Effect of Sulfur Autotrophic Denitrification Sludge on the Start-Up Characteristics of Anaerobic Ammonia Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Start-Up ANAMMOX Using a Two-Stage Experiment
2.1.1. The S0-SADN Operation Stage
2.1.2. The ANAMMOX Start-Up Stage
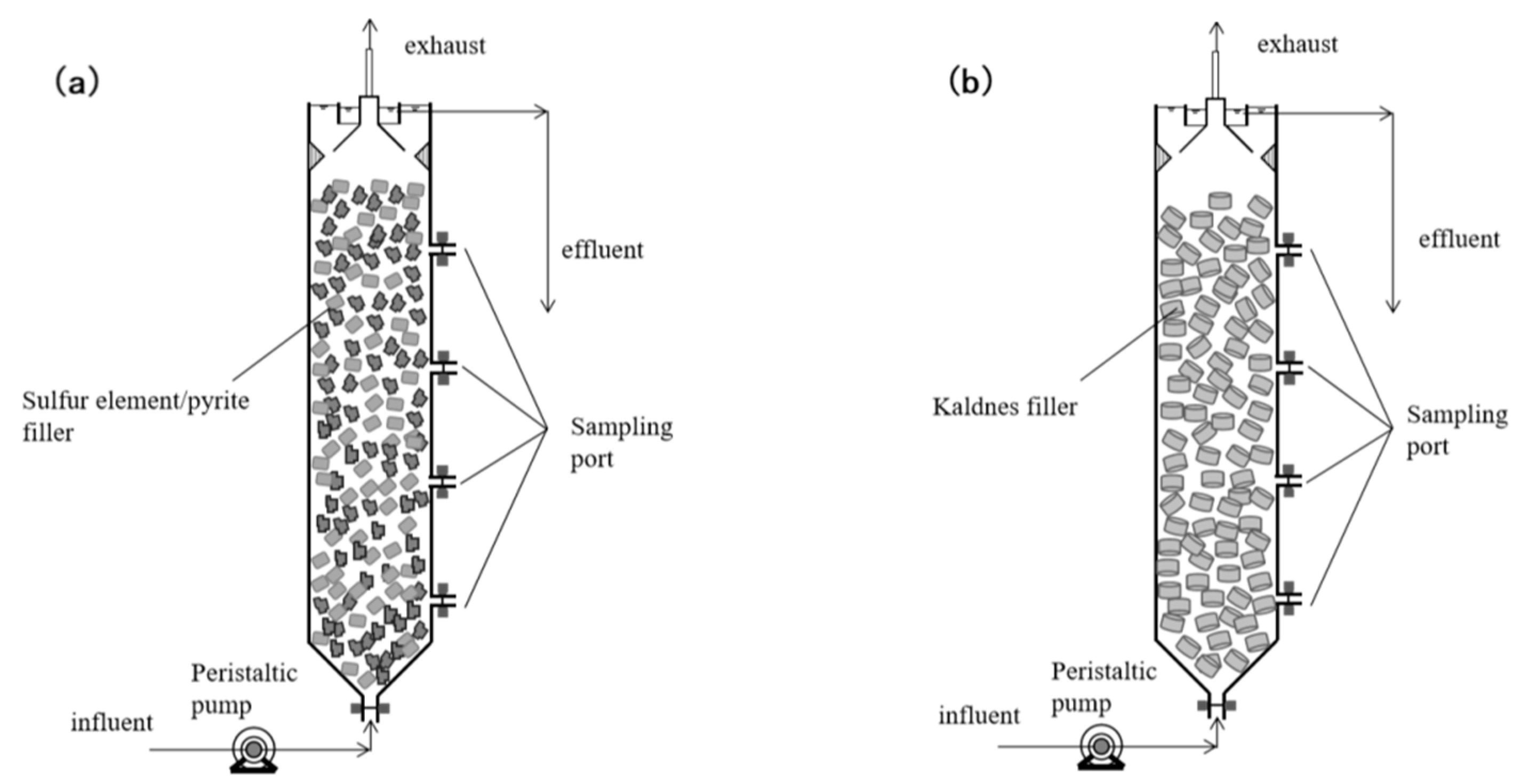
2.2. Test Apparatus
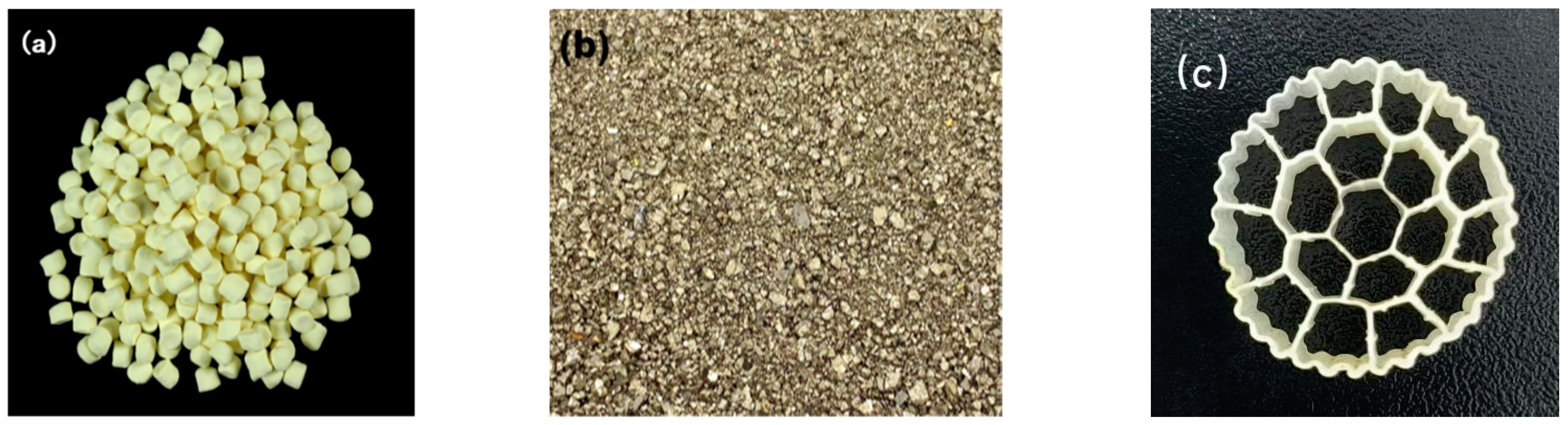
2.3. Filler
2.3.1. The S0-SADN Operation Stage
2.3.2. The ANAMMOX Start-Up Stage
2.4. Synthetic Wastewater Composition
2.5. Water Quality Measurement
2.6. High-Throughput Sequencing
2.7. Batch Beaker Test
2.8. Statistical Treated of Data
2.9. TIN Measurement Method
3. Results
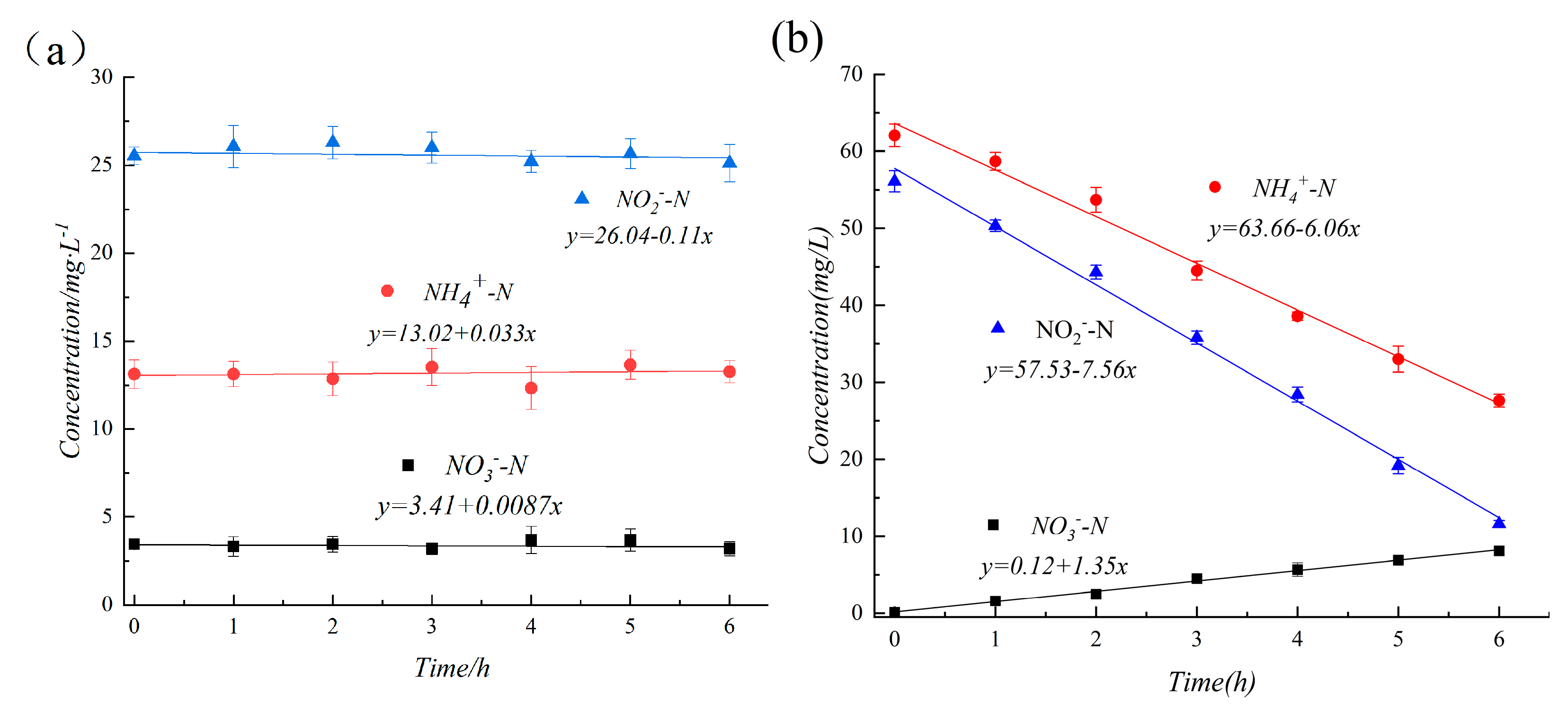
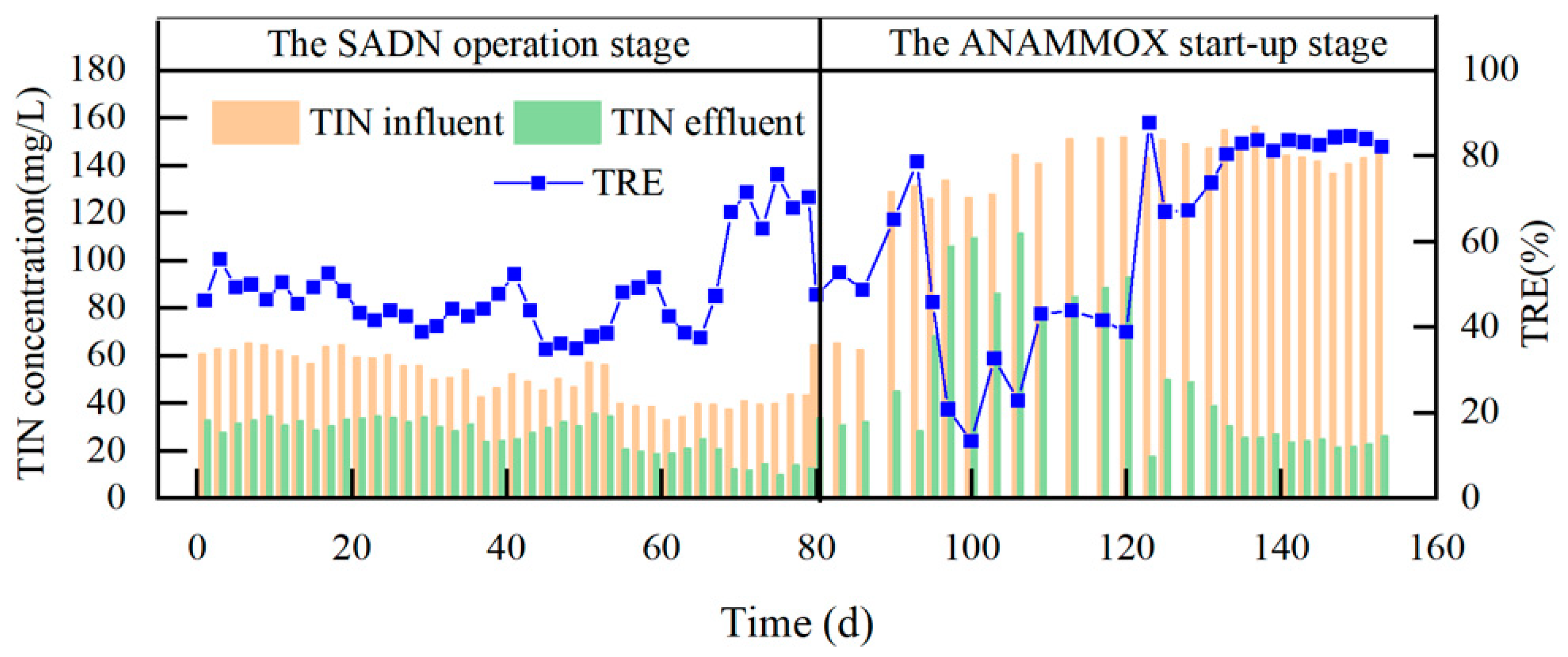
3.1. S0-SADN Operation Stage
3.2. ANAMMOX Start-Up Stage
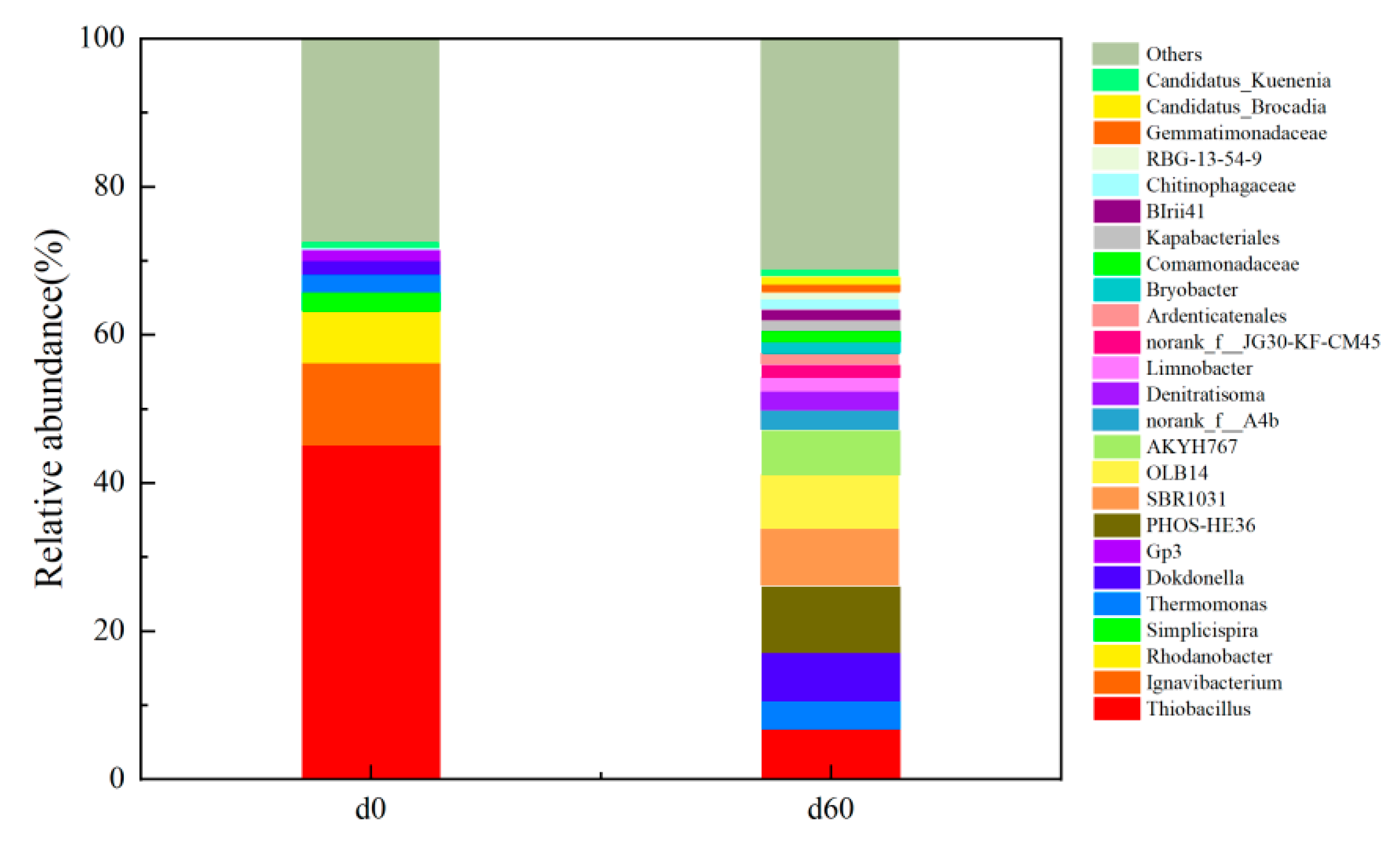
3.3. Microbial Communities
3.4. Batch Beaker Test Results
3.5. Morphology Change of Sludge
4. Discussion
4.1. S0-SADN Operation Stage
4.2. ANAMMOX Start-Up Stage
4.3. The Mass Balance of Nitrogen Species in Both Systems
4.4. Matrix and Product Metering Ratio
4.5. High-Throughput Result Analysis
- (1)
- ANAMMOX bacteria: Candidatus Brocadia and Candidatus Kuenenia are considered to be the main ANAMMOX bacteria in laboratory reactors [30]. At the end of the S0-SADN stage, the ANAMMOX bacteria in the system were mainly Candidatus Kuenenia. After the ANAMMOX process was successfully started and stabilized, the abundance of Candidatus Kuenenia did not change significantly, while that of Candidatus Brocadia increased from 0% to 1.08%. This different growth rate at different stages can be explained by the fact that Candidatus Kuenenia can utilize a low concentration of nitrite nitrogen and is more tolerant to inhibition [44], while Candidatus Brocadia has a weak substrate affinity but a strong reproductive capacity [43].At the S0-SADN stage, the addition of ammonia nitrogen in the influent water stimulated the growth of the ANAMMOX to some extent but the growth process was slow and the activity of the ANAMMOX bacteria was inhibited as the system was dominated by the autotrophic denitrification culture. At the end of this stage, despite the 0.80% abundance of Candidatus Kuenenia in the system, there was no ANAMMOX process at the macroscopic level.
- (2)
- S0-SADN bacteria: this group dominated the S0-SADN operation stage. After 60 days of the ANAMMOX start-up stage, there were still some functional bacteria of this group in the system, proving that it is possible to initiate the ANAMMOX process using inoculation with sulfur autotrophic denitrifying bacteria. First, the ANAMMOX can be started using inoculating sulfur autotrophic denitrifying bacteria, and then the sulfur autotrophic denitrifying process can be coupled.
- (3)
- Heterotrophic denitrifying bacteria: although in the S0-SADN operation stage there was a certain amount of heterotrophic denitrification process in the system, as no organic matter was added to the influent water, endogenous denitrification mainly occurred after the ANAMMOX process was successfully started and stabilized. The number of heterotrophic denitrification bacteria increased after the death of the original S0-SADN bacteria in the reactor, as this produced a large amount of organic matter.
- (1)
- A small amount of ANAMMOX bacteria were enriched in the first stage of the S0-SADN operation. The ANAMMOX process takes NO2−-N as the electron acceptor, while the S0-SADN takes NO3−-N or NO2−-N as the electron acceptor. During the S0-SADN process, NO3−-N will be first reduced to NO2−-N; on the other hand, ANAMMOX needs to use NH4+-N as the electron donor, while S0-SADN uses a sulfur simple substance or a sulfur ion as the electron donor, and the two are different. However, some ANAMMOX bacteria can conduct a DNRA reaction [45], directly reducing NO2−-N or NO3−-N to NH4+-N, and the microorganisms will also generate part of the NH4+-N after autolysis of the bacteria. Therefore, sulfur autotrophic denitrifying bacteria and ANAMMOX bacteria have certain similarities in growth conditions, which make the sulfur autotrophic denitrifying sludge form a micro-concentration of ANAMMOX bacteria during the operation process.
- (2)
- The autolysis process of the bacteria in the reactor is slow. In other research reports [21,46,47,48], at the initial stage of the start-up of the anaerobic ammonia oxidation process, the accumulation of NH4+-N and the removal of a large amount of NO2−-N occurred in the reactor due to the influence of the bacterial autolysis and endogenous denitrification and denitrification processes. In the studies by Chen et al. [46] and Wang et al. [47], this process lasted about 20 days. In the second stage, during the start-up of the ANAMMOX using inoculating sulfur autotrophic denitrifying sludge in this experiment, there was no large accumulation of NH4+-N at the initial stage, and NH4+-N consumption began to occur at the initial stage of the reactor start-up. It was analyzed that the sulfur autotrophic denitrifying bacteria had a strong ability to adapt to the new environment without sulfur sources, the amount of NH4+-N produced by the autolysis of the bacteria was less, and the oxidation consumption of NH4+-N by AOB was presented. At the same time, the slowing down of the autolysis process also inhibits the endogenous denitrification process to a certain extent and reduces the competition between the endogenous denitrifying bacteria and ANAMMOX bacteria. Moreover, the slow autolysis of sulfur autotrophic denitrifying bacteria is conducive to the retention of some sulfur autotrophic denitrifying bacteria in the system after the start of the ANAMMOX, creating the possibility of reintroducing the sulfur autotrophic denitrifying process without the re-culture or inoculation of the sulfur autotrophic denitrifying bacteria.
5. Conclusions
- (1)
- When pyrite was used as a filler and elemental sulfur was used for autotrophic denitrification, a certain amount of NH4+-N was added to the influent water. Through high-throughput sequencing, it was established that although there were a certain amount of ANAMMOX bacteria, they did not have the characteristics of an ANAMMOX process at the macroscopic level.
- (2)
- Under the condition that there is no sulfur source in the system, the start-up time required to initiate the ANAMMOX process by inoculating the S0-SADN sludge was short, so this type of sludge can reduce the cultivation time of the ANAMMOX bacteria. After 60 days without a sulfur source in the system, there were also sulfur autotrophic denitrifying functional bacteria with an abundance of 7.22% in the system, belonging to Thiobacillus.
- (3)
- When the influent nitrogen source components were NH4+-N and NO2−-N, with no external sulfur source, at day 44 of the ANAMMOX start-up stage, the total nitrogen removal rate reached 87.66%, and the TIN removal load reached 0.30 kg(m3·d)−1. After 55 days, the removal rates of NH4+-N and NO2−-N in the reactor were 99.1% and 99.0%, respectively, and the removal rates of TIN were 83.0%. Candidatus Brocadia and Candidatus Kuenenia were the main genera of ANAMMOX bacteria, with abundances of 1.08% and 0.96%, respectively.
- (4)
- The experimental results indicate that it is feasible to initiate the ANAMMOX process by inoculating the S0-SADN sludge. However, simultaneous initiation of the ANAMMOX and the stable operation of the S0-SADN is not feasible. It was recommended to start-up the ANAMMOX under the condition of no sulfur source, which can be accomplished in a short period. Despite the successful start-up of the ANAMMOX, there remained an excessive abundance of S0-SADN bacteria within the system, which suggest that is worth considering reinitiating the coupled S0-SADN process at a later time; this is greatly beneficial for further removing TIN effluent.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raboni, M.; Viotti, P.; Rada, E.C.; Conti, F.; Boni, M.R. The sensitivity of a specific denitrification rate under the dissolved oxygen pressure. Int. J. Environ. Res. Public Health 2020, 17, 9366. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Zhou, Y. Coupling anammox with heterotrophic denitrification for enhanced nitrogen removal: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2260–2293. [Google Scholar] [CrossRef]
- Jetten, M.S.; Cirpus, I.; Kartal, B.; van Niftrik, L.; van de Pas-Schoonen, K.T.; Sliekers, O.; Haaijer, S.; van der Star, W.; Schmid, M.; van de Vossenberg, J.; et al. 1994-2004: 10 years of research on the anaerobic oxidation of ammonium. Biochem. Soc. Trans. 2005, 33, 119–123. [Google Scholar] [CrossRef]
- Van de Graaf, A.A.; de Bruijn, P.; Robertson, L.A.; Jetten, M.S.M.; Kuenen, J.G. Metabolic pathway of anaerobic ammonium oxidation on the basis of (15)N studies in a fluidized bed reactor. Microbiology 1997, 143, 2415–2421. [Google Scholar] [CrossRef]
- Van de Graaf, A.A.; Mulder, A.; de Bruijn, P.; Jetten, M.S.; Robertson, L.A.; Kuenen, J.G. Anaerobic oxidation of ammonium is a biologically mediated process. Appl. Environ. Microbiol. 1995, 61, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, Q.; Huang, B.; Fan, N.; Jin, R. A review on anammox process for the treatment of antibiotic-containing wastewater: Linking effects with corresponding mechanisms. Front. Environ. Sci. Eng. 2020, 15, 1–15. [Google Scholar] [CrossRef]
- Strous, M.; Pelletier, E.; Mangenot, S.; Rattei, T.; Lehner, A.; Taylor, M.W.; Horn, M.; Daims, H.; Bartol-Mavel, D.; Wincker, P.; et al. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 2006, 440, 790–794. [Google Scholar] [CrossRef]
- Pedrouso, A.; Río, N.V.D.; Morales, N.; Vázquez-Padín, J.; Mosquera-Corral, A. Nitrite oxidizing bacteria suppression based on in-situ free nitrous acid production at mainstream conditions. Sep. Purif. Technol. 2017, 186, 55–62. [Google Scholar] [CrossRef]
- Capua, F.D.; Papirio, S.; Lens, P.N.L.; Esposito, G. Chemolithotrophic denitrification in biofilm reactors. Chem. Eng. J. 2015, 280, 643–657. [Google Scholar] [CrossRef]
- Sierra-Alvarez, R.; Beristain-Cardoso, R.; Salazar, M.; Gómez, J.; Razo-Flores, E.; Field, J.A. Chemolithotrophic denitrification with elemental sulfur for groundwater treatment. Water Res. 2007, 41, 1253–1262. [Google Scholar] [CrossRef]
- Chen, F.; Li, X.; Gu, C.; Huang, Y.; Yuan, Y. Selectivity control of nitrite and nitrate with the reaction of S0 and achieved nitrite accumulation in the sulfur autotrophic denitrification process. Bioresour. Technol. 2018, 266, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, D.; Xiao, Z.; Wang, H.; Yang, K. Nitrate removal in a combined bioelectrochemical and sulfur autotrophic denitrification system under high nitrate concentration: Effects of pH. Bioprocess Biosyst. Eng. 2018, 41, 449–455. [Google Scholar] [CrossRef]
- Sun, Y.; Nemati, M. Evaluation of sulfur-based autotrophic denitrification and denitritation for biological removal of nitrate and nitrite from contaminated waters. Bioresour. Technol. 2012, 114, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Fang, W.Y.; Li, X.; Huang, Y.; Guo, C.R.; Hu, Y.T.; Tao, R.J. Improved on Nitrogen Removal of Anaerobic Ammonia Oxidation by Coupling Element Sulfur-based Autotrophic Short-cut Denitrification. Huan Jing Ke Xue Huanjing Kexue 2020, 41, 3699–3706. [Google Scholar] [PubMed]
- Huo, D.; Dang, Y.; Sun, D.; Holmes, D.E. Efficient nitrogen removal from leachate by coupling Anammox and sulfur-siderite-driven denitrification. Sci. Total Environ. 2022, 829, 154683. [Google Scholar] [CrossRef]
- Chen, F.; Li, X.; Yuan, Y.; Huang, Y. An efficient way to enhance the total nitrogen removal efficiency of the Anammox process by S0-based short-cut autotrophic denitrification. J. Environ. Sci. 2019, 81, 214–224. [Google Scholar] [CrossRef]
- Ni, B.J.; Hu, B.L.; Fang, F.; Xie, W.M.; Kartal, B.; Liu, X.W.; Sheng, G.P.; Jetten, M.; Zheng, P.; Yu, H.Q. Microbial and physicochemical characteristics of compact anaerobic ammonium-oxidizing granules in an upflow anaerobic sludge blanket reactor. Appl. Environ. Microbiol. 2010, 76, 2652–2656. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, Y.Y.; Tang, C.J.; Chai, L.Y.; Xu, K.Q.; Song, Y.X.; Ali, M.; Zheng, P. Start-up characteristics of a granule-based anammox UASB reactor seeded with anaerobic granular sludge. BioMed Res. Int. 2013, 2013, 396487. [Google Scholar] [CrossRef]
- Hsu, S.C.; Lai, Y.C.; Hsieh, P.H.; Cheng, P.J.; Wong, S.S.; Hung, C.H. Successful enrichment of rarely found Candidatus Anammoxoglobus propionicus from leachate sludge. J. Microbiol. Biotechnol. 2014, 24, 879–887. [Google Scholar] [CrossRef]
- Bi, Z.; Qiao, S.; Zhou, J.; Tang, X.; Zhang, J. Fast start-up of Anammox process with appropriate ferrous iron concentration. Bioresour. Technol. 2014, 170, 506–512. [Google Scholar] [CrossRef]
- Chen, H.; Hu, H.Y.; Chen, Q.Q.; Shi, M.L.; Jin, R.C. Successful start-up of the anammox process: Influence of the seeding strategy on performance and granule properties. Bioresour. Technol. 2016, 211, 594–602. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, J.; Peng, Y.; Wang, S. An improved start-up strategy for mainstream anammox process through inoculating ordinary nitrification sludge and a small amount of anammox sludge. J. Hazard. Mater. 2020, 384, 121325. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Li, W.; Wang, Y.; Hao, T.; Mao, F.; Wang, T.; Li, J. Low strength wastewater anammox start-up and stable operation by inoculating sponge-iron sludge: Cooperation of biological iron and iron bacteria. J. Environ. Manag. 2022, 322, 116086. [Google Scholar] [CrossRef]
- Ali, M.; Okabe, S. Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues. Chemosphere 2015, 141, 144–153. [Google Scholar] [CrossRef]
- Graaf, A.A.v.d.; Bruijn, P.d.; Robertson, L.A.; Jetten, M.S.M.; Kuenen, J.G. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 1996, 142, 2187–2196. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 1st ed.; American Public Health Association, Ed.; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Chernousova, E.; Gridneva, E.; Grabovich, M.; Dubinina, G.; Akimov, V.; Rossetti, S.; Kuever, J. Thiothrix caldifontis sp. nov. and Thiothrix lacustris sp. nov.; gammaproteobacteria isolated from sulfide springs. Int. J. Syst. Evol. Microbiol. 2009, 59, 3128–3135. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhang, M.; Shang, H.; Liu, Z.; Zhou, W. Microbial community succession, species interactions and metabolic pathways of sulfur-based autotrophic denitrification system in organic-limited nitrate wastewater. Bioresour. Technol. 2020, 315, 123826. [Google Scholar] [CrossRef]
- Huang, Z.; Wei, Z.; Xiao, X.; Tang, M.; Li, B.; Zhang, X. Nitrification/denitrification shaped the mercury-oxidizing microbial community for simultaneous Hg(0) and NO removal. Bioresour. Technol. 2019, 274, 18–24. [Google Scholar] [CrossRef]
- Prakash, O.; Green, S.J.; Singh, P.; Jasrotia, P.; Kostka, J.E. Stress-related ecophysiology of members of the genus Rhodanobacter isolated from a mixed waste contaminated subsurface. Front. Environ. Sci. Eng. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Xing, W.; Li, J.; Li, P.; Wang, C.; Cao, Y.; Li, D.; Yang, Y.; Zhou, J.; Zuo, J. Effects of residual organics in municipal wastewater on hydrogenotrophic denitrifying microbial communities. J. Environ. Sci. 2018, 65, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, J.; Cnockaert, M.C.; Swings, J. Thermomonas fusca sp. nov. and Thermomonas brevis sp. nov.; two mesophilic species isolated from a denitrification reactor with poly(epsilon-caprolactone) plastic granules as fixed bed, and emended description of the genus Thermomonas. Int. J. Syst. Evol. Microbiol. 2003, 53, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Pishgar, R.; Dominic, J.A.; Sheng, Z.; Tay, J.H. Denitrification performance and microbial versatility in response to different selection pressures. Bioresour. Technol. 2019, 281, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Dabert, P.; Sialve, B.; Delgenès, J.P.; Moletta, R.; Godon, J.J. Characterisation of the microbial 16S rDNA diversity of an aerobic phosphorus-removal ecosystem and monitoring of its transition to nitrate respiration. Appl. Microbiol. Biotechnol. 2001, 55, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guan, M.; Wang, W. Simultaneous arsenite and nitrate removal from simulated groundwater based on pyrrhotite autotrophic denitrification. Water Res. 2021, 189, 116662. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Q.; Zhao, L.; Zhao, L.; Wu, W. Enhanced simultaneous removal of nitrate and phosphate using novel solid carbon source/zero-valent iron composite. J. Clean. Prod. 2021, 289, 125757. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Gao, D. The threshold of influent ammonium concentration for nitrate over-accumulation in a one-stage deammonification system with granular sludge without aeration. Sci. Total Environ. 2018, 634, 843–852. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, K.; Li, J.; Sun, S.; Jia, T.; Huang, Y.; Peng, Y.; Zhang, L. Pilot-scale evaluation of partial denitrification/anammox on nitrogen removal from low COD/N real sewage based on a modified process. Bioresour. Technol. 2021, 338, 125580. [Google Scholar] [CrossRef]
- Fangfang, L.I.; Shi, C.; Zhou, B.; Haibo, L.I. Enhanced Treatment of Secondary Effluent via Autotrophic Denitrification Biofilter Process Using Sulfur and Pyrite as Fillings. Res. Environ. Sci. 2016, 29, 1693–1700. [Google Scholar]
- Adams, M.; Xie, J.; Xie, J.; Chang, Y.; Guo, M.; Chen, C.; Zhang, T.C. The effect of carrier addition on Anammox start-up and microbial community: A review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 355–368. [Google Scholar] [CrossRef]
- Bae, H.; Choi, M.; Lee, C.; Chung, Y.-C.; Yoo, Y.J.; Lee, S. Enrichment of ANAMMOX bacteria from conventional activated sludge entrapped in poly(vinyl alcohol)/sodium alginate gel. Chem. Eng. J. 2015, 281, 351–540. [Google Scholar] [CrossRef]
- Zhang, L.; Narita, Y.; Gao, L.; Ali, M.; Oshiki, M.; Ishii, S.; Okabe, S. Microbial competition among anammox bacteria in nitrite-limited bioreactors. Water Res. 2017, 125, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Puyol, D.; Carvajal-Arroyo, J.M.; Garcia, B.; Sierra-Alvarez, R.; Field, J.A. Kinetic characterization of Brocadia spp.-dominated anammox cultures. Bioresour. Technol. 2013, 139, 94–100. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Z.; Azari, M.; Kartal, B.; Du, H.; Cai, M.; Gu, J.D. Discovery of a new genus of anaerobic ammonium oxidizing bacteria with a mechanism for oxygen tolerance. Water Res. 2022, 226, 119165. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Huang, X.X.; Lei, C.X.; Zhu, W.J.; Chen, Y.X.; Wu, W.X. Improving Anammox start-up with bamboo charcoal. Chemosphere. 2012, 89, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, X.; Yuan, L.; Luo, Z.; Indira, H.K. Start-up and operational performance of Anammox process in an anaerobic baffled biofilm reactor (ABBR) at a moderate temperature. Bioresour. Technol. 2019, 279, 1–9. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Yang, F. Performance of Anammox process and low-oxygen adaptability of Anammox biofilms in a FBR with small ring non-woven carriers. Ecol. Eng. 2016, 86, 126–134. [Google Scholar] [CrossRef]


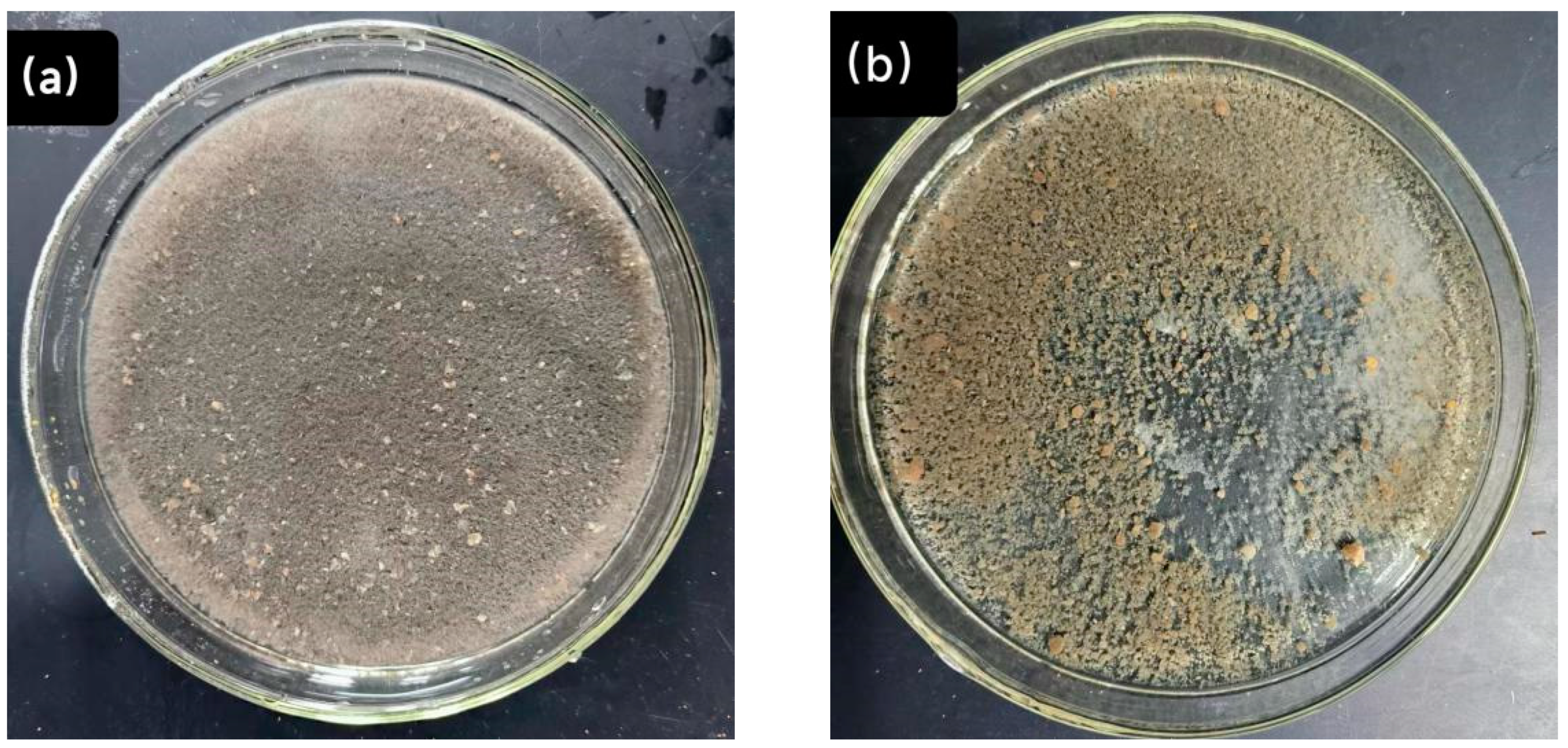

| Operation Stage | Time (d) | NH4+-N (mg·L−1) | NO2−-N (mg·L−1) | NO3−-N (mg·L−1) |
|---|---|---|---|---|
| S0-SADN operation stage | 1–66 | 22 ± 5 | - | 12–35 |
| 67–79 | 18 ± 2 | 25 ± 5 | - | |
| ANAMMOX start-up stage | 1–10 | 27 ± 3 | 33 ± 2 | - |
| 11–30 | 50 ± 5 | 75 ± 10 | - | |
| 31–75 | 65 ± 5 | 75 ± 5 | - |
| Sludge Sample | Sequences | OTUs | Shannon | Chao | ACE | Simpson |
|---|---|---|---|---|---|---|
| 0 d | 25.115 | 318 | 3.05 | 367 | 359 | 0.019 |
| 60 d | 23.159 | 467 | 4.58 | 467 | 467 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, K.; Zeng, Z.; Huang, S. Effect of Sulfur Autotrophic Denitrification Sludge on the Start-Up Characteristics of Anaerobic Ammonia Oxidation. Water 2023, 15, 1275. https://doi.org/10.3390/w15071275
Fu K, Zeng Z, Huang S. Effect of Sulfur Autotrophic Denitrification Sludge on the Start-Up Characteristics of Anaerobic Ammonia Oxidation. Water. 2023; 15(7):1275. https://doi.org/10.3390/w15071275
Chicago/Turabian StyleFu, Kunming, Zhixue Zeng, and Shaowei Huang. 2023. "Effect of Sulfur Autotrophic Denitrification Sludge on the Start-Up Characteristics of Anaerobic Ammonia Oxidation" Water 15, no. 7: 1275. https://doi.org/10.3390/w15071275
APA StyleFu, K., Zeng, Z., & Huang, S. (2023). Effect of Sulfur Autotrophic Denitrification Sludge on the Start-Up Characteristics of Anaerobic Ammonia Oxidation. Water, 15(7), 1275. https://doi.org/10.3390/w15071275






