Effects of Elevated Fe (III) on Anaerobic Ammonia Oxidation Biofilm Process: Inhibition and Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Synthetic Wastewater
2.3. Analytical Methods
2.3.1. Sampling and Analytical Methods
2.3.2. Substrate Degradation Rate of Anammox Analysis
2.3.3. Specific Anammox Activity Analysis
2.3.4. Extraction and Analysis of EPS
2.3.5. Continuous Impact of Fe (III) on Anammox
- Reactor start-up
- Reactor operation strategy
- Reactor recovery
3. Results and Discussion
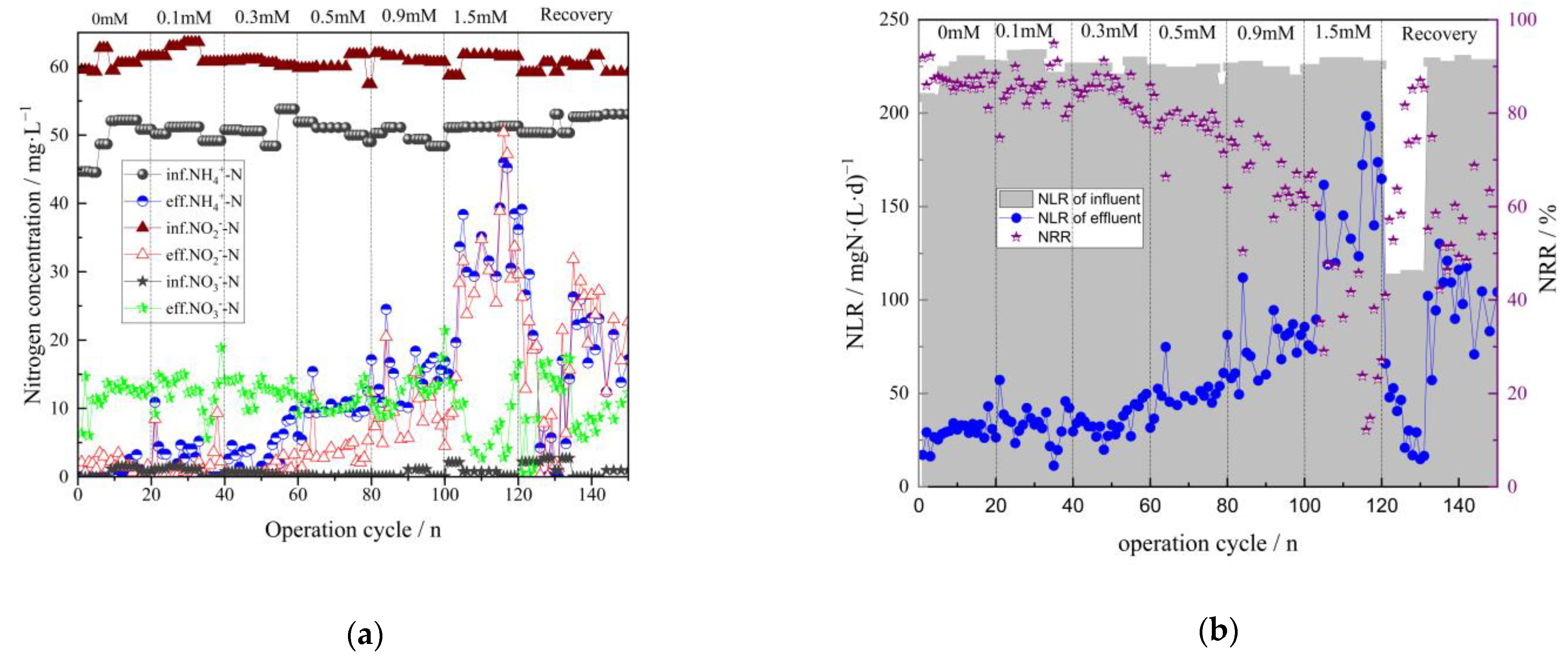
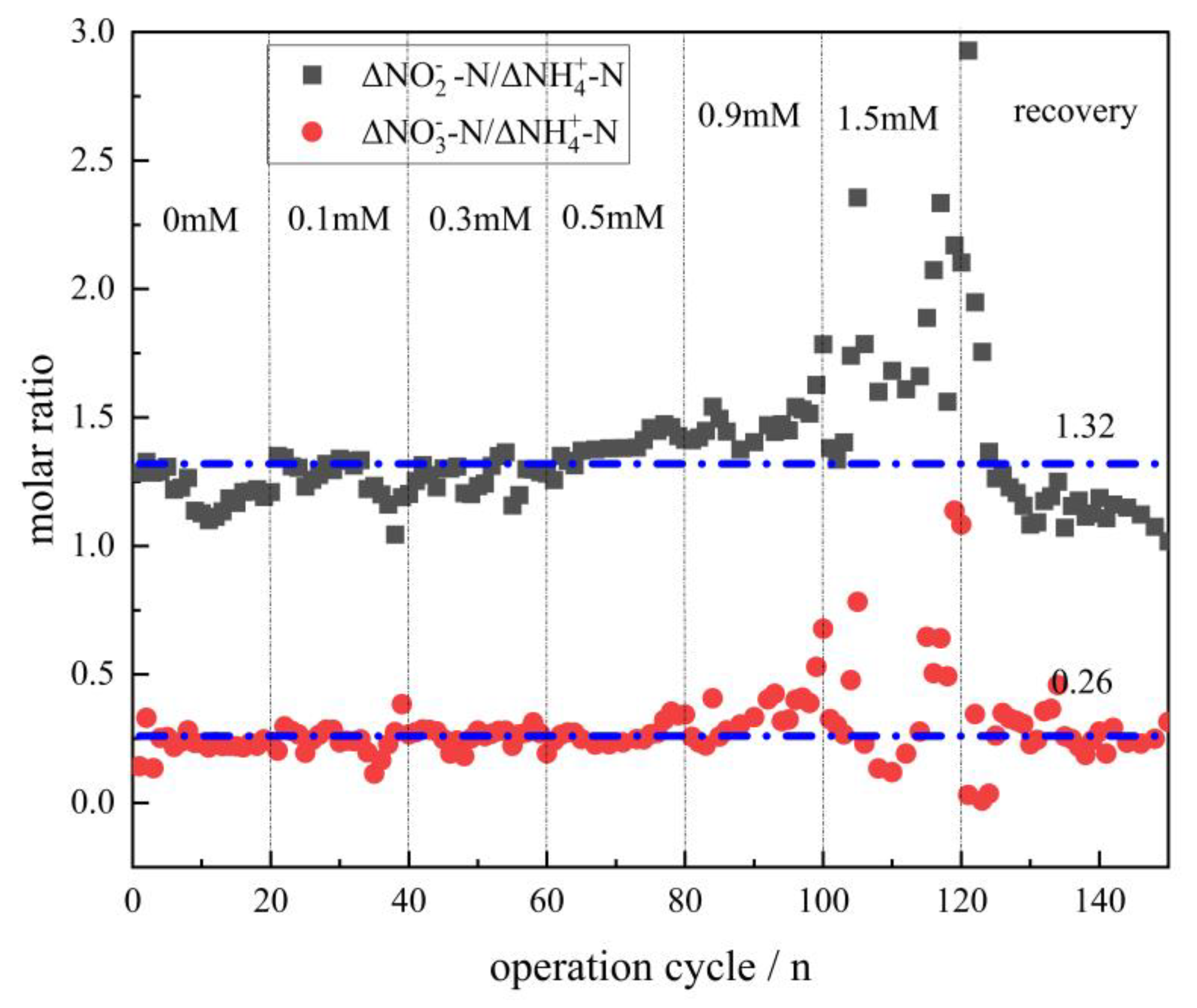
3.1. Effect of Fe (III) on the Removal of Nitrogen in the Anammox Biofilm Process
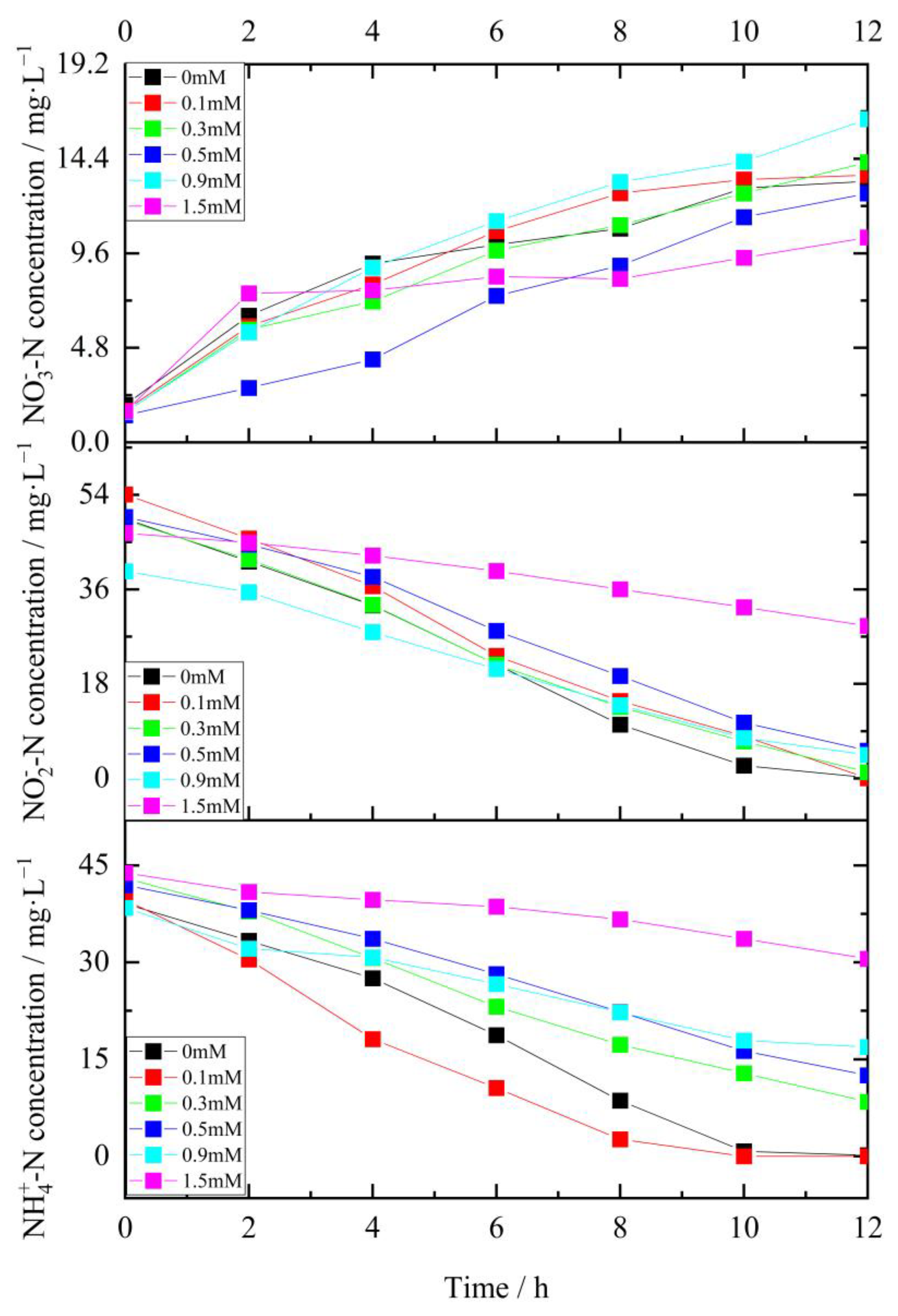
3.2. Kinetics of Various Fe (III) Concentrations on Periodical Substrate Degradation
3.3. Kinetics of Various Fe (III) Concentrations on Periodical Substrate Degradation
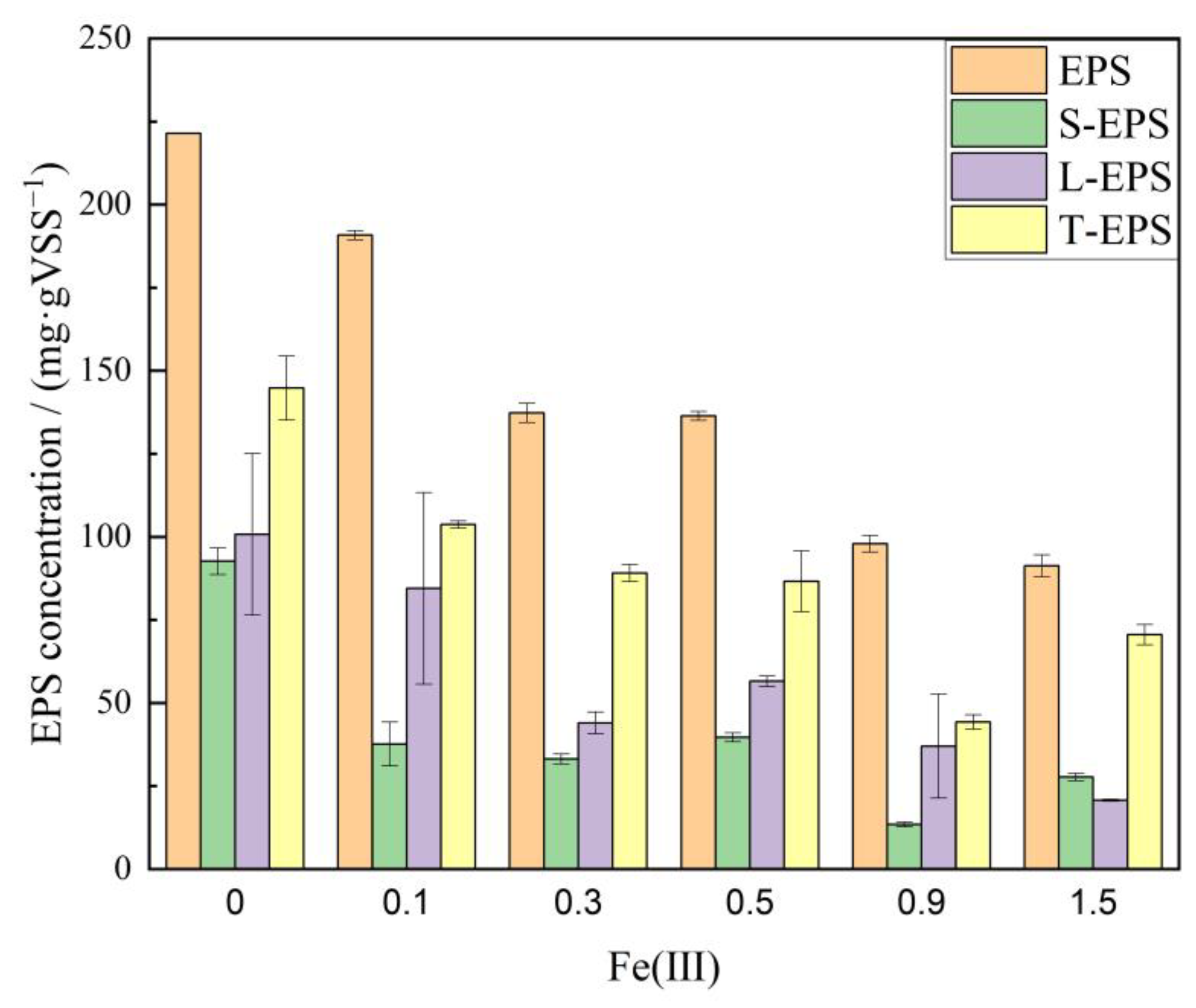
3.4. Biofilm Extracellular Polymer Analysis
3.5. Characteristics of Total Iron Migration
4. Conclusions
- (1)
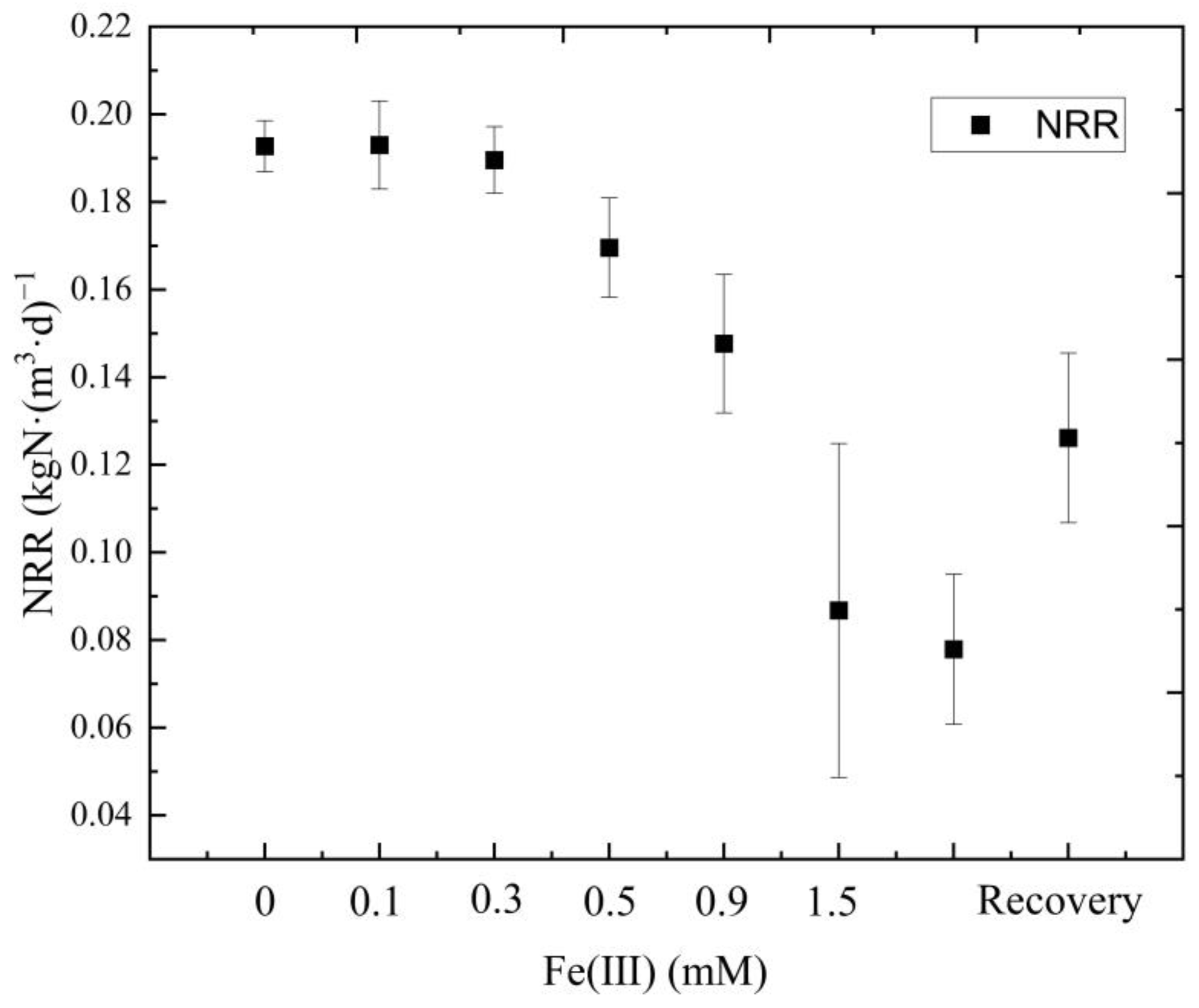
- The effects of Fe (III) content (ranging from 0 to 1.5 mM) on the anammox biofilm process were studied. Results indicated that the average NRR slightly increased at a low concentration of Fe (III) (lower than 0.1 mM). With the addition of Fe (III) doses ranging from 0.3 mM to 1.5 mM, the average TNRE declined from 86.9% to a minimum of 38.3%, indicating severe inhibition of anammox at high levels of Fe (III). Nevertheless, when adding EDTA·2Na to the reactor, the TNRR in the system gradually restored to 0.126 kgN·(m3·d)−1, and the TNRE maintained at about 60%, indicating that the anammox bacteria’s activity could be recovered. Additionally, the anammox bacteria exhibited a strong ability to resist the continuous loading of Fe (III).
- (2)
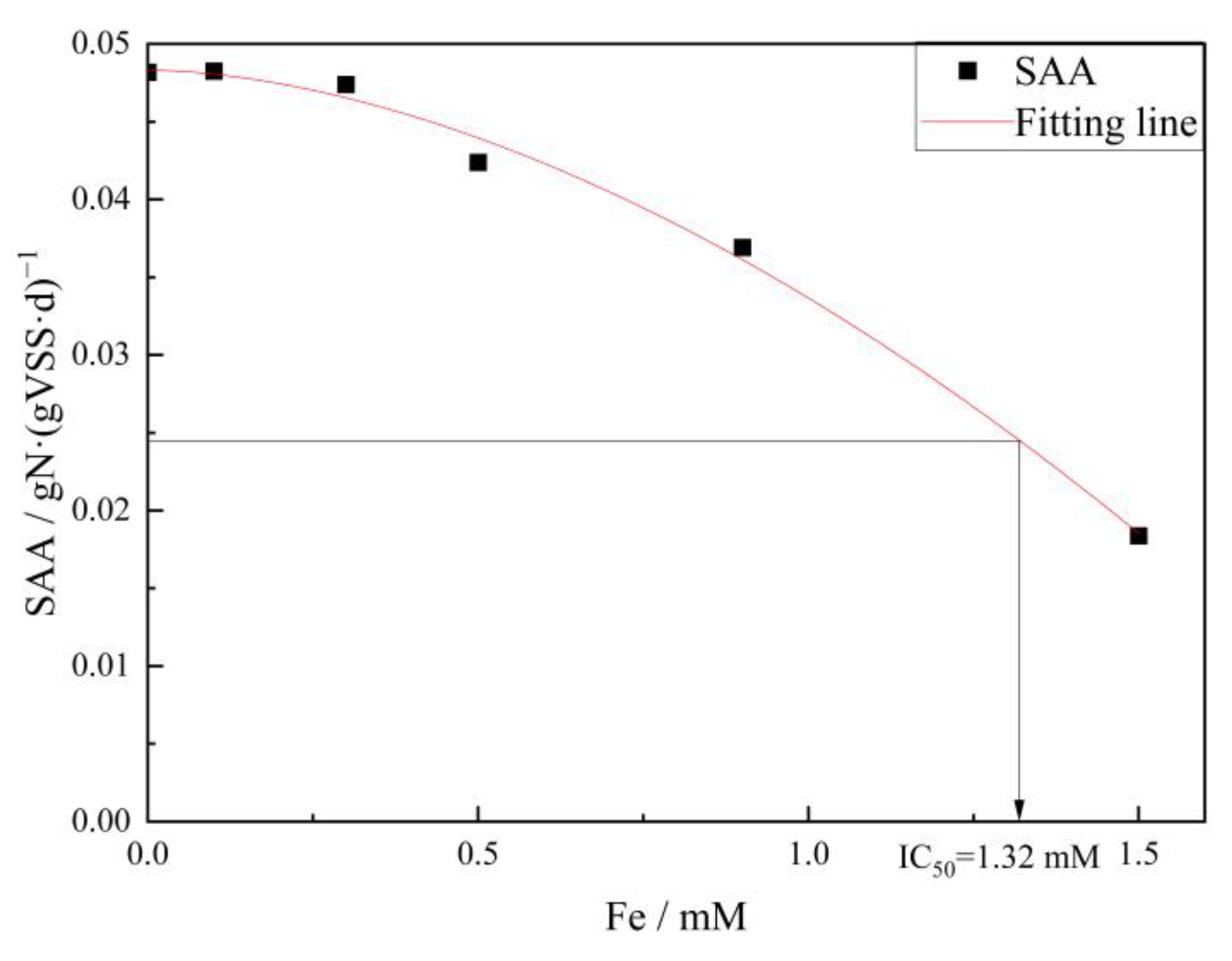
- The results of the kinetic fitting analysis showed that parameter K increased slightly from 8.77 to 8.93 at a low level of Fe (III) (lower than 0.1 mM) and decreased from 8.93 to 2.35 at a high level of Fe (III) (more than 0.1 mM), indicating that the lower concentrations of Fe (III) promote the anammox reaction, while higher concentrations of Fe (III) inhibited it. Additionally, the anammox reaction exhibited an IC50 of 1.32 mM for Fe (III) under the conditions investigated.
- (3)
- Under exposure to elevated Fe (III), a notable accumulation of Fe (III) was observed in the bacteria. The total iron content in anammox sludge increased from 30 mgFe·gVSS−1 to 300 mgFe·gVSS−1 and gradually reached a saturation point. EPS produced by cell metabolism decreased from 221.4 mg·gVSS−1 to 91.3 mg·gVSS−1, indicating that the anammox biofilm was destructed gradually. The SAA of anammox bacteria declined from 0.047 gN·(gVSS·d)−1 to 0.018 gN·(gVSS·d)−1 as the Fe (III) doses rose from 0.3 mM to 1.5 mM. Consequently, the anammox reaction was completely inhibited in the system, leading to a significant degradation in nitrogen removal efficiency.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.; Yang, B.; Yi, Y.; Feng, Q.; Liu, D. Synergistic activation of smithsonite with copper-ammonium species for enhancing surface reactivity and xanthate adsorption. Int. J. Min. Sci. Technol. 2023, 33, 519–527. [Google Scholar] [CrossRef]
- Shen, Z.; Wen, S.; Wang, H.; Miao, Y.; Wang, X.; Meng, S.; Feng, Q. Effect of dissolved components of malachite and calcite on surface properties and flotation behavior. Int. J. Miner. Metall. Mater. 2023, 30, 1297–1309. [Google Scholar] [CrossRef]
- Sun, W.; Xu, X.; Lv, Z.; Mao, H.; Wu, J. Environmental impact assessment of wastewater discharge with multi-pollutants from iron and steel industry. J. Environ. Manag. 2019, 245, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Sliekers, A.O. Completely autotrophic nitrogen removal over nitrite in one single reactor. Water Res. 2002, 36, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Kartal, B.; Kuenen, J.G.; van Loosdrecht, M.C.M. Sewage Treatment with Anammox. Science 2010, 328, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Kartal, B.; Maalcke, W.J.; de Almeida, N.M.; Cirpus, I.; Gloerich, J.; Geerts, W.; Op den Camp, H.J.M.; Harhangi, H.R.; Janssen-Megens, E.M.; Francoijs, K.-J.; et al. Molecular mechanism of anaerobic ammonium oxidation. Nature 2011, 479, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Webeck, E.; Li, Y.-Y. Towards more efficient nitrogen removal and phosphorus recovery from digestion effluent: Latest developments in the anammox-based process from the application perspective. Bioresour. Technol. 2020, 299, 122560. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.; Miao, L.; Cao, T.; Zhang, F.; Wang, S.; Han, J. Continuous-flow combined process of nitritation and ANAMMOX for treatment of landfill leachate. Bioresour. Technol. 2016, 214, 514–519. [Google Scholar] [CrossRef]
- Miao, L.; Wang, K.; Wang, S.; Zhu, R.; Li, B.; Peng, Y.; Weng, D. Advanced nitrogen removal from landfill leachate using real-time controlled three-stage sequence batch reactor (SBR) system. Bioresour. Technol. 2014, 159, 258–265. [Google Scholar] [CrossRef]
- Li, X.; Lu, M.-Y.; Qiu, Q.-C.; Huang, Y.; Li, B.-L.; Yuan, Y.; Yuan, Y. The effect of different denitrification and partial nitrification-Anammox coupling forms on nitrogen removal from mature landfill leachate at the pilot-scale. Bioresour. Technol. 2020, 297, 122430. [Google Scholar] [CrossRef]
- Tang, C.-J.; Zheng, P.; Chen, T.-T.; Zhang, J.-Q.; Mahmood, Q.; Ding, S.; Chen, X.-G.; Chen, J.-W.; Wu, D.-T. Enhanced nitrogen removal from pharmaceutical wastewater using SBA-ANAMMOX process. Water Res. 2011, 45, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zuo, J.; Wang, X.; Lin, J.; Yang, Y.; Zhou, J.; Chu, H.; Li, P. GeoChip-based analysis of microbial community of a combined nitritation-anammox reactor treating anaerobic digestion supernatant. Water Res. 2014, 67, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Peng, Y.; Han, X.; Gan, Y. Nitrogen removal performance and microbial distribution in pilot- and full-scale integrated fixed-biofilm activated sludge reactors based on nitritation-anammox process. Bioresour. Technol. 2015, 196, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, B.; Garcia, M.; Karakashev, D.; Angelidaki, I. Anammox for ammonia removal from pig manure effluents: Effect of organic matter content on process performance. Bioresour. Technol. 2009, 100, 2171–2175. [Google Scholar] [CrossRef]
- Huang, D.-Q.; Fu, J.-J.; Li, Z.-Y.; Fan, N.-S.; Jin, R.-C. Inhibition of wastewater pollutants on the anammox process: A review. Sci. Total Environ. 2022, 803, 150009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Z.; Zhou, Y.; Ma, Y.; Ma, C.; Li, Y.; Liang, Y.; Jia, J. Impacts of the heavy metals Cu (II), Zn (II) and Fe (II) on an Anammox system treating synthetic wastewater in low ammonia nitrogen and low temperature: Fe (II) makes a difference. Sci. Total Environ. 2019, 648, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Y.; Liu, Y.; Niu, J.; Zhao, T.; Bai, X.; Hussain, A.; Li, Y.-Y. Insights into heavy metals shock on anammox systems: Cell structure-based mechanisms and new challenges. Water Res. 2023, 239, 120031. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, F.; Liu, Y.; Yu, W.; Cai, W.; Zhang, X.; He, H.; Yao, H. The effects of Fe(III) and Fe(II) on anammox process and the Fe–N metabolism. Chemosphere 2021, 285, 131322. [Google Scholar] [CrossRef]
- Ferousi, C.; Lindhoud, S.; Baymann, F.; Kartal, B.; Jetten, M.S.M.; Reimann, J. Iron assimilation and utilization in anaerobic ammonium oxidizing bacteria. Curr. Opin. Chem. Biol. 2017, 37, 129–136. [Google Scholar] [CrossRef]
- Kartal, B.; de Almeida, N.M.; Maalcke, W.J.; Op den Camp, H.J.M.; Jetten, M.S.M.; Keltjens, J.T. How to make a living from anaerobic ammonium oxidation. FEMS Microbiol. Rev. 2013, 37, 428–461. [Google Scholar] [CrossRef]
- Kartal, B.; Keltjens, J.T. Anammox Biochemistry: A Tale of Heme c Proteins. Trends Biochem. Sci. 2016, 41, 998–1011. [Google Scholar] [CrossRef]
- Allen, J.W.A.; Barker, P.D.; Daltrop, O.; Stevens, J.M.; Tomlinson, E.J.; Sinha, N.; Sambongi, Y.; Ferguson, S.J. Why isn’t ‘standard’ heme good enough for c-type and d1-type cytochromes? Dalton Trans. 2005, 21, 3410–3418. [Google Scholar] [CrossRef] [PubMed]
- Poulos, T.L. Heme Enzyme Structure and Function. Chem. Rev. 2014, 114, 3919–3962. [Google Scholar] [CrossRef] [PubMed]
- Kleingardner, J.G.; Bren, K.L. Biological Significance and Applications of Heme c Proteins and Peptides. Acc. Chem. Res. 2015, 48, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, L.; Biswal, B.K.; Chen, G.-H.; Wu, D. Bioaugmentation of marine anammox bacteria (MAB)-based anaerobic ammonia oxidation by adding Fe(III) in saline wastewater treatment under low temperature. Bioresour. Technol. 2020, 295, 122292. [Google Scholar] [CrossRef]
- Chen, H.; Yu, J.-J.; Jia, X.-Y.; Jin, R.-C. Enhancement of anammox performance by Cu(II), Ni(II) and Fe(III) supplementation. Chemosphere 2014, 117, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, L.; Mao, N.; Geng, J.; Ren, H.; Xu, K. Effects of Fe3+ on microbial communities shifts, functional genes expression and nitrogen transformation during the start-up of Anammox process. Bioresour. Technol. 2021, 320, 124326. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Yang, Y.; Wang, Z.; Wang, J.; Yang, L.; Cai, X.; Wang, Z.; Xia, S. Enhancement and mechanisms of iron-assisted anammox process. Sci. Total Environ. 2023, 858, 159931. [Google Scholar] [CrossRef]
- Feng, L.; Li, J.; Ma, H.; Chen, G. Effect of Fe(II) on simultaneous marine anammox and Feammox treating nitrogen-laden saline wastewater under low temperature: Enhanced performance and kinetics. Desalination 2020, 478, 114287. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Yao, H.; Rong, H.; Li, S. Responses of anammox process to elevated Fe(III) stress: Reactor performance, microbial community and functional genes. J. Hazard. Mater. 2021, 414, 125051. [Google Scholar] [CrossRef]
- Ahmed, M.; Anwar, R.; Deng, D.; Garner, E.; Lin, L.-S. Functional Interrelationships of Microorganisms in Iron-Based Anaerobic Wastewater Treatment. Microorganisms 2021, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Saup, C.M.; Wilkins, M.J.; Lin, L.-S. Continuous ferric iron-dosed anaerobic wastewater treatment: Treatment performance, sludge characteristics, and microbial composition. J. Environ. Chem. Eng. 2020, 8, 103537. [Google Scholar] [CrossRef]
- Ahmed, M.; Lin, O.; Saup, C.M.; Wilkins, M.J.; Lin, L.-S. Effects of Fe/S ratio on the kinetics and microbial ecology of an Fe(III)-dosed anaerobic wastewater treatment system. J. Hazard. Mater. 2019, 369, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ji, M.; Wang, F.; Tian, Z.; Wang, T.; Wang, S.; Wang, S.; Yan, Z. Insight into the short-term effect of fulvic acid on nitrogen removal performance and N-acylated-L-homoserine lactones (AHLs) release in the anammox system. Sci. Total Environ. 2020, 704, 135285. [Google Scholar] [CrossRef]
- Belser, L.W.; Schmidt, E.L. Diversity in the Ammonia-Oxidizing Nitrifier Population of a Soil. Appl. Environ. Microbiol. 1978, 36, 584–588. [Google Scholar] [CrossRef]
- Jenkins, S.H. Standard Methods for the Examination of Water and Wastewater, 15th ed.; Greenberg, A.E., Connors, J.J., Jenkins, D., Eds.; American Public Health Association: Washington, DC, USA, 1980. [Google Scholar]
- Meng, L.; Xi, J.; Yeung, M. Degradation of extracellular polymeric substances (EPS) extracted from activated sludge by low-concentration ozonation. Chemosphere 2016, 147, 248–255. [Google Scholar] [CrossRef]
- Dapena-Mora, A.; Fernández, I.; Campos, J.L.; Mosquera-Corral, A.; Méndez, R.; Jetten, M.S.M. Evaluation of activity and inhibition effects on Anammox process by batch tests based on the nitrogen gas production. Enzym. Microb. Technol. 2007, 40, 859–865. [Google Scholar] [CrossRef]
- Bashir, M.J.K.; Ng, C.A.; Chen, W.H.; Lin, J.G.; Mak, C.Y. The short- and long-term inhibitory effects of Fe (II) on anaerobic ammonium oxidizing (anammox) process. Water Sci. Technol. 2019, 79, 1860–1867. [Google Scholar] [CrossRef]
- Brown, M.J.; Lester, J.N. Comparison of Bacterial Extracellular Polymer Extraction Methods. Appl. Environ. Microbiol. 1980, 40, 179–185. [Google Scholar] [CrossRef]
- Li, X.Y.; Yang, S.F. Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Res. 2007, 41, 1022–1030. [Google Scholar] [CrossRef]
- Gerhardt, P.; Murray, R.G.E.; Wood, W.A.; Krieg, N.R. Methods for General and Molecular Bacteriology; American Society for Microbiology: Washington, DC, USA, 1994. [Google Scholar]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, T.; Wang, J.; Shen, Y.; Ji, X.; Yang, D. A new concept of waste iron recycling for the enhancement of the anammox process. Chemosphere 2022, 307, 136151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-W.; Yang, X.-R.; Li, H.; Marshall, C.W.; Zheng, B.-X.; Yan, Y.; Su, J.-Q.; Zhu, Y.-G. Electron Shuttles Enhance Anaerobic Ammonium Oxidation Coupled to Iron(III) Reduction. Environ. Sci. Technol. 2016, 50, 9298–9307. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chi, Z.; Yan, B. Insight into the impact of Fe3O4 nanoparticles on anammox process of subsurface-flow constructed wetlands under long-term exposure. Environ. Sci. Pollut. Res. 2018, 25, 29584–29592. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, B.; Yu, C.; Zhang, Z.; Yao, J.; Jin, R. The effects of magnetite on anammox performance: Phenomena to mechanisms. Bioresour. Technol. 2021, 337, 125470. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yan, Y.; Zhao, Y.; Shi, Q.; Wang, Y. Characterization of stratified EPS and their role in the initial adhesion of anammox consortia. Water Res. 2020, 169, 115223. [Google Scholar] [CrossRef]
- Li, N.; Wei, D.; Wang, S.; Hu, L.; Xu, W.; Du, B.; Wei, Q. Comparative study of the role of extracellular polymeric substances in biosorption of Ni(II) onto aerobic/anaerobic granular sludge. J. Colloid Interface Sci. 2017, 490, 754–761. [Google Scholar] [CrossRef]
- Jia, F.; Yang, Q.; Liu, X.; Li, X.; Li, B.; Zhang, L.; Peng, Y. Stratification of Extracellular Polymeric Substances (EPS) for Aggregated Anammox Microorganisms. Environ. Sci. Technol. 2017, 51, 3260–3268. [Google Scholar] [CrossRef]
- Hou, X.; Liu, S.; Zhang, Z. Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge. Water Res. 2015, 75, 51–62. [Google Scholar] [CrossRef]
- Peeters, S.H.; van Niftrik, L. Trending topics and open questions in anaerobic ammonium oxidation. Curr. Opin. Chem. Biol. 2019, 49, 45–52. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Metal Binding Affinity and Selectivity in Metalloproteins: Insights from Computational Studies. Annu. Rev. Biophys. 2008, 37, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-F.; Ma, W.-J.; Cheng, Y.-F.; Li, S.-T.; Zhao, J.-W.; Li, J.-P.; Liu, Q.; Fan, N.-S.; Huang, B.-C.; Jin, R.-C. A spectra metrology insight into the binding characteristics of Cu2+ onto anammox extracellular polymeric substances. Chem. Eng. J. 2020, 393, 124800. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Deng, R.; Cheng, Y.-F.; Zhou, Y.-H.; Buayi, X.; Zhang, X.; Wang, H.-Z.; Jin, R.-C. Behavior and fate of copper ions in an anammox granular sludge reactor and strategies for remediation. J. Hazard. Mater. 2015, 300, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fang, F.; Chen, Y.-P.; Shen, Y.; Zhang, W.; Yang, J.-X.; Li, C.; Guo, J.-S.; Liu, S.-Y.; Huang, Y.; et al. Composition of EPS fractions from suspended sludge and biofilm and their roles in microbial cell aggregation. Chemosphere 2014, 117, 59–65. [Google Scholar] [CrossRef]
- Tang, S.-M.; Xu, Z.-H.; Liu, Y.-L.; Yang, G.-F.; Mu, J.; Jin, R.-C.; Yang, Q.; Zhang, X.-L. Performance, kinetics characteristics and enhancement mechanisms in anammox process under Fe(II) enhanced conditions. Biodegradation 2020, 31, 223–234. [Google Scholar] [CrossRef]
- Long, Y.-Y.; Du, Y.; Fang, Y.; Xu, J.; He, Y.-N.; Shen, D.-S. Effect of migration and transformation of iron on the endogenous reduction of H2S in anaerobic landfill. Waste Manag. 2016, 53, 76–81. [Google Scholar] [CrossRef]



| Compound | Concentration (mg·L−1) |
|---|---|
| (NH4)2SO4 and NaNO2 | As required |
| KH2PO4 | 30 |
| MgSO4·7H2O | 300 |
| NaHCO3 | 500 |
| CaCl2·2H2O | 150 |
| Trace elements solution Ⅰ | 1 |
| Trace elements solution Ⅱ | 1 |
| Analysis Items | Detection Methods | Instrument |
|---|---|---|
| -N | Sodium reagent spectrophotometry | UV2000 (SHIMADZU, Kyoto, Japan) |
| -N | N-(1-naphthyl)-ethylenediamine spectrophotometry | UV2000 (SHIMADZU, Kyoto, Japan) |
| -N | Ultraviolent spectroscopy | UV2000 (SHIMADZU, Kyoto, Japan) |
| pH | Glass electrode method | 6309PDT, JENCO (Shanghai, China) |
| DO | Membrane electrode method | HQ30d, HACH (Loveland, CO, USA) |
| MLSS, MLVSS | Gravimetry | Analytical balance (SHIMADZU, Shanghai, China) |
| Total iron | o-Phenanthroline spectrophotometry | UV2000 (SHIMADZU, Kyoto, Japan) |
| Fe(Ⅲ)/mM | K | R2 | Model |
|---|---|---|---|
| 0 | 8.7734 ± 0.35 | 0.9920 | C = C0 − K*t |
| 0.1 | 8.9336 ± 0.49 | 0.9848 | |
| 0.3 | 7.5216 ± 0.28 | 0.9933 | |
| 0.5 | 6.6017 ± 0.32 | 0.9885 | |
| 0.9 | 5.2394 ± 0.09 | 0.9985 | |
| 1.5 | 2.3539 ± 0.12 | 0.9874 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, F.; Li, R.; Ji, M. Effects of Elevated Fe (III) on Anaerobic Ammonia Oxidation Biofilm Process: Inhibition and Recovery. Water 2023, 15, 4080. https://doi.org/10.3390/w15234080
Wang S, Wang F, Li R, Ji M. Effects of Elevated Fe (III) on Anaerobic Ammonia Oxidation Biofilm Process: Inhibition and Recovery. Water. 2023; 15(23):4080. https://doi.org/10.3390/w15234080
Chicago/Turabian StyleWang, Shuya, Fen Wang, Ruying Li, and Min Ji. 2023. "Effects of Elevated Fe (III) on Anaerobic Ammonia Oxidation Biofilm Process: Inhibition and Recovery" Water 15, no. 23: 4080. https://doi.org/10.3390/w15234080
APA StyleWang, S., Wang, F., Li, R., & Ji, M. (2023). Effects of Elevated Fe (III) on Anaerobic Ammonia Oxidation Biofilm Process: Inhibition and Recovery. Water, 15(23), 4080. https://doi.org/10.3390/w15234080








