Abstract
Clean water is very important for the good health of society. In South Africa, it is estimated that people need 20 to 50 litres of safe water daily for basic hygiene, drinking, and cooking. In recent times, water bodies have harboured harmful pollutants, including oil, heavy metal ions, and dyes. As a result, this has become a major global concern. Societies with limited clean water are often forced to utilise contaminated water or buy filtered water, which might be a problem for poor residents. The health consequences that are related to contaminated water include Guinea worm disease, dysentery, cholera, etc. The side effects associated with the utilisation of unclean water are gastrointestinal diseases such as cramps, vomiting, and diarrhoea. The wastewater disposed of by chemical industries contains toxic elements such as arsenic. Wastewater that is released directly without treatment causes serious damage to the environment. Chronic arsenic poisoning can lead to keratinisation of the skin and even cancer. Cellulose biomass materials have the potential to become the greatest bio-based materials used in wastewater treatment applications. There are two major reasons that validate this statement: firstly, cellulose is a low-cost material that is abundant in nature, and, secondly, cellulose is an environmentally friendly material. However, these are not the only reasons that validate cellulose as a good candidate for wastewater treatment applications. Cellulose has a unique structure a large surface area, good mechanical properties and is degradable, renewable, and biocompatible. Cellulose also has an abundance of hydroxyl groups on its surface. These hydroxyl functional groups allow cellulose to be chemically modified in various ways, which results in the fabrication of nanocomposites with tunable characteristics. Since arsenic pollution has become a serious global concern, this review uniquely provides a broad discussion of the work that has been accomplished recently on the fabrication of functionalised cellulose-based materials designed specifically for the removal of arsenic heavy metal species from wastewater treatment facilities. Furthermore, the functionalised cellulose materials’ arsenic adsorption capacities are also discussed. These adsorption capacities can reach up to a maximum of 350 mg/g, depending on the system used. Factors such as pH and temperature are discussed in relation to the adsorption of arsenic in wastewater. The removal of As(V) was found to be effective in the pH range of 3.0–8.8, with a removal efficiency of 95%. Moreover, the removal efficiency of As(III) was reported to be effective in the pH range of 6–9. However, the effective pH range also depends on the system used. The selective extraction of cellulose from various sources is also discussed in order to verify the percentage of cellulose in each source. Future work should be focused on how the chemical modification of cellulose affects the toxicity, efficiency, selectivity, and mechanical stability of cellulose materials. The use of cheaper and environmentally friendly chemicals during cellulose functionalisation should be considered.
1. Introduction
In the current century, the key issue is the development and/or utilisation of renewable and biodegradable materials in order to decrease the burden of the environmental crisis [1]. The purpose is to use these renewable materials in various applications to counteract the demolition of the planet by industry. Due to its eco-friendliness, the cellulose extracted from various natural fibres provides solutions for the current environmental problems caused by the non-biodegradable materials that are used in different applications [2]. Cellulose is normally found in trees and plants, and it is inserted in lignins and hemicellulose. It is one of the most common natural organic materials, making up one-third of vegetables globally [3]. The cellulose structure is held up by a hydrogen bond, due to the multiple hydroxyl groups on its structure. The advantages of cellulose include its (i) biodegradability, (ii) low cost (iii), abundance (iv), sustainability, and (v) high aspect ratio [4]. Cellulose-based materials heavily contribute to the field of nanotechnology, including applications in medicine [5,6,7,8], food packaging [9,10,11,12,13,14,15,16], water treatment, which includes the removal of dyes and heavy metal ions from wastewater [17,18,19] and supercapacitor applications [20,21,22,23,24]. Figure 1 illustrates the applications of cellulose and cellulose-based materials.
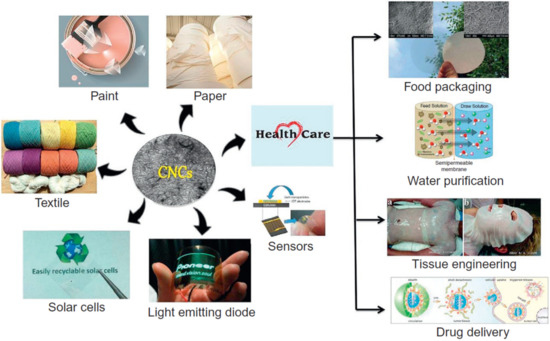
Figure 1.
Summarised applications of cellulose-based materials [25]. Copyrights: Elsevier, 2018.
Amongst all the applications of cellulose-based materials, the one that seems to currently require a lot of attention is wastewater treatment. Various contaminants such as detergents, heavy metal ions, food additives, dyes, pesticides, biomolecules, and polycyclic aromatic hydrocarbons are responsible for contaminating water [25]. Amongst all the treatments utilised for the removal of contaminants from water, cellulose based membranes are preferred due to their chemical inertness, hydrophilic surface, high strength [26,27,28,29], and ability to undergo surface modification; as a result, they ease the selective adsorption of the pollutant. Besides the primary original cellulose, cellulose may be divided into microfibrillated cellulose (MFC) and nanofibrillated cellulose (NFC). The diameter of MFC is normally in the region of 10–100 nm, and its length is roughly between 0.5 and 10 mm, while nanofibrillated cellulose has a diameter in the range of 4–20 nm with a length of 500–2000 nm [30]. It is documented in pieces of the literature that the functionalisation of cellulose takes place through the hydroxyl groups, and, once cellulose is functionalised, its applications are extended [30,31,32,33]. There are two categories for the functionalisation of cellulose viz, chemical and physical functionalisation [30]. The removal of unwanted material in an aqueous medium is normally accomplished with chemically modified cellulose. Various chemicals have been utilised for the chemical modification of cellulose, i.e., aminoethanethiol, diethylenetriamine, carboxymethylation, and 2,2,6,6-tetramethylpiperidine-1-oxylradical (TEMPO) [34,35,36,37,38,39,40,41,42,43]. This current review discusses the effect of various chemical functionalisations on cellulose for the removal of arsenic materials in wastewater. Since cellulose is being functionalised, the morphology of unfunctionalised and functionalised cellulose is also discussed in relation to water-purification efficiency. The sources of cellulose extraction are also discussed, since it is important to understand the percentage of cellulose in their original sources.
The development of the metallurgical and chemical industries as well as the exploitation of ores has led to an increased discharge of arsenic and its compounds into our mainstream water outlets [44]. The presence of copious amounts of arsenic in water bodies, especially wastewater, is due to numerous factors that include ore mining, fossil fuel combustion, the smelting of non-ferrous metals, the preparation of drugs containing arsenic, volcanic eruptions, and the usage of pesticides [44]. Arsenic is a toxic and carcinogenic element that exists in various forms in the environment [45]. The two most common forms of arsenic found in contaminated water are arsenate (As(V)) and arsenite (As(III)) [45]. As(III) is more resistant and has higher health risks upon exposure [45]. Arsenic pollution has become a huge problem for many countries [44]. The discharge of arsenic-containing water into the main water sources can inflict severely harmful effects on human health and may also cause an environmental imbalance [44]. Arsenic can alter major metabolic processes in cells and, thus, cause cell death [44]. Chronic arsenic poisoning can lead to keratinisation of the skin and even cancer [44]. Therefore, it is important to control arsenic concentrations in water environments to ensure safe drinking water [44]. The treatment of arsenic-containing drinking water is one of the best ways of ensuring that arsenic concentrations are kept in check in our drinking water [44]. In 2011, the World Health Organization (WHO) stipulated that the arsenic concentration in drinking water should not exceed 10 ppb [45].
Cellulose is an ideal low-cost source for the extraction of water purification materials. This is because cellulose is the most abundant and renewable natural polymer in the world [45,46,47]. Cellulose also contains numerous hydroxyl functional groups on its surface. These functional groups enable the modification of cellulose into nanocomposites, with tunable properties that may involve the removal of arsenic from wastewater [48]. Modified cellulose-based materials have a history of being used to remove toxic metal ions from water. Succinic anhydride-modified nanocellulose was used to effectively remove heavy metal ions, e.g., Cr(VI) and Pd(II), and dyes from water [49,50]. A carbon aerogel prepared from microcrystalline cellulose was also used to effectively remove Cr(V) and Pb(II) ions [51]. Carboxylated cellulose nanofibre (CNF), an oxidised form of nanocellulose, was discovered to possess an excellent adsorption capacity towards Uranium ions (UO22+) [52,53], Pb(II) ions [54], and Cd(II) ions [55]. Ferric hydroxide-coated CNFs also proved to be excellent candidates for the removal of phosphate from wastewater [56]. Furthermore, three-dimensional porous cellulose was discovered to be an effective adsorbent with a high capacity for the removal of cationic dyes [57]. It was also demonstrated that nanocomposites based on regenerated microfibrillated cellulose decorated with zinc oxide nanocrystals had a very high maximum capacity for the removal of As(V) [58].
2. Selective Studies on the Extraction of Cellulose from Various Sources
Cellulose fibres may be extracted into nano or micro cellulose by chemical and mechanical techniques [59,60,61,62,63,64,65,66,67,68]. There are different sources of cellulose fibres, which include wood, non-woody fibres, tunicates, agro-wastes or biomass, algae, etc. [69,70,71,72,73,74,75,76]. Figure 2 illustrates selective sources of cellulose fibres. Generally, the extraction route of cellulose consists of pre-hydrolysis, pulping, and bleaching (Figure 3). Generally, it is known that pre-hydrolysis is utilized to open the matrix with an acid or alkali material. Chemical pulping is the process whereby the lignin and hemicellulose are degraded into small as well as water-soluble molecules, which can be easily removed from the cellulose without depolymerising the fibres. Morán et al. [77] extracted cellulose from sisal fibres, with sisal fibre consisting of cellulose (50–74%), hemicellulose (10–14%), lignin (8–11%), pectin (1%), and wax (2%). As a standard in most extractions, when the fibre is received, it is washed with distilled water several times and dried in an oven. The pre-treatment of the sisal fibre was accomplished with sodium hydroxide in a solution of ethanol. The bleaching of the fibre was accomplished with sodium chlorite (NaClO2). The nanofibres were produced by acid hydrolysis using sulphuric acid (H2SO4). After acid hydrolysis, the obtained cellulose nanofibres were reported to be in the range of 30.9 ± 12.5 nm.
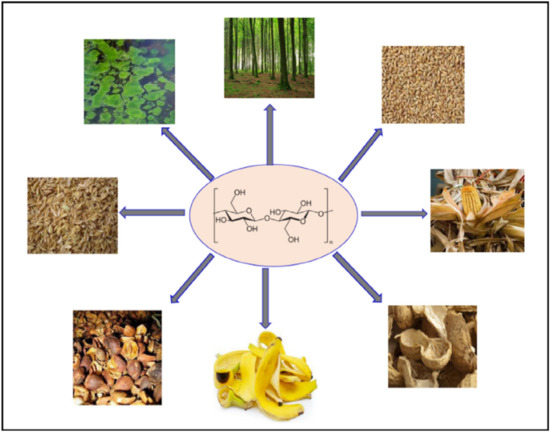
Figure 2.
Selective sources of cellulose extraction [69]. Copyrights: Elsevier, 2022.
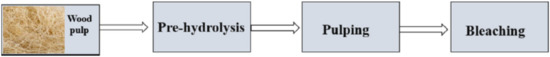
Figure 3.
Extraction route for cellulose extraction from sources [69]. Copyrights: Elsevier, 2022.
The cellulose was extracted from Calotropis procera fibre (CPF) [78]. The CPF was found to have 64.1% cellulose, 9.7% lignin, and 19.5% hemicellulose. As reported by previous studies, the extraction of cellulose in this case also took place through pre-hydrolysis, pulping, and bleaching. The cellulose nanocrystals were also fabricated by acid hydrolysis with 63 wt% sulphuric acid (H2SO4). TEM revealed uniform needle-like CNCs with a length of 250 nm and a diameter of 12 nm. The extraction of microcrystalline cellulose from Washington fibre was reported by Azum and co-workers [79]. The production yield of the WMCC was found to consist of 82.5% α-Cellulose, 5.2% hemicellulose, and 2.1% lignin. The length and diameter reported in this study were in the ranges of 300–500 μm and 60–90 μm, respectively. The acid hydrolysis in this study was found to be responsible for rupturing the cellulose structure into various individual fibrils of smaller size. Song et al. [80] proved the effectiveness of microwave technology with regard to the extraction of lignin. The aim of that study was to make certain that the lignin was completely removed by microwave technology, and then nanocellulose extraction was further completed by acid hydrolysis using an ultrasonic device. Microwave technology was used due to its various advantages such as energy conservation, cleanness, speediness, and high efficiency [80,81]. There was a decrease in the content of lignin and hemicellulose after microwave treatment, which obviously led to an increase in the content of the cellulose. For example, there was 69.28% lignin extracted from the original kenaf fibre by microwave treatment, while there was 24.49% lignin content extracted by bleaching [80]. The extracted cellulose by microwave treatment and acid hydrolysis revealed a needle-like type of morphology with a length of around 300 to 600 nm, while the diameter was reported to be in the range of 10–60 nm. Luzi et al. [82] reported on the extraction of cellulose nanocrystals from pristine and carded hemp fibres. Fibre carding is the process whereby pristine fibre undergoes a mechanical process to disentangle and clean the fibre for further processing. During the process of carding, there is a separation of tufts into fibres, an alignment of the fibres, and the removal of any impurities. The aim of the study was to investigate the effect of the carding process in relation to the pre-treatment competency and the percentage yield of cellulose nanocrystals. The extraction method followed the well-known route that involves chemical treatment with the gradual removal of lignin and subsequent acid hydrolysis for the formation of cellulose nanocrystals. In this study, both chemical and enzymatic treatments of non-carded and carded hemp fibres were reported. Based on the visual images, the neat hemp fibres were found to be unorganised (Figure 4a), while, on the contrary, the carded hemp fibres were revealed to be well-aligned, with no dust or impurities (Figure 4b). A similar observation was seen in the FESEM images, whereby the non-carded hemp fibres showed unaligned fibres (Figure 4c), whereas the carded hemp fibres were well-separated and aligned without any impurities (Figure 4d). The fibre diameters for the uncarded and carded hemp were reported as 21 and 19 μm, respectively.
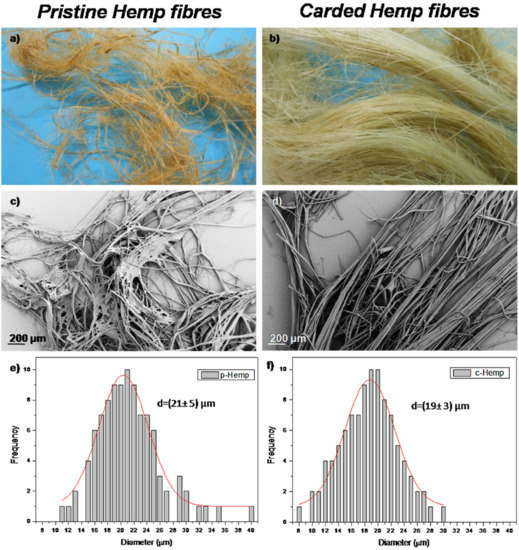
Figure 4.
Optical images (a,b) and FESEM images (c–f) of diameter distribution of pristine and carded hemp fibres, respectively [67].
The chemically extracted cellulose from both the neat and carded fibres was reported to be acicular, which symbolised the nanocrystals or nanowhiskers. Furthermore, it was reported that there was no difference in the shape or dimension between the extracted cellulose from the two fibres (i.e., the neat and carded hemp fibres). A similar observation was reported for the enzymatic treatments of the fibres, whereby both fibres revealed nanocellulose with the same shape and dimension. XRD results revealed that the carding process produced a high conversion of cellulose I. The effect of the reaction parameters, such as temperature, catalyst, time, and alkali treatment, were studied to find the optimum reaction conditions for cellulose extraction from agricultural waste [83]. Cellulose was extracted from rice husk (RH) with a montmorillonite K-10/LiOH solution followed by peroxide bleaching. The concentration range of the catalyst/alkali system varied from 0.1 g/4% to 0.5/12%. The maleic acid concentration was in the range of 5–20%, while the investigated temperatures were kept in the range of 50–90 °C. The investigated time range used in this study varied between 3 to 7 h. The optimum conditions were found to be 6 h, 80 °C, and 20% maleic acid as well as 10% of LiOH (in water). The percentage yield of cellulose was reported to be 68%. The effect of alkaline treatment on the extraction of cellulose from various agricultural by products such as rice straw, Phulae pineapple leaves, corncob, and Phulae pineapple peels was reported in a study [84]. The extracted cellulose from agricultural waste was compared with commercial cellulose. The treatment of the fibres, which included both the alkaline and bleaching processes, was performed by employing 1.4% of acidified sodium chlorite and 5% potassium hydroxide. The percentage yield of cellulose from various agricultural wastes, i.e., RS, CC, PL, and PP, was reported to be 32.26%, 38.18%, 16.60%, and 9.05%, respectively. The extraction of cellulose from bamboo pulp by mechanochemical activation and phosphotungstic acid hydrolysis was reported by Lu et al. [85]. The mechanochemical method is preferred because it is environmentally friendly, without the utilisation of solvents. The concentration of phosphotungstic acid and its reaction were found to play a key role in the yield of cellulose nanocrystals. The yield percentage of the cellulose nanocrystals increased with the reaction time. The yield percentage was found to be high when the reaction time was in the range of 4.5–5 h with the phosphotungstic acid in the concentration range of 10–15%. The lower yield percentage of the cellulose nanocrystals at lower acid concentrations is associated with fewer acid sites to break up the β-1,4-glycosidic bonds of the cellulose fibres. The mechanochemical process was also found to play a critical role in the production of cellulose, with the yield of the cellulose nanocrystals increasing between 1.5 and 2.5 h at various instances in the reactions. The mechanochemical process, by utilising the ball milling process, is able to promote a lot of stress, which was transferred into the cellulose, enhancing the cleavage of the chemical bonds in the cellulose and reducing the size of the cellulose. In summary, the optimum conditions to obtain the highest cellulose nanocrystals (CNC) are: a 13.5% concentration of phosphotungstic acid, 4.7 h reaction time, and ball time of 2.2 h, which yielded 88.4% CNC. In addition to the well-known sources of cellulose such as kenaf, bamboo, sisal, jute, sugarcane bagasse, hemp, etc., there are emerging sources of cellulose such as the halophytes. Singh et al. [86] utilised halophytes (viz, Tamarix aphylla, Juncus rigidus, and Thespesia populnea) as an emerging source of cellulose. Halophytes are sources of cellulose that are salt-loving plants. Cellulose extraction was completed from the source using various concentrations of acid, alkaline, and bleaching completed at different temperatures. The extraction of cellulose from the plant source was completed by the following treatment: (i) sodium chlorite, (ii) sodium hydroxide, (iii), and (iv) hydrogen peroxide. The extracted samples were further fractionated into α-cellulose and β-cellulose. Generally, the halophytes produced crude cellulose in the range of 39–48%. The key findings reported by the study were, for example, that T. aphylla needs a low concentration of sodium chlorite (NaClO2), hydrochloric (HCl) acid, and more heating time in order to remove the lignin and hemicellulose when compared with other halophytes. Furthermore, T.aphylla recorded the highest extraction of α-cellulose with the highest percentage crystallinity index, i.e., CI = 61.8%, whereas the lowest crystallinity was recorded to be 48%, for the extraction from T. populnea. In summary, the extraction of cellulose and the fabrication of cellulose nanocrystals (CNCs) depend on the source(s) of the cellulose, extraction method, reaction time, and type of acid utilised. Table 1 illustrates selective studies on the extraction of cellulose from various sources.

Table 1.
Selective studies on the extraction of cellulose from various sources.
3. Brief History of Arsenic
Arsenic is a substance that has been around for thousands of years [102]. The notion regarding arsenic as an infamous substance used for suicide and homicide began to gain popularity in the Middle Ages and the Renaissance period [103,104]. It became one of the most notorious poisons leading up to the mid-1850s, due to its toxic and discreet nature. Moreover, analytical testing methods for arsenic were only discovered in the mid-1700s, but it was not until the trial of Marie Lafarge in 1840 that arsenic-detection techniques, pioneered by James Marsh, were proven to be admissible in court. Despite the toxic nature of arsenic, it gained significant attention due to its widespread use across various industries such as the (i) pharmaceutical industries, (ii) agricultural sector (as a pesticide and insecticide), (iii) commercial industries (in the manufacturing of pigment products, glass, and semiconductors), and (iv) copper smelting industry (where it is released as a by-product) [103,105]. It was documented that Hippocrates, the “Father of Medicine”, was believed to have utilised arsenic paste to treat abscesses and ulcers [103,106]. Furthermore, in 1786, Fowler’s solution, used to treat diseases such as asthma, eczema, psoriasis, malaria, and syphilis, was reported to contain 1% potassium arsenite. In 1910, Paul Ehrlich presented the “magic bullet”, which was an arsenic-based drug used for the treatment of syphilis, until penicillin gained popularity in the 1940s. Following the success of Fowler’s solution in the treatment of leukemia in 1878, by lowering the white blood cell count, the investigation into arsenic trioxide as a viable chemotherapeutic drug for other cancers has been ongoing [103]. The agricultural use of arsenic began with the introduction of Paris Green, between 1867 and 1900, as an effective insecticide against the beetles and mosquitoes that destroyed the Colorado potato. The use of Paris Green declined as lead arsenates (less-toxic arsenic-containing pesticides) were introduced in the 1800s. However, by 1960, the use of lead arsenate-based pesticides also declined due to the health complications expressed by orchard workers that were associated with the arsenic remnants found in fruits. This eventually led to the ban of lead arsenate by the United States in 1988. Nonetheless, some arsenic-based pesticides are still used today, such as chromated copper arsenate (CCA), which is used as a wood preservative. Much like for the agricultural sector, the adverse health effects of arsenic in commercial products such as wallpaper pigments as well as in the copper smelting industry have been identified [105]. Studies have shown exposure to arsenic in an occupational setting as well as during commercial use can increase the burden of lung cancer, neurological abnormalities, and dermatitis. Reports by Bartolomco Gosio in 1893 illustrated how the volatised arsenic used in wallpaper pigments can emit arsenic-containing compounds such as Paris Green [103].
The dangers of ingesting Arsenic in high dosages has been well-known for many years. Ancient Chinese farmers used arsenic trioxide to kill fungi and rats in their rice fields in order to protect their crops and increase crop yields [107]. Napoleon Bonaparte’s death was suspected to be caused by arsenic poisoning [104,108]. However, its effects at low dosages only became clear in the 1980s [102]. The full impact of this element on society is only becoming clear now. Nowadays, it is known to be the most dangerous substance ever to be regulated by the US Environmental Protection Agency (EPA). High concentrations of Arsenic have been found in many drinking water sources around the world [109]. For example, in 2008, the number of Bangladeshi people affected by overexposure to Arsenic vastly exceeded the number of people affected by the Chernobyl disaster that occurred on 26 April 1986 [102].
Arsenic in the environment has become a global concern because of the widespread chronic arsenic poisoning found in many countries affecting large numbers of people [104]. In 1963, the World Health Organization (WHO) set the arsenic standard in drinking water as 50 ppb. However, in 1993, the standard was reduced to 10 ppb [104,105,110]. Based on analysis, the 50 ppb level was considered to cause arsenic poisoning and was not protective of overall public health [104]. Therefore, in 2006, U.S. water treatment techniques were required to meet the new 10 ppb maximum contaminant level (MCL) for arsenic, which is still being used today [111]. Since arsenic toxicity depends on its oxidation states and chemical forms, the measurement of all arsenic species in natural water is essential for future environmental monitoring and regulatory considerations [111].
Water containing high concentrations of arsenic has been treated using several techniques. The lime softening technique is one of the oldest effective methods used to reduce lime concentration. In 2015, Klerk et al. [112] used lime neutralisation to conduct a continuous circuit co-precipitation of As(V) ions with ferric ions. The two stages of the continuous experiments were operated at a pH of 4 and 8, respectively. The lowest residual concentration of arsenic was achieved when the Fe/As molar ratio was kept at 4.
Ion exchange technology has been considered as another effective way of removing arsenic from water, by using anionic exchange resins. However, this technique was only effective in the removal of As(V) from water, and it was not effective in the removal of uncharged As(III) [113]. One major drawback with the development of these systems is that they turned out to be expensive. During ion exchange water purification, the adsorption capacity was limited by the interference and competitive adsorption of other co-existing anions. The adsorbent regeneration process in this technology created a sludge disposal problem [113].
In recent times, membrane technologies such as nanofiltration and reverse osmosis are increasingly becoming popular for the removal of arsenic from water [114]. The advantages of such techniques are their high removal efficiency, their easy operation, and the reduced amount of sludge generated during the water purification process [115]. However, the disadvantage is that the initial investment and operating costs of these membrane technologies are relatively high. During membrane water purification, high pressure is applied to force the contaminated water through the membranes. Discharge of the concentrate, membrane fouling, and flux decline are usually unavoidable in the membrane process [116]. Electrodialysis was also capable of removing both arsenic and other contaminants. The problem is that large amounts of insoluble coagulants were deposited on the cathode [117].
The adsorption process is considered one of the most promising techniques, of the many that are used to remove arsenic from water. This is because the adsorption technique is cheaper, highly efficient, and easy to operate [118]. Iron-based adsorbents for the effective removal of arsenic species from water have been extensively developed [119]. Some commercial-grade adsorbents such as granular ferric hydroxide (GFH) and zero-valent iron have been developed and produced on an industrial scale for commercial purposes. Unfortunately, most of the reported adsorbents do not make it to the practical field, in spite of their effective arsenic removal capacity, due to the interfering ions present in water [120]. Due to the specific chemical reaction between arsenic and iron in iron-based adsorbents, anions such as , , , and were not considered as an interference to arsenic adsorption [121]. However, phosphate was found to strongly compete with arsenic for the adsorption sites and, thus, reduce the arsenic adsorption capacity of iron-based adsorbents [122]. Organic matter such as humic acid and fulvic acid were also shown to have negative effects on the adsorption of arsenic by slowing down the adsorption equilibrium [123].
4. Functionalised Cellulose for Removal of Arsenic
4.1. Preparation of Functionalised Cellulose
Arsenic as a metal is a toxic as well as carcinogenic element, which occurs in various forms such as the atmosphere, soil, and water. Water, especially drinking water, is a direct track for human beings to intake arsenic, which has become a major issue globally [124]. The diseases associated with arsenic include bladder cancer, skin cancer, and kidney cancer. According to the World Health Organization (WHO), in the year 2011, the finite arsenic concentration of 10 ppb in drinking water was prescribed. The various types of arsenic found in water include arsenate (As(V)) and arsenite (As(III)) [124]. Based on the above chronic diseases that are associated with arsenic, urgent methods are needed for the removal of arsenic from water. The well-known methods include coagulation, membrane separation, adsorption, and ion exchange [124,125,126,127]. The adsorption method is the most favoured method since it is cheap and has high efficiency and low energy consumption. However, the adsorbents materials used in the adsorption method pose various challenges that include a high amount of preparation, a complex preparation method, and low biocompatibility, which might result in secondary contamination in the water. As a result, there is a need for the development of an eco-friendly and inexpensive adsorbent to avoid the problems associated with the adsorption methods. Therefore, the cellulose materials extracted from various plants are ideal sources to remove unwanted materials such as arsenic from water. A novel adsorbent system containing cellulose with thiol groups, with the cellulose extracted from wood pulp, was utilised to remove arsenic As(III) [124]. Two biomass-based cellulose structured materials, i.e., (i) microscale dialdehyde cellulose-cysteine (MDAC-cys) and nanoscale dialdehyde cellulose-cysteine (NDAC-cys), were reported in this study. The morphology of the wood pulp cellulose, per SEM images, showed fibres with an average diameter of 34 μm, with a long and thick microscale. However, after periodate oxidation, the cellulose fibres became thinner with an average diameter of 30 μm. The two nanostructured cellulose-based adsorbents were reported to show a similar but excellent behaviour towards As(III) removal efficiency. A slight decrease was observed in the adsorption capacity of NDAC-cys for the removal As(III), when compared with MDAC-cys, due to the agglomeration of NDAC-cys. Magnetic iron-based nanoparticles were utilised for functionalising cellulose nanoparticles for the removal of arsenic. The nanosized iron nanoparticles arise from the inherent properties of the nanoparticles such as interfacial reactivity, a high surface area, and magnetism [50]. Hokkanen and co-workers [128] reported on the fabrication of the magnetic iron nanoparticles that modified microfibrillated cellulose (FeNP/MFC) for the removal of arsenate (As(V)) in an aqueous medium. The effects of various parameters such as the pH, initial As(V) concentration, and contact time were reported. According to Figure 5, the adsorption of As(V) was found to be strong in the acidic medium (viz, pH = 2). In an acidic medium, the charge of the adsorbent was positive; as a result, it was able to attract As(V), which was in the form of H2AsO4- in the pH range of 2–5. However, beyond a pH of 5.2, there was more domination of the negative sites, as evidenced by the low adsorption efficiency, which is associated with the repulsion attraction.
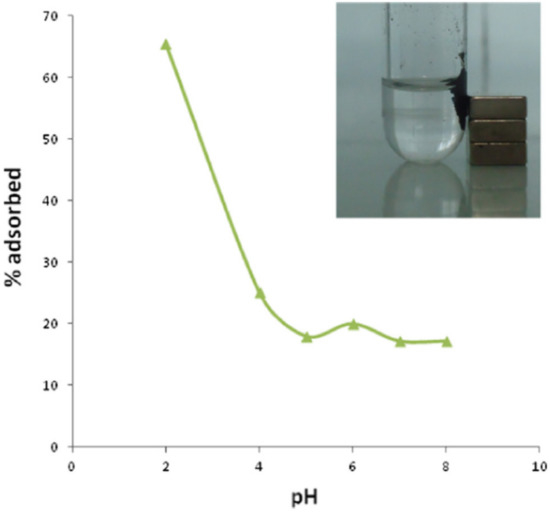
Figure 5.
The effect of the initial pH for the adsorption of arsenate As(V) by FeNP/MFC at room temperature, with the adsorbent dosage of 0.067 g, while inset is an illustration of magnetic separation [128]. Copyrights: Elsevier, 2015.
The very same study [128] investigated the effect of the contact time for As(V) removal by the modified MFC. There was an initial increase in the adsorption of AS(V) after 75 min, after which the system reached equilibrium (Figure 6).
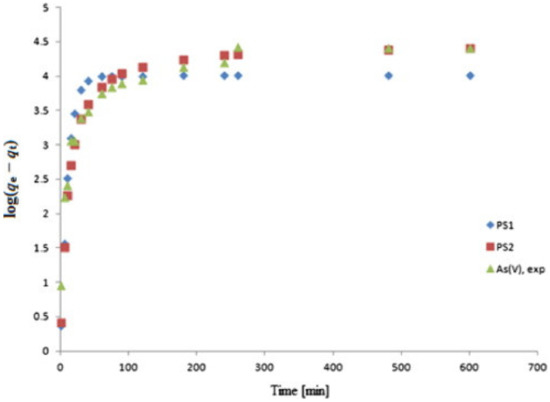
Figure 6.
Illustration of the kinetic plots in relation: pseudo-first-order and pseudo-second-order models [128]. Copyrights: Elsevier, 2015.
Furthermore, the pseudo-first-order and pseudo-second-order models were utilised to verify the experimental results. The pseudo-first-order (PS1) model is employed for simulating sorption in liquid or solid systems [128,129], while the pseudo-second-order (PS2) model is used for the assumption of the rate-determining step.
The PS2 model was found to be better-fitted to the experimental results, since the qe in the equation was close to the experimental value. PS2 was based on the rate-determining step, which was assumed to be the chemical sorption, with valence forces being involved in the sharing of electrons between both the adsorbate and the adsorbent. To further enhance the removal efficiency of arsenic by cellulose, functionalised iron nanoparticles and magnesium-doped iron oxide nanoparticles were produced and confined on the surface of the cellulose nanofibrils [130]. The immobilisation of the cellulose fibrils by the iron oxide nanoparticles is to avoid the aggregation of the nanoparticles. The synthesis of the Mg-doped iron oxide nanoparticles was synthesised and immobilised on the cellulose nanofibrils in a porous aerogel. The iron oxide nanoparticles (IONPs) were incorporated into the cellulose nanofibrils aerogels at up to 12.5 wt.% of the IONPs. IONP dosages of 63, 31, and 16 mg/L were reported in this study. The As(III) and As(V) concentrations were reduced in the first 2 h, depending on the concentration of the sorbent due to more IONP sites. However, as the time increases, there seems to be less removal of As(III) and As(V); for example, an IONP dosage of 63 mg/L removed less than 10 µg/L of As(III) and As(V) after 12 h of contact time. Another important parameter that was reported to affect the removal of arsenic is the pH. There was a high removal rate for As(III) and As(V) in the pH range of 3–7; however, the adsorption drops above a pH of 7.
Furthermore, the zeta potentials of CNFs and IONPs were reported (Figure 7B). CNFs were found to be negatively charged in the pH range of 3–11 because of the carboxylate groups on the CNFs’ surface. In summary, there seems to be a better adsorption of arsenic at a lower pH due to the electrostatic charges between arsenic and IONP at a lower pH. At a higher pH, there is a possible electrostatic repulsion that may result in low adsorption. Another type of nanoparticle that has been utilised together with cellulose for arsenic removal is nanoscale zero-valent iron (nZVI). The nZVI was favoured for the removal of arsenic due to a high affinity towards arsenic, low cost, and ease of availability [131]. The reason that nZVI cannot be employed alone but is accompanied by cellulose is because of the ease of aggregates and rapid deactivation. The study conducted by Chai and co-workers [131] investigated the removal of arsenic (III)/V) from ground water by employing nZVI functionalised cellulose. In order to improve the affinity between the two components, i.e., nZVI and cellulose, nZVI was tied up into the polydopamine-coated cellulose nanocrystals. The composite was synthesised by the in situ reduction method (Figure 8a).
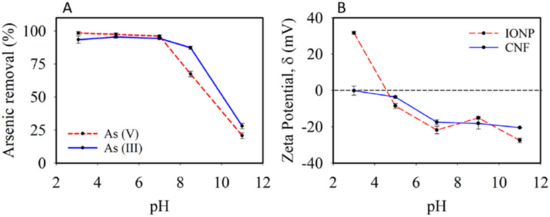
Figure 7.
The effect of pH on the removal of arsenic: (A) initial arsenic ion concentration and (B) zeta potential measurements [130]. Open access: MDPI.
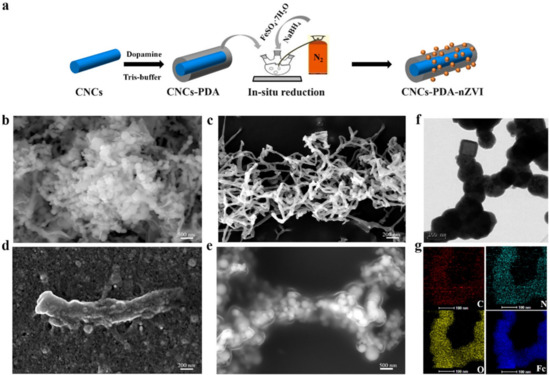
Figure 8.
(a) Schematic representation of the fabrication of the CNCs–PDA–nZVI and SEM images of: (b) nZVI, (c) CNCs, (d) CNCs–PDA, (e) CNCs–PDA–nZVI, (f) TEM image of CNCs–PDA–nZVI, and (g) elemental mapping of CNCs–PDA–nZVI. Copyrights: Elsevier, 2022 [131].
4.2. Adsorption Capacity of Functionalised Cellulose Materials
The SEM images of the neat CNCs showed smooth rods with an average size of 16–30 nm in diameter and 200–1000 nm in length (Figure 8c). The functionalised cellulose with PDA kept the rod-like shape of the nanocrystal (Figure 8d); however, the size of the CNCs was found to be large due to a thin layer of PDA that was created on the surface of the CNCs. The incorporation of nZVI into the PDA-modified CNCs showed bright particles of nZVI evenly spread on the surface of CNC–PDA. Several factors such as pH and temperature were found to be the other factors affecting the removal of As. The effect of pH was investigated in the range of 3–11. The neat cellulose nanocrystals (CNCs) recorded a zeta potential value of −37.2~−18.6 mV, while CNCs–PDA recorded a zeta value of +0.85~−27 mV. In the investigated pH range (viz, 3–11), neat CNCs revealed a low adsorption capacity for both As(III) and As(V), and this behaviour was attributed to a low zeta potential. With the functionalisation of cellulose with PDA, there was a steady improvement in the adsorption of arsenic, i.e., 1.77 mg g−1 for As(III) and 2.20 mg g−1 for As(V), when compared with neat CNCs. The effect of temperature was investigated with the following temperatures: 25, 35, and 45 °C. The temperature had an effect on the removal of As(III) and As(V). For example, the CNCs–PDA–nZVI had an enhancement for As(III), with an adsorption capacity increase from 285 mg g−1 to 318 mg g−1 with a temperature increase from 25 to 35 °C. Furthermore, it was revealed that the maximum adsorption capacity was recorded at the temperature of 35 °C; above this temperature, there was a reduction in the adsorption capacity. It was further revealed that the As(V) removal by CNCs–PDA–nZVI and nZVI was more affected by the temperature than the As(III) removal. The removal of As(III) and As(V) was investigated by the one-step synthesis of cellulose–iron oxide [132]. The adsorption capacity of As(V) was found to decrease with an increase in pH; however, the adsorption capacity of As(III) had the highest adsorption in the pH range of 6–9. At low pH, the adsorption is low due partially to the dissolving of Fe2O3, which might be an indication of the decrease in the active sites as a result of the low adsorption capacity of As(III). At a pH between 6 and 9, the active sites are not dissolved nor destroyed, and, as a result, there is a high adsorption capacity of As(III). Similarly, Fe(III)-loaded ligand exchange cotton cellulose adsorbent (Fe(III) LECCA) was fabricated for the adsorption of arsenate anions [133]. The SEM image of Fe(III) LECCA revealed large pores (viz, 5–30 μm) on their surface and in the inner part, which is advantageous for enhancing the hydrophilic surface in water solutions. Parameters such as time and pH were discussed in this study in relation to the adsorption of As(V). The removal efficiency of As(V) were reported to be above 95% in the pH range of 3.0–8.8, which is contrary to the previous study [133], whereby the adsorption was very high at a pH of 6–9. Furthermore, the effect of time on the adsorption of Fe(III) LECCA was also analysed and reported. There is rapid adsorption of the As(V), and the removal is almost complete at around 15 min (Figure 9). The increase in the adsorption was monotone as well as continuous. Based on the above observations, it was assumed the type of adsorption for As(V) was the monolayer adsorption. Table 2 summarises various studies on the adsorption of arsenic by functionalised cellulose membranes.
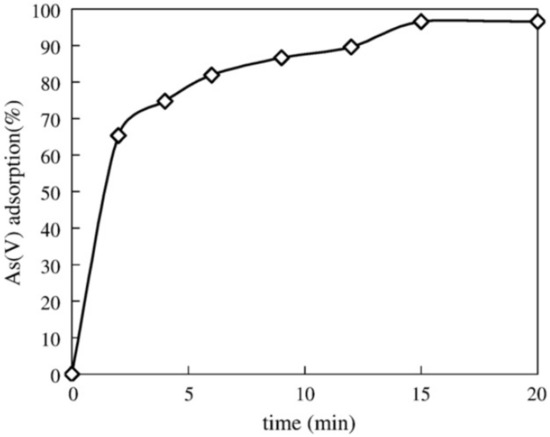
Figure 9.
The effect of time on the adsorption of As(V) by Fe(III) LECCA at As(V) concentration: 1 mg/L; pH: 7.0; room temperature: 25 °C [133]. Copyright: Elsevier.

Table 2.
Various studies on the adsorption of arsenic by functionalised cellulose materials.
5. Conclusions and Future Recommendations
Hazardous arsenic concentrations, most notably above 10 µg/L, threaten millions of people’s health globally. The use of environmentally friendly adsorption technologies is considered a solution for arsenic adsorption. Cellulose-based materials are preferred for the adsorption of arsenic due to their renewability, abundance, biodegradability, and low cost. Cellulose also has numerous hydroxyl functional groups on its surface. These hydroxyl functional groups enable the modification of cellulose in various ways, which leads to the fabrication of cellulose-based materials with tunable characteristics. One of the tunable characteristics is the adsorption of arsenic from wastewater. Various functionalised materials such as magnetite-loaded amino, 3-mercapto-propanoic acid, diethylene triamine grafted dialdehyde, and magnetic iron nanoparticles are some of the materials utilised for the functionalisation of cellulose for arsenic removal. Factors such as pH, reaction time, and temperature were found to affect the removal of arsenic, depending on the cellulose system used. The pH’s efficiency in arsenic removal depends on the type of material used for functionalising the cellulose (i.e., the cellulose system used for the removal of arsenic) and the type of arsenic to be removed. For example, in one study, it was proven that when Fe2O3 was used as a functionalising material for cellulose-based materials, the removal of As(V) was found to be effective at a pH of 3.0–8.8, with a removal efficiency of 95%. However, the removal efficiency of As(III) was reported to be effective in the pH range of 6–9. In some other cases, the effective efficiency for removal of As(V) was reported in the pH = 2–5, which emphasises the variation in pH in relation to the various systems used. For future recommendations, there is a need for the incorporation of two or more adsorbents materials for cellulose functionalisation, in order to enhance the removal efficiency of arsenic and selectivity. Furthermore, there is a need for more investigation into the recycling and reuse of functionalised cellulose systems for arsenic removal, as this is important for the environment and economy. In spite of the work that has been done, wastewater treatment technologies involving functionalised cellulose-based biomaterials still face a number of challenges that need to be addressed. Unfortunately, most of the current research is focused on the fabrication, functionalisation, and mechanism of the wastewater treatment of cellulose-based materials. Some of the chemicals used to modify the cellulose could be harmful to the environment and human health. Therefore, future work should be focused on how cellulose’s chemical modification affects its toxicity to the environment and human health. Another focus area could be the efficiency of the removal of these functionalised cellulose-based materials. The selectivity of the cellulose materials is important for the complete removal of all toxic pollutants from wastewater. Furthermore, research could be channeled towards studying the longevity of these cellulose materials by investigating their mechanical stability under various media conditions. The use of cheaper and environmentally friendly chemicals during cellulose functionalisation should be considered.
Author Contributions
Conceptualization, M.T.M. and S.I.M.; writing—original draft preparation, M.T.M., S.I.M., A.K., T.S.S.; writing—review and editing, M.T.M., S.I.M., T.S.S., K.L., M.J.M.; visualization, S.I.M.; supervision, S.I.M. and M.J.M.; project administration, S.I.M. and M.J.M.; funding acquisition, M.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation [NRF] grant number 129347.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Razali, N.A.M.; Sohaimi, R.M.; Othman, R.N.I.R.; Abdullah, N.; Demon, S.Z.N.; Jasmani, L.; Yunus, W.M.Z.W.; Ya’Acob, W.M.H.W.; Salleh, E.M.; Norizan, M.N.; et al. Comparative Study on Extraction of Cellulose Fiber from Rice Straw Waste from Chemo-Mechanical and Pulping Method. Polymers 2022, 14, 387. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Karim, Z.; Hakalahti, M.; Tammelin, T.; Mathew, A.P. In situ TEMPO surface functionalization of nanocellulose membranes for enhanced adsorption of metal ions from aqueous medium. RSC Adv. 2017, 7, 5232–5241. [Google Scholar] [CrossRef]
- Pettignano, A.; Charlot, A.; Fleury, E. Carboxyl-functionalized derivatives of carboxymethyl cellulose: Towards advanced biomedical applications. Polym. Rev. 2019, 59, 510–560. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced functional materials based on nanocellulose for pharmaceutical/medical applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef]
- Long, W.; Ouyang, H.; Hu, X.; Liu, M.; Zhang, X.; Feng, Y.; Wei, Y. State-of-art review on preparation, surface functionalization and biomedical applications of cellulose nanocrystals-based materials. Int. J. Biol. Macromol. 2021, 186, 591–615. [Google Scholar] [CrossRef]
- Koshani, R.; Zhang, J.; van de Ven, T.G.M.; Lu, X.; Wang, Y. Modified Hairy Nanocrystalline Cellulose as Photobactericidal Nanofillers for Food Packaging Application. ACS Sustain. Chem. Eng. 2021, 9, 10513–10523. [Google Scholar] [CrossRef]
- Yu, H.; Yan, C.; Yao, J. Fully biodegradable food packaging materials based on functionalized cellulose nanocrystals/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites. RSC Adv. 2014, 4, 59792–59802. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopol-ymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Jiang, Z.; Ngai, T. Recent Advances in Chemically Modified Cellulose and Its Derivatives for Food Packaging Applications: A Review. Polymers 2022, 14, 1533. [Google Scholar] [CrossRef]
- Li, F.; Biagioni, P.; Bollani, M.; Maccagnan, A.; Piergiovanni, L. Multi-functional coating of cellulose nanocrystals for flexible packaging applications. Cellulose 2013, 20, 2491–2504. [Google Scholar] [CrossRef]
- Saedi, S.; Garcia, C.V.; Kim, J.T.; Shin, G.H. Physical and chemical modifications of cellulose fibers for food packaging ap-plications. Cellulose 2021, 28, 8877–8897. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Bethke, K.; Palantöken, S.; Andrei, V.; Roß, M.; Raghuwanshi, V.S.; Kettemann, F.; Greis, K.; Ingber, T.T.K.; Stückrath, J.B.; Valiyaveettil, S.; et al. Functionalized Cellulose for Water Purification, Antimicrobial Applications, and Sensors. Adv. Funct. Mater. 2018, 28, 1800409. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.; Chen, W.; Pastel, G.; Guo, X.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H.; et al. Nanocellulose-enabled, all-nanofiber, high-performance supercapacitor. ACS Appl. Mater. Interfaces 2019, 11, 5919–5927. [Google Scholar]
- Jiang, Q.; Kacica, C.; Soundappan, T.; Liu, K.K.; Tadepalli, S.; Biswas, P.; Singamaneni, S. An in situ grown bacterial nano-cellulose/graphene oxide composite for flexible supercapacitors. J. Mater. Chem. 2017, 5, 13976–13982. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Jiang, K.; Thundat, T. Carbonized nanocellulose sustainably boosts the performance of activated carbon in ionic liquid supercapacitors. Nano Energy 2016, 25, 161–169. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar] [CrossRef]
- Virtanen, J.; Pammo, A.; Keskinen, J.; Sarlin, E.; Tuukkanen, S. Pyrolysed cellulose nanofibrils and dandelion pappus in su-percapacitor application. Cellulose 2017, 24, 3387–3397. [Google Scholar] [CrossRef]
- Shankaran, D.R. Cellulose nanocrystals for health care applications. In Applications of Nanomaterials: Advances and Key Technologies; Woodhead Publishing: Sawston, UK, 2018; pp. 415–459. [Google Scholar]
- Heinze, T. Cellulose: Structure and properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer: Berlin/Heidelberg, Germany, 2016; Volume 271, pp. 1–52. [Google Scholar]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Nishiyama, Y. Structure and properties of the cellulose microfibril. J. Wood Sci. 2009, 55, 241–249. [Google Scholar] [CrossRef]
- Naderi, A. Nanofibrillated cellulose: Properties reinvestigated. Cellulose 2017, 24, 1933–1945. [Google Scholar] [CrossRef]
- Peng, B.L.; Yao, Z.L.; Wang, X.C.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Liimatainen, H.; Sirviö, J.; Sundman, O.; Visanko, M.; Hormi, O.; Niinimäki, J. Flocculation performance of a cationic bi-opolymer derived from a cellulosic source in mild aqueous solution. Bioresour. Technol. 2011, 102, 9626–9632. [Google Scholar] [CrossRef]
- Yan, M.; Li, S.; Zhang, M.; Li, C.; Dong, F.; Li, W. Characterization of Surface Acetylated Nanocrystalline Cellulose by Single-Step Method. Bioresources 2013, 8, 6330–6341. [Google Scholar] [CrossRef]
- Zhang, Z.; Sèbe, G.; Rentsch, D.; Zimmermann, T.; Tingaut, P. Ultralightweight and Flexible Silylated Nanocellulose Sponges for the Selective Removal of Oil from Water. Chem. Mater. 2014, 26, 2659–2668. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S. Chemical modification of cellulose extracted from sugarcane bagasse: Preparation of hydroxyethyl cel-lulose. Arab. J. Chem. 2014, 7, 362–371. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Salon-Brochier, M.C.; Krouit, M.; Bras, J. Recent advances in surface chemical modification of cellulose fibres. J. Adhes. Sci. Technol. 2011, 25, 661–684. [Google Scholar] [CrossRef]
- Yagyu, H.; Saito, T.; Isogai, A.; Koga, H.; Nogi, M. Chemical Modification of Cellulose Nanofibers for the Production of Highly Thermal Resistant and Optically Transparent Nanopaper for Paper Devices. ACS Appl. Mater. Interfaces 2015, 7, 22012–22017. [Google Scholar] [CrossRef] [PubMed]
- Heise, K.; Delepierre, G.; King, A.W.T.; Kostiainen, M.A.; Zoppe, J.; Weder, C.; Kontturi, E. Chemical Modification of Reducing End-Groups in Cellulose Nanocrystals. Angew. Chem. Int. Ed. 2020, 60, 66–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Li, X.; Gibril, M.E.; Yu, M. Chemical modification of cellulose by in situ reactive extrusion in ionic liq-uid. Carbohydr. Polym. 2014, 99, 126–131. [Google Scholar] [CrossRef]
- Durán, V.L.; Larsson, P.A.; Wågberg, L. Chemical modification of cellulose-rich fibres to clarify the influence of the chemical structure on the physical and mechanical properties of cellulose fibres and thereof made sheets. Carbohydr. Polym. 2018, 182, 1–7. [Google Scholar] [CrossRef]
- Zhou, L.; Ke, K.; Yang, M.B.; Yang, W. Recent progress on chemical modification of cellulose for high mechanical-performance Poly (lactic acid)/Cellulose composite: A review. Compos. Commun. 2021, 23, 100548. [Google Scholar] [CrossRef]
- Taczała, J.; Sawicki, J.; Pietrasik, J. Chemical Modification of Cellulose Microfibres to Reinforce Poly(methyl methacrylate) Used for Dental. Appl. Mater. 2020, 13, 3807. [Google Scholar] [CrossRef]
- Aguado, R.; Lourenço, A.F.; Ferreira, P.J.T.; Moral, A.; Tijero, A. The relevance of the pretreatment on the chemical modification of cellulosic fibers. Cellulose 2019, 26, 5925–5936. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Heiskanen, J.P. Room-temperature dissolution and chemical modification of cellulose in aqueous tetrae-thylammonium hydroxide–carbamide solutions. Cellulose 2020, 27, 1933–1950. [Google Scholar] [CrossRef]
- Sun, L.; Lu, M.; Li, Q.; Jiang, H.; Yin, S. Research progress of arsenic removal from wastewater. IOP Conf. Ser. Earth Environ. Sci. 2019, 218, 012142. [Google Scholar]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Yeh, H.; Johnson, K.; Hsiao, B.S. Arsenic(III) Removal by Nanostructured Dialdehyde Cellulose–Cysteine Microscale and Nanoscale Fibers. ACS Omega 2019, 4, 22008–22020. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, M.; Lin, X.; Huang, P.; Huang, Y. Synthesis of magnetic wheat straw for arsenic adsorption. J. Hazard. Mater. 2011, 193, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw materi-al. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, S.; Repo, E.; Sillanpää, M. Removal of heavy metals from aqueous solutions by succinic anhydride modified mercerized nanocellulose. J. Chem. Eng. 2013, 223, 40–47. [Google Scholar] [CrossRef]
- Yang, R.; Aubrecht, K.B.; Ma, H.; Wang, R.; Grubbs, R.B.; Hsiao, B.S.; Chu, B. Thiol-modified cellulose nanofibrous composite membranes for chromium (VI) and lead (II) adsorption. Polymer 2014, 55, 1167–1176. [Google Scholar] [CrossRef]
- Alatalo, S.-M.; Pileidis, F.; Mäkilä, E.; Sevilla, M.; Repo, E.; Salonen, J.; Sillanpää, M.; Titirici, M.-M. Versatile Cellulose-Based Carbon Aerogel for the Removal of Both Cationic and Anionic Metal Contaminants from Water. ACS Appl. Mater. Interfaces 2015, 7, 25875–25883. [Google Scholar] [CrossRef]
- Ma, H.; Hsiao, B.S.; Chu, B. Ultrafine cellulose nanofibers as efficient adsorbents for removal of UO22+ in water. ACS Macro Lett. 2012, 1, 213–216. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient Removal of UO22+ from Water Using Carboxycellulose Nanofibers Prepared by the Nitro-Oxidation Method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S. Lead removal from water using carboxycel-lulose nanofibers prepared by nitro-oxidation method. Cellulose 2018, 25, 1961–1973. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Cui, G.; Liu, M.; Chen, Y.; Zhang, W.; Zhao, J. Synthesis of a ferric hydroxide-coated cellulose nanofiber hybrid for effective removal of phosphate from wastewater. Carbohydr. Polym. 2016, 154, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Zhao, L.; Meng, G.; Wu, J.; Liu, Z. Cellulose-based porous adsorbents with high capacity for methylene blue adsorption from aqueous solutions. Fibers Polym. 2017, 18, 891–899. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Antoine, R.; Hsiao, B.S. Efficient Removal of Arsenic Using Zinc Oxide Nanocrystal-Decorated Regenerated Microfibrillated Cellulose Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151. [Google Scholar] [CrossRef]
- Cullen, L.E.; Macfarlane, C. Comparison of cellulose extraction methods for analysis of stable isotope ratios of carbon and oxygen in plant material. Tree Physiol. 2005, 25, 563–569. [Google Scholar] [CrossRef]
- Ohwoavworhua, F.; Adelakun, T.; Okhamafe, A. Processing pharmaceutical grade microcrystalline cellulose from groundnut husk: Extraction methods and characterization. Int. J. Green Pharm. 2009, 3, 97–104. [Google Scholar] [CrossRef]
- Vu, N.D.; Tran, H.T.; Bui, N.D.; Vu, C.D.; Nguyen, H.V. Lignin and Cellulose Extraction from Vietnam’s Rice Straw Using Ultrasound-Assisted Alkaline Treatment Method. Int. J. Polym. Sci. 2017, 2017, 1063695. [Google Scholar]
- Menon, M.P.; Selvakumar, R.; Kumar, P.S.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef]
- Park, N.-M.; Choi, S.; Oh, J.E.; Hwang, D.Y. Facile extraction of cellulose nanocrystals. Carbohydr. Polym. 2019, 223, 115114. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Huang, Y.; Yu, W. Effects of extraction methods on morphology, structure and properties of bamboo cellulose. Ind. Crop. Prod. 2021, 169, 113640. [Google Scholar] [CrossRef]
- He, C.; Li, H.; Hong, J.; Xiong, H.; Ni, H.; Zheng, M. Characterization and Functionality of Cellulose from Pomelo Fruitlets by Different Extraction Methods. Polymers 2022, 14, 518. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.N.M.; Hosseinmardi, A.; Martin, D.J.; Annamalai, P.K. A mixed acid methodology to produce thermally stable cellulose nanocrystal at high yield using phosphoric acid. J. Bioresour. Bioprod. 2022, 7, 99–108. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic coatings on cellulose-based materials: Fabrication, properties, and applications. Adv. Mater. Interfaces 2014, 1, 1300026. [Google Scholar] [CrossRef]
- Chopra, L. Manikanika Extraction of cellulosic fibers from the natural resources: A short review. Mater. Today Proc. 2021, 48, 1265–1270. [Google Scholar]
- de Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; El Halal, S.L.M.; da Rosa, G.S.; Dias, A.R.G.; da Rosa Zavareze, E. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Recent developments in the production and applications of bacterial cellulose fibers and nanocrystals. Crit. Rev. Biotechnol. 2016, 37, 510–524. [Google Scholar] [CrossRef]
- Iglesias, M.C.; Gomez-Maldonado, D.; Via, B.K.; Jiang, Z.; Peresin, M.S. Pulping Processes and Their Effects on Cellulose Fibers and Nanofibrillated Cellulose Properties: A Review. For. Prod. J. 2020, 70, 10–21. [Google Scholar] [CrossRef]
- Harini, K.; Ramya, K.; Sukumar, M. Extraction of nano cellulose fibers from the banana peel and bract for production of acetyl and lauroyl cellulose. Carbohydr. Polym. 2018, 201, 329–339. [Google Scholar] [CrossRef]
- Ilangovan, M.; Guna, V.; Hu, C.; Nagananda, G.; Reddy, N. Curcuma longa L. plant residue as a source for natural cellulose fibers with antimicrobial activity. Ind. Crop. Prod. 2018, 112, 556–560. [Google Scholar] [CrossRef]
- Orelma, H.; Hokkanen, A.; Leppänen, I.; Kammiovirta, K.; Kapulainen, M.; Harlin, A. Optical cellulose fiber made from regenerated cellulose and cellulose acetate for water sensor applications. Cellulose 2019, 27, 1543–1553. [Google Scholar] [CrossRef]
- Lavanya, D.K.P.K.; Kulkarni, P.K.; Dixit, M.; Raavi, P.K.; Krishna, L.N.V. Sources of cellulose and their applications—A review. Int. J. Drug Dev. Res. 2011, 2, 19–38. [Google Scholar]
- Morán, J.I.; Álvarez, V.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2007, 15, 149–159. [Google Scholar] [CrossRef]
- Song, K.; Zhu, X.; Zhu, W.; Li, X. Preparation and characterization of cellulose nanocrystal extracted from Calotropis procera biomass. Bioresour. Bioprocess 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Azum, N.; Jawaid, M.; Kian, L.K.; Khan, A.; Alotaibi, M.M. Extraction of Microcrystalline Cellulose from Washingtonia Fibre and Its Characterization. Polymers 2021, 13, 3030. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jiang, W.; Zhang, Y.; Wang, H.; Zou, F.; Yu, K.; Han, G. A novel process of nanocellulose extraction from kenaf bast. Mater. Res. Express 2018, 5, 085032. [Google Scholar] [CrossRef]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.; Luong, J.H. Applications of functionalized and nanoparticle-modified nano-crystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Puglia, D.; Lavorgna, M.; Santulli, C.; Kenny, J.M.; Torre, L. Optimized extraction of cellulose nano-crystals from pristine and carded hemp fibres. Ind. Crops Prod. 2014, 56, 175–186. [Google Scholar] [CrossRef]
- Das, A.M.; Hazarika, M.P.; Goswani, M.; Yadav, A.; Khound, P. Extraction of cellulose from agricultural waste using mont-morillonite K-10/LiOH and its conversion to renewable energy: Biofuel by using myrothecium gramineum. Carbohydr. Polym. 2016, 141, 20–27. [Google Scholar] [CrossRef]
- Romruen, O.; Karbowiak, T.; Tongdeesoontorn, W.; Shiekh, K.A.; Rawdkuen, S. Extraction and Characterization of Cellulose from Agricultural By-Products of Chiang Rai Province, Thailand. Polymers 2022, 14, 1830. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Cai, Z.; Lin, F.; Tang, L.; Wang, S.; Huang, B. Extraction of cellulose nanocrystals with a high yield of 88% by simul-taneous mechanochemical activation and phosphotungstic acid hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2165–2172. [Google Scholar] [CrossRef]
- Singh, A.; Ranawat, B.; Meena, R. Extraction and characterization of cellulose from halophytes: Next generation source of cellulose fibre. SN Appl. Sci. 2019, 1, 1311. [Google Scholar] [CrossRef]
- Wahib, S.A.; Da’Na, D.A.; Al-Ghouti, M.A. Insight into the extraction and characterization of cellulose nanocrystals from date pits. Arab. J. Chem. 2022, 15, 103650. [Google Scholar] [CrossRef]
- Al-Ghamdi, Y.O. Immobilization of cellulose extracted from Robinia Pseudoacacia seed fibers onto chitosan: Chemical characterization and study of methylene blue removal. Arab. J. Chem. 2022, 15, 104066. [Google Scholar] [CrossRef]
- Judith, R.B.D.; Pámanes-Carrasco, G.A.; Delgado, E.; Rodríguez-Rosales, M.D.J.; Medrano-Roldán, H.; Reyes-Jáquez, D. Ex-traction optimization and molecular dynamic simulation of cellulose nanocrystals obtained from bean forage. Biocatal. Agric. Biotechnol. 2022, 43, 102443. [Google Scholar] [CrossRef]
- Mohamad, N.A.N.; Jai, J. Response surface methodology for optimization of cellulose extraction from banana stem using NaOH-EDTA for pulp and papermaking. Heliyon 2022, 8, e09114. [Google Scholar] [CrossRef]
- Gao, A.; Chen, H.; Tang, J.; Xie, K.; Hou, A. Efficient extraction of cellulose nanocrystals from waste Calotropis gigantea fiber by SO42-/TiO2 nano-solid superacid catalyst combined with ball milling exfoliation. Ind. Crops Prod. 2020, 152, 112524. [Google Scholar] [CrossRef]
- Chin, K.-M.; Sam, S.T.; Ong, H.L.; Wong, Y.S.; Tan, W.K.; Vannaladsaysy, V. Bioinspired Crosslinked Nanocomposites of Polyvinyl Alcohol-Reinforced Cellulose Nanocrystals Extracted from Rice Straw with Ethanedioic Acid. J. Nanomater. 2022, 2022, 3225211. [Google Scholar] [CrossRef]
- Khan, A.; Raghunathan, V.; Singaravelu, D.L.; Sanjay, M.; Siengchin, S.; Jawaid, M.; Alamry, K.A.; Asiri, A.M. Extraction and Characterization of Cellulose Fibers from the Stem of Momordica Charantia. J. Nat. Fibers 2020, 19, 2232–2242. [Google Scholar] [CrossRef]
- Lemita, N.; Deghboudj, S.; Rokbi, M.; Rekbi, F.M.L.; Halimi, R. Characterization and analysis of novel natural cellulosic fiber extracted from Strelitzia reginae plant. J. Compos. Mater. 2021, 56, 99–114. [Google Scholar] [CrossRef]
- Pota, G.; Salerno, A.S.; Costantini, A.; Silvestri, B.; Passaro, J.; Califano, V. Co-immobilization of Cellulase and β-Glucosidase into Mesoporous Silica Nanoparticles for the Hydrolysis of Cellulose Extracted from Eriobotrya japonica Leaves. Langmuir 2022, 38, 5481–5493. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Zhou, Y.; Yan, A.; Liu, Y. Extraction cellulose from corn-stalk taking advantage of pretreatment technology with immobilized enzyme. RSC Adv. 2021, 12, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ma, T.; Hu, X.; Zhao, J.; Liao, X.; Song, Y.; Hu, X. Facile extraction and characterization of cellulose nanocrystals from agricultural waste sugarcane straw. J. Sci. Food Agric. 2021, 102, 312–321. [Google Scholar] [CrossRef]
- Almutairi, F.M.; El-Ghoul, Y.; Jabli, M. Extraction of Cellulose Polymeric Material from Populus tremula Fibers: Character-ization and Application to the Adsorption of Methylene Blue and Crystal Violet. Polymers 2021, 13, 3334. [Google Scholar] [CrossRef]
- Nasution, H.; Yahya, E.B.; Khalil, H.P.S.A.; Shaah, M.A.; Suriani, A.B.; Mohamed, A.; Alfatah, T.; Abdullah, C.K. Extraction and Isolation of Cellulose Nanofibers from Carpet Wastes Using Supercritical Carbon Dioxide Approach. Polymers 2022, 14, 326. [Google Scholar] [CrossRef]
- Mitbumrung, W.; Rungraung, N.; Muangpracha, N.; Akanitkul, P.; Winuprasith, T. Approaches for Extracting Nanofibrillated Cellulose from Oat Bran and Its Emulsion Capacity and Stability. Polymers 2022, 14, 327. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Sadiku, E.R.; Li, X.; He, Y. Extraction of cellulose nanocrystals from areca waste and its application in eco-friendly biocomposite film. Chemosphere 2021, 287, 132084. [Google Scholar] [CrossRef]
- Sambu, S.; Wilson, R. Arsenic in food and water–a brief history. Toxicol. Ind. Health 2008, 24, 217–226. [Google Scholar] [CrossRef]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic Exposure and Toxicology: A Historical Perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef]
- Vahidnia, A.; van der Voet, G.B.; de Wolff, F.A. Arsenic neurotoxicity—A review. Hum. Exp. Toxicol. 2007, 26, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ye, Z.; Huang, L.; Zhang, C.; Guo, Y.; Zhang, W. Arsenic Occurrence and Cycling in the Aquatic Environment: A Comparison between Freshwater and Seawater. Water 2022, 15, 147. [Google Scholar] [CrossRef]
- Waxman, S.; Anderson, K.C. History of the Development of Arsenic Derivatives in Cancer Therapy. Oncologist 2001, 6, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Wai, C.M. Arsenic in Drinking Water—A Global Environmental Problem. J. Chem. Educ. 2004, 81, 207. [Google Scholar] [CrossRef]
- Cullen, W.R. Is Arsenic an Aphrodisiac? The Sociochemistry of an Element; Royal Society of Chemistry: London, UK, 2008; p. 428. [Google Scholar]
- Arifullah Changsheng, H.; Akram, W.; Rashid, A.; Ullah, Z.; Shah, M.; Alrefaei, A.F.; Kamel, M.; Aleya, L.; Abdel-Daim, M.M. Quality Assessment of Groundwater Based on Geochemical Modelling and Water Quality Index (WQI). Water 2022, 14, 3888. [Google Scholar] [CrossRef]
- Kumar, A.; Joshi, H.; Kumar, A. Arsenate Removal from the Groundwater Employing Maghemite Nanoparticles. Water 2022, 14, 3617. [Google Scholar] [CrossRef]
- 40 CFR 141.11; Code of Federal Regulations. U.S. Government Publishing Office (GPO): Washington, DC, USA, 1992.
- De Klerk, R.J.; Feldmann, T.; Daenzer, R.; Demopoulos, G.P. Continuous circuit coprecipitation of arsenic (V) with ferric iron by lime neutralization: The effect of circuit staging, co-ions and equilibration pH on long-term arsenic reten-tion. Hydrometallurgy 2015, 151, 42–50. [Google Scholar] [CrossRef]
- Laatikainen, M.; Sillanpää, M.; Sainio, T. Comparison of ion exchange process configurations for arsenic removal from natural waters. Desalination Water Treat. 2016, 57, 13770–13781. [Google Scholar] [CrossRef]
- Bahmani, P.; Maleki, A.; Daraei, H.; Khamforoush, M.; Rezaee, R.; Gharibi, F.; Tkachev, A.G.; Burakov, A.E.; Agarwal, S.; Gupta, V.K. High-flux ultrafiltration membrane based on electrospun polyacrylonitrile nanofibrous scaffolds for arsenate removal from aqueous solutions. J. Colloid Interface Sci. 2017, 506, 564–571. [Google Scholar] [CrossRef]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Hao, L.; Wang, N.; Wang, C.; Li, G. Arsenic removal from water and river water by the combined adsorption—UF membrane process. Chemosphere 2018, 202, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Yang, Z.; Zeng, G.; Yang, X.; Xu, H.; Wang, L.; Xu, R.; Xiong, W.; Ahmad, K. Electrocoagulation treatment of arsenic in wastewaters: A comprehensive review. Chem. Eng. J. 2017, 317, 707–725. [Google Scholar] [CrossRef]
- Chowdhury, R. Using adsorption and sulphide precipitation as the principal removal mechanisms of arsenic from a con-structed wetland–a critical review. Chem. Ecol. 2017, 33, 560–571. [Google Scholar] [CrossRef]
- Driehaus, W.; Dupont, F. Arsenic removal: Solutions for a world wide health problem using iron based adsorbents. Eur. J. Water Qual. 2005, 36, 119–132. [Google Scholar] [CrossRef]
- Pintor, A.M.; Vieira, B.R.; Santos, S.C.; Boaventura, R.A.; Botelho, C.M. Arsenate and arsenite adsorption onto iron-coated cork granulates. Sci. Total Environ. 2018, 642, 1075–1089. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Y.; Song, S. Competitive adsorption of As(V) with co-existing ions on porous hematite in aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 1497–1503. [Google Scholar] [CrossRef]
- Lalley, J.; Han, C.; Li, X.; Dionysiou, D.D.; Nadagouda, M.N. Phosphate adsorption using modified iron oxide-based sorbents in lake water: Kinetics, equilibrium, and column tests. Chem. Eng. J. 2016, 284, 1386–1396. [Google Scholar] [CrossRef]
- Fakour, H.; Pan, Y.-F.; Lin, T.-F. Effect of Humic Acid on Arsenic Adsorption and Pore Blockage on Iron-Based Adsorbent. Water Air Soil Pollut. 2015, 226, 14. [Google Scholar] [CrossRef]
- Feng, Z.; Ning, Y.; Yang, S.; Yu, J.; Ouyang, W.; Li, Y. A novel strategy for arsenic removal from acid wastewater via strong reduction processing. Environ. Sci. Pollut. Res. 2023, 30, 1–15. [Google Scholar] [CrossRef]
- Akin, I.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Removal of arsenate [As(V)] and arsenite [As(III)] from water by SWHR and BW-30 reverse osmosis. Desalination 2011, 281, 88–92. [Google Scholar] [CrossRef]
- Gecol, H.; Ergican, E.; Fuchs, A. Molecular level separation of arsenic (V) from water using cationic surfactant micelles and ultrafiltration membrane. J. Membr. Sci. 2004, 241, 105–119. [Google Scholar] [CrossRef]
- Figoli, A.; Cassano, A.; Criscuoli, A.; Mozumder, M.S.I.; Uddin, M.T.; Islam, M.A.; Drioli, E. Influence of operating parameters on the arsenic removal by nanofiltration. Water Res. 2010, 44, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, S.; Repo, E.; Lou, S.; Sillanpää, M. Removal of arsenic (V) by magnetic nanoparticle activated microfibrillated cellulose. Chem. Eng. J. 2015, 260, 886–894. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Liu, Z.; Huang, Q. Characteristics of equilibrium, kinetics studies for adsorption of Hg (II), Cu (II), and Ni (II) ions by thiourea-modified magnetic chitosan microspheres. J. Hazard. Mater. 2009, 161, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Hafez, I.; Tajvidi, M.; Amirbahman, A. Highly Efficient Iron Oxide Nanoparticles Immobilized on Cellulose Nanofibril Aerogels for Arsenic Removal from Water. Nanomaterials 2021, 11, 2818. [Google Scholar] [CrossRef]
- Chai, F.; Zhang, R.; Min, X.; Yang, Z.; Chai, L.; Zhao, F. Highly efficient removal of arsenic (III/V) from groundwater using nZVI functionalized cellulose nanocrystals fabricated via a bioinspired strategy. Sci. Total Environ. 2022, 842, 156937. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. One-step synthesis of magnetic composites of cellulose@ iron oxide nano-particles for arsenic removal. J. Mater. Chem. A 2013, 1, 959–965. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, M.; Wu, W.; Jin, W. Synthesis of the cotton cellulose based Fe(III)-loaded adsorbent for arsenic(V) removal from drinking water. Desalination 2009, 249, 1006–1011. [Google Scholar] [CrossRef]
- Taleb, K.; Markovski, J.; Veličković, Z.; Rusmirović, J.; Rančić, M.; Pavlović, V.; Marinković, A. Arsenic removal by magnet-ite-loaded amino modified nano/microcellulose adsorbents: Effect of functionalization and media size. Arab. J. Chem. 2019, 12, 4675–4693. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, A.A.; Sudhakar, C.; Kumar, R.; Ahuja, T.; Mondal, B.; Srikrishnarka, P.; Philip, L.; Pradeep, T. Sustainable and Affordable Composites Built Using Microstructures Performing Better than Nanostructures for Arsenic Removal. ACS Sustain. Chem. Eng. 2018, 7, 3222–3233. [Google Scholar] [CrossRef]
- Deng, S.; Chen, S.; Xue, Y.; Du, Z.; Wang, P. Rapid and effective preparation of a HPEI modified biosorbent based on cellulose fiber with a microwave irradiation method for enhanced arsenic removal in water. J. Mater. Chem. A 2016, 441, 15851–15860. [Google Scholar] [CrossRef]
- Chai, F.; Wang, R.; Yan, L.; Li, G.; Cai, Y.; Xi, C. Facile fabrication of pH-sensitive nanoparticles based on nanocellulose for fast and efficient As(V) removal. Carbohydr. Polym. 2020, 245, 116511. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.; Nima, J.; Sandeep, S.; Ratheesh, V.R.N. Development of an amino functionalized glycidylmethacry-late-grafted-titanium dioxide densified cellulose for the adsorptive removal of arsenic (V) from aqueous solutions. Chem. Eng. J. 2012, 209, 362–371. [Google Scholar] [CrossRef]
- Pramanik, K.; Sarkar, P.; Bhattacharyay, D. 3-Mercapto-propanoic acid modified cellulose filter paper for quick removal of arsenate from drinking water. Int. J. Biol. Macromol. 2018, 122, 185–194. [Google Scholar] [CrossRef]
- Nagarajan, D.; Venkatanarasimhan, S. Magnetite microparticles decorated cellulose sponge as an efficacious filter for im-proved arsenic (V) removal. J. Environ. Chem. Eng. 2019, 7, 103386. [Google Scholar] [CrossRef]
- Singh, K.; Sinha, T.; Srivastava, S. Functionalized nanocrystalline cellulose: Smart biosorbent for decontamination of arsenic. Int. J. Miner. Process. 2015, 139, 51–63. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Senan, P.; Suchithra, P.S. Evaluation of iron (III)-coordinated amino-functionalized poly (glycidyl methac-rylate)-grafted cellulose for arsenic (V) adsorption from aqueous solutions. Wat. Air Soil Pollut. 2011, 220, 101–116. [Google Scholar] [CrossRef]
- Nakakubo, K.; Endo, M.; Sakai, Y.; Biswas, F.B.; Wong, K.H.; Mashio, A.S.; Taniguchi, T.; Nishimura, T.; Maeda, K.; Hasegawa, H. Cross-linked dithiocarbamate-modified cellulose with enhanced thermal stability and dispersibility as a sorbent for ar-senite removal. Chemosphere 2022, 307, 135671. [Google Scholar] [CrossRef]
- Tewatia, P.; Kumar, V.; Samota, S.; Singhal, S.; Kaushik, A. Sensing and annihilation of ultra-trace level arsenic (III) using fluoranthene decorated fluorescent nanofibrous cellulose probe. J. Hazard. Mater. 2022, 424, 127722. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).