Effect of Potassium Dihydrogen Phosphate Combined with Thermally Activated Nano Serpentine and Thermally Activated Nano Zeolite on Cadmium in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Soils and Material
2.2. Experimental Design
2.3. Sample Analysis
2.4. Data Processing and Analysis
3. Results and Analysis
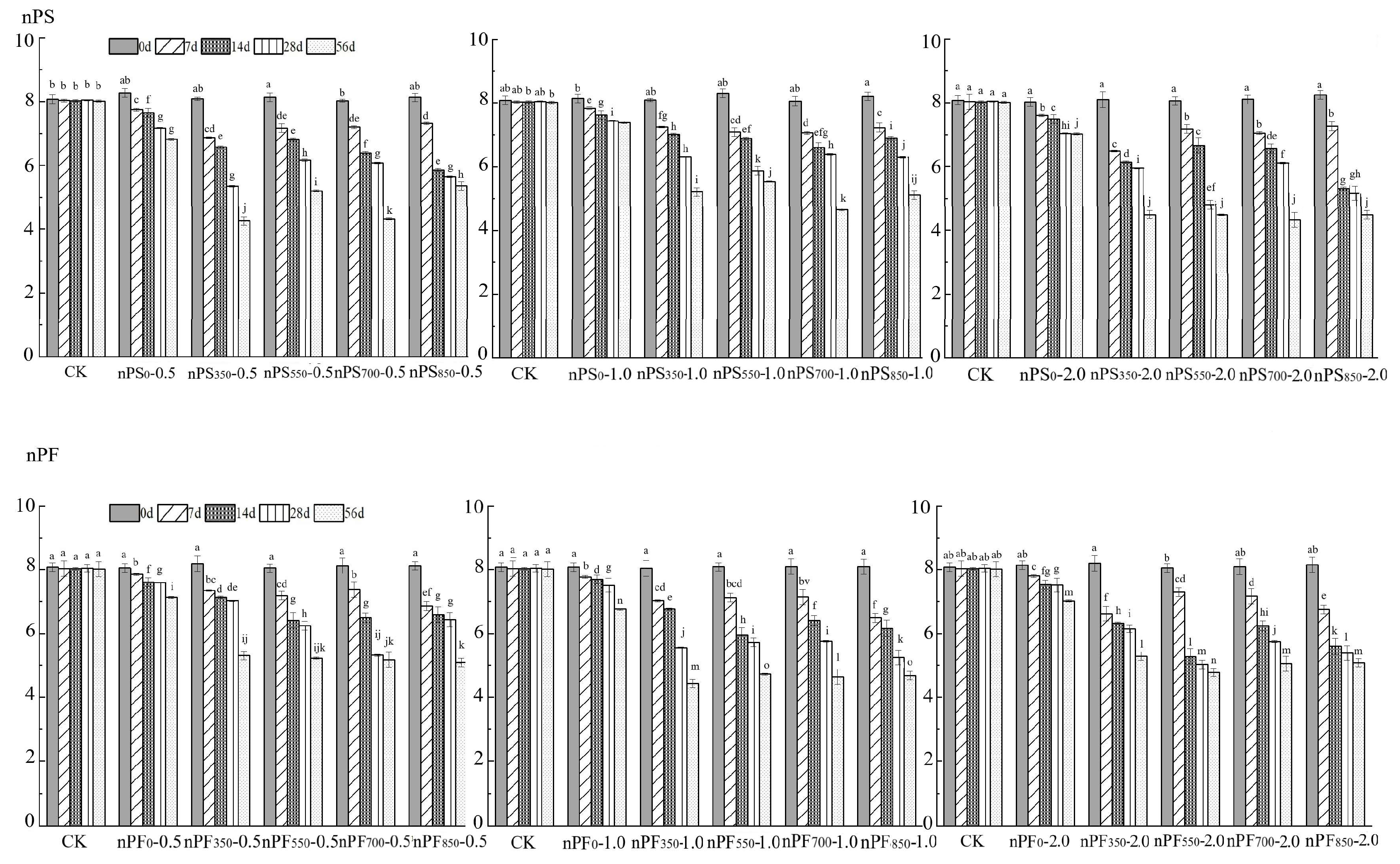
3.1. Effect on Soil pH
3.2. Effect on Soil DTPA-Cd and Exchangeable Cd by Different Modifiers
4. Discussion
4.1. pH
4.2. Cd Content
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, Y.W.; Liu, H.J.; Jiang, J.J.; Chen, C.K.; Liang, T.X.; Li, H.B. Study on determination of cadmium in rice by absorption spectrometry. Food Ferment. Sci. Technol. 2019, 54, 111–114. [Google Scholar]
- Demchenkov, E.L.; Nagdalian, A.A.; Budkevich, R.O.; Oboturova, N.P.; Okolelova, A.I. Usage of atomic force microscopy for detection of the damaging effect of CdCl2 on red blood cells membrane. Ecotoxicol. Environ. Saf. 2021, 208, 111683. [Google Scholar] [CrossRef] [PubMed]
- Browar, A.W.; Leavitt, L.L.; Prozialeck, W.C.; Edwards, J.R. Levels of Cadmium in Human Mandibular Bone. Toxics 2019, 7, 31. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Buha Djordjevic, A.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. Toxic Effect of Acute Cadmium and Lead Exposure in Rat Blood, Liver, and Kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- Kazuo, N.; Hiroko, N. Cadmium-induced renal dysfunction: New mechanism, treatment and prevention. J. Trace Elem. Exp. Med. 1998, 11, 275–288. [Google Scholar] [CrossRef]
- Lu, R.K.; Xiong, L.M.; Shi, Z.Y. Study on cadmium in soil-crop system. Soils 1992, 24, 129–132. [Google Scholar]
- Huang, Y.Z.; Hu, Y.; Liu, Y.X. Combined toxicity of copper and cadmium to six rice genotypes (Oryza sativa L.). J. Environ. Sci. 2009, 21, 647–653. [Google Scholar] [CrossRef]
- Liao, Z. Environmental Chemistry and Biological Effects of Trace Elements; Environmental Science Publishing House: Beijing, China, 1993; pp. 301–303. [Google Scholar]
- Hu, N.J.; Li, Z.Q.; Huang, P.; Cheng, W.Y. The pollution, prevention and remediation of heavy metals in infield land in some suburb areas, China. Bull. Mineral. Petrol. Geochem. 2003, 22, 251–254. [Google Scholar]
- Cui, L.T.; Geng, S.G.; Li, Z.W. Current situation and Prevention Countermeasures of cadmium pollution in farmland soil in China. Mod. Agric. Sci. Technol. 2006, 21, 184–186. [Google Scholar] [CrossRef]
- Yang, K.B. Heavy metal pollution and its phytoremediation in farmland soils in China. World Agric. 2007, 8, 58–61. [Google Scholar] [CrossRef]
- Zhao, M.L.; Wang, H.J.; Sun, J.X.; Tang, R.; Cai, B.Y.; Song, X.Y.; Huang, X.M.; Huang, J.; Fan, Z.Q. Spatio-temporal characteristics of soil Cd pollution and its influencing factors: A Geographically and temporally weighted regression (GTWR) method. J. Hazard. Mater. 2023, 446, 130613. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Zhu, Y.G. A review on cadmium contamination in forest ecosystem. Acta Ecol. Sin. 2004, 24, 101–108. [Google Scholar] [CrossRef]
- Hashem, A.; Adam, E.; Hussein, H.A.; Sanousy, M.A.; Ayoub, A. Bioadsorption of Cd (II) from contaminated water on treated sawdust: Adsorption mechanism and optimization. J. Water Resour. Prot. 2013, 5, 82–90. [Google Scholar] [CrossRef]
- Sun, Y.B.; Xu, Y.; Xu, Y.M.; Wang, L.; Liang, X.F.; Li, Y. Reliability and stability of immobilization remediation of Cd polluted soils using sepiolite under pot and field trials. Environ. Pollut. 2016, 208, 739–746. [Google Scholar] [CrossRef]
- Komrek, M.; Vaněk, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.C.; Huang, Z.B.; Tang, K.; Zhang, Y. Research progress of chemical solidification on administering soil heavy metal pollution. Environ. Eng. 2014, 32, 158–161. [Google Scholar] [CrossRef]
- Veysel, T. Confident performance of chitosan and pistachio shell biochar on reducing Ni bioavailability in soil and plant plus improved the soil enzymatic activities, antioxidant defense system and nutritional quality of lettuce. Ecotoxicology and Environmental. Safety 2019, 183, 109594. [Google Scholar] [CrossRef]
- Veysel, T. Arbuscular mycorrhizal fungi and pistachio husk biochar combination reduces Ni distribution in mungbean plant and improves plant antioxidants and soil enzymes. Physiol. Plant. 2021, 173, 418–429. [Google Scholar] [CrossRef]
- Liang, X.; Xu, Y.; Wang, L.; Sun, G.; Sun, Y. In-situ immobilization of cadmium and lead in a contaminated agricultural fields by adding natural clays combined with phosphate fertilizer. Acta Sci. Circumstantiae 2011, 31, 1011–1018. [Google Scholar]
- Chang, Y.T.; His, H.C.; Hseu, Z.Y.; Jheng, S.L. Chemical stabilization of cadmium in acidic soil using alkaline agronomic and industrial by-products. J. Environ. Sci. Health 2013, 48, 1748–1756. [Google Scholar] [CrossRef]
- Homa, G.; Mehrnoosh, A.; Tazkieh, G.; Behzad, A.; Saeed, M. Rapid and effective removal of heavy metal ions from aqueous solution using nanostructured clay particles. Results Surf. Interfaces 2023, 10, 100097. [Google Scholar] [CrossRef]
- Hamidpour, M.; Afyuni, M.; Kalbasi, M.; Khoshgoftarmanes, A.H.; Inglezakis, V.J. Mobility and plant-availability of Cd(II) and Pb(II) adsorbed on zeolite and bentonite. Appl. Clay Sci. 2010, 48, 342–348. [Google Scholar] [CrossRef]
- Taparcevska, J.; Markovska, L.; Koumanova, B.; Meshko, V. Diffusion models for adsorption kinetics of Zn(2+), Cd(2+) and Pb(2+) onto natural zeolite. Water Sci. Technol. 2010, 62, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Zhang, Y.; Du, L.Y.; Su, X.N.; Dong, S.P.; Lan, X.P. Effect of biocarbon and zeolite mixing on morphological conversion of soil Cd. J. Soil Water Conserv. 2014, 28, 248–252. [Google Scholar] [CrossRef]
- Guo, J.X.; Yuan, C.G. Experimental study on the treatment of heavy metals in sewage by serpentine adsorption. Fine Chem. 2000, 17, 586–589. [Google Scholar] [CrossRef]
- Luo, W.H. Research on Mineralogy Characteristics and Adsorption Capability of Serpentine from Jianchaling. Master’s Thesis, Chang’an University, Xi’an, China, 2008. [Google Scholar] [CrossRef]
- Cao, C.Y.; Yu, B.; Wang, M.; Zhao, Y.Y.; Wan, X.; Zhao, S. Immobilization of cadmium in simulated contaminated soils using thermal-activated serpentine. Soil Sci. Plant Nutr. 2020, 66, 499–505. [Google Scholar] [CrossRef]
- Raicevic, S.; Kaludjerovic-Radoicic, T.; Zouboulis, A.I. In situ stabilization of toxic metals in polluted soils using phosphates: Theoretical prediction and experimental verification. J. Hazard. Mater. 2005, 117, 41–53. [Google Scholar] [CrossRef]
- Bao, S.D. Agrochemical Analysis of Soil, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 25–200. [Google Scholar]
- Cao, C.Y.; Liang, C.H.; Yin, Y.; Du, L.Y. Thermal Activation of Serpentine for Adsorption of Cadmium. J. Hazard. Mater. 2017, 329, 222–229. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil Agrochemistry; China Agricultural Science and Technology Press: Beijing, China, 1999; pp. 12–139. [Google Scholar]
- Liu, M.; Liu, F.Z.; Liu, B.F. Determination of available lead and cadmium in Soil. J. Agro-Environ. Sci. 2007, 26, 300–302. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Majdan, M.; Pikus, S.; Kowalska-Ternes, M.; Gładysz-Płaska, A.; Staszczuk, P.; Fuks, L.; Skrzypek, H. Equilibrium study of selected divalent d-electron metals adsorption on A-type zeolite. J. Colloid Interface Sci. 2003, 262, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.S.; Chao, C.Y.H.; Kot, S.C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Sheikhhosseini, A.; Shirvani, M.; Shariatmadari, H. Competitive sorption of nickel, cadmium, zinc and copper on palygorskite and sepiolite silicate clay minerals. Geoderma 2013, 192, 249–253. [Google Scholar] [CrossRef]
- Li, Y.M.; Wang, K.Q.; Liu, Z.Q.; Wang, J.Y.; Zhou, X. Effect of measure of engineering preparation to soil water in Yunnan Dry-Hot River Valley. J. Soil Water Conserv. 2006, 20, 15–19. [Google Scholar] [CrossRef]
- Ren, L.X. Soil heavy metal morphology and solubility organic matter environmental behavior. Environ. Sci. Technol. 2008, 31, 69–73. [Google Scholar] [CrossRef]
- Li, X.J.; Qiu, S.; Zhao, J.; Zhang, C.P.; Zeng, D.J.; Liu, S.H. Studies on manganese absorption and soil remediation of four ornamental plants. J. South. Agric. 2010, 41, 951–954. [Google Scholar] [CrossRef]
- Liang, X.J.; Han, Y.; Xu, Y.; Sun, L.; Wang, X. In Situ Field-scale Remediation of Cd Polluted Paddy Soil Using Sepiolite and Palygorskite. Geoderma 2014, 235–236, 9–18. [Google Scholar] [CrossRef]
- Jafarnejadi, A.R.; Sayyad, G.; Homaee, M.; Davamei, A.H. Spatial Variability of Soil Total and DTPA-extractable Cadmium Caused by Long-term Application of Phosphate Fertilizers, Crop Rotation, and Soil Characteristics. Environ. Monit. Assess. 2013, 185, 4087–4096. [Google Scholar] [CrossRef]
- Hooda, P.S.; Alloway, B.J. Cadmium and lead sorption behavior of selected English and Indian soils. Geoderma 1998, 84, 121–134. [Google Scholar] [CrossRef]
- Ding, J.H.; Wen, Y.M.; Shu, Q. Fraction Transformation of cadmium and zinc in soils. Urban Environ. Urban Ecol. 2001, 14, 47–49. [Google Scholar]
- Chen, M.; Chen, Y.H.; Shen, Z.G.; Shen, Q.R. Amelioration of aluminum toxicity on wheat plants grown in acid red soil by pig manure. J. Plant Nutr. Fertil. 2002, 8, 173–176. [Google Scholar] [CrossRef]
- Zhang, J.; Sui, Q.; Zhong, H.; Meng, X.; Wang, Z.; Wang, Y.; Wei, Y. Impacts of zero valent iron, natural zeolite and Dnase on the fate of antibiotic resistance genes during thermophilic and mesophilic anaerobic digestion of swine manure. Bioresour. Technol. 2018, 258, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Wang, L.J.; Lu, A.H.; Wang, C.Q. A Discussion on Activation Mechanism of Atom Groups in Serpentine. Acta Petrol. Mineral. 2003, 22, 386–390. [Google Scholar] [CrossRef]


| Soil Type | pH | Organic Matter (g·kg−1) | Alkali- Hydrolyzable Nitrogen (mg·kg−1) | Available Phosphorus (mg·kg−1) | Available Potassium (mg·kg−1) | Total Phosphorus (g·kg−1) | Total Cd (mg·kg−1) |
|---|---|---|---|---|---|---|---|
| Brown soil | 6.73 | 26.93 | 50.02 | 71.27 | 183.59 | 0.63 | 0.18 |
| Composition | SiO2 | Al2O3 | MgO | K2O | CaO | Na2O | Fe2O3 |
|---|---|---|---|---|---|---|---|
| Content in zeolite (wt. %) | 76.32 | 12.49 | 3.78 | 3.24 | 2.30 | 1.29 | 0.58 |
| Composition | SiO2 | Al2O3 | MgO | CaO | Na2O | Fe2O3 |
|---|---|---|---|---|---|---|
| Content in serpentine (wt. %) | 57.24 | 0.11 | 37.97 | 3.20 | 0.18 | 1.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, H.; Zou, H. Effect of Potassium Dihydrogen Phosphate Combined with Thermally Activated Nano Serpentine and Thermally Activated Nano Zeolite on Cadmium in Soil. Water 2023, 15, 538. https://doi.org/10.3390/w15030538
Wang X, Liu H, Zou H. Effect of Potassium Dihydrogen Phosphate Combined with Thermally Activated Nano Serpentine and Thermally Activated Nano Zeolite on Cadmium in Soil. Water. 2023; 15(3):538. https://doi.org/10.3390/w15030538
Chicago/Turabian StyleWang, Xiuli, Hongdou Liu, and Hongtao Zou. 2023. "Effect of Potassium Dihydrogen Phosphate Combined with Thermally Activated Nano Serpentine and Thermally Activated Nano Zeolite on Cadmium in Soil" Water 15, no. 3: 538. https://doi.org/10.3390/w15030538
APA StyleWang, X., Liu, H., & Zou, H. (2023). Effect of Potassium Dihydrogen Phosphate Combined with Thermally Activated Nano Serpentine and Thermally Activated Nano Zeolite on Cadmium in Soil. Water, 15(3), 538. https://doi.org/10.3390/w15030538








