Magnetic Natural Coagulants for Plastic Recycling Industry Wastewater Treatability
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
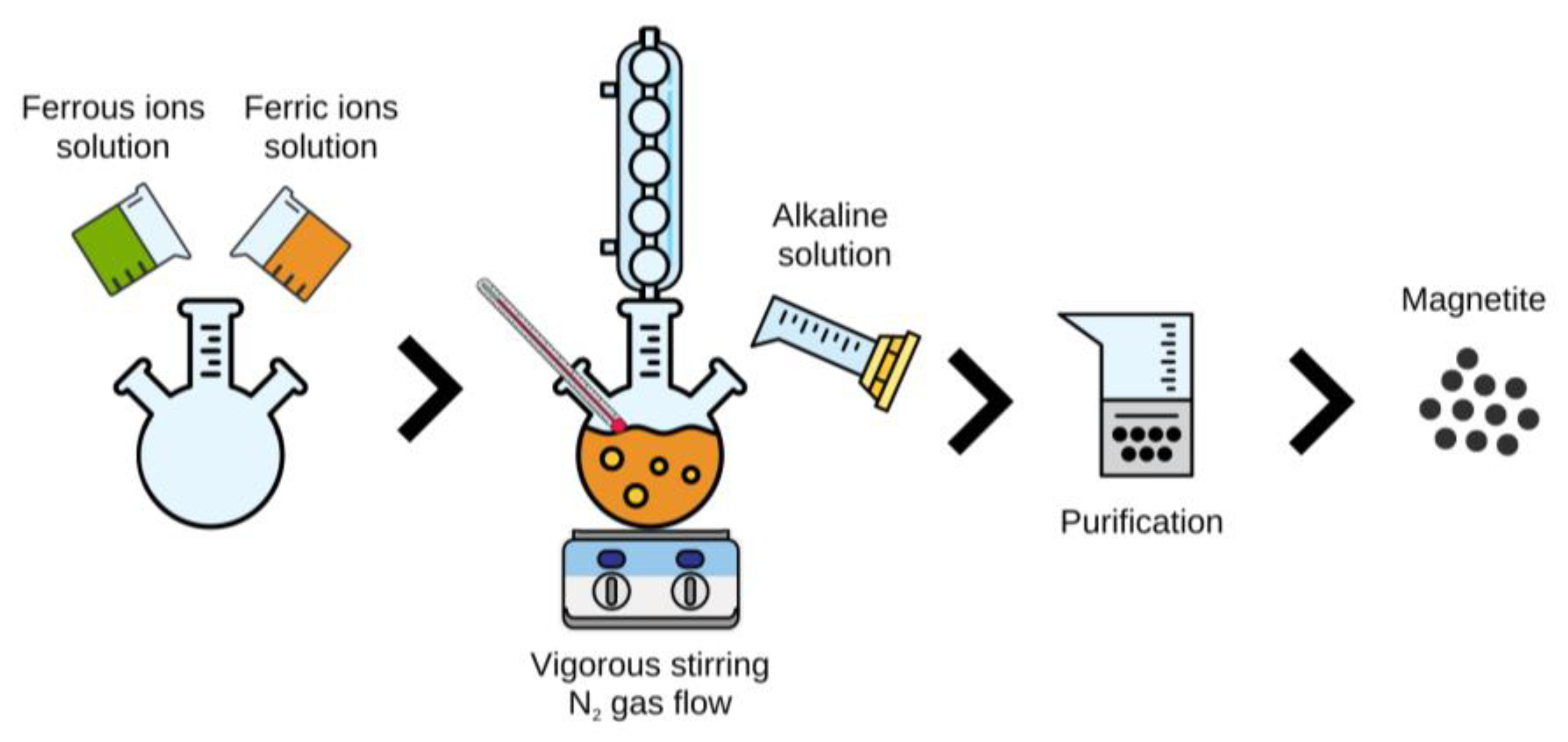
2.2. Synthesis of Magnetite Nanoparticles
2.3. Natural Coagulants Preparation
2.4. C/F/S Experiments
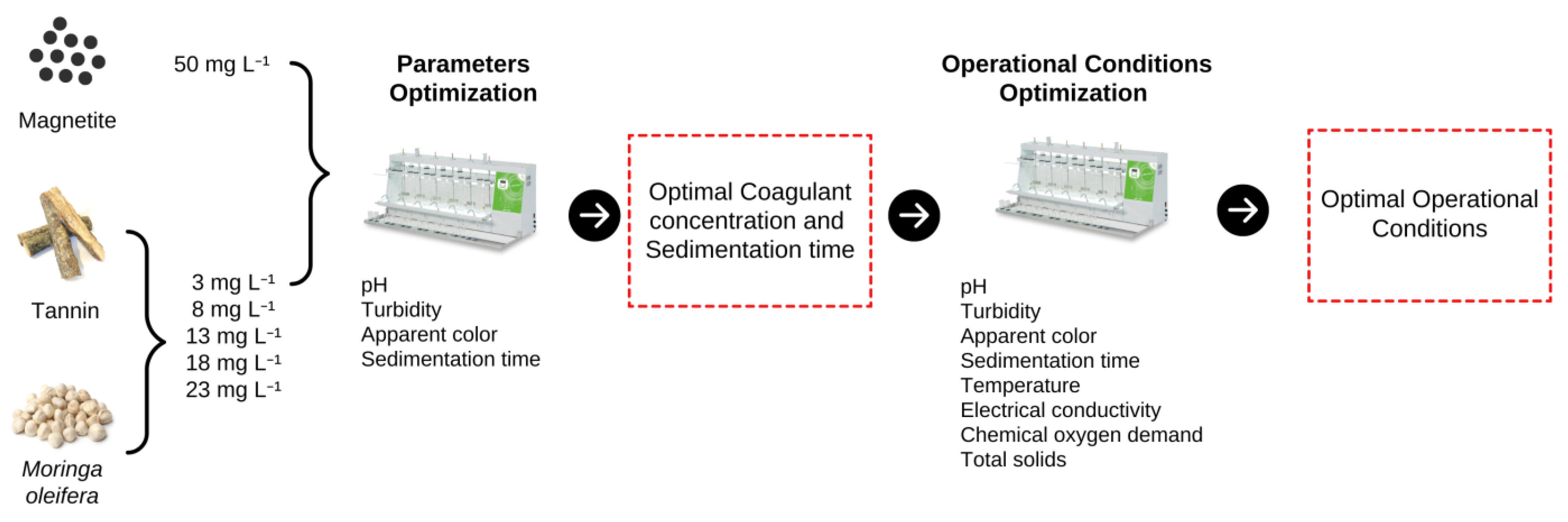
2.5. Experimental Design for Parameters Optimization
2.6. Optimization of Operational Conditions
3. Results and Discussion
3.1. Parameters Optimization
3.2. Optimization of Operational Conditions
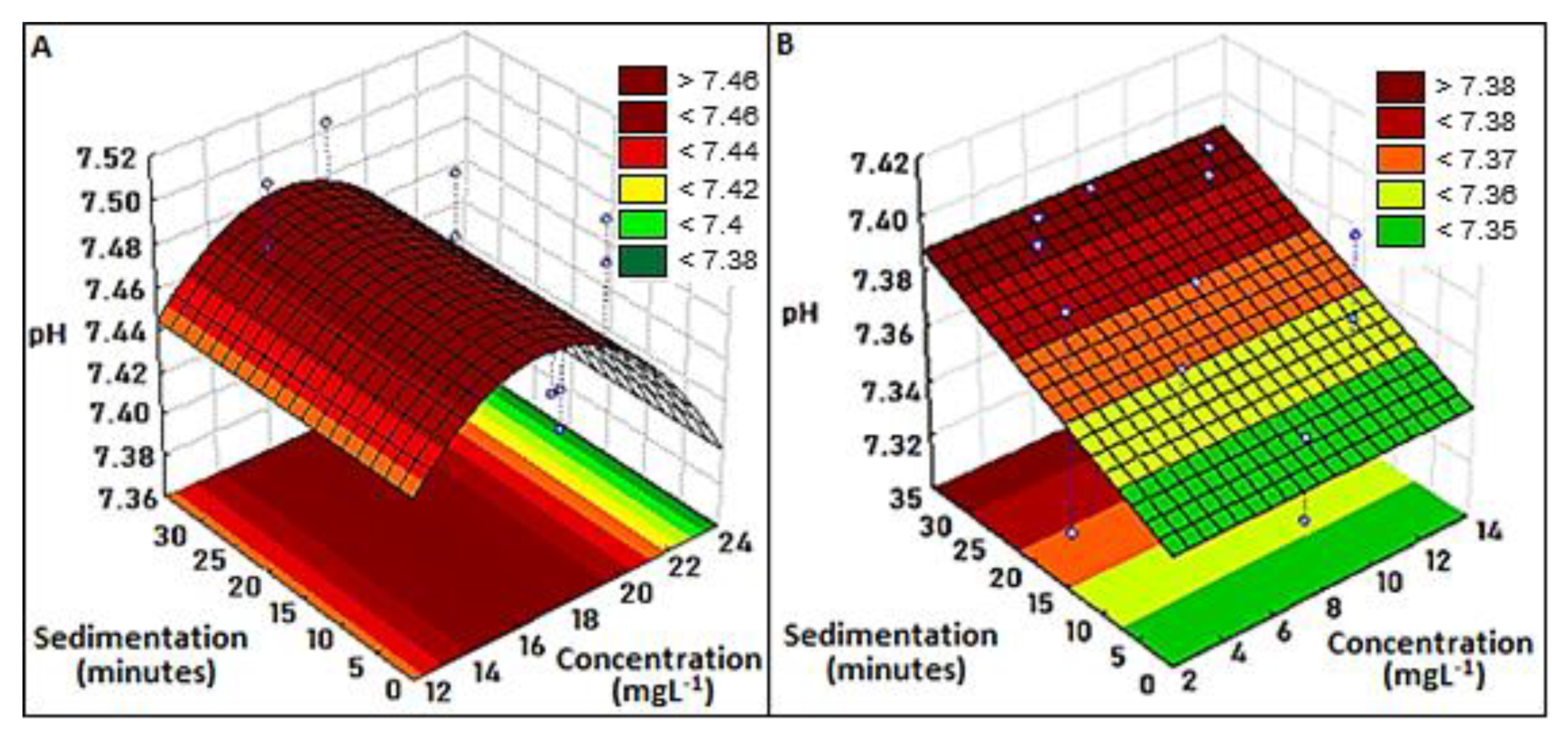
3.2.1. pH
3.2.2. Temperature
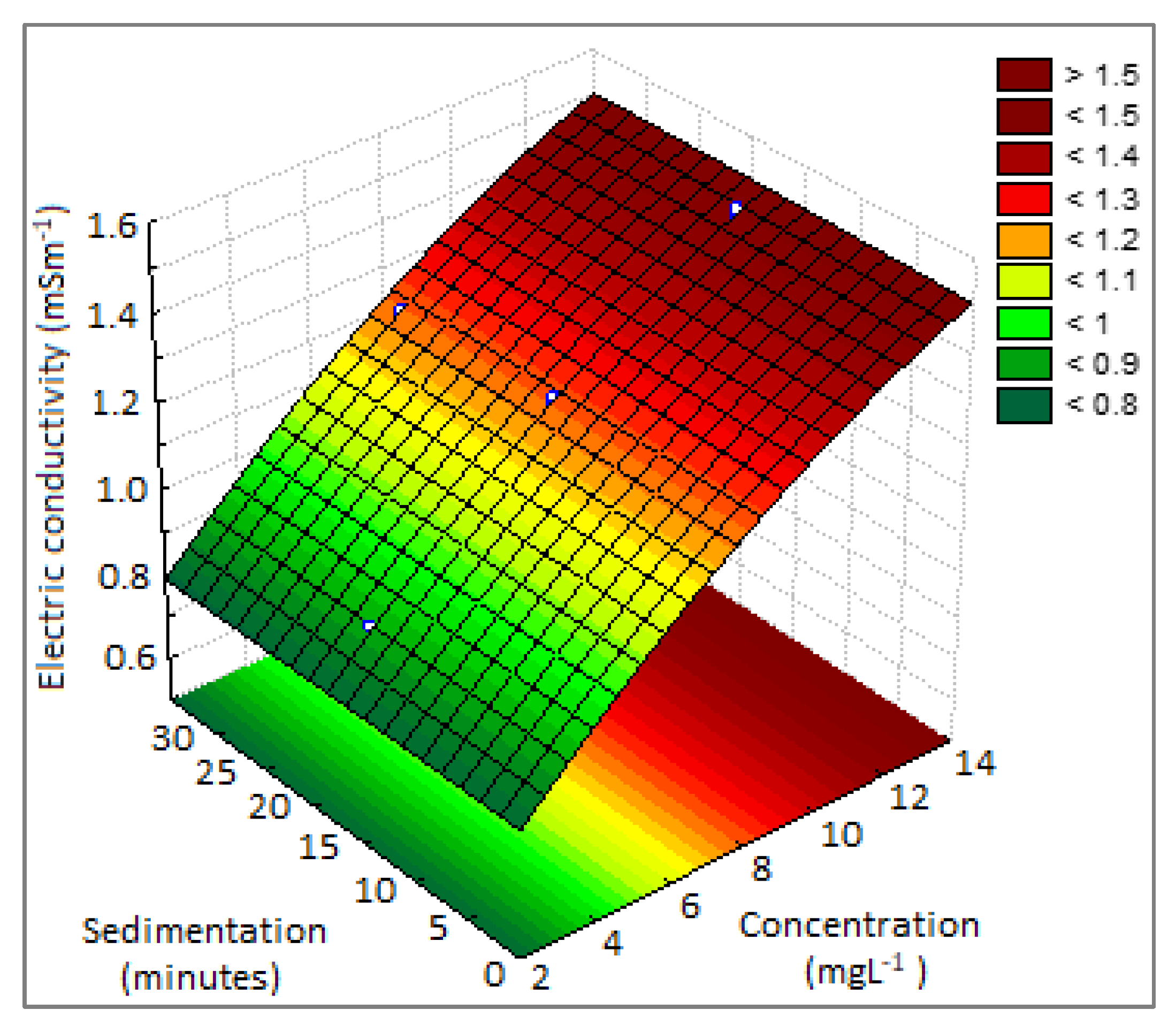
3.2.3. Electrical Conductivity
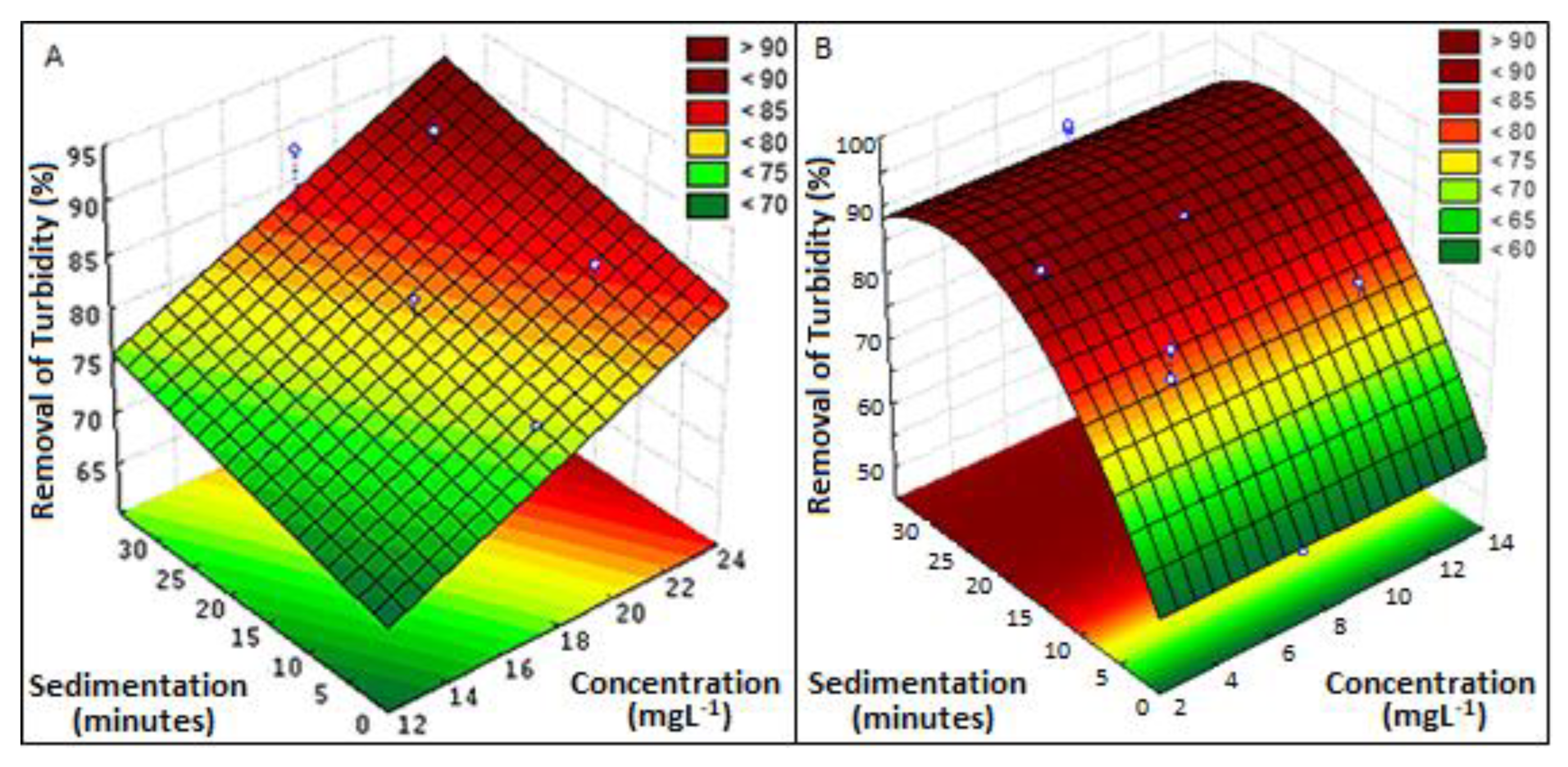
3.2.4. Turbidity
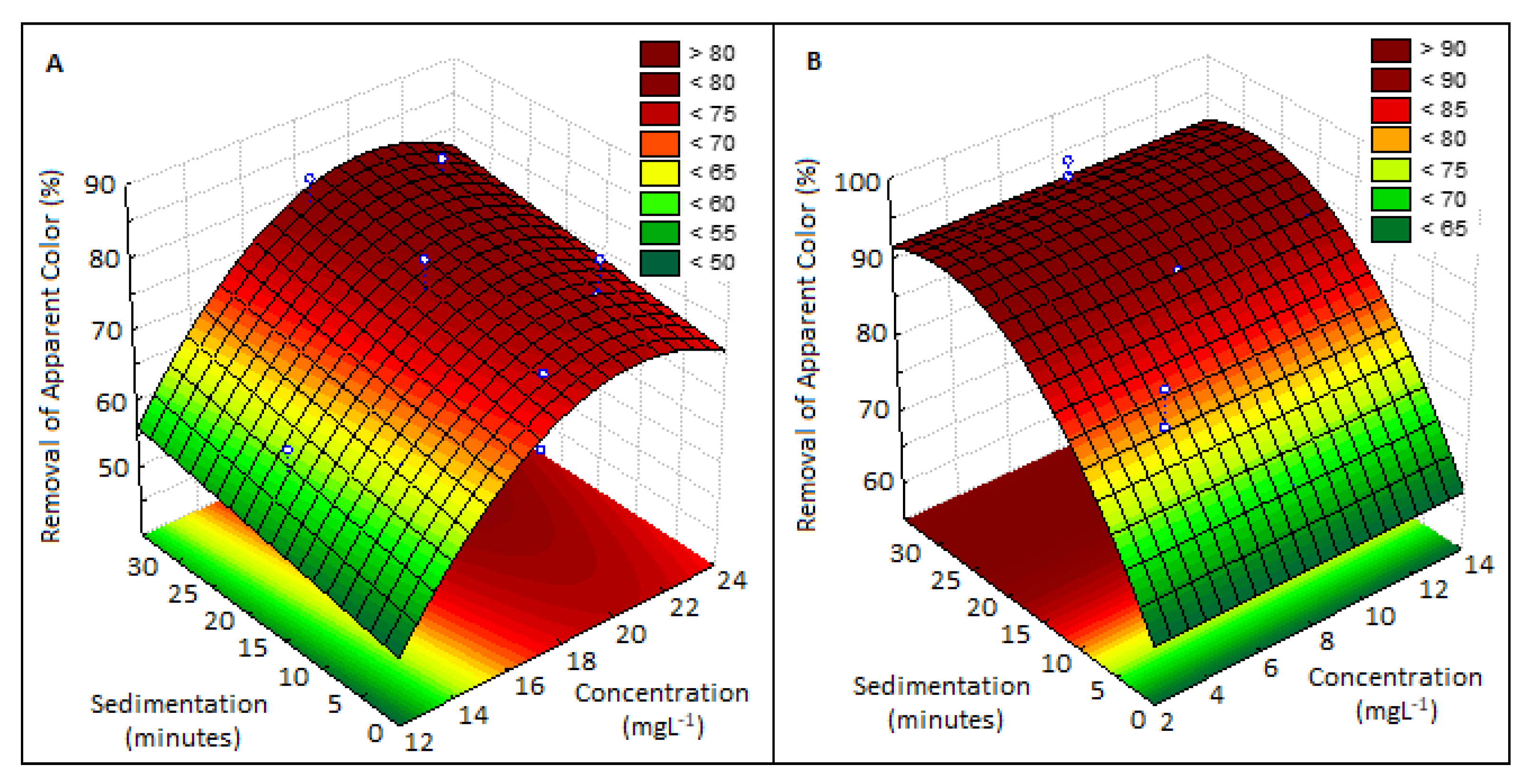
3.2.5. Color Removal
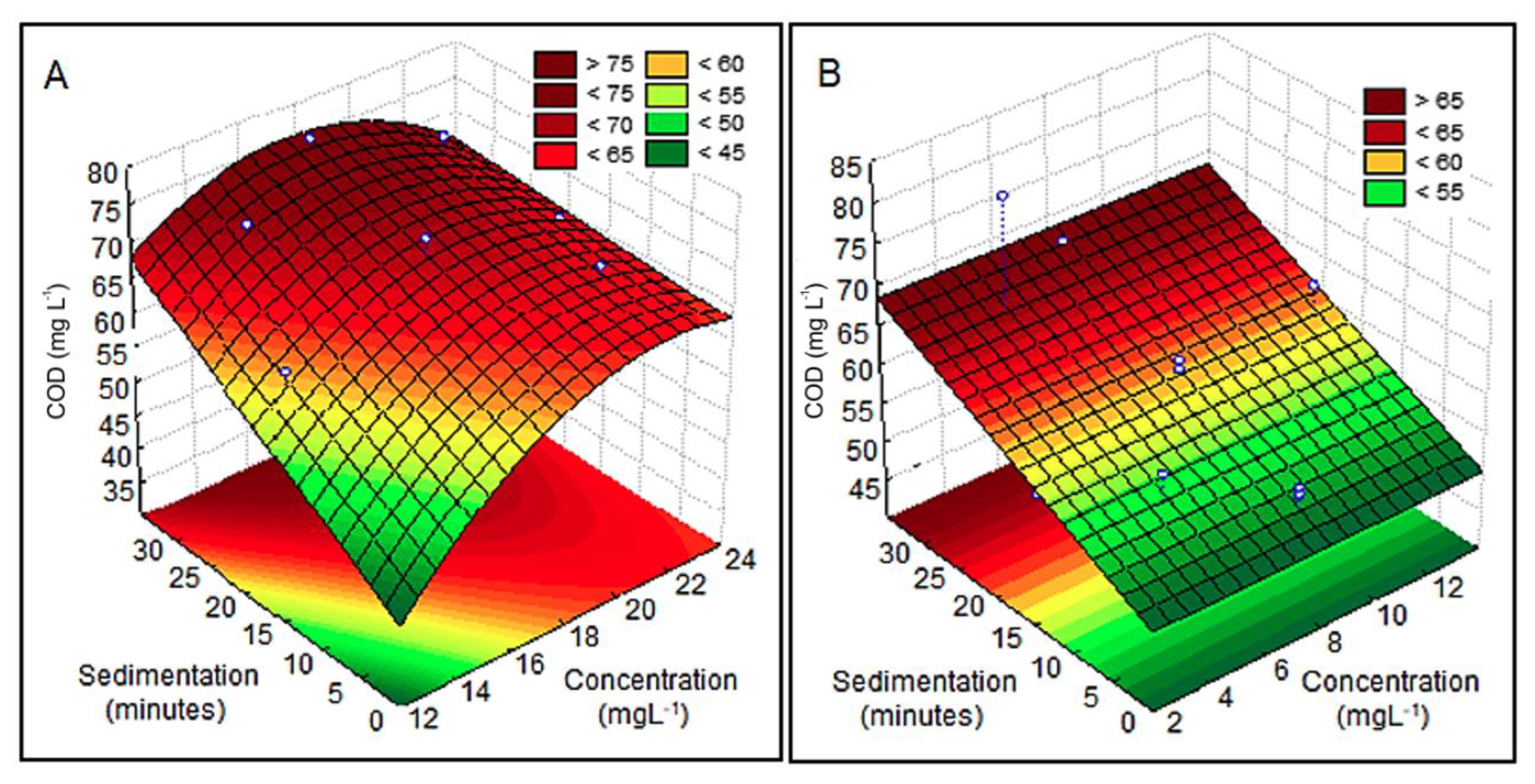
3.2.6. COD
3.2.7. Total Solids
3.3. Other Natural Coagulants Associated with Magnetite Nanoparticles
4. Conclusions and Future Perspectives
- Different magnetic nanoparticles can be tested, especially with Moringa oleifera, which obtained the best removals efficiencies.
- The adsorption mechanism and interaction with magnetic nanoparticles can be further studied.
- Other real effluents, pilot, and large-scale experiments are necessary.
- Leaching and loss experiments related to the stability of the nanoparticles.
- Ecotoxicity analysis caused by magnetite nanoparticles and by-products.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozdemir, N.C.; Yel, E. Synthesis of a New Flocculant from Waste Polystyrene: Plastic Recycling Industry Wastewater Treatability. Water Air Soil Pollut. 2023, 234, 88. [Google Scholar] [CrossRef]
- Walker, T.R. (Micro)Plastics and the UN Sustainable Development Goals. Curr. Opin. Green Sustain. Chem. 2021, 30, 100497. [Google Scholar] [CrossRef]
- Tejaswini, M.S.S.R.; Pathak, P.; Ramkrishna, S.; Ganesh, P.S. A Comprehensive Review on Integrative Approach for Sustainable Management of Plastic Waste and Its Associated Externalities. Sci. Total Environ. 2022, 825, 153973. [Google Scholar] [CrossRef] [PubMed]
- d’Ambrières, W. Plastics Recycling Worldwide: Current Overview and Desirable Changes. Field Actions Sci. Rep. J. Field Actions 2019, 19, 12–21. [Google Scholar]
- Mancini, S.D.; de Medeiros, G.A.; Paes, M.X.; de Oliveira, B.O.S.; Antunes, M.L.P.; de Souza, R.G.; Ferraz, J.L.; Bortoleto, A.P.; de Oliveira, J.A.P. Circular Economy and Solid Waste Management: Challenges and Opportunities in Brazil. Circ. Econ. Sustain. 2021, 1, 261–282. [Google Scholar] [CrossRef]
- Altieri, V.G.; De Sanctis, M.; Sgherza, D.; Pentassuglia, S.; Barca, E.; Di Iaconi, C. Treating and Reusing Wastewater Generated by the Washing Operations in the Non-Hazardous Plastic Solid Waste Recycling Process: Advanced Method vs. Conventional Method. J. Environ. Manag. 2021, 284, 112011. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Yaser, A.Z.; Lamaming, J. Synthesising Tannin-Based Coagulants for Water and Wastewater Application: A Review. J. Environ. Chem. Eng. 2021, 9, 105007. [Google Scholar] [CrossRef]
- Niu, Q. (Ed.) Overview of the Relationship between Aluminum Exposure and Health of Human Being. In Neurotoxicity of Aluminum; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; pp. 1–31. ISBN 9789811313707. [Google Scholar]
- Mold, M.; Chmielecka, A.; Rodriguez, M.R.R.; Thom, F.; Linhart, C.; King, A.; Exley, C. Aluminium in Brain Tissue in Multiple Sclerosis. Int. J. Environ. Res. Public Health 2018, 15, 1777. [Google Scholar] [CrossRef]
- Shaw, C.A.; Tomljenovic, L. Aluminum in the Central Nervous System (CNS): Toxicity in Humans and Animals, Vaccine Adjuvants, and Autoimmunity. Immunol. Res. 2013, 56, 304–316. [Google Scholar] [CrossRef]
- Bahrodin, M.B.; Zaidi, N.S.; Hussein, N.; Sillanpää, M.; Prasetyo, D.D.; Syafiuddin, A. Recent Advances on Coagulation-Based Treatment of Wastewater: Transition from Chemical to Natural Coagulant. Curr. Pollut. Rep. 2021, 7, 379–391. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Gamelas, J.A.F.; Rasteiro, M.G. Up-Scaling of Tannin-Based Coagulants for Wastewater Treatment: Performance in a Water Treatment Plant. Environ. Sci. Pollut. Res. 2020, 27, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Ueda Yamaguchi, N.; Cusioli, L.F.; Quesada, H.B.; Camargo Ferreira, M.E.; Fagundes-Klen, M.R.; Salcedo Vieira, A.M.; Gomes, R.G.; Vieira, M.F.; Bergamasco, R. A Review of Moringa Oleifera Seeds in Water Treatment: Trends and Future Challenges. Process. Saf. Environ. Prot. 2021, 147, 405–420. [Google Scholar] [CrossRef]
- da Conceição, V.M.; Yamaguchi, N.U.; de Jesus Bassetti, F.; Bergamasco, R. Process Performance Combining Natural Coagulant Moringa Oleifera Lam and Ultrafiltration for Groundwater Defluoridation. Water Air Soil Pollut. 2021, 232, 222. [Google Scholar] [CrossRef]
- Valverde, K.C.; de Souza Paccola, E.A.; Pomini, A.M.; Yamaguchi, N.U.; Bergamasco, R. Combined Water Treatment with Extract of Natural Moringa oleifera Lam and Synthetic Coagulant. Rev. Ambient. Água 2018, 13, e2135. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Hayder, G.; Latiff, A.A.A.; Aziz, N.A.A.; Umaru, I.; Ghaleb, A.A.S.; Abubakar, S.; Lawal, I.M.; Nasara, M.A. Sustainable Use of Natural and Chemical Coagulants for Contaminants Removal from Palm Oil Mill Effluent: A Comparative Analysis. Ain. Shams. Eng. J. 2020, 11, 951–960. [Google Scholar] [CrossRef]
- Lopes, E.C.; Santos, S.C.R.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Evaluation of a Tannin-Based Coagulant on the Decolorization of Synthetic Effluents. J. Environ. Chem. Eng. 2019, 7, 103125. [Google Scholar] [CrossRef]
- Kristianto, H. Recent Advances on Magnetic Natural Coagulant: A Mini Review. Environ. Technol. Rev. 2021, 10, 255–270. [Google Scholar] [CrossRef]
- Mateus, G.A.P.; Paludo, M.P.; dos Santos, T.R.T.; Silva, M.F.; Nishi, L.; Fagundes-Klen, M.R.; Gomes, R.G.; Bergamasco, R. Obtaining Drinking Water Using a Magnetic Coagulant Composed of Magnetite Nanoparticles Functionalized with Moringa Oleifera Seed Extract. J. Environ. Chem. Eng. 2018, 6, 4084–4092. [Google Scholar] [CrossRef]
- Santos, T.R.T.; Silva, M.F.; Nishi, L.; Vieira, A.M.S.; Klein, M.R.F.; Andrade, M.B.; Vieira, M.F.; Bergamasco, R. Development of a Magnetic Coagulant Based on Moringa Oleifera Seed Extract for Water Treatment. Environ. Sci. Pollut. Res. 2016, 23, 7692–7700. [Google Scholar] [CrossRef]
- Marcuello, C.; Chambel, L.; Rodrigues, M.S.; Ferreira, L.P.; Cruz, M.M. Magnetotactic Bacteria: Magnetism beyond Magnetosomes. IEEE Trans. Nanobiosci. 2018, 17, 555–559. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Noor, A.; Affam, A.C.; Ghfar, A.A.; Usman, A.K.; Lawal, I.M.; Birniwa, A.H.; Kankia, M.U.; Afolabi, H.K.; et al. Parametric Optimization and Kinetic Modelling for Organic Matter Removal from Agro-Waste Derived Paper Packaging Biorefinery Wastewater. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Naushad, M.; Lawal, I.M.; Noor, A.; Affam, A.C.; Birniwa, A.H.; Abubakar, S.; Soja, U.B.; Abioye, K.J.; et al. Removal of Nutrients from Pulp and Paper Biorefinery Effluent: Operation, Kinetic Modelling and Optimization by Response Surface Methodology. Environ. Res. 2022, 214, 114091. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Transforming Our World: The 2030 Agenda For Sustainable Development; United Nations: New York, NY, USA, 2015; p. 41. [Google Scholar]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large PH Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; Pharmabooks: Washington, DC, USA; New York, NY, USA, 2017; ISBN 10-087553287X. [Google Scholar]
- CONAMA. Resolução No 357-Dispõe Sobre a Classificação Dos Corpos de Água e Diretrizes Ambientais Para o Seu Enquadramento [...]. CONAMA 2005, 27. [Google Scholar]
- Ahmad, N.N.R.; Ang, W.L.; Leo, C.P.; Mohammad, A.W.; Hilal, N. Current Advances in Membrane Technologies for Saline Wastewater Treatment: A Comprehensive Review. Desalination 2021, 517, 115170. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, F.; Wang, D.; Xu, X.; Zhou, Q. Comparison of Novel Magnetic Polyaluminum Chlorides Involved Coagulation with Traditional Magnetic Seeding Coagulation: Coagulant Characteristics, Treating Effects, Magnetic Sedimentation Efficiency and Floc Properties. Sep. Purif. Technol. 2017, 182, 118–127. [Google Scholar] [CrossRef]
- Valverde, K.C.; COldebella, P.F.; Vieira, A.M.S.; Nishi, L.; Bongiovani, M.C.; Baptista, A.T.A.; Fagundes-Klen, M.R.; dos Santos, O.A.A. Rosângela Bergamasco Preparation of Moringa Oleifera Seeda as Coagulant in Water Treatment. Environ. Eng. Manag. J. 2018, 17, 1123–1129. [Google Scholar] [CrossRef]
- Wydra, R.J.; Oliver, C.E.; Anderson, K.W.; Dziubla, T.D.; Hilt, J.Z. Accelerated Generation of Free Radicals by Iron Oxide Nanoparticles in the Presence of an Alternating Magnetic Field. RSC Adv. 2015, 5, 18888–18893. [Google Scholar] [CrossRef] [PubMed]
- Gerbin, E.; Frapart, Y.-M.; Marcuello, C.; Cottyn, B.; Foulon, L.; Pernes, M.; Crônier, D.; Molinari, M.; Chabbert, B.; Ducrot, P.-H.; et al. Dual Antioxidant Properties and Organic Radical Stabilization in Cellulose Nanocomposite Films Functionalized by In Situ Polymerization of Coniferyl Alcohol. Biomacromolecules 2020, 21, 3163–3175. [Google Scholar] [CrossRef]
- Mohamed Noor, M.H.; Mohd Azli, M.F.Z.; Ngadi, N.; Mohammed Inuwa, I.; Anako Opotu, L.; Mohamed, M. Optimization of Sonication-Assisted Synthesis of Magnetic Moringa Oleifera as an Efficient Coagulant for Palm Oil Wastewater Treatment. Environ. Technol. Innov. 2022, 25, 102191. [Google Scholar] [CrossRef]
- De Oliveira, A.M.; Mateus, G.A.P.; dos Santos, T.R.T.; de Abreu Filho, B.A.; Gomes, R.G.; Bergamasco, R. Functionalized Magnetite Nanoparticles with Moringa Oleifera with Potent Antibacterial Action in Wastewater. Environ. Technol. 2021, 42, 4296–4305. [Google Scholar] [CrossRef] [PubMed]
- Miyashiro, C.S.; Mateus, G.A.P.; dos Santos, T.R.T.; Paludo, M.P.; Bergamasco, R.; Fagundes-Klen, M.R. Synthesis and Performance Evaluation of a Magnetic Biocoagulant in the Removal of Reactive Black 5 Dye in Aqueous Medium. Mater. Sci. Eng. C 2021, 119, 111523. [Google Scholar] [CrossRef] [PubMed]
- Praveena, S.M.; Xin-Yi, C.K.; Liew, J.Y.C.; Khan, M.F. Functionalized Magnetite Nanoparticle Coagulants with Tropical Fruit Waste Extract: A Potential for Water Turbidity Removal. Arab. J. Sci. Eng. 2022. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Amo-Duodu, G.; Tetteh, E.K.; Rathilal, S. Effect of Magnetized Coagulants on Wastewater Treatment: Rice Starch and Chitosan Ratios Evaluation. Polymers 2022, 14, 4342. [Google Scholar] [CrossRef]
- Kristianto, H.; Tanuarto, M.Y.; Prasetyo, S.; Sugih, A.K. Magnetically Assisted Coagulation Using Iron Oxide Nanoparticles-Leucaena Leucocephala Seeds’ Extract to Treat Synthetic Congo Red Wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 3561–3570. [Google Scholar] [CrossRef]
- Hermawan, E.; Carmen, L.U.; Kristianto, H.; Prasetyo, S.; Sugih, A.K.; Arbita, A.A. A Synthesis of Magnetic Natural Coagulant and Its Application to Treat Congo Red Synthetic Wastewater. Water Air Soil Pollut. 2022, 233, 443. [Google Scholar] [CrossRef]
| Parameters | Results |
|---|---|
| Temperature (°C) | 25.3 |
| pH | 7.26 |
| Electrical conductivity (mS m−1) | 0.682 |
| Turbidity (NTU) | 379 |
| Apparent color (×103 uH) | 1.34 |
| Chemical oxygen demand (mg L−1) | 596 |
| Total solids (mg L−1) | 14.3 |
| Test | Variable | CCRD 1 | |||
|---|---|---|---|---|---|
| Time (min) | Tannin Concentration (mg L−1) | Moringa oleifera Concentration (mg L−1) | Time | Coagulant Concentration | |
| 1 | 8 | 14.45 | 4.63 | −1 | −1 |
| 2 | 8 | 21.55 | 11.37 | −1 | 1 |
| 3 | 28 | 14.45 | 4.63 | 1 | −1 |
| 4 | 28 | 21.55 | 11.37 | 1 | 1 |
| 5 | 18 | 13 | 3 | 0 | −1.41 |
| 6 | 18 | 23 | 13 | 0 | 1.41 |
| 7 | 3 | 18 | 8 | −1.41 | 0 |
| 8 | 33 | 18 | 8 | 1.41 | 0 |
| 9 | 18 | 18 | 8 | 0 | 0 |
| 10 | 18 | 18 | 8 | 0 | 0 |
| 11 | 18 | 18 | 8 | 0 | 0 |
| Coagulant | Effects | Coefficient | Standard Error | T | p-Value | R2 |
|---|---|---|---|---|---|---|
| Tannin | Intercept | 7.459 | 0.008 | 950.002 | 0.000 | 0.297 |
| Concentration | −0.028 | 0.013 | −2.138 | 0.046 | ||
| Quadratic concentration | −0.041 | 0.015 | −2.709 | 0.014 | ||
| Moringa oleifera | Intercept | 7.365 | 0.005 | 1393.791 | 0.000 | 0.321 |
| Block | 0.025 | 0.011 | 2.409 | 0.026 | ||
| Time | 0.027 | 0.012 | 2.251 | 0.036 |
| Effects | Coefficient | Standard Error | T | p-Value | R2 |
|---|---|---|---|---|---|
| Intercept | 1.238 | 0.009 | 141.574 | 0.000 | 0.986 |
| Block | 0.023 | 0.009 | 2.480 | 0.024 | |
| Concentration | 0.398 | 0.010 | 37.969 | 0.000 | |
| Quadratic concentration | −0.056 | 0.012 | −4.639 | 0.000 | |
| Quadratic time | −0.030 | 0.012 | −2.518 | 0.022 |
| Coagulant | Effects | Coefficient | Standard Error | T | p-Value | R2 |
|---|---|---|---|---|---|---|
| Tannin | Intercept | 79.547 | 0.460 | 172.793 | 0.000 | 0.827 |
| Block | 3.828 | 0.921 | 4.158 | 0.001 | ||
| Concentration | 8.988 | 1.082 | 8.308 | 0.000 | ||
| Time | 4.349 | 1.047 | 4.152 | 0.001 | ||
| Moringa oleifera | Intercept | 88.930 | 0.910 | 97.719 | 0.000 | 0.769 |
| Time | 15.588 | 1.514 | 10.293 | 0.000 | ||
| Quadratic time | −7.475 | 1.606 | −4.654 | 0.000 |
| Coagulant | Effects | Coefficient | Standard Error | T | p-Value | R2 |
|---|---|---|---|---|---|---|
| Tannin | Intercept | 76.001 | 0.823 | 92.337 | 0.000 | 0.878 |
| Block | 7.069 | 1.180 | 5.991 | 0.000 | ||
| Concentration | 12.622 | 1.387 | 9.103 | 0.000 | ||
| Quadratic concentration | −8.647 | 1.585 | −5.457 | 0.000 | ||
| Time | 3.626 | 1.342 | 2.701 | 0.015 | ||
| Moringa oleifera | Intercept | 88.930 | 0.910 | 97.719 | 0.000 | 0.857 |
| Time | 15.588 | 1.514 | 10.293 | 0.000 | ||
| Quadratic time | −7.475 | 1.606 | −4.654 | 0.000 |
| Coagulant | Effects | Coefficient | Standard Error | T | p-Value | R2 |
|---|---|---|---|---|---|---|
| Tannin | Intercept | 68.252 | 0.847 | 80.588 | 0.000 | 0.786 |
| Block | 4.348 | 1.214 | 3.581 | 0.002 | ||
| Concentration | 5.708 | 1.427 | 4.001 | 0.001 | ||
| Quadratic concentration | −5.917 | 1.631 | −3.629 | 0.002 | ||
| Time | 8.330 | 1.381 | 6.031 | 0.000 | ||
| Concentration and time interaction | −3.956 | 2.014 | −1.965 | 0.067 | ||
| Moringa oleifera | Intercept | 59.433 | 1.313 | 45.257 | 0.000 | 0.347 |
| Time | 10.417 | 2.988 | 3.487 | 0.002 |
| Coagulant | Wastewater | Coagulant Dosage (mg L−1) | Magnetite Dosage (mg L−1) | Raw Water Parameters | Sedimentation Time (min) | Removal (%) | Reference |
|---|---|---|---|---|---|---|---|
| Moringa oleifera seeds | Surface water | 400 | 10 | Turbidity: 79 NTU a, color: 265 uH b | 30 | 90 (turbidity), 85 (color) | [20] |
| Moringa oleifera seeds | Surface water | 200 | 67 | Turbidity: 143 NTU, Color: 509 uH | 10 | 97 (color), 97 (turbidity) | [19] |
| Moringa oleifera seeds | Palm oil wastewater | 1000 | ~10 | TSS c: 120 mg L−1, color: 4 uH, turbidity: 65 NTU, COD d: 16,405 mg L−1 | 15 | 83 (TSS), 28 (color), 85 (COD) | [33] |
| Moringa oleifera seeds | Synthetic dairy effluent | 16,000 | 1000 | Color: 1336 uH, turbidity: 200 NTU, bacterial load 103 CFU ml−1 | 30 | 82 (color), 50 (turbidity), 99 (S aureus) | [34] |
| Moringa oleifera seeds | Synthetic textile wastewater | 100 | - | 10 mg L−1 RB5 e, color: 162 uH | 20 | 94 (color), 96 (RB5) | [35] |
| Banana extract | Synthetic turbid water | 2600 | 144 | 150 NTU | 25 | 93 (turbidity) | [36] |
| Rice starch | Real wastewater | 4000 | 4000 | Color: 315 uH, turbidity: 46 NTU, COD: 352 mg L−1 | 20 | 85 (turbidity), 85 (color), 54 (COD) | [37] |
| Leucaena seeds | Synthetic textile wastewater | - | 3 | 50 mg L−1 CR f | 30 | 90 (CR, pH 3) | [38] |
| Leucaena seeds | Synthetic wastewater | 100 | - | 10 mg L−1 CR | 5 | 89 (CR, pH 2) | [39] |
| Acacia mearnsii bark | Plastic recycling industry wastewater | 22 | 50 | Turbidity: 379 NTU, color: 1340 uH, COD: 596, TS g: 14 mg L−1 | 28 | 89 (turbidity), 84 (color), 74 (COD), 94 (TS) | This study |
| Moringa oleifera seeds | 5 | 28 | 92 (turbidity), 92 (color), 76 (COD), 96 (TS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, T.; Ladeia Janz, F.J.; Vizibelli, D.; Borges, J.C.Â.; Borssoi, J.A.; Fukumoto, A.A.F.; Bergamasco, R.; Ueda Yamaguchi, N.; Pereira, E.R. Magnetic Natural Coagulants for Plastic Recycling Industry Wastewater Treatability. Water 2023, 15, 1276. https://doi.org/10.3390/w15071276
Ribeiro T, Ladeia Janz FJ, Vizibelli D, Borges JCÂ, Borssoi JA, Fukumoto AAF, Bergamasco R, Ueda Yamaguchi N, Pereira ER. Magnetic Natural Coagulants for Plastic Recycling Industry Wastewater Treatability. Water. 2023; 15(7):1276. https://doi.org/10.3390/w15071276
Chicago/Turabian StyleRibeiro, Thais, Fellipe Jhordã Ladeia Janz, Dandley Vizibelli, Julio Cesar Ângelo Borges, Joelmir André Borssoi, Amanda Alcaide Francisco Fukumoto, Rosângela Bergamasco, Natália Ueda Yamaguchi, and Edilaine Regina Pereira. 2023. "Magnetic Natural Coagulants for Plastic Recycling Industry Wastewater Treatability" Water 15, no. 7: 1276. https://doi.org/10.3390/w15071276
APA StyleRibeiro, T., Ladeia Janz, F. J., Vizibelli, D., Borges, J. C. Â., Borssoi, J. A., Fukumoto, A. A. F., Bergamasco, R., Ueda Yamaguchi, N., & Pereira, E. R. (2023). Magnetic Natural Coagulants for Plastic Recycling Industry Wastewater Treatability. Water, 15(7), 1276. https://doi.org/10.3390/w15071276








