Use of Extracted Proteins from Oak Leaves as Bio-Coagulant for Water and Wastewater Treatment: Optimization by a Fractional Factorial Design
Abstract
1. Introduction
2. Materials and Methods
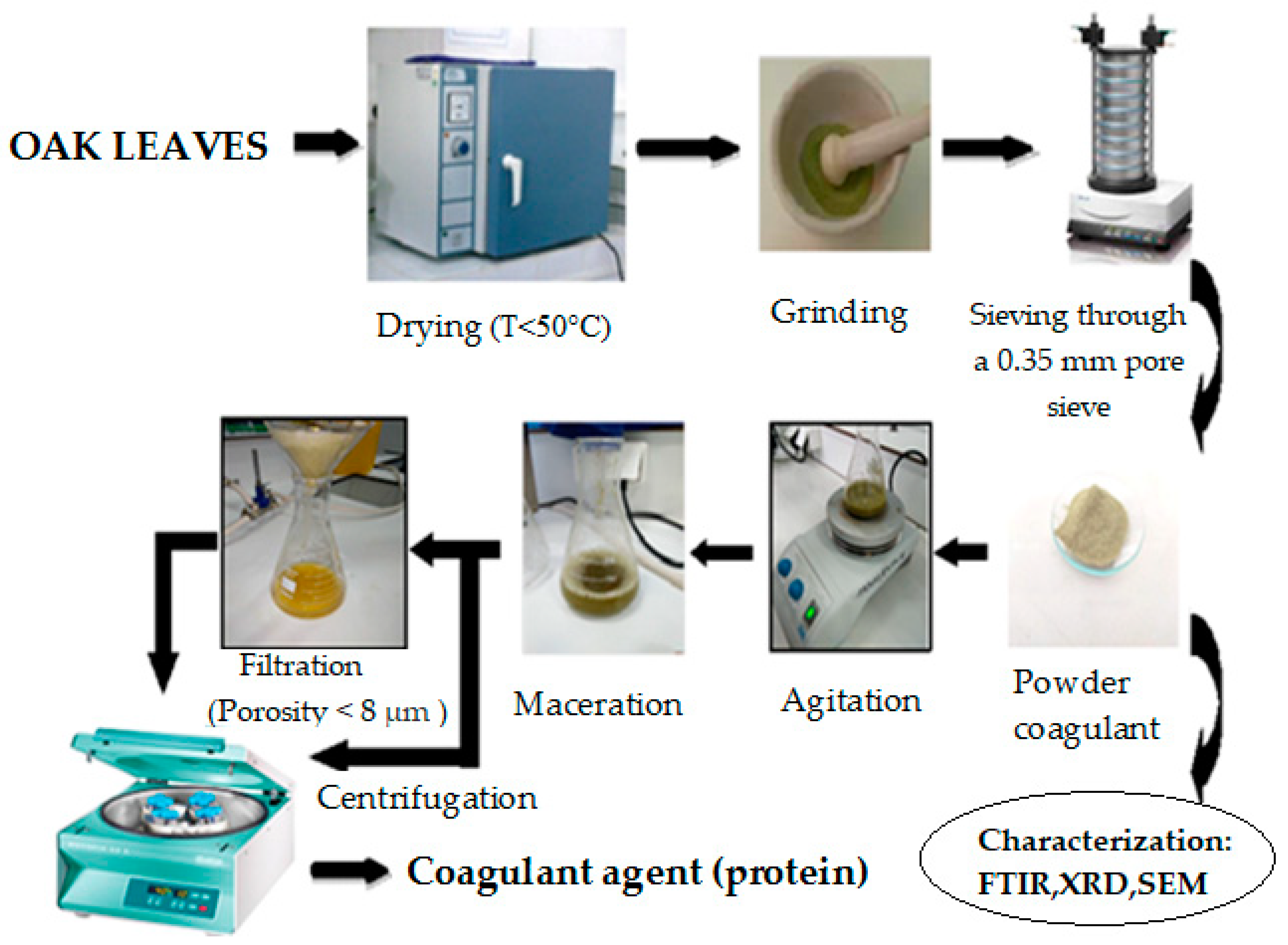
2.1. Raw Material for Bio-Coagulant Agent Extraction
2.2. Extraction and Purification of Proteins from Oak Leaves
- i.
- Washing with tap water;
- ii.
- Drying at a relatively low temperature of 50 °C for 24 h to avoid denaturation of the active compounds responsible for coagulation [32];
- iii.
- Grinding for particle size reduction and sieving up to obtain a homogeneous fine powder;
- iv.
- Sieving through a 0.35 mm pore sieve.
2.3. Water Samples Collection
2.4. Jar Test Experiments
2.5. Analytical Methods
3. Results and Discussions
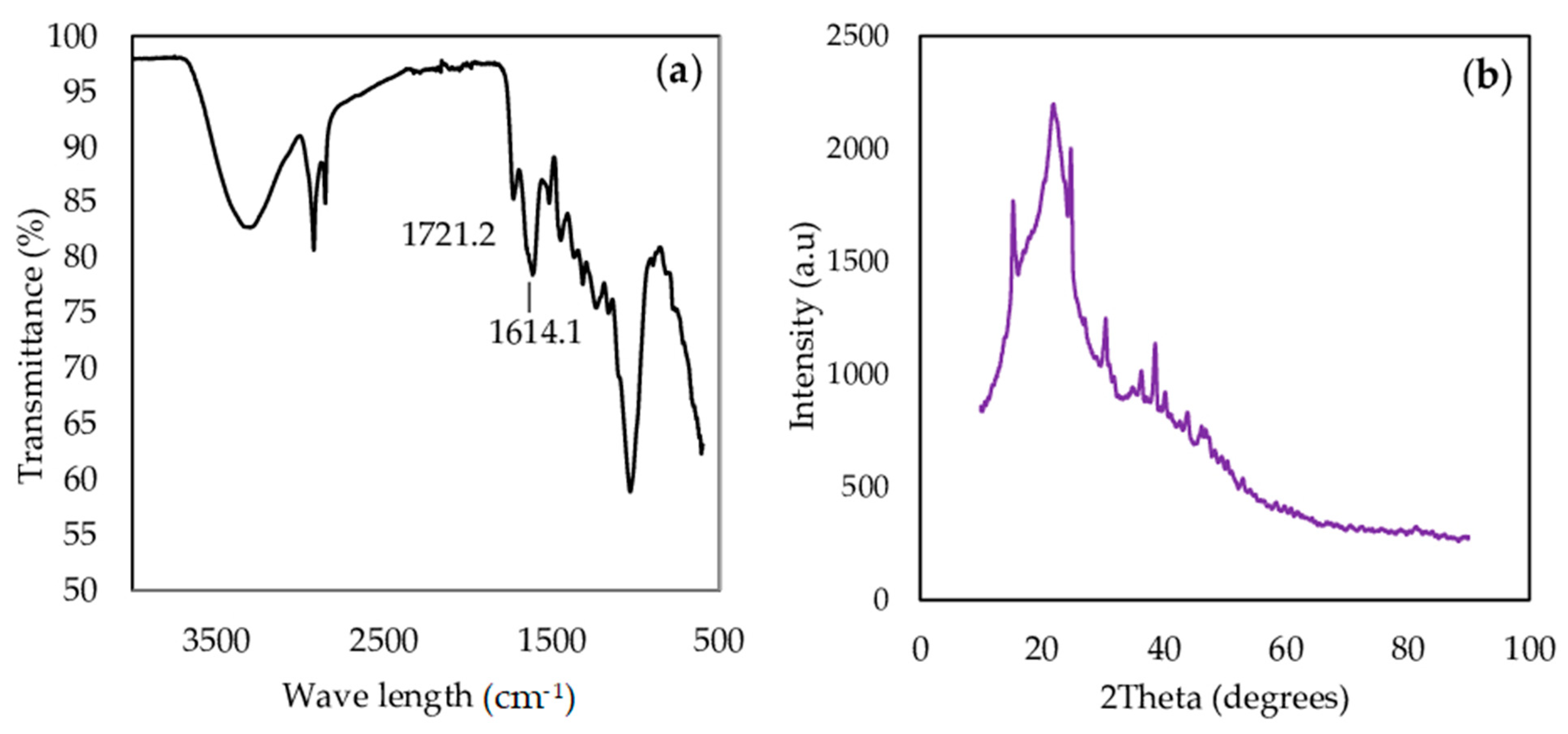
3.1. Characterization of Oak Leaves Powder
3.1.1. FTIR and XRD Analysis
3.1.2. SEM Analysis
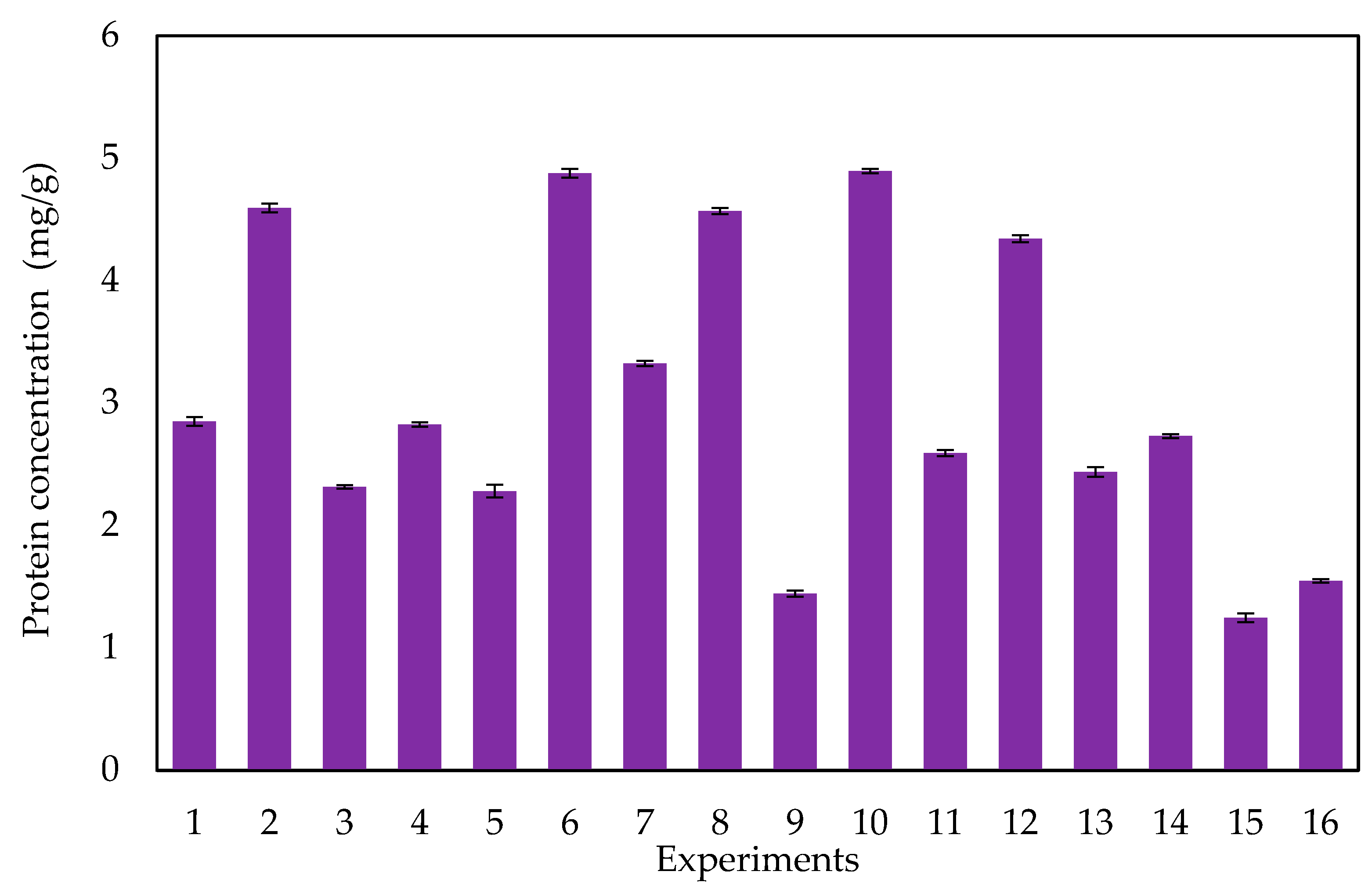
3.2. Factorial Design
3.2.1. Effect of Operational Parameters on Protein Extraction
3.2.2. Optimization of Protein Extraction
3.3. Application for Water and Wastewater Treatment
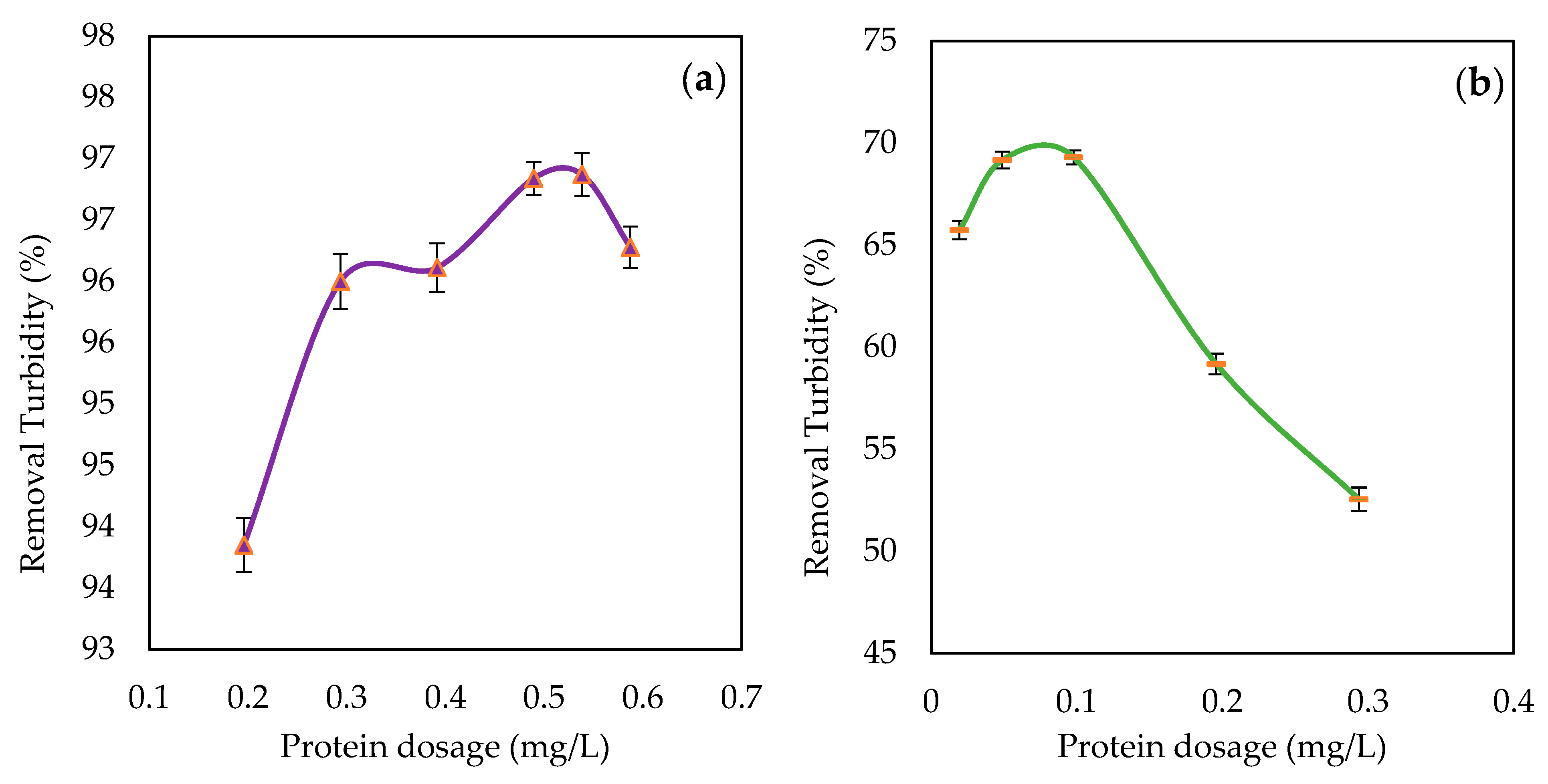
3.3.1. Effect of the Coagulant Protein Dosage on Turbidity Removal from Water
3.3.2. Effect of Coagulant Protein Dosage on Other Water Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BOD5 | Biochemical oxygen demand |
| COD | Chemical oxygen demand |
| DOE | Design of experiences |
| E | East |
| Ec | Electrical conductivity |
| FTIR | Fourier-transform infrared spectrophotometry |
| N | North |
| NTU | Nephelometric turbidity unit |
| OM | Organic matter |
| pH | Hydrogen potential |
| pHi | Isoelectric pH |
| rpm | Revolutions per minute |
| SEM | Scanning electron microscope |
| T | Temperature |
| TA | Total alkalinity |
| TSS | Total suspended solids |
| XRD | X-ray diffractometer |
References
- Moulin, M. Biochemical, Biophysical, and Structural Studies of Seed Proteins from Moringa Oleifera and Implications for Traditional Water Purification. Ph.D. Thesis, Keele University, Keele, UK, 2019. [Google Scholar]
- Penchev, P.E. Étude Des Procédés D’extraction et de Purification de Produits Bioactifs à Partir de Plantes Par Couplage de Techniques Séparatives à Basses et Hautes Pressions. Ph.D. Thesis, Institut National Polytechnique de Toulouse, Toulouse, France, 2010. [Google Scholar]
- Jovanovic, A.; Petrovic, P.; Ðordjevic, V.; Zdunic, G.; Savikin, K.; Bugarski, B. Polyphenols Extraction from Plant Sources. Lek. Sirovine 2017, 37, 45–49. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical Fluid Extraction in Plant Essential and Volatile Oil Analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Morro Pérez, N. Distillation and Extraction of Herbs from Lamiaceae Family. Master’s Thesis, Valladolid University, Valladolid, Spain, 2021. [Google Scholar]
- Gray, A.I.; Satyajit, D.S.; Zahid, L. Natural Products Isolation, 2nd ed.; Institute, A.S., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2006; ISBN 1588294471. [Google Scholar]
- Revutska, A.; Belava, V.; Golubenko, A.; Taran, N.; Chen, M. Plant Secondary Metabolites as Bioactive Substances for Innovative Biotechnologies. E3S Web Conf. 2021, 280, 07014. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Fatombi, J.K.; Lartiges, B.; Aminou, T.; Barres, O.; Caillet, C. A Natural Coagulant Protein from Copra (Cocos Nucifera): Isolation, Characterization, and Potential for Water Purification. Sep. Purif. Technol. 2013, 116, 35–40. [Google Scholar] [CrossRef]
- Chandrashekar, S.; Vijayakumar, R.; Chelliah, R. Identification and Purification of Potential Bioactive Peptide of Moringa Oleifera Seed Extracts. Plants 2020, 9, 1445. [Google Scholar] [CrossRef] [PubMed]
- Vishali, S.; Roshini, S.K.; Samyuktha, M.R.; Ashish, K. Towards Zero Waste Production in the Paint Industry Wastewater Using an Agro-Based Material in the Treatment Train. Environ. Monit. Assess. 2018, 190, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Klašnja, M.; Antov, M.; Škrbic, B.; Šc, M. Removal of Water Turbidity by Natural Coagulants Obtained from Chestnut and Acorn. Bioresour. Technol. 2009, 100, 6639–6643. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Pizzi, A.; Medjahdi, G. The Use of Aloe Vera as Natural Coagulant in Algerian Drinking Water Treatment Plant. J. Renew. Mater. 2022, 10, 625–637. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-klen, M.R.; Veit, M.T.; Palácio, S.M.; Bergamasco, R. Performance of Different Coagulants in the Coagulation/Flocculation Process of Textile Wastewater. J. Clean. Prod. 2018, 20, 656–665. [Google Scholar] [CrossRef]
- Vishali, S.; Karthikeyan, R. Cactus Opuntia (Ficus-Indica): An Eco-Friendly Alternative Coagulant in the Treatment of Paint Effluent. Desalin. Water Treat. 2015, 56, 1489–1497. [Google Scholar] [CrossRef]
- Megersa, M.; Gach, W.; Beyene, A.; Ambelu, A.; Triest, L. Effect of Salt Solutions on Coagulation Performance of Moringa Stenopetala and Maerua Subcordata for Turbid Water Treatment. Sep. Purif. Technol. 2019, 221, 319–324. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Panico, A.; Pirozzi, F. Use of Acorn Leaves as a Natural Coagulant in a Drinking Water Treatment Plant. Water 2019, 11, 57. [Google Scholar] [CrossRef]
- Subramonian, W.; Wu, T.Y.; Chai, S. A Comprehensive Study on Coagulant Performance and Floc Characterization of Natural Cassia Obtusifolia Seed Gum in Treatment of Raw Pulp and Paper Mill Effluent. Ind. Crop. Prod. 2014, 61, 317–324. [Google Scholar] [CrossRef]
- Marina, B.S.; Prodanovi, J.M. Extracts of Fava Bean (Vicia faba L.) Seeds as Natural Coagulants. Ecol. Eng. 2015, 84, 229–232. [Google Scholar] [CrossRef]
- Yin, C. Emerging Usage of Plant-Based Coagulants for Water and Wastewater Treatment. Process Biochem. 2010, 45, 1437–1444. [Google Scholar] [CrossRef]
- Hameed, Y.T.; Idris, A.; Hussain, S.A.; Abdullah, N.; Che Man, H. Effect of Pre-Treatment with a Tannin-Based Coagulant and Flocculant on a Biofilm Bacterial Community and the Nitrification Process in a Municipal Wastewater Biofilm Treatment Unit. J. Environ. Chem. Eng. 2020, 8, 103679. [Google Scholar] [CrossRef]
- Trivedi, P.; Schaller, J.; Gustafsson, J.; Fardim, P. Supramolecular Design of Cellulose Hydrogel Beads. J. Renew. Mater. 2017, 5, 400–409. [Google Scholar] [CrossRef]
- Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Raghunandan, M.E.; Ramanan, R.N. Utilization of Plant-Based Natural Coagulants as Future Alternatives towards Sustainable Water Clarification. J. Environ. Sci. 2014, 26, 2178–2189. [Google Scholar] [CrossRef]
- Petrovic, N.J.; Antov, M.G.; Šc, M.B. Proteins from Common Bean (Phaseolus vulgaris) Seed as a Natural Coagulant for Potential Application in Water Turbidity Removal. Bioresour. Technol. 2010, 101, 2167–2172. [Google Scholar] [CrossRef]
- Rasool, M.A.; Tavakoli, B.; Chaibakhsh, N.; Reza, A.; Aazam Sadat, M. Use of a Plant-Based Coagulant in Coagulation—Ozonation Combined Treatment of Leachate from a Waste Dumping Site. Ecol. Eng. 2016, 90, 431–437. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Narasiah, K.S.; Talbot, B.G. Active Agents and Mechanism of Coagulation of Turbid Waters Using Moringa oleifera. Water Res. 1995, 29, 703–710. [Google Scholar] [CrossRef]
- Taiwo, A.S.; Adenike, K.; Aderonke, O. Efficacy of a Natural Coagulant Protein from Moringa oleifera (Lam) Seeds in Treatment of Opa Reservoir Water, Ile-Ife, Nigeria. Heliyon 2020, 6, e03335. [Google Scholar] [CrossRef]
- Bouchareb, R.; Derbal, K.; Benalia, A. Optimization of Active Coagulant Agent Extraction Method from Moringa Oleifera Seeds for Municipal Wastewater Treatment. Water Sci. Technol. 2021, 84, 393–403. [Google Scholar] [CrossRef]
- Bello, A.; Virtanen, V.; Salminen, J.P.; Leiviskä, T. Aminomethylation of Spruce Tannins and Their Application as Coagulants for Water Clarification. Sep. Purif. Technol. 2020, 242, 116765. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K. Etude Expérimentale et Modélisation Du Processus de La Coagulation Floculation: Application Aux Eaux Destinée à La Consommation. Master’s Thesis, Université Constantine 3, El Khroub, Algeria, 2015. [Google Scholar]
- Desjardins, R. Traitement Des Eaux.Pdf, 2nd ed.; Montréal, Presses Polytechnique de de Montréal: Montréal, QC, Canada, 1997. [Google Scholar]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Gisonni, C.; Crispino, G.; Pirozzi, F.; Pizzi, A. Use of Aloe Vera as an Organic Coagulant for Improving Drinking Water Quality. Water 2021, 13, 2024. [Google Scholar] [CrossRef]
- Muthuraman, G.; Sasikala, S.; Prakash, N. Proteins from Natural Coagulant for Potential Application of Turbidity Removal in Water. Int. J. Eng. Innov. Technol. 2013, 3, 278–283. [Google Scholar]
- Landázuri-Rojas, A.C.; Villarreal, J.S.; Núñez, E.R.; Pico, M.M.; Lagos, A.S.; Caviedes, M. Experimental Evaluation of Crushed Moringa oleifera Lam. Seeds and Powder Waste during Coagulation-Flocculation Processes. J. Environ. Chem. Eng. 2018, 6, 5443–5451. [Google Scholar] [CrossRef]
- Wang, W.; Scali, M.; Vignani, R.; Spadafora, A.; Sensi, E.; Mazzuca, S.; Cresti, M. Protein Extraction for Two-Dimensional Electrophoresis from Olive Leaf, a Plant Tissue Containing High Levels of Interfering Compounds. Electrophoresis 2003, 24, 2369–2375. [Google Scholar] [CrossRef]
- Rodhe, A.; Bella, N. Alkaline Protein Extraction of Oat Bran and Oat Endosperm Flour the Effect of PH during Extraction and Precipitation. Master’s Thesis, Lund University, Lund, Sweden, 2021. [Google Scholar]
- Bouteflika, A. Valeurs Limites Des Paramètres de Rejets d’effluents Liquides Industriels. J. Off. République Algérienne 2006, 26, 1–27. [Google Scholar]
- Boutteflika, A. Paramètres de Qualité de L’eau de Consommation Humaine. J. Off. 2011, 18, 6–9. [Google Scholar]
- Benalia, A.; Derbal, K. Comparative Study between Aluminum Sulfate and Ferric Chloride in Water Treatment: Turbidity Removal. J. Dr. 2015, 1, 4–9. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. ; American Public Health Association (APHA). (Eds.) Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Water Works Association (AWWA) and Water Environment Federation (WEF): Washington, DC, USA, 2012; ISBN 978-087553-0130. [Google Scholar]
- Padhiyar, H.; Thanki, A.; Kumar, N.; Pandey, S.; Yadav, M. Parametric and Kinetic Investigations on Segregated and Mixed Textile Effluent Streams Using Moringa Oleifera Seed Powders of Different Sizes. J. Water Process Eng. 2020, 34, 101159–101170. [Google Scholar] [CrossRef]
- Magalhães, E.R.B.; Fonseca de Menezes, N.N.; Silva, F.L.; Alves Garrido, J.W.; Angélica dos Santos Bezerra Sousa, M.; dos Santos, E.S. Effect of Oil Extraction on the Composition, Structure, and Coagulant Effect of Moringa Oleifera Seeds. J. Clean. Prod. 2021, 279, 123902. [Google Scholar] [CrossRef]
- Saritha, V.; Karnena, M.K.; Dwarapureddi, B.K. “Exploring Natural Coagulants as Impending Alternatives towards Sustainable Water Clarification”—A Comparative Studies of Natural Coagulants with Alum. J. Water Process Eng. 2019, 32, 100982. [Google Scholar] [CrossRef]
- Baquerizo-Crespo, R.J.; Nuñez, Y.; Albite, J.; Macías-Alcívar, J.A.; Cedeño-Zambrano, N.; Dueñas-Rivadeneira, A.A.; Gómez-Salcedo, Y.; Rodríguez-Díaz, J.M. Biocoagulants as an Alternative for Water Treatment. In Environmental and Microbial Biotechnology; Prasad, R., Ed.; Springer: New York, NY, USA, 2021; pp. 313–334. ISBN 9789811589997. [Google Scholar]
- Kebede, T.G.; Dube, S.; Mhuka, V.; Nindi, M.M. Bioremediation of Cd (II), Pb (II) and Cu (II) from Industrial Effluents by Moringa Stenopetala Seed Husk. J. Environ. Sci. Health Part A 2019, 54, 337–351. [Google Scholar] [CrossRef]
- Dao, M.; Nguyen, V.; Tran, T.; Nguyen, X.; Vo, D.; Nguyen, V.; Hoang, L. Pilot-Scale Study of Real Domestic Textile Wastewater Treatment Using Cassia Fistula Seed-Derived Coagulant. J. Chem. 2021, 2021, 7608856. [Google Scholar] [CrossRef]
- Selmane, D. Etude de l’extraction Des Protéines de Coproduits d’abattage et de Leur Valorisation Comme Ingrédients Fonctionnels. Ph.D. Thesis, Université Blaise Pascal-Clermont-Ferrand II, Clermont-Ferrand, France, 2010. [Google Scholar]
- Preece, K.E.; Hooshyar, N.; Zuidam, N.J. Whole Soybean Protein Extraction Processes: A Review. Innov. Food Sci. Emerg. Technol. 2017, 43, 163–172. [Google Scholar] [CrossRef]
- Potin, F. Effet de l’extraction Des Protéines de Tourteaux de Chanvre Sur Leurs Fonctionnalités. Ph.D. Thesis, Université Bourgogne Franche-Comté, Besançon, France, 2019. [Google Scholar]
- Ragab, D.D.M.; Babiker, E.E.; Eltinay, A.H. Fractionation, Solubility and Functional Properties of Cowpea (Vigna unguiculata) Proteins as Affected by PH and/or Salt Concentration. Food Chem. 2004, 84, 207–212. [Google Scholar] [CrossRef]
- Arntfield, S.D. Effects of Divalent Cations, Phytic Acid, and Phenolic Compounds on the Gelation of Ovalbumin and Canola Proteins. In Macromolecular Interactions in Food Technology; American Chemical Society: Washington, DC, USA, 1996; Volume 650, pp. 80–92. [Google Scholar]
- Akyüz, A.; Ersus, S. Optimization of Enzyme Assisted Extraction of Protein from the Sugar Beet (Beta vulgaris L.) Leaves for Alternative Plant Protein Concentrate Production. Food Chem. 2021, 335, 127673. [Google Scholar] [CrossRef] [PubMed]
- Kristianto, H.; Rahman, H.; Prasetyo, S.; Sugih, A.K. Removal of Congo Red Aqueous Solution Using Leucaena Leucocephala Seed’s Extract as Natural Coagulant. Appl. Water Sci. 2019, 9, 88. [Google Scholar] [CrossRef]
- Bodlund, I.; Pavankumar, A.R.; Chelliah, R.; Kasi, S.; Sankaran, K.; Rajarao, G.K. Coagulant Proteins Identified in Mustard: A Potential Water Treatment Agent. Int. J. Environ. Sci. Technol. 2014, 11, 873–880. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W. State of the Art and Sustainability of Natural Coagulants in Water and Wastewater Treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Shan, T.C.; Al Matar, M.; Makky, E.A.; Ali, E.N. The Use of Moringa Oleifera Seed as a Natural Coagulant for Wastewater Treatment and Heavy Metals Removal. Appl. Water Sci. 2017, 7, 1369–1376. [Google Scholar] [CrossRef]
- Jacques, F.K.; Josse, R.G.; Mama, D.; Aminou, T. Étude de l’activité Floculante de La Caséine Acide Extraite de La Crème de Cocos Nucifera Dans La Clarification Des Eaux de Surface. Rev. Sci. L’eau 2009, 22, 93–101. [Google Scholar] [CrossRef]

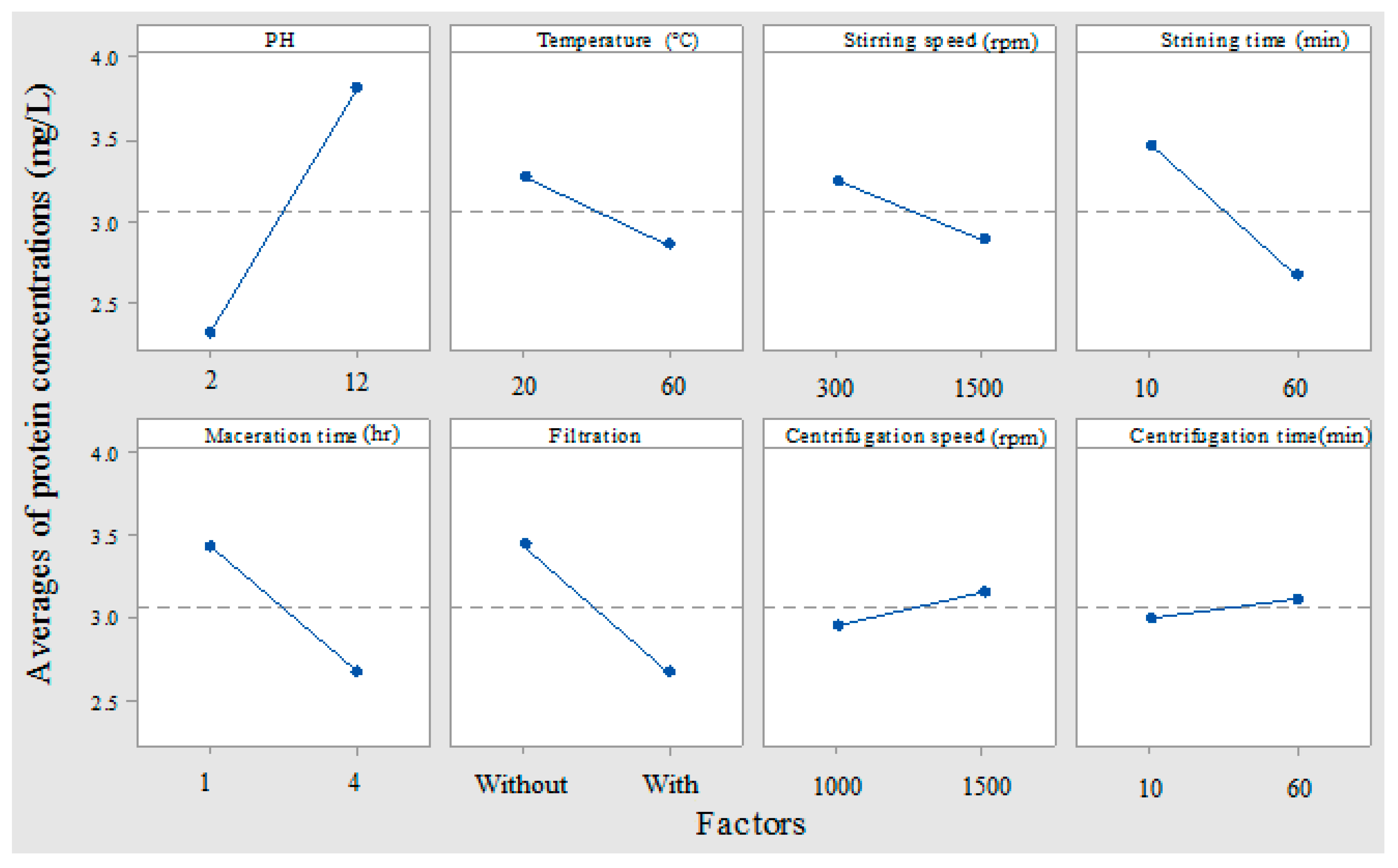
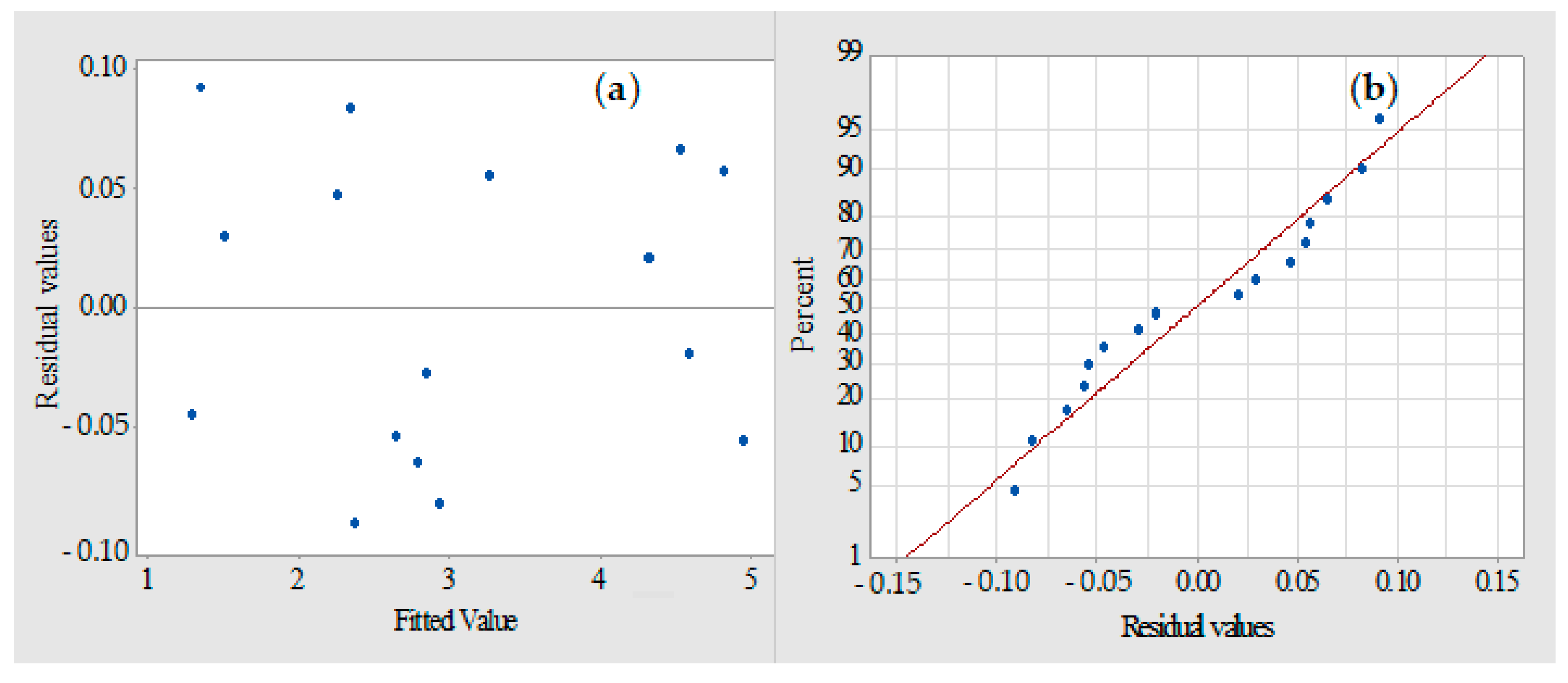
| Parameters | Unit | Minimum | Maximum |
|---|---|---|---|
| pH (X1) | / | 2 | 12 |
| Temperature(X2) | °C | 20 | 60 |
| Stirring speed(X3) | rpm | 300 | 1500 |
| Stirring time(X4) | min | 10 | 60 |
| Maceration time(X5) | hr | 1 | 4 |
| Filtration(X6) | / | Without | With |
| Centrifugation speed(X7) | rpm | 1000 | 4000 |
| Centrifugation time(X8) | min | 10 | 60 |
| Standard Run | pH | Temperature (°C) | Stirring Speed (rpm) | Stirring Time (min) | Maceration Time (h) | Filtration * | Centrifugation Speed (rpm) | Centrifugation Time (min) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 20 | 300 | 10 | 1 | 0 | 1000 | 10 |
| 2 | 12 | 20 | 300 | 10 | 1 | 1 | 4000 | 60 |
| 3 | 2 | 60 | 300 | 10 | 4 | 0 | 4000 | 60 |
| 4 | 12 | 60 | 300 | 10 | 4 | 1 | 1000 | 10 |
| 5 | 2 | 20 | 1500 | 10 | 4 | 1 | 4000 | 10 |
| 6 | 12 | 20 | 1500 | 10 | 4 | 0 | 1000 | 60 |
| 7 | 2 | 60 | 1500 | 10 | 1 | 1 | 1000 | 60 |
| 8 | 12 | 60 | 1500 | 10 | 1 | 0 | 4000 | 10 |
| 9 | 2 | 20 | 300 | 60 | 4 | 1 | 1000 | 60 |
| 10 | 12 | 20 | 300 | 60 | 4 | 0 | 4000 | 10 |
| 11 | 2 | 60 | 300 | 60 | 1 | 1 | 4000 | 10 |
| 12 | 12 | 60 | 300 | 60 | 1 | 0 | 1000 | 60 |
| 13 | 2 | 20 | 1500 | 60 | 1 | 0 | 4000 | 60 |
| 14 | 12 | 20 | 1500 | 60 | 1 | 1 | 1000 | 10 |
| 15 | 2 | 60 | 1500 | 60 | 4 | 0 | 1000 | 10 |
| 16 | 12 | 60 | 1500 | 60 | 4 | 1 | 4000 | 60 |
| Min | 2 | 20 °C | 300 RPM | 10 min | 1 h | 0 | 1000 RPM | 10 min |
| Max | 12 | 60 °C | 1500 RPM | 60 min | 4 h | 1 | 4000 RPM | 60 min |
| Parameters | Industrial Oily Wastewater | Algerian Standards [37] | Drinking Water | Algerian Standards [38] |
|---|---|---|---|---|
| Turbidity (NTU) | 187 | 20 | 15.7 | 5 |
| pH | 9 | 6.5–8.5 | 7.75 | 6.5–9 |
| OM (mgO2/L) | 17.5 | / | 2.1 | 5 |
| TSS (mg/L) | 969 | 35 | / | / |
| COD (mg/L) | 784.45 | 120 | / | / |
| TA (mg CaCO3/L) | / | / | 134 | 500 |
| Salinity (g/L) | / | / | 0.6 | / |
| Ec (µs/cm) | / | / | 782 | 2800 |
| Parameters | Industrial Oily Wastewater | Drinking Water | ||
|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | Pre-Treatment | Post-Treatment | |
| Protein dosage (mg/L) | / | 0.538 | / | 0.098 |
| Turbidity (NTU) | 187 | 5.85 | 15.7 | 4.82 |
| Turbidity removal (%) | / | 96.87 | / | 69.29 |
| pH | 9 | 9.68 | 7.75 | 8.46 |
| OM (mgO2/L) | 17.5 | 9.4 | 2.1 | 5.2 |
| TSS (mg/L) | 969 | 100 | / | / |
| TSS removal (%) | / | 89.86 | / | / |
| COD (mg/L) | 784.45 | 28.278 | / | / |
| COD removal (%) | / | 96.395 | / | / |
| TA (mg CaCO3/L) | / | / | 134 | 142 |
| Salinity (g/L) | / | / | 0.6 | 0.6 |
| Ec (µs/cm) | / | / | 782 | 935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benalia, A.; Chaibraa, W.; Djeghar, S.; Derbal, K.; Khalfaoui, A.; Mahfouf, A.; Bouchareb, R.; Panico, A.; Pizzi, A. Use of Extracted Proteins from Oak Leaves as Bio-Coagulant for Water and Wastewater Treatment: Optimization by a Fractional Factorial Design. Water 2023, 15, 1984. https://doi.org/10.3390/w15111984
Benalia A, Chaibraa W, Djeghar S, Derbal K, Khalfaoui A, Mahfouf A, Bouchareb R, Panico A, Pizzi A. Use of Extracted Proteins from Oak Leaves as Bio-Coagulant for Water and Wastewater Treatment: Optimization by a Fractional Factorial Design. Water. 2023; 15(11):1984. https://doi.org/10.3390/w15111984
Chicago/Turabian StyleBenalia, Abderrezzaq, Walid Chaibraa, Sara Djeghar, Kerroum Derbal, Amel Khalfaoui, Asma Mahfouf, Raouf Bouchareb, Antonio Panico, and Antonio Pizzi. 2023. "Use of Extracted Proteins from Oak Leaves as Bio-Coagulant for Water and Wastewater Treatment: Optimization by a Fractional Factorial Design" Water 15, no. 11: 1984. https://doi.org/10.3390/w15111984
APA StyleBenalia, A., Chaibraa, W., Djeghar, S., Derbal, K., Khalfaoui, A., Mahfouf, A., Bouchareb, R., Panico, A., & Pizzi, A. (2023). Use of Extracted Proteins from Oak Leaves as Bio-Coagulant for Water and Wastewater Treatment: Optimization by a Fractional Factorial Design. Water, 15(11), 1984. https://doi.org/10.3390/w15111984









