Hydrochemical Characteristics and Ion Source Analysis of the Yarlung Tsangpo River Basin
Abstract
1. Introduction
2. Study Area and Methods
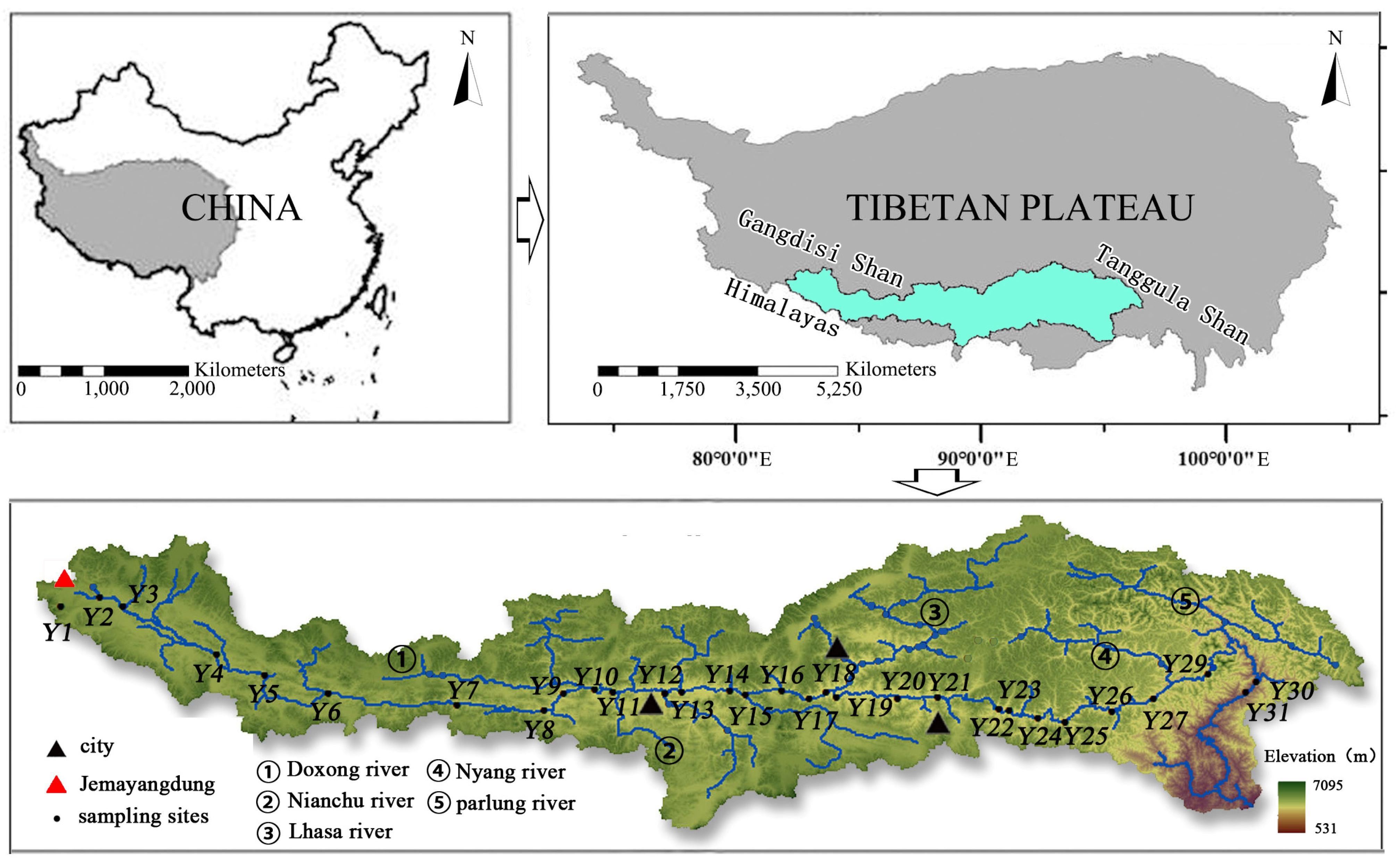
2.1. River Catchments
2.2. Water Sample Collection and Analysis
2.2.1. Sampling and Measurement
2.2.2. Data Processing and Analysis
3. Results and Discussion
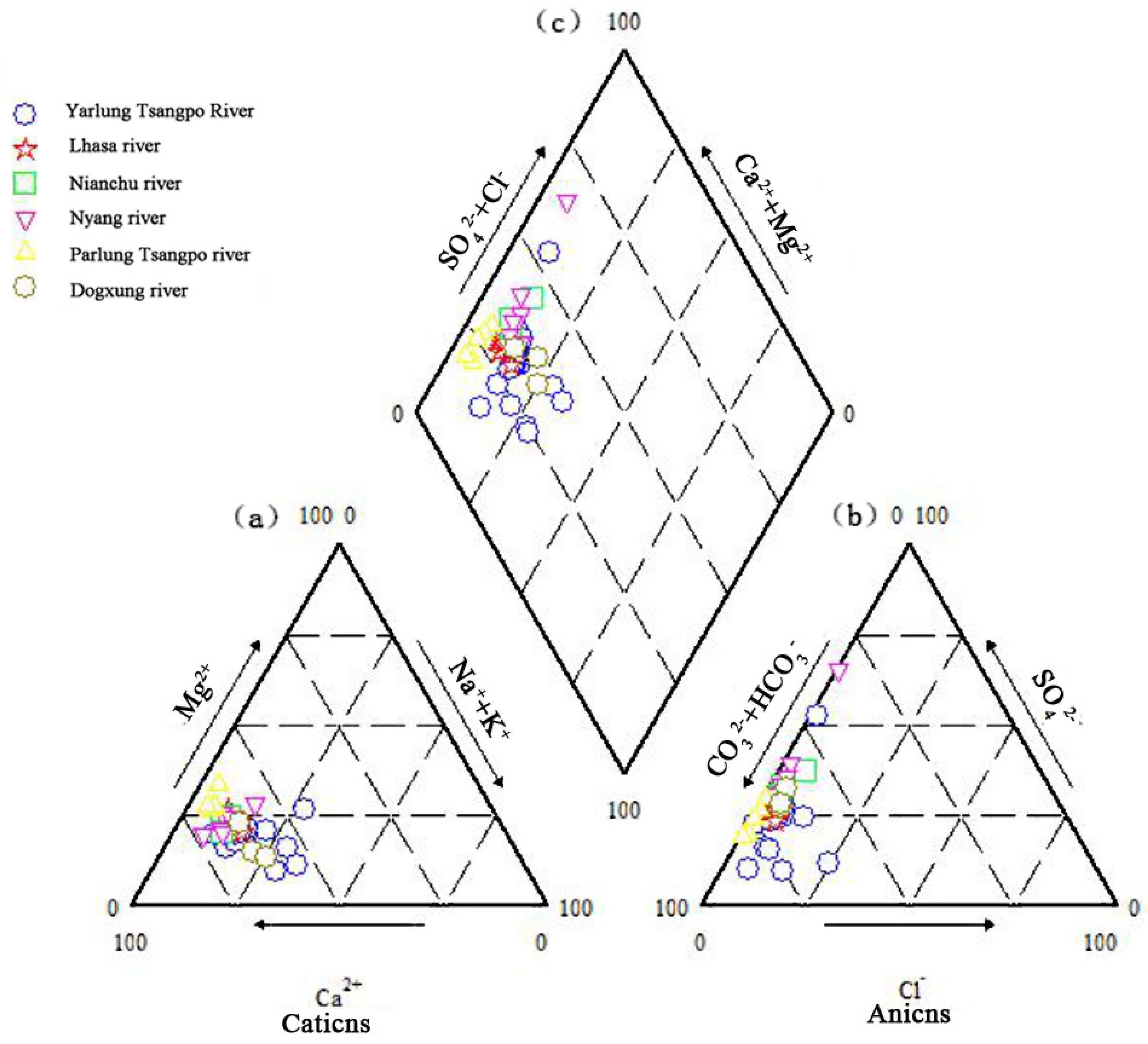
3.1. General Hydrochemical Characteristics
3.1.1. Basic Physical and Chemical Properties of the Main Stream and Tributaries of the Yarlung Tsangpo River
3.1.2. Heavy Metal Analysis
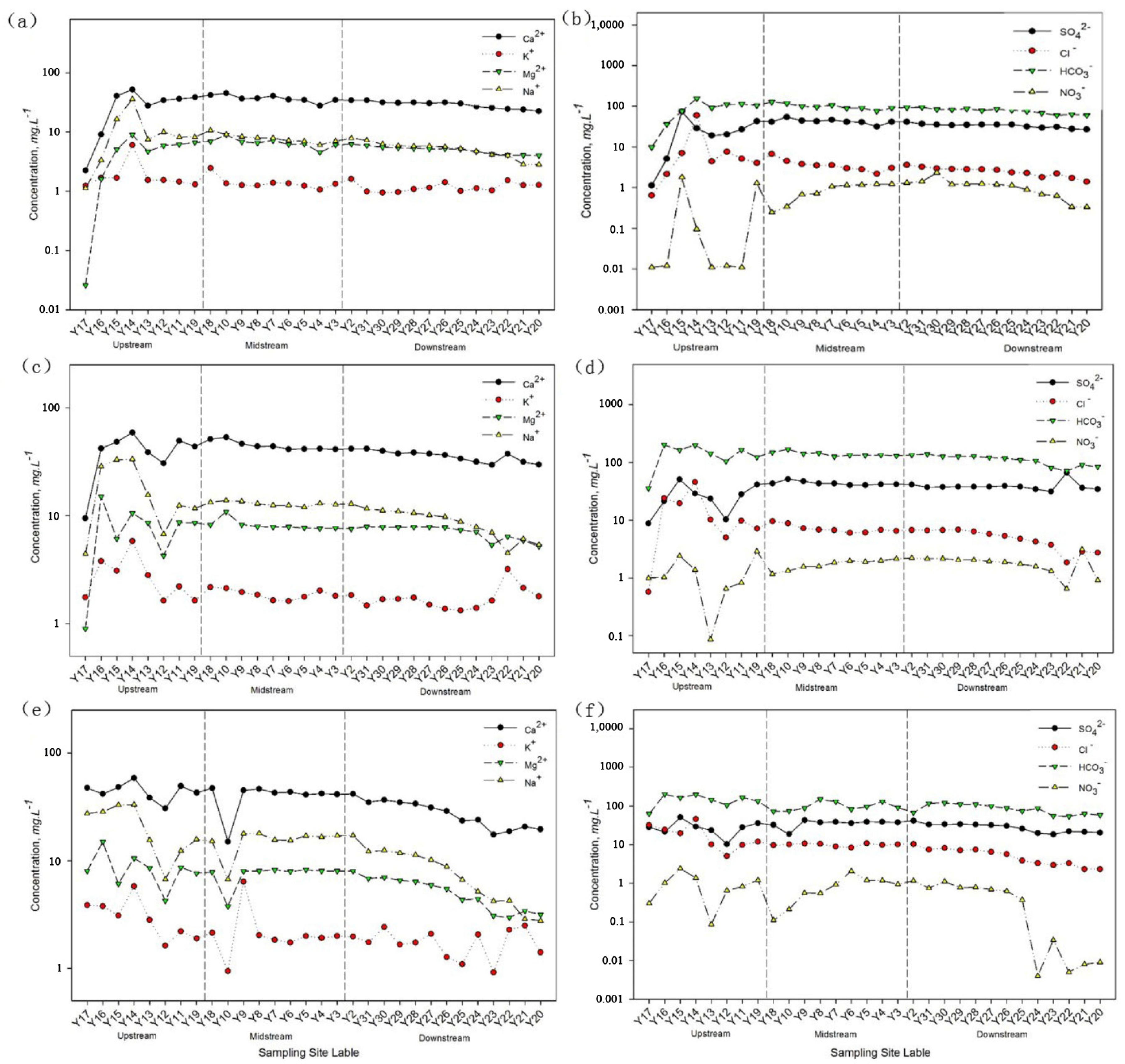
3.2. Spatial and Seasonal Variation Characteristics of Major Ions in the Main Stream of the Yarlung Tsangpo River
3.3. Ion Source Analysis
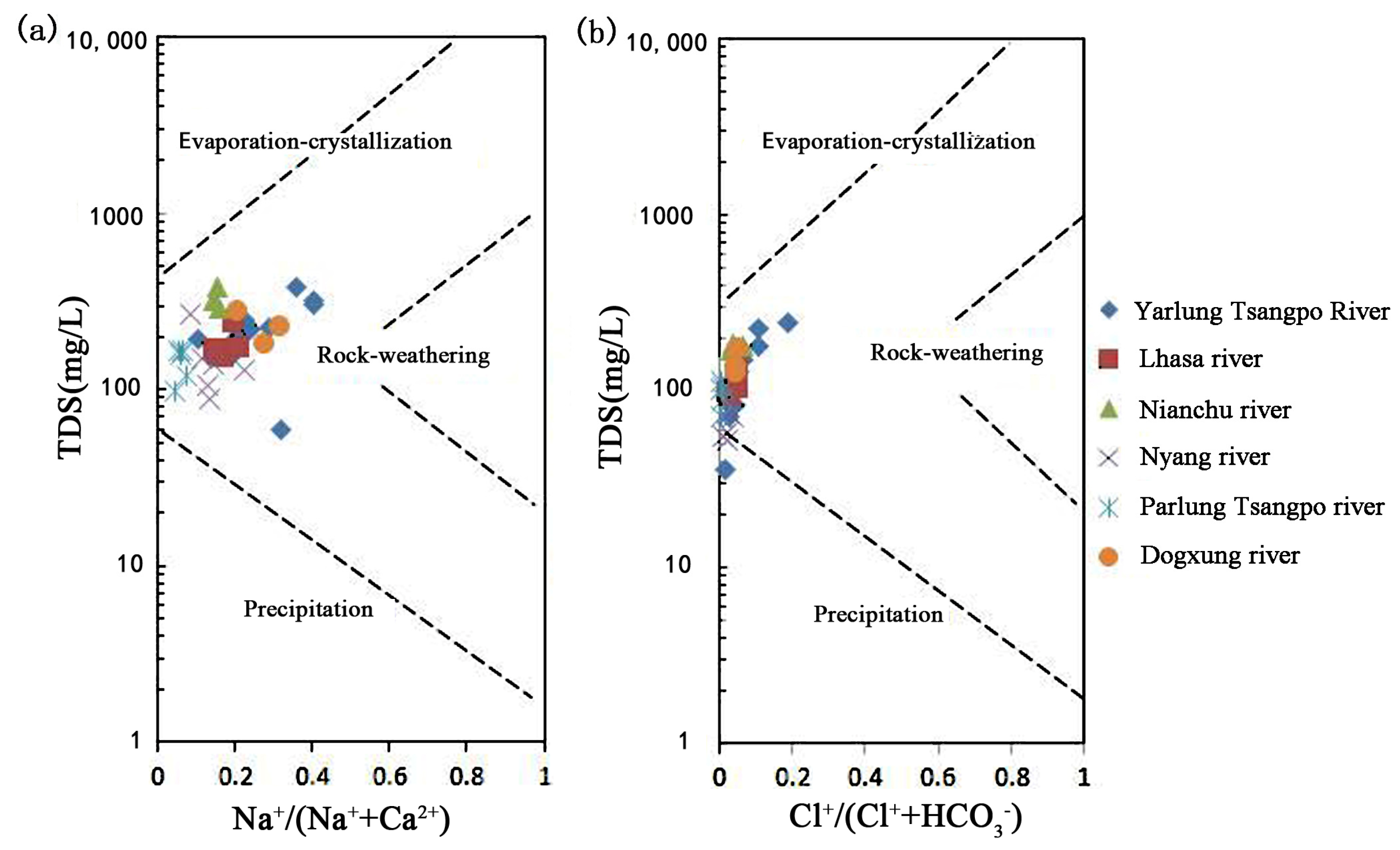
3.3.1. Gibbs Analysis
3.3.2. Analysis of the Rock Weathering Process
3.3.3. Principal Component Analysis
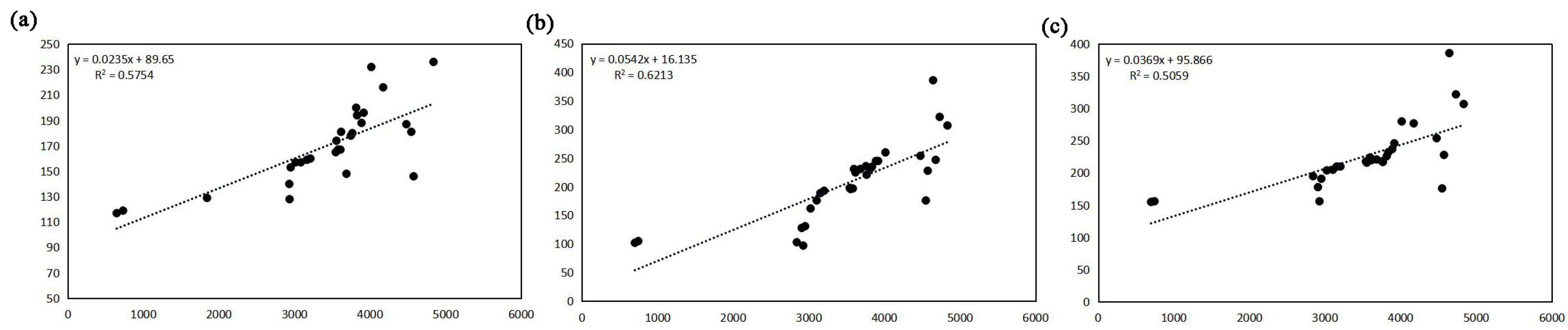
3.3.4. Analysis of Elevation Effect
- High water period: TDS = 0.0235H + 89.65 (R2 = 0.57);
- Dry season: TDS = 0.0542H + 16.135 (R2 = 0.62);
- Level water period: TDS = 0.0369H + 95.866 (R2 = 0.50).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewey, J.; Shackleton, R.; Chengfa, C. The tectonic evolution of the Tibetan Plateau. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 1988, 327, 379–413. [Google Scholar]
- Tian, L.; Yao, T.; Shen, Y. Study on stable isotope in river water and precipitation in Naqu River basin, Tibetan Plateau. Adv. Water Sci. 2002, 13, 206–210. [Google Scholar]
- Paudyal, R.; Kang, S.; Sharma, C. Variations of the physicochemical parameters and metal levels and their risk assessment in 2 urbanized Bagmati River, Kathmandu, Nepal. J. Chem. 2016, 2016, 6025905. [Google Scholar] [CrossRef]
- Huang, X.; Sillanpaa, M.; Gjessing, E. Water quality in the Tibetan Plateau: Major ions and trace elements in the headwaters of four major asian rivers. Sci. Total Environ. 2009, 407, 6242–6254. [Google Scholar] [CrossRef] [PubMed]
- Hren, M.; Chamberlain, C.P.; Hilley, G.E.; Blisniuk, P.M.; Bookhagen, B. Major ion chemistry of the Yarlung Tsangpo–Brahmaputra river: Chemical weathering, erosion, and CO2 consumption in the southern Tibetan Plateau and eastern syntaxis of the Himalaya. Geochim. Cosmochim. Acta 2007, 71, 2907–2935. [Google Scholar] [CrossRef]
- Wu, W.; Xu, S.; Yang, J.; Yin, H. Silicate weathering and CO2 consumption deduced from the seven Chinese rivers originating in the Qinghai–Tibet Plateau. Chem. Geol. 2008, 249, 307–320. [Google Scholar] [CrossRef]
- Liu, Y.; Vick-Majors, T.; Priscu, J. Biogeography of cryoconite bacterial communities on glaciers of the Tibetan Plateau. FEMS Microbiol. Ecol. 2017, 93, fix072. [Google Scholar] [CrossRef]
- Huang, X.; Sillanpää, M.; Gjessing, E. Water quality in the southern Tibetan Plateau: Chemical evaluation of the Yarlung Tsangpo (Brahmaputra). River Res. Appl. 2011, 27, 113–121. [Google Scholar] [CrossRef]
- Liu, J.; Xie, J.; Gong, T. Impacts of winter warming and permafrost degradation on water variability, upper Lhasa River, Tibet. Quat. Int. 2011, 244, 178–184. [Google Scholar] [CrossRef]
- Niu, H.; He, Y.; Lu, X. Characteristics of modern atmospheric dust deposition in snow in the Mt. Yulong region, southeastern Tibetan Plateau. J. Asian Earth Sci. 2017, 94, 45–54. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Zhang, W. Characteristics of water chemistry and its indication of chemical weathering in Jinshajiang, Lancangjiang and Nujiang drainage basins. Environ. Earth Sci. 2016, 75, 506. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Subramani, T.; Roy, P. Impact of groundwater contamination on human health. Environ. Geochem. Health 2021, 43, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, I.; Marini, L.; De Rosa, R.; Figoli, A.; Gabriele, B.; Apollaro, C. Use of reaction path modelling to investigate the evolution of water chemistry in shallow to deep crystalline aquifers with a special focus on fluoride. Sci. Total Environ. 2022, 830, 154566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sillanpää, M.; Li, C. River water quality across the Himalayan regions: Elemental concentrations in headwaters of Yarlung Tsangbo, Indus and Ganges River. Environ. Earth Sci. 2015, 73, 4151–4163. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, J. Two new species of Garra (Cypriniformes: Cyprinidae) from the lower Yarlung Tsangpo River drainage in southeastern Tibet, China. Zootaxa 2018, 4532, 367–384. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Li, Z. Quantitative source apportionment of water solutes and CO2 consumption of the whole Yarlung Tsangpo River basin in Tibet, China. Environ. Sci. Pollut. Res. 2019, 26, 28243–28255. [Google Scholar] [CrossRef]
- Scherler, D.; Bookhagen, B.; Strecker, M. Spatially variable response of Himalayan glaciers to climate change affected by debris cover. Nat. Geosci. 2011, 4, 156–159. [Google Scholar] [CrossRef]
- Gong, T.; Liu, C.; Liu, J. Hydrological response of Lhasa River to climate change and permafrost degradation in Xizang. Acta Geogr. Sin. 2006, 61, 519–526. [Google Scholar]
- Gaillardet, J.; Dupre, B.; Louvat, P. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Pant, R.; Zhang, F.; Rehman, F. Spatiotemporal variations of hydrogeochemistry and its controlling factors in the Gandaki River Basin, Central Himalaya Nepal. Sci. Total Environ. 2017, 770, 622–623. [Google Scholar] [CrossRef]
- Paudyal, R.; Kang, S.; Sharma, C. Major ions and trace elements of two selected rivers near Everest region, southern Himalayas, Nepal. Environ. Earth Sci. 2016, 75, 46. [Google Scholar] [CrossRef]
- Abbas, N.; Subramanian, V. Erosion and sediment trans-port in the Ganges river basin, India. J. Hydrol. 1984, 69, 173–182. [Google Scholar] [CrossRef]
- Varol, M.; Gokot, B.; Bekleyen, A.; Şen, B. Geochemistry of the Tigris river basin, Turkey: Spatial and seasonal variations of major ion compositions and their controlling factors. Quat. Int. 2013, 304, 22–32. [Google Scholar] [CrossRef]
- Kennedy, V.; Zellweger, G. Filter pore-size effects on the analysis of Al, Fe, Mn, and Ti in water. Water Resour. Res. 1974, 56, 785–790. [Google Scholar] [CrossRef]
- Laxen, D.; Chandler, I. Comparison of filtration techniques for size distribution in freshwater. Anal. Chem. 1982, 54, 1350–1355. [Google Scholar] [CrossRef]
- Fuoco, I.; De Rosa, R.; Barca, D.; Figoli, A.; Gabriele, B. Arsenic polluted waters: Application of geochemical modelling as a tool to understand the release and fate of the pollutant in crystalline aquifers. J. Environ. Manag. 2022, 301, 113796. [Google Scholar] [CrossRef]
- Apollaro, C.; Tripodi, V.; Vespasiano, G.; De Rosa, R.; Dotsika, E.; Fuoco, I.; Critelli, S. Chemical, isotopic and geotectonic relations of the warm and cold waters of the Galatro and Antonimina thermal areas, southern Calabria, Italy. Mar. Pet. Geol. 2019, 109, 469–483. [Google Scholar] [CrossRef]
- Gibbs, R. Mechanisms controlling world water chemistry. Science 1971, 170, 1088–1090. [Google Scholar] [CrossRef]
- Mohamed, A.; Asmoay, A.; Alshehri, F.; Abdelrady, A. Hydro-geochemical applications and multivariate analysis to assess the water–rock interaction in arid environments. Appl. Sci. 2022, 12, 6340. [Google Scholar] [CrossRef]
- Raymo, M.; Ruddiman, W. Tectonic forcing of late Cenozoic climate. Nature 1992, 359, 117–122. [Google Scholar] [CrossRef]
- Moya, C.; Raiber, M.; Taulis, M. Hydrochemical evolution and groundwater flow processes in the Galilee and Eromanga basins, Great Artesian Basin, Australia: A multivariate statistical approach. Sci. Total Environ. 2015, 508, 411–426. [Google Scholar] [CrossRef] [PubMed]




| River | pH | TDS | Ca2+ | Mg2+ | K+ | Na+ | SiO2 | CL− | NO3− | SO42− | HCO3− | F− | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry period of Yarlung Tsangpo | Min | 7.97 | 13.30 | 2.11 | 0.04 | 0.51 | 0.81 | 0.00 | 0.08 | 0.00 | 0.95 | 7.54 | 0.00 | This study |
| Max | 9.03 | 451.00 | 54.43 | 28.9 | 8.42 | 49.76 | 0.04 | 46.82 | 10.94 | 76.85 | 303.25 | 0.75 | ||
| Mean | 8.67 | 183.54 | 26.72 | 6.02 | 1.52 | 9.58 | 0.01 | 7.46 | 0.79 | 30.00 | 79.84 | 0.26 | ||
| SD | 0.17 | 72.72 | 13.57 | 3.82 | 0.42 | 9.58 | 0.32 | 7.29 | 1.44 | 17.54 | 40.32 | 0.324 | ||
| Normal period of Yarlung Tsangpo | Min | 7.62 | 59.6 | 9.46 | 0.9 | 0.76 | 0.98 | 3.15 | 0.33 | 0.08 | 8.81 | 35.54 | 0 | |
| Max | 8.91 | 386 | 77.95 | 18.26 | 9.53 | 49.99 | 34.03 | 60 | 3.15 | 99.44 | 201.4 | 1.67 | ||
| Mean | 8.46 | 204.51 | 36.02 | 7.25 | 1.98 | 11.15 | 8.64 | 7.32 | 1.58 | 36.86 | 115.57 | 0.20 | ||
| SD | 0.65 | 68.03 | 12.19 | 3.01 | 1.33 | 9.28 | 3.96 | 10.08 | 0.83 | 16.12 | 36.56 | 0.26 | ||
| Wet period of Yarlung Tsangpo | Min | 8.08 | 15.20 | 2.24 | 0.03 | 0.59 | 0.72 | 2.66 | 0.42 | 0.01 | 1.13 | 9.81 | 0.01 | |
| Max | 8.98 | 375.00 | 61.08 | 13.45 | 6.00 | 35.73 | 10.43 | 59.65 | 2.61 | 105.31 | 155.32 | 0.49 | ||
| Mean | 8.58 | 154.14 | 29.22 | 5.29 | 1.37 | 5.93 | 6.32 | 3.70 | 0.75 | 34.05 | 78.15 | 0.01 | ||
| SD | 0.17 | 61.21 | 11.33 | 2.35 | 0.77 | 5.14 | 1.42 | 7.51 | 0.61 | 18.73 | 27.31 | 0.08 | ||
| Dudhkoshi, Nepal | 7.52 | 37 | 7.90 | 0.4 | 0.70 | 0.80 | - | 0.60 | 1.20 | 3.70 | 22 | - | [21] | |
| Indus, India | 8.55 | 260 | 24 | 4.50 | 1.60 | 7.30 | 2.89 | 4.80 | 1.70 | 11.9 | 81.2 | - | [22] | |
| Tigris, Turkey | 8.45 | 276 | 46.61 | 9.14 | 1.44 | 6.43 | 5.92 | 20.70 | 2.49 | 23.20 | 153.80 | - | [23] | |
| Upper Yangtze, China | 7.98 | 778 | 53.40 | 22.90 | 5.50 | 157.70 | - | 233.70 | 1.30 | 114.90 | 188.50 | - | [16] | |
| Upper Mekong, China | 8.42 | 302 | 49 | 14 | 1 | 12.00 | 1.86 | 14 | - | 69.01 | 138.00 | - | [16] | |
| Yellow, China | 9.30 | 486 | 44.90 | 22.40 | 3.50 | 60 | 8.40 | 46.90 | 7.40 | 8.20 | 200.10 | - | [16] | |
| Global mean | 8 | 120 | 15 | 4.10 | 2.30 | 6.30 | 7.63 | 7.80 | 1 | 11.20 | 58.40 | - | [19] | |
| ELE | Fe | Cu | Ni | Co | Mn | Cr | Li | Ti | Al | Mo | Ag | Cd | Pb | Zn | Se | As | Hg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | Dry period | mg/L | ug/L | ug/L | ug/L | ug/L | ug/L | ug/L | ug/L | ug/L | ug/L | ug/L | ug/L | ug/L | ug/L | ug/L | ug/L | ug/L |
| Min | 13.86 | 2.11 | 74.18 | 1.87 | 13.50 | 6.60 | 300.00 | 3.34 | 75.91 | 10.60 | 0.21 | 6.64 | 7.37 | 122.73 | 0.04 | 182.09 | 0.07 | |
| Max | 0.00 | 0.43 | 1.40 | 0.22 | 1.71 | 3.78 | 21.60 | 0.31 | 0.31 | 0.64 | 0.21 | 0.04 | 0.08 | 0.25 | 0.01 | 0.15 | 0.00 | |
| Mean | 2.36 | 1.24 | 23.67 | 1.35 | 6.54 | 5.45 | 138.75 | 1.01 | 21.14 | 2.58 | 0.21 | 1.38 | 4.32 | 64.96 | 0.02 | 12.51 | 0.03 | |
| SD | 4.27 | 0.60 | 19.62 | 0.38 | 3.70 | 0.97 | 97.90 | 0.80 | 29.72 | 1.67 | 0.00 | 1.32 | 2.38 | 44.40 | 0.01 | 32.83 | 0.02 | |
| CV% | 0.54 | 2.31 | 1.35 | 3.51 | 1.77 | 5.60 | 1.42 | 1.25 | 0.71 | 1.54 | 0.21 | 1.03 | 1.79 | 1.48 | 1.59 | 0.38 | 1.38 | |
| Min | Wet period | 10.39 | 4.51 | 3.36 | 0.77 | 86.74 | 8.83 | 203.33 | 2.11 | 70.58 | 4.58 | 0.00 | 0.17 | 0.24 | 5.09 | 0.00 | 217.56 | 0.13 |
| Max | 4.94 | 0.51 | 0.55 | 0.27 | 1.09 | 2.83 | 12.33 | 2.11 | 2.68 | 0.44 | 0.00 | 0.17 | 0.07 | 0.42 | 0.00 | 0.19 | 0.01 | |
| Mean | 6.97 | 1.46 | 2.35 | 0.45 | 6.40 | 5.07 | 42.02 | 2.11 | 40.33 | 1.67 | 0.00 | 0.17 | 0.12 | 1.27 | 0.00 | 25.86 | 0.05 | |
| SD | 0.96 | 0.87 | 0.55 | 0.23 | 15.05 | 1.11 | 35.69 | 0.00 | 17.17 | 0.69 | 0.00 | 0.00 | 0.04 | 1.12 | 0.00 | 67.79 | 0.04 | |
| CV% | 7.20 | 1.66 | 3.58 | 1.98 | 0.43 | 4.09 | 1.17 | 0.29 | 0.28 | 2.28 | 0.00 | 0.00 | 0.69 | 1.10 | 0.00 | 0.38 | 1.34 | |
| Min | Normal period | 0.18 | 1.64 | 6.88 | 0.80 | 22.90 | 6.60 | 300.00 | 12.10 | 597.00 | 10.60 | 0.00 | 0.04 | 0.40 | 1.64 | 0.12 | 182.09 | 0.07 |
| Max | 0.01 | 0.29 | 0.49 | 0.20 | 1.71 | 1.30 | 16.50 | 2.91 | 1.03 | 0.64 | 0.00 | 0.04 | 0.05 | 0.24 | 0.00 | 0.28 | 0.00 | |
| Mean | 0.03 | 0.76 | 2.40 | 0.37 | 5.43 | 4.01 | 75.65 | 7.51 | 42.37 | 1.83 | 0.00 | 0.04 | 0.13 | 0.53 | 0.05 | 12.20 | 0.03 | |
| SD | 0.04 | 0.30 | 1.07 | 0.21 | 4.35 | 1.14 | 57.32 | 4.60 | 104.68 | 1.68 | 0.00 | 0.00 | 0.09 | 0.36 | 0.03 | 31.73 | 0.02 | |
| CV% | 0.71 | 2.50 | 2.21 | 1.79 | 1.23 | 3.53 | 1.32 | 1.63 | 0.40 | 1.07 | 0.00 | 0.00 | 1.35 | 1.46 | 1.61 | 0.38 | 1.21 |
| Variable | Wet Period | Normal Period | Dry Period | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pc1 | pc2 | pc3 | pc1 | pc2 | pc3 | pc1 | pc2 | pc3 | |
| Ca2+ | −0.446 | 0.875 | −0.039 | 0.272 | 0.880 | 0.203 | 0.046 | 0.667 | 0.254 |
| K+ | 0.662 | 0.588 | 0.148 | 0.897 | −0.034 | 0.120 | 0.703 | 0.323 | −0.076 |
| Mg2+ | 0.688 | −0.614 | −0.040 | 0.069 | 0.860 | 0.074 | 0.226 | 0.789 | −0.027 |
| Na+ | 0.910 | 0.356 | 0.111 | 0.976 | 0.018 | 0.004 | 0.907 | 0.290 | −0.088 |
| SO42− | 0.599 | −0.645 | 0.179 | 0.021 | 0.646 | 0.632 | 0.065 | 0.661 | 0.595 |
| HCO3− | 0.724 | 0.512 | 0.191 | 0.487 | 0.778 | −0.158 | 0.909 | 0.115 | −0.114 |
| CL− | 0.862 | −0.255 | −0.245 | 0.934 | 0.014 | 0.026 | 0.196 | 0.622 | −0.322 |
| SiO2 | −0.355 | 0.635 | −0.055 | 0.775 | −0.336 | 0.042 | 0.089 | −0.707 | 0.098 |
| Mn | −0.410 | 0.478 | 0.033 | 0.031 | −0.242 | 0.929 | −0.042 | −0.696 | 0.144 |
| Al | 0.750 | 0.482 | 0.141 | −0.074 | −0.618 | 0.038 | 0.948 | −0.100 | −0.048 |
| Zn | −0.184 | −0.247 | 0.885 | −0.195 | −0.300 | 0.868 | −0.069 | −0.070 | 0.874 |
| The variance contribution rate | 30.57% | 28.31% | 13.41% | 30.57% | 28.31% | 13.41% | 30.52% | 21.49% | 14.64% |
| The cumulative variance contribution rate% | 30.57% | 58.89% | 72.31% | 30.57% | 58.89% | 72.31% | 30.52% | 52.02% | 66.67% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Guo, H. Hydrochemical Characteristics and Ion Source Analysis of the Yarlung Tsangpo River Basin. Water 2023, 15, 537. https://doi.org/10.3390/w15030537
Liu J, Guo H. Hydrochemical Characteristics and Ion Source Analysis of the Yarlung Tsangpo River Basin. Water. 2023; 15(3):537. https://doi.org/10.3390/w15030537
Chicago/Turabian StyleLiu, Jiaju, and Huaicheng Guo. 2023. "Hydrochemical Characteristics and Ion Source Analysis of the Yarlung Tsangpo River Basin" Water 15, no. 3: 537. https://doi.org/10.3390/w15030537
APA StyleLiu, J., & Guo, H. (2023). Hydrochemical Characteristics and Ion Source Analysis of the Yarlung Tsangpo River Basin. Water, 15(3), 537. https://doi.org/10.3390/w15030537





