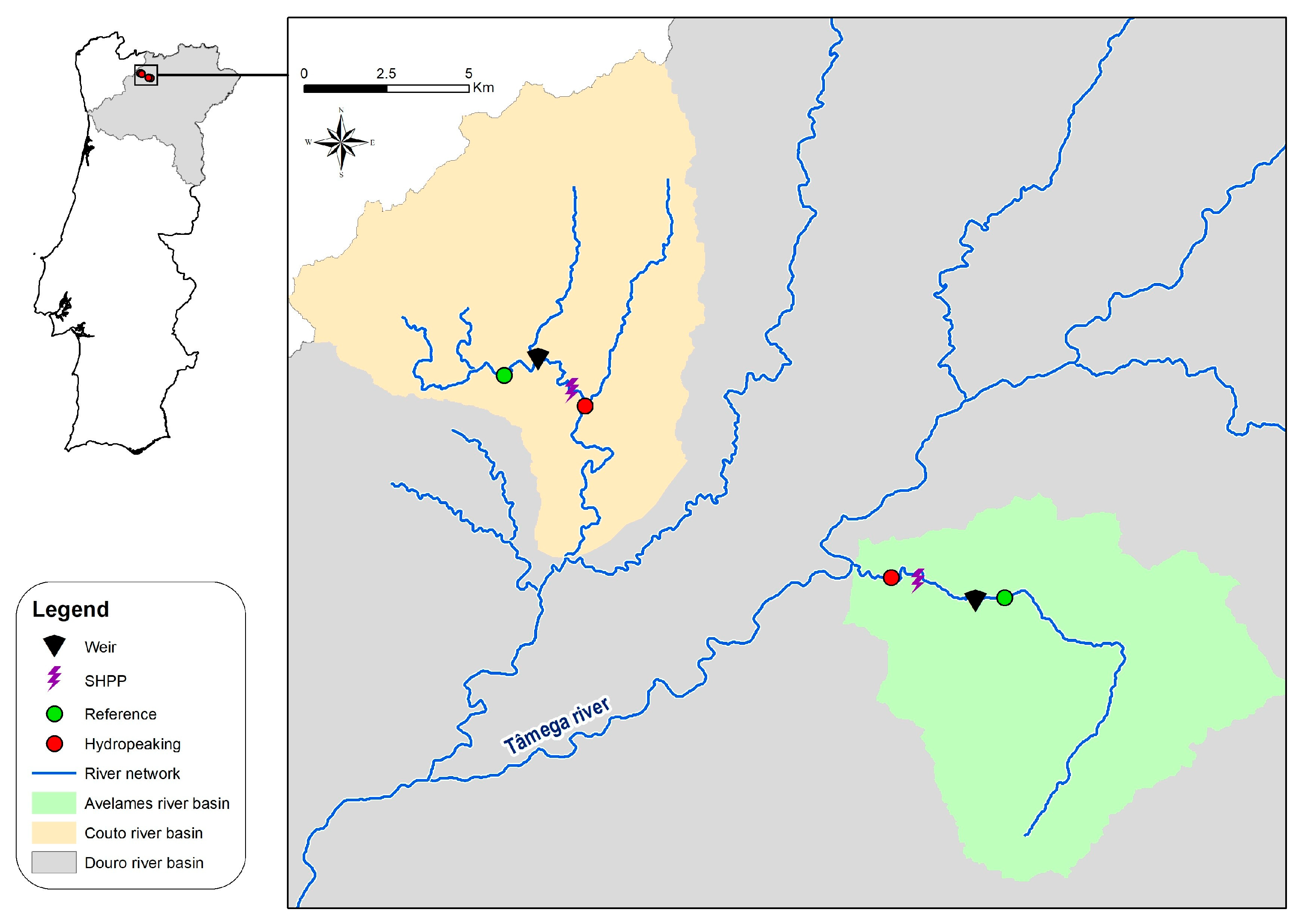
2.1. Study Area
2.2. Fish Sampling
2.3. Microhabitat Use and Availability Measurements
2.3.1. Microhabitat Use
2.3.2. Microhabitat Availability
2.4. Data Analysis
3. Results
3.1. Microhabitat Availability and Use
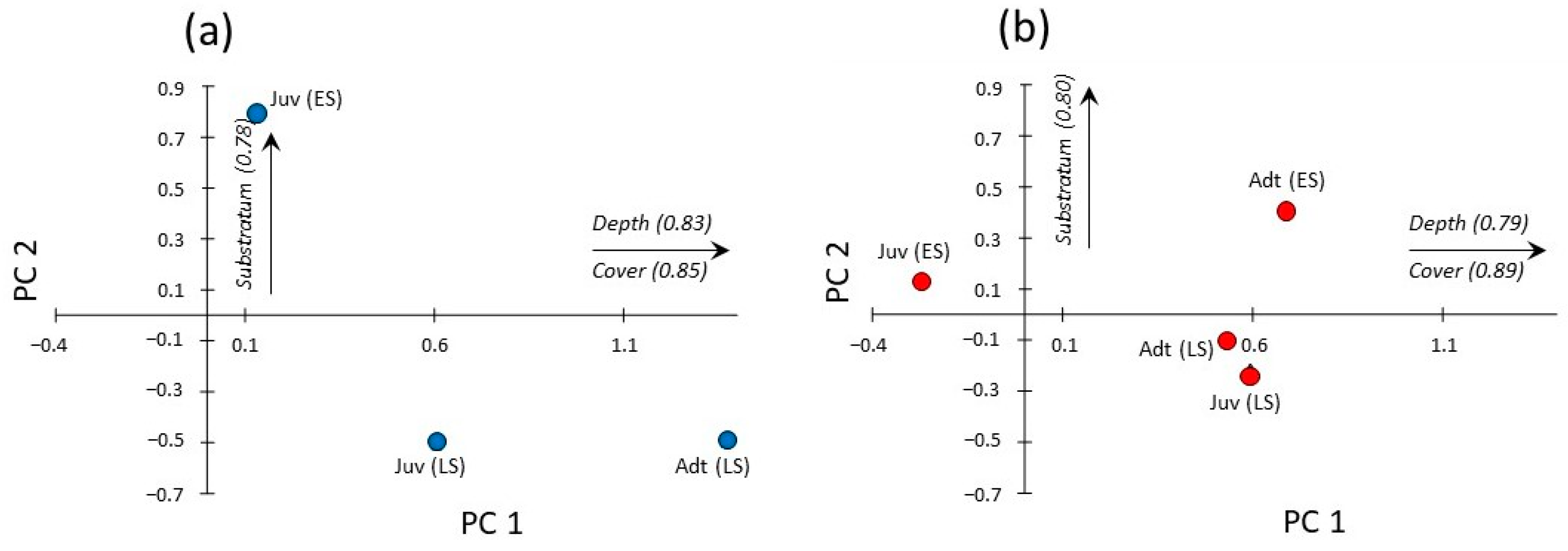
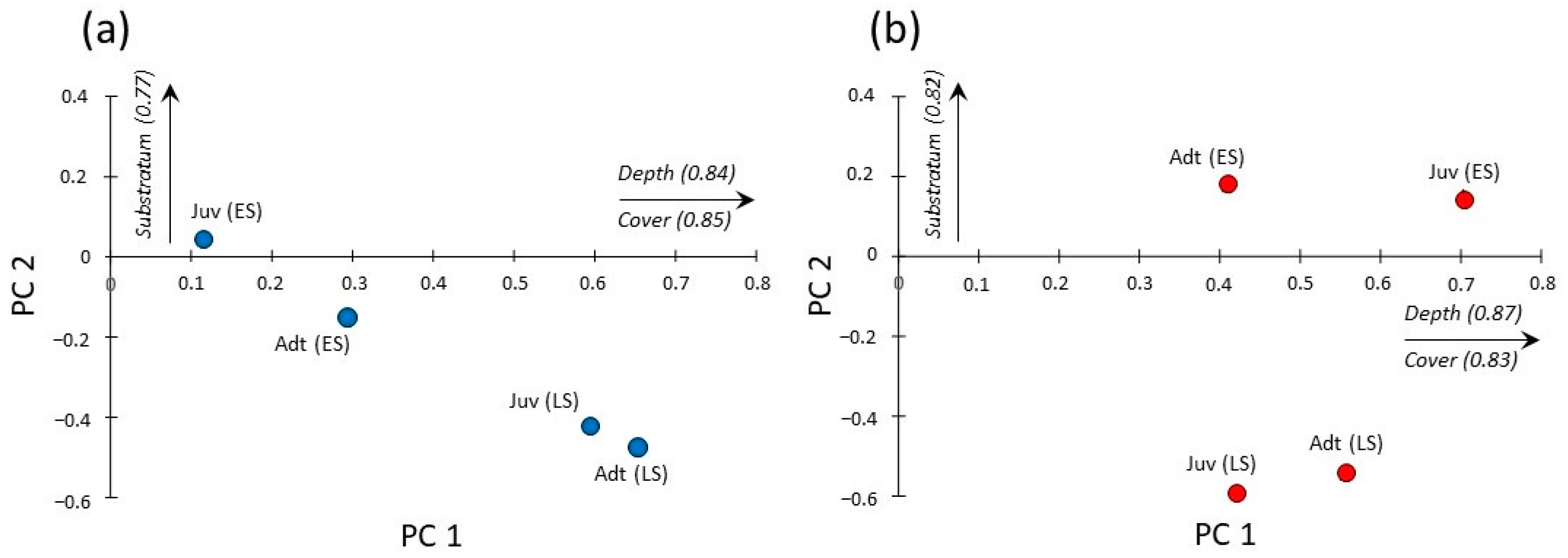
3.2. Non-Random (Selective) Microhabitat Use
3.3. Seasonal and Size-Related Variations in Microhabitat Use
4. Discussion
4.1. Driving Variables of Microhabitat Use
4.2. Non-Random (Selective) Microhabitat Use
4.3. Seasonal and Size-Related Variation in Microhabitat Use
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ocko, I.B.; Hamburg, S.P. Climate Impacts of Hydropower: Enormous Differences among Facilities and over Time. Environ. Sci. Technol. 2019, 53, 14070–14082. [Google Scholar] [CrossRef] [PubMed]
- Geist, J. Editorial: Green or Red: Challenges for Fish and Freshwater Biodiversity Conservation Related to Hydropower. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1551–1558. [Google Scholar] [CrossRef]
- Tsiropoulos, I.; Nijs, W.; Tarvydas, D.; Ruiz, P. Towards Net-Zero Emissions in the EU Energy System by 2050: Insights from Scenarios in Line with the 2030 and 2050 Ambitions of the European Green Deal; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-13096-3. [Google Scholar]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Twardek, W.M.; Cowx, I.G.; Lapointe, N.W.R.; Paukert, C.; Beard, T.D.; Bennett, E.M.; Browne, D.; Carlson, A.K.; Clarke, K.D.; Hogan, Z.; et al. Bright Spots for Inland Fish and Fish000eries to Guide Future Hydropower Development. Water Biol. Secur. 2022, 1, 100009. [Google Scholar] [CrossRef]
- Barbarossa, V.; Schmitt, R.J.P.; Huijbregts, M.A.J.; Zarfl, C.; King, H.; Schipper, A.M. Impacts of Current and Future Large Dams on the Geographic Range Connectivity of Freshwater Fish Worldwide. Proc. Natl. Acad. Sci. USA 2020, 117, 3648–3655. [Google Scholar] [CrossRef]
- Santos, J.M.; Ferreira, M.T.; Pinheiro, A.N.; Bochechas, J.H. Effects of Small Hydropower Plants on Fish Assemblages in Medium-Sized Streams in Central and Northern Portugal. Aquat. Conserv. Mar. Freshw. Ecosyst. 2006, 16, 373–388. [Google Scholar] [CrossRef]
- WSHDR. World Small Hydropower Development Report; United Nations Industrial Development Organization: Vienna, Austria; International Center on Small Hydro Power: Hangzhou, China, 2016. [Google Scholar]
- Lange, K.; Meier, P.; Trautwein, C.; Schmid, M.; Robinson, C.T.; Weber, C.; Brodersen, J. Basin-Scale Effects of Small Hydropower on Biodiversity Dynamics. Front. Ecol. Environ. 2018, 16, 397–404. [Google Scholar] [CrossRef]
- Xu, R.; Zeng, Z.; Pan, M.; Ziegler, A.D.; Holden, J.; Spracklen, D.V.; Brown, L.E.; He, X.; Chen, D.; Ye, B.; et al. A Global-Scale Framework for Hydropower Development Incorporating Strict Environmental Constraints. Nat. Water 2023, 1, 113–122. [Google Scholar] [CrossRef]
- Couto, T.B.; Olden, J.D. Global Proliferation of Small Hydropower Plants—Science and Policy. Front. Ecol. Environ. 2018, 16, 91–100. [Google Scholar] [CrossRef]
- Boavida, I.; Santos, J.M.; Ferreira, T.; Pinheiro, A. Barbel Habitat Alterations Due to Hydropeaking. J. Hydro-Environ. Res. 2015, 9, 237–247. [Google Scholar] [CrossRef]
- Nagrodski, A.; Raby, G.D.; Hasler, C.T.; Taylor, M.K.; Cooke, S.J. Fish Stranding in Freshwater Systems: Sources, Consequences, and Mitigation. J. Environ. Manag. 2012, 103, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Boavida, I.; Harby, A.; Clarke, K.D.; Heggenes, J. Move or Stay: Habitat Use and Movements by Atlantic Salmon Parr (Salmo salar) during Induced Rapid Flow Variations. Hydrobiologia 2017, 785, 261–275. [Google Scholar] [CrossRef]
- Vehanen, T.; Louhi, P.; Huusko, A.; Mäki-Petäys, A.; van der Meer, O.; Orell, P.; Huusko, R.; Jaukkuri, M.; Sutela, T. Behaviour of Upstream Migrating Adult Salmon (Salmo salar L.) in the Tailrace Channels of Hydropeaking Hydropower Plants. Fish. Manag. Ecol. 2020, 27, 41–51. [Google Scholar] [CrossRef]
- Jones, N.E.; Haxton, T.J. Spatial Patterns of Stable Isotopes and Trophic Ecology in a Hydropeaking River. River Res. Appl. 2022, 38, 873–883. [Google Scholar] [CrossRef]
- Bejarano, M.D.; Jansson, R.; Nilsson, C. The Effects of Hydropeaking on Riverine Plants: A Review. Biol. Rev. 2018, 93, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, D.; Siviglia, A.; Carolli, M.; Zolezzi, G. Characterization of Sub-Daily Thermal Regime in Alpine Rivers: Quantification of Alterations Induced by Hydropeaking. Hydrol. Process. 2016, 30, 1052–1070. [Google Scholar] [CrossRef]
- Béjar, M.; Vericat, D.; Batalla, R.J.; Gibbins, C.N. Variation in Flow and Suspended Sediment Transport in a Montane River Affected by Hydropeaking and Instream Mining. Geomorphology 2018, 310, 69–83. [Google Scholar] [CrossRef]
- Hayes, D.S.; Moreira, M.; Boavida, I.; Haslauer, M.; Unfer, G.; Zeiringer, B.; Greimel, F.; Auer, S.; Ferreira, T.; Schmutz, S. Life Stage-Specific Hydropeaking Flow Rules. Sustainability 2019, 11, 1547. [Google Scholar] [CrossRef]
- Boavida, I.; Costa, M.J.; Portela, M.M.; Godinho, F.; Tuhtan, J.; Pinheiro, A. Do Cyprinid Fish Use Lateral Flow-Refuges during Hydropeaking? River Res. Appl. 2023, 39, 554–560. [Google Scholar] [CrossRef]
- Bartoň, D.; Bretón, F.; Blabolil, P.; Souza, A.T.; Vejřík, L.; Sajdlová, Z.; Kolařík, T.; Kubečka, J.; Šmejkal, M. Effects of Hydropeaking on the Attached Eggs of a Rheophilic Cyprinid Species. Ecohydrology 2021, 14, e2280. [Google Scholar] [CrossRef]
- Hayes, D.S.; Auer, S.; Fauchery, E.; Graf, D.; Hasler, T.; Mameri, D.; Schmutz, S.; Führer, S. The Interactive Effect of River Bank Morphology and Daytime on Downstream Displacement and Stranding of Cyprinid Larvae in Hydropeaking Conditions. Ecohydrol. Hydrobiol. 2023, 23, 152–161. [Google Scholar] [CrossRef]
- Auer, S.; Zeiringer, B.; Führer, S.; Tonolla, D.; Schmutz, S. Effects of River Bank Heterogeneity and Time of Day on Drift and Stranding of Juvenile European Grayling (Thymallus thymallus L.) Caused by Hydropeaking. Sci. Total Environ. 2017, 575, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Capra, H.; Plichard, L.; Bergé, J.; Pella, H.; Ovidio, M.; McNeil, E.; Lamouroux, N. Fish Habitat Selection in a Large Hydropeaking River: Strong Individual and Temporal Variations Revealed by Telemetry. Sci. Total Environ. 2017, 578, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Hohensinner, S.; Hauer, C.; Muhar, S. River Morphology, Channelization, and Habitat Restoration. In Riverine Ecosystem Management: Science for Governing towards a Sustainable Future; Schmutz, S., Sendzimir, J., Eds.; Aquatic Ecology Series; Springer International Publishing: Cham, Switzerland, 2018; pp. 41–65. ISBN 978-3-319-73250-3. [Google Scholar]
- Judes, C.; Capra, H.; Gouraud, V.; Pella, H.; Lamouroux, N. Past Hydraulics Influence Microhabitat Selection by Invertebrates and Fish in Hydropeaking Rivers. River Res. Appl. 2023, 39, 375–388. [Google Scholar] [CrossRef]
- Schmutz, S.; Bakken, T.H.; Friedrich, T.; Greimel, F.; Harby, A.; Jungwirth, M.; Melcher, A.; Unfer, G.; Zeiringer, B. Response of Fish Communities to Hydrological and Morphological Alterations in Hydropeaking Rivers of Austria. River Res. Appl. 2015, 31, 919–930. [Google Scholar] [CrossRef]
- Rato, A.S.; Alexandre, C.M.; de Almeida, P.R.; Costa, J.L.; Quintella, B.R. Effects of Hydropeaking on the Behaviour, Fine-Scale Movements and Habitat Selection of an Iberian Cyprinid Fish. River Res. Appl. 2021, 37, 1365–1375. [Google Scholar] [CrossRef]
- Judes, C.; Gouraud, V.; Capra, H.; Maire, A.; Barillier, A.; Lamouroux, N. Consistent but Secondary Influence of Hydropeaking on Stream Fish Assemblages in Space and Time. J. Ecohydraulics 2021, 6, 157–171. [Google Scholar] [CrossRef]
- Nestler, J.M.; Milhous, R.T.; Payne, T.R.; Smith, D.L. History and Review of the Habitat Suitability Criteria Curve in Applied Aquatic Ecology. River Res. Appl. 2019, 35, 1155–1180. [Google Scholar] [CrossRef]
- Conallin, J.; Boegh, E.; Jensen, J.K. Instream Physical Habitat Modelling Types: An Analysis as Stream Hydromorphological Modelling Tools for EU Water Resource Managers. Int. J. River Basin Manag. 2010, 8, 93–107. [Google Scholar] [CrossRef]
- Santos, J.M.; Rivaes, R.; Boavida, I.; Branco, P. Structural Microhabitat Use by Endemic Cyprinids in a Mediterranean-Type River: Implications for Restoration Practices. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 26–36. [Google Scholar] [CrossRef]
- Bätz, N.; Judes, C.; Weber, C. Nervous Habitat Patches: The Effect of Hydropeaking on Habitat Dynamics. River Res. Appl. 2023, 39, 349–363. [Google Scholar] [CrossRef]
- Santos, J.M.; Godinho, F.N.; Ferreira, M.T. Microhabitat Use by Iberian Nase Chondrostoma Polylepis and Iberian Chub Squalius Carolitertii in Three Small Streams, North-West Portugal. Ecol. Freshw. Fish 2004, 13, 223–230. [Google Scholar] [CrossRef]
- Katano, I.; Negishi, J.N.; Minagawa, T.; Doi, H.; Kawaguchi, Y.; Kayaba, Y. Effects of Sediment Replenishment on Riverbed Environments and Macroinvertebrate Assemblages Downstream of a Dam. Sci. Rep. 2021, 11, 7525. [Google Scholar] [CrossRef] [PubMed]
- Boavida, I.; Ambrósio, F.; Costa, M.J.; Quaresma, A.; Portela, M.M.; Pinheiro, A.; Godinho, F. Habitat Use by Pseudochondrostoma Duriense and Squalius Carolitertii Downstream of a Small-Scale Hydropower Plant. Water 2020, 12, 2522. [Google Scholar] [CrossRef]
- Alexandre, C.M.; Ferreira, M.T.; Almeida, P.R. Life-Cycle Responses of a Mediterranean Non-Migratory Cyprinid Species, the Northern Iberian Chub (Squalius Carolitertii Doadrio, 1988), to Streamflow Regulation. Ecohydrology 2018, 11, e1998. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Sousa, L.; Santos, J.M.; Reino, L.; Oliveira, J.; Almeida, P.R.; Cortes, R.V. Regional and Local Environmental Correlates of Native Iberian Fish Fauna. Ecol. Freshw. Fish 2007, 16, 504–514. [Google Scholar] [CrossRef]
- Teixeira, A.; Cortes, R.M.V. Diet of Stocked and Wild Trout, Salmo Trutta: Is There Competition for Resources? Czschoslovak Academy of Sciences of Praha: Prague, Czech Republic, 2006. [Google Scholar]
- Doadrio, I.; Perea, S.; Garzón-Heydt, P.; González, J. Ictiofauna Continental Española. Bases Para Su Seguimiento; Ministerio de Medio Ambiente y Medio Rural y Marino, Centro de Publicaciones: Madrid, Spain, 2011; ISBN 978-84-491-1158-7. [Google Scholar]
- Magalhães, M.F.; Schlosser, I.J.; Collares-Pereira, M.J. The Role of Life History in the Relationship between Population Dynamics and Environmental Variability in Two Mediterranean Stream Fishes. J. Fish Biol. 2003, 63, 300–317. [Google Scholar] [CrossRef]
- Krimmer, A.N.; Paul, A.J.; Hontela, A.; Rasmussen, J.B. Behavioural and Physiological Responses of Brook Trout Salvelinus Fontinalis to Midwinter Flow Reduction in a Small Ice-Free Mountain Stream. J. Fish Biol. 2011, 79, 707–725. [Google Scholar] [CrossRef]
- Heggenes, J.; Brabrand, Å.; Saltveit, S. Comparison of Three Methods for Studies of Stream Habitat Use by Young Brown Trout and Atlantic Salmon. Trans. Am. Fish Soc. 1990, 119, 101–111. [Google Scholar] [CrossRef]
- CEN-EN 14011—Water Quality—Sampling of Fish with Electricity|GlobalSpec. Available online: https://standards.globalspec.com/std/282064/en-14011 (accessed on 6 September 2023).
- Garner, P. Sample Sizes for Length and Density Estimation of 0+ Fish When Using Point Sampling by Electrofishing. J. Fish Biol. 1997, 50, 95–106. [Google Scholar] [CrossRef]
- Santos, J.M.; Reino, L.; Porto, M.; Oliveira, J.; Pinheiro, P.; Almeida, P.R.; Cortes, R.; Ferreira, M.T. Complex Size-Dependent Habitat Associations in Potamodromous Fish Species. Aquat. Sci. 2011, 73, 233–245. [Google Scholar] [CrossRef]
- Collares-Pereira, M.J.; Alves, M.J.; Ribeiro, F.; Domingos, I.; Almeida, P.R.; Costa, L.; Gante, H.; Filipe, A.F.; Aboim, M.A.; Rodrigues, P.M.; et al. Guia dos Peixes de Água Doce e Migradores de Portugal Continental; Edições Afrontamento: Porto, Portugal, 2021; ISBN 978-972-36-1849-5. [Google Scholar]
- Nicola, G.G.; Almodóvar, A. Reproductive Traits of Stream-Dwelling Brown Trout Salmo Trutta in Contrasting Neighbouring Rivers of Central Spain. Freshw. Biol. 2002, 47, 1353–1365. [Google Scholar] [CrossRef]
- Bovee, K.D. Development and Evaluation of Habitat Suitability Criteria for Use in the Instream Flow Incremental Methodology; USDI Fish and Wildlife Service: Fairfax County, VA, USA, 1986. [Google Scholar]
- Beyer, H.L.; Haydon, D.T.; Morales, J.M.; Frair, J.L.; Hebblewhite, M.; Mitchell, M.; Matthiopoulos, J. The Interpretation of Habitat Preference Metrics under Use–Availability Designs. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2245–2254. [Google Scholar] [CrossRef]
- Boavida, I.; Santos, J.M.; Cortes, R.V.; Pinheiro, A.N.; Ferreira, M.T. Assessment of Instream Structures for Habitat Improvement for Two Critically Endangered Fish Species. Aquat. Ecol. 2011, 45, 113–124. [Google Scholar] [CrossRef]
- Ayllón, D.; Almodóvar, A.; Nicola, G.G.; Elvira, B. Interactive Effects of Cover and Hydraulics on Brown Trout Habitat Selection Patterns. River Res. Appl. 2009, 25, 1051–1065. [Google Scholar] [CrossRef]
- Kramer, D.L.; Rangeley, R.W.; Chapman, L.J. Habitat Selection: Patterns of Spatial Distribution from Behavioural Decisions. In Behavioural Ecology of Teleost Fishes; Godin, J.G.J., Ed.; Oxford University Press: Oxford, UK, 1997; pp. 37–80. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists. Available online: https://www.cambridge.org/highereducation/books/experimental-design-and-data-analysis-for-biologists/BAF276114278FF40A7ED1B0FE77D691A (accessed on 7 September 2023).
- Alexandre, C.M.; Almeida, P.R.; Neves, T.; Mateus, C.S.; Costa, J.L.; Quintella, B.R. Effects of Flow Regulation on the Movement Patterns and Habitat Use of a Potamodromous Cyprinid Species. Ecohydrology 2016, 9, 326–340. [Google Scholar] [CrossRef]
- Grossman, G.D.; de Sostoa, A. Microhabitat Use by Fish in the Upper Rio Matarraña, Spain, 1984–1987. Ecol. Freshw. Fish 1994, 3, 141–152. [Google Scholar] [CrossRef]
- Grossman, G.D.; De Sostoa, A. Microhabitat Use by Fish in the Lower Rio Matarraña, Spain, 1984–1987. Ecol. Freshw. Fish 1994, 3, 123–136. [Google Scholar] [CrossRef]
- Grossman, G.D.; Freeman, M.C. Microhabitat Use in a Stream Fish Assemblage. J. Zool. 1987, 212, 151–176. [Google Scholar] [CrossRef]
- Copp, G.H.; Spathari, S.; Turmel, M. Consistency of Diel Behaviour and Interactions of Stream Fishes and Invertebrates during Summer. River Res. Appl. 2005, 21, 75–90. [Google Scholar] [CrossRef]
- Heggenes, J.; Northcote, T.G.; Peter, A. Seasonal Habitat Selection and Preferences by Cutthroat Trout (Oncorhynchus clarki) in a Small Coastal Stream. Can. J. Fish Aquat. Sci. 1991, 48, 1364–1370. [Google Scholar] [CrossRef]
- Allouche, S. Nature and Functions of Cover for Riverine Fish. Bull. Fr. Pêche Piscic. 2002, 365–366, 297–324. [Google Scholar] [CrossRef]
- Heggenes, J. Effects of Short-Term Flow Fluctuations on Displacement of, and Habitat Use by, Brown Trout in a Small Stream. Trans. Am. Fish Soc. 1988, 117, 336–344. [Google Scholar] [CrossRef]
- Moreira, M.; Costa, M.J.; Valbuena-Castro, J.; Pinheiro, A.N.; Boavida, I. Cover or Velocity: What Triggers Iberian Barbel (Luciobarbus bocagei) Refuge Selection under Experimental Hydropeaking Conditions? Water 2020, 12, 317. [Google Scholar] [CrossRef]
- Vesipa, R.; Camporeale, C.; Ridolfi, L. Recovery Times of Riparian Vegetation. Water Resour. Res. 2016, 52, 2934–2950. [Google Scholar] [CrossRef]
- Stromberg, J.C.; Beauchamp, V.B.; Dixon, M.D.; Lite, S.J.; Paradzick, C. Importance of Low-Flow and High-Flow Characteristics to Restoration of Riparian Vegetation along Rivers in Arid South-Western United States. Freshw. Biol. 2007, 52, 651–679. [Google Scholar] [CrossRef]
- Smith, C.D.; Quist, M.C.; Hardy, R.S. Fish Assemblage Structure and Habitat Associations in a Large Western River System. River Res. Appl. 2016, 32, 622–638. [Google Scholar] [CrossRef]
- Allan, J.D.; Castillo, M.M. Stream Ecology; Springer: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-5582-9. [Google Scholar]
- Howell, T.D.; Arthington, A.H.; Pusey, B.J.; Brooks, A.P.; Creese, B.; Chaseling, J. Responses of Fish to Experimental Introduction of Structural Woody Habitat in Riffles and Pools. Restor. Ecol. 2012, 20, 43–55. [Google Scholar] [CrossRef]
- Acheampong, J.N.; Gyamfi, C.; Arthur, E. Impacts of Retention Basins on Downstream Flood Peak Attenuation in the Odaw River Basin, Ghana. J. Hydrol. Reg. Stud. 2023, 47, 101364. [Google Scholar] [CrossRef]
- Ayllón, D.; Almodóvar, A.; Nicola, G.G.; Elvira, B. Ontogenetic and Spatial Variations in Brown Trout Habitat Selection. Ecol. Freshw. Fish 2010, 19, 420–432. [Google Scholar] [CrossRef]
- Heggenes, J. Habitat Selection by Brown Trout (Salmo trutta) and Young Atlantic Salmon (S. salar) in Streams: Static and Dynamic Hydraulic Modelling. Regul. Rivers Res. Manag. 1996, 12, 155–169. [Google Scholar] [CrossRef]
- Fausch, K.D. Profitable Stream Positions for Salmonids: Relating Specific Growth Rate to Net Energy Gain. Can. J. Zool. 1984, 62, 441–451. [Google Scholar] [CrossRef]
- Facey, D.E.; Grossman, G.D. The Metabolic Cost of Maintaining Position for Four North American Stream Fishes: Effects of Season and Velocity. Physiol. Zool. 1990, 63, 757–776. [Google Scholar] [CrossRef]
- Haddadchi, A.; Booker, D.J.; Measures, R.J. Predicting River Bed Substrate Cover Proportions across New Zealand. Catena 2018, 163, 130–146. [Google Scholar] [CrossRef]
- Venus, T.E.; Smialek, N.; Pander, J.; Harby, A.; Geist, J. Evaluating Cost Trade-Offs between Hydropower and Fish Passage Mitigation. Sustainability 2020, 12, 8520. [Google Scholar] [CrossRef]
- Dodds, W.K.; Whiles, M.R. Chapter 23—Fish Ecology and Fisheries. In Freshwater Ecology, 2nd ed.; Dodds, W.K., Whiles, M.R., Eds.; Aquatic Ecology; Academic Press: London, UK, 2010; pp. 611–633. ISBN 978-0-12-374724-2. [Google Scholar]
- Brownscombe, J.W.; Griffin, L.P.; Brooks, J.L.; Danylchuk, A.J.; Cooke, S.J.; Midwood, J.D. Applications of Telemetry to Fish Habitat Science and Management. Can. J. Fish Aquat. Sci. 2022, 79, 1347–1359. [Google Scholar] [CrossRef]
- Glowa, S.E.; Watkinson, D.A.; Jardine, T.D.; Enders, E.C. Evaluating the Risk of Fish Stranding Due to Hydropeaking in a Large Continental River. River Res. Appl. 2023, 39, 444–459. [Google Scholar] [CrossRef]
- Tuhtan, J.A.; Noack, M.; Wieprecht, S. Estimating Stranding Risk Due to Hydropeaking for Juvenile European Grayling Considering River Morphology. KSCE J. Civ. Eng. 2012, 16, 197–206. [Google Scholar] [CrossRef]
- Lobón-Cerviá, J. Why, When and How Do Fish Populations Decline, Collapse and Recover? The Example of Brown Trout (Salmo trutta) in Rio Chaballos (Northwestern Spain). Freshw. Biol. 2009, 54, 1149–1162. [Google Scholar] [CrossRef]
- Railsback, S.F.; Harvey, B.C. Analysis of Habitat-Selection Rules Using an Individual-Based Model. Ecology 2002, 83, 1817–1830. [Google Scholar] [CrossRef]
- Harvey, B.C.; White, J.L. Use of Cover for Concealment Behavior by Rainbow Trout: Influences of Cover Structure and Area. N. Am. J. Fish Manag. 2016, 36, 1308–1314. [Google Scholar] [CrossRef]
- Saltveit, S.J.; Brabrand, Å.; Juárez, A.; Stickler, M.; Dønnum, B.O. The Impact of Hydropeaking on Juvenile Brown Trout (Salmo trutta) in a Norwegian Regulated River. Sustainability 2020, 12, 8670. [Google Scholar] [CrossRef]
- Vardakas, L.; Kalogianni, E.; Smeti, E.; Economou, A.N.; Skoulikidis, N.T.; Koutsoubas, D.; Dimitriadis, C.; Datry, T. Spatial Factors Control the Structure of Fish Metacommunity in a Mediterranean Intermittent River. Ecohydrol. Hydrobiol. 2020, 20, 346–356. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Habitat Use. In Ecology of Atlantic Salmon and Brown Trout: Habitat as a Template for Life Histories; Jonsson, B., Jonsson, N., Eds.; Fish & Fisheries Series; Springer: Dordrecht, The Netherlands, 2011; pp. 67–135. ISBN 978-94-007-1189-1. [Google Scholar]
- Rocaspana, R.; Aparicio, E.; Palau-Ibars, A.; Guillem, R.; Alcaraz, C. Hydropeaking Effects on Movement Patterns of Brown Trout (Salmo trutta L.). River Res. Appl. 2019, 35, 646–655. [Google Scholar] [CrossRef]
- Blanco-Garrido, F.; Prenda, J.; Narvaez, M. Eurasian Otter (Lutra lutra) Diet and Prey Selection in Mediterranean Streams Invaded by Centrarchid Fishes. Biol. Invasions 2008, 10, 641–648. [Google Scholar] [CrossRef]
- Hayes, D.S.; Lautsch, E.; Unfer, G.; Greimel, F.; Zeiringer, B.; Höller, N.; Schmutz, S. Response of European Grayling, Thymallus Thymallus, to Multiple Stressors in Hydropeaking Rivers. J. Environ. Manag. 2021, 292, 112737. [Google Scholar] [CrossRef]
- Hermoso, V. Freshwater Ecosystems Could Become the Biggest Losers of the Paris Agreement. Glob. Chang. Biol. 2017, 23, 3433–3436. [Google Scholar] [CrossRef]
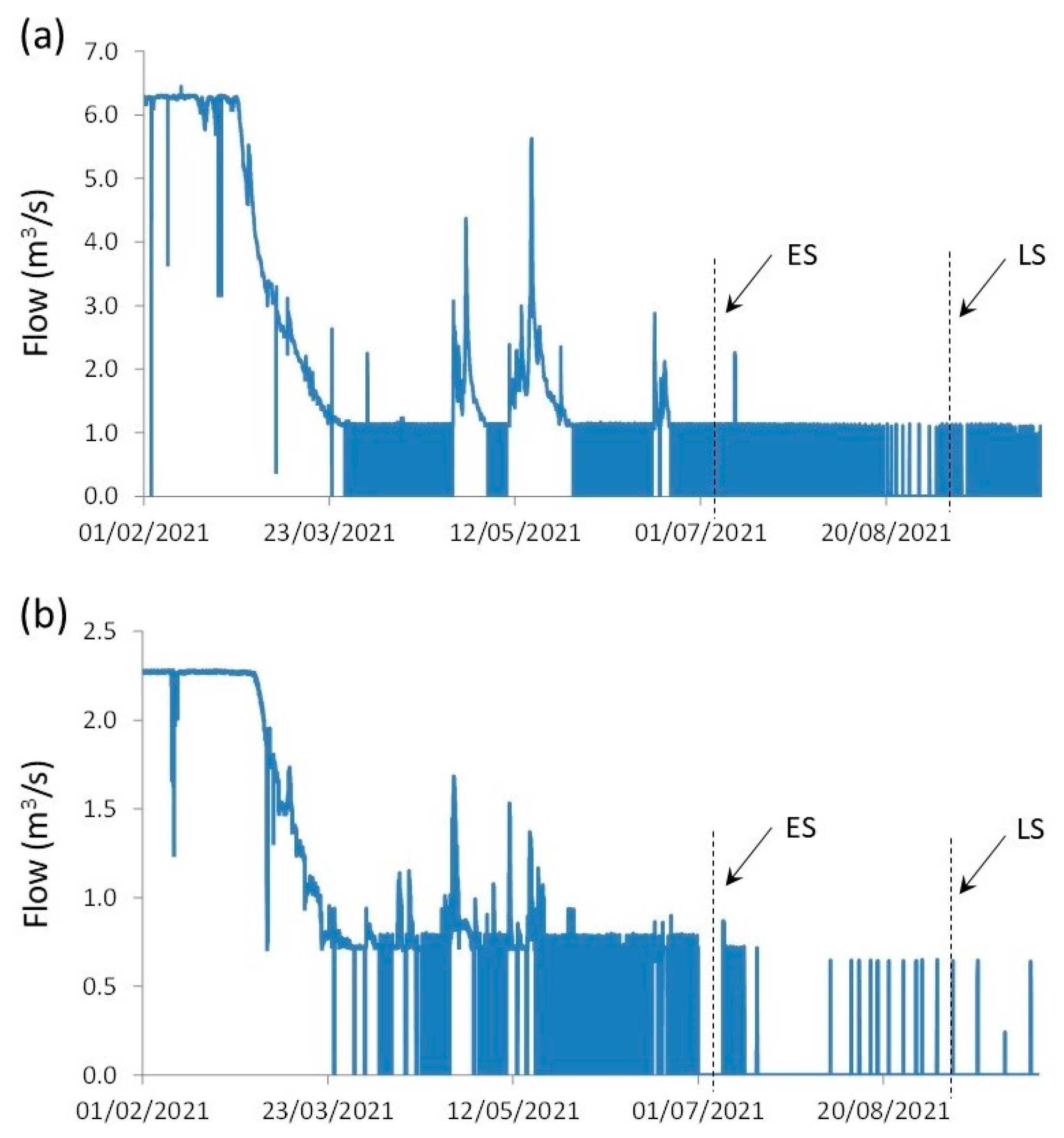
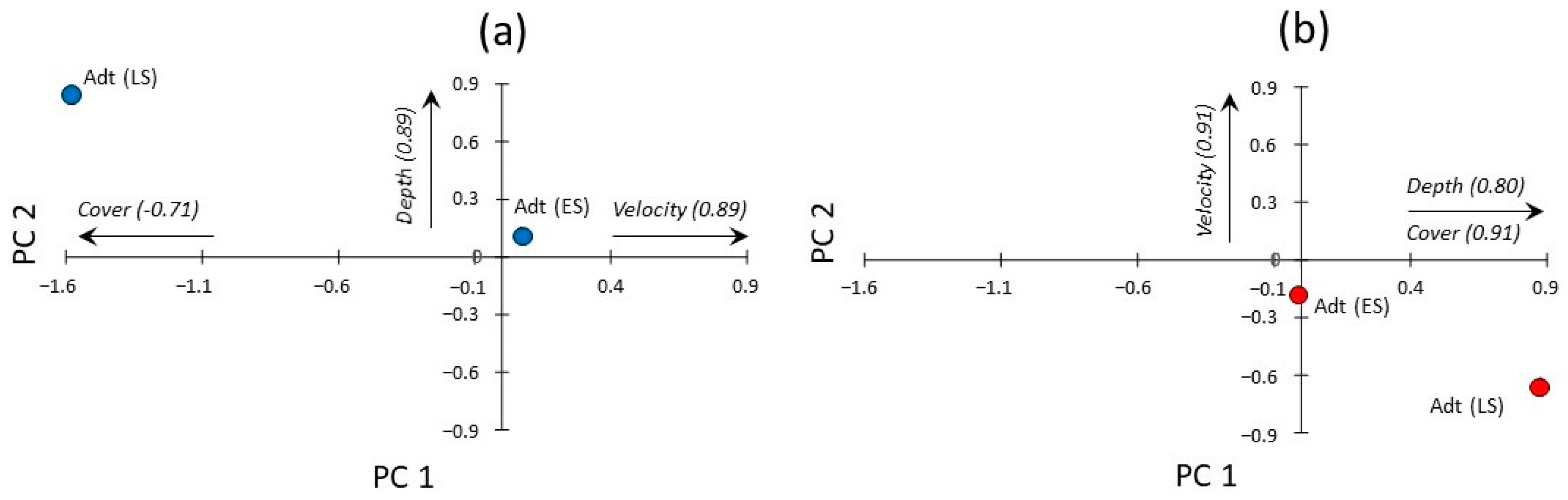
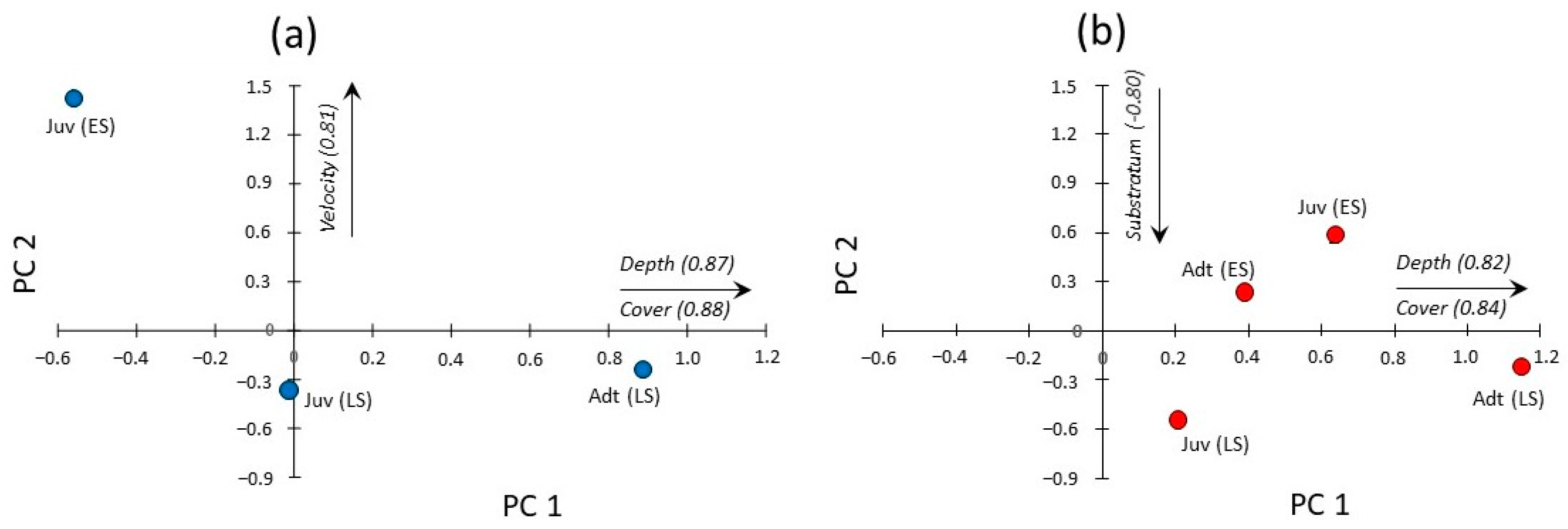
| Season | Mean TL (Range) (cm) | Depth (cm) | Water Velocity (cm/s) | Substratum (Class) | Cover | N | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | Dis. | Ref. | Dis. | Ref. | Dis. | Ref. | Dis. | Ref. | Dis. | Ref. | Dis. | |||
| Early summer | Availability | 47.7 (5–150) | 46.5 (4–106) | 13.9 (0–106) | 21.7 (0–106) | 8 (3–8) | 8 (3–8) | 30 (0–90) | 30 (0–90) | 177 | 118 | |||
| Use | ||||||||||||||
| Nase | ||||||||||||||
| juvenile | 6.7 (5.0–10.0) | 8.0 (3.0–11.0) | 54.6 (5.5) | 45.8 (1.2) | 14.4 (3.3) | 9.6 (1.5) | 8 (3–8) | 8 (3–8) | 35 (10–80) | 30 (0–90) | 20 | 103 | ||
| adult | - | - | - | - | - | - | - | - | - | - | 0 | 7 | ||
| Chub | ||||||||||||||
| juvenile | 5.8 (4.5–7.0) | 5.2 (4.0–7.0) | 38.6 (2.7) | 57.1 (5.8) | 3.7 (1.9) | 6.5 (2.3) | 8 (3–8) | 8 (6–8) | 20 (0–90) | 50 (20–80) | 27 | 20 | ||
| adult | 10.8 (8.0–15.0) | 10.0 (8.0–13.0) | 46 (3.5) | 47.6 (2.6) | 11.1 (5.9) | 9.7 (2.0) | 8 (3–8) | 8 (3–8) | 35 (20–80) | 40 (10–80) | 16 | 27 | ||
| Calandino | ||||||||||||||
| juvenile | - | - | - | - | - | - | - | - | - | - | 1 | 1 | ||
| adult | 6.7 (6.0–8.0) | 6.5 (6.0–7.5) | 35.3 (1.3) | 43.3 (2.7) | 4.9 (1.8) | 4.8 (1.5) | 8 (8–8) | 6 (3–8) | 40 (10–50) | 30 (20–60) | 12 | 12 | ||
| Trout | ||||||||||||||
| juvenile | 5.4 (4.0–7.0) | 6.3 (5.0–8.0) | 21.5 (1.2) | 57.9 (3.6) | 42.1 (10.3) | 31.8 (6.6) | 8 (6–8) | 8 (3–8) | 30 (20–50) | 50 (30–50) | 13 | 16 | ||
| adult | - | - | - | - | - | - | - | - | - | - | 0 | 6 | ||
| Late summer | Availability | 32.6 (4–79) | 32.7 (4–84) | 3.3 (0–67) | 6.7 (0–81) | 7 (3–8) | 7 (3–8) | 30 (10–100) | 30 (10–90) | 189 | 150 | |||
| Use | ||||||||||||||
| Nase | ||||||||||||||
| juvenile | 7.5 (4.0–11.0) | 7.7 (3.0–11.0) | 36.9 (3.3) | 51.5 (0.9) | 2.6 (0.8) | 1.8 (0.4) | 7 (3–8) | 7 (3–7) | 60 (20–100) | 70 (10–100) | 35 | 299 | ||
| adult | 17.3 (12.0–25.0) | 12.4 (11.5–15.0) | 49.6 (0.4) | 51.1 (3.5) | 0 (0.0) | 1.9 (0.9) | 7 (6–7) | 7 (7–7) | 85 (50–100) | 65 (20–100) | 12 | 18 | ||
| Chub | ||||||||||||||
| juvenile | 5.7 (3.0–7.0) | 5.9 (2.5–7.0) | 38.1 (2.1) | 39.1 (3.6) | 0.9 (0.4) | 0.6 (0.6) | 7 (3–8) | 7 (3–7) | 50 (10–100) | 55 (20–70) | 34 | 14 | ||
| adult | 9.9 (7.5–16.0) | 9.5 (7.5–12.5) | 39.2 (4.2) | 42.9 (3.3) | 3.2 (1.1) | 1.2 (0.8) | 7 (3–8) | 7 (3–7) | 65 (40–100) | 50 (30–100) | 20 | 16 | ||
| Calandino | ||||||||||||||
| juvenile | - | - | - | - | - | - | - | - | - | - | 0 | 2 | ||
| adult | 7.2 (5.0–9.0) | 6.9 (6.0–8.5) | 34.9 (3.9) | 45.6 (4.0) | 0 (0.0) | 1.6 (1.1) | 5.5 (3–8) | 7 (3–7) | 90 (70–100) | 70 (10–90) | 14 | 18 | ||
| Trout | ||||||||||||||
| juvenile | 7.4 (3.0–9.5) | 8.3 (7.0–10.0) | 30.7 (2.5) | 49.0 (3.9) | 1.4 (0.7) | 1.8 (0.8) | 7 (3–7) | 7 (7–8) | 40 (20–70) | 50 (20–60) | 23 | 14 | ||
| adult | 18.7 (12.0–26.0) | 26.1 (22.0–31.0) | 45.7 (1.8) | 53.4 (1.8) | 0.6 (0.3) | 4.4 (1.0) | 7 (6–7) | 7 (3–8) | 70 (20–90) | 75 (20–100) | 19 | 14 | ||
| Species | PC | Source of Variation | Reference | Disturbed | ||
|---|---|---|---|---|---|---|
| Z | p | Z | p | |||
| Nase | 1 | Season | −2.93 | <0.01 | −8.09 | <0.001 |
| Size-class | −3.83 | <0.001 | −0.72 | 0.471 | ||
| 2 | Season | 4.67 | <0.001 | 4.82 | <0.001 | |
| Size-class | 1.82 | 0.070 | 0.71 | 0.478 | ||
| Chub | 1 | Season | −2.27 | <0.05 | 0.06 | 0.950 |
| Size-class | −1.22 | 0.222 | 0.59 | 0.552 | ||
| 2 | Season | 4.07 | <0.001 | 4.35 | <0.001 | |
| Size-class | 0.29 | 0.774 | −0.58 | 0.559 | ||
| Calandino | 1 | Season | 4.32 | <0.001 | −2.62 | 0.01 |
| Size-class | -- | -- | -- | -- | ||
| 2 | Season | −1.85 | 0.064 | 1.41 | 0.059 | |
| Size-class | -- | -- | -- | -- | ||
| Trout | 1 | Season | −3.76 | <0.001 | −1.17 | 0.241 |
| Size-class | −4.86 | <0.001 | −2.75 | <0.01 | ||
| 2 | Season | 4.58 | <0.001 | 2.99 | <0.01 | |
| Size-class | 1.08 | 0.063 | 0.89 | 0.373 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.M.; Leite, R.; Costa, M.J.; Godinho, F.; Portela, M.M.; Pinheiro, A.N.; Boavida, I. Seasonal and Size-Related Fish Microhabitat Use Upstream and Downstream from Small Hydropower Plants. Water 2024, 16, 37. https://doi.org/10.3390/w16010037
Santos JM, Leite R, Costa MJ, Godinho F, Portela MM, Pinheiro AN, Boavida I. Seasonal and Size-Related Fish Microhabitat Use Upstream and Downstream from Small Hydropower Plants. Water. 2024; 16(1):37. https://doi.org/10.3390/w16010037
Chicago/Turabian StyleSantos, José M., Renan Leite, Maria J. Costa, Francisco Godinho, Maria M. Portela, António N. Pinheiro, and Isabel Boavida. 2024. "Seasonal and Size-Related Fish Microhabitat Use Upstream and Downstream from Small Hydropower Plants" Water 16, no. 1: 37. https://doi.org/10.3390/w16010037
APA StyleSantos, J. M., Leite, R., Costa, M. J., Godinho, F., Portela, M. M., Pinheiro, A. N., & Boavida, I. (2024). Seasonal and Size-Related Fish Microhabitat Use Upstream and Downstream from Small Hydropower Plants. Water, 16(1), 37. https://doi.org/10.3390/w16010037












