Abstract
Lake Uiam is situated midway through a basin with dams at both the upstream and downstream ends; considerable environmental fluctuations have been observed here. However, studies on changes in environmental factors and plankton community fluctuations remain limited. This study analyzed the seasonal physicochemical factors and changes in the phytoplankton community structure in Lake Uiam (2015–2016). Organic matter, phosphorus, total suspended solids (TSS), and Chl-a concentrations were high in the summer. Seasonal changes in the dominant taxa followed the typical succession pattern of temperate phytoplankton, with Bacillariophyceae (Ulnaria acus) being dominant in spring and Cyanophyceae (Pseudanabaena limnetica) dominant in summer. However, Cryptophyceae (Rhodomonas sp.) showed unusually high dominance in autumn. Cell abundance showed no seasonal differences. Rhodomonas sp. was negatively correlated with water temperature, suggesting its dominance in spring and autumn. Cryptophyceae showed a significant correlation with Chl-a (0.708 **), indicating its contribution to spring Chl-a concentrations. Cryptophyceae (Rhodomonas sp. and Cryptomonas spp.) commonly appear in spring but are dominant in autumn in Lake Uiam. Despite disturbances from various environmental factors, they showed higher adaptability than other algae, resulting in their consistent appearance and dominance, differing from the general succession patterns of temperate phytoplankton.
1. Introduction
Artificial lakes, created by building dams on rivers, dominate South Korea and are mainly used for drinking and agriculture. These expanded water surfaces are easily affected by nearby pollution sources. Eutrophication is progressing in most artificial lakes in South Korea, and population increases and urbanization in the surrounding catchment areas are accelerating this process [1,2,3]. These lakes are influenced by the monsoon climate, with summer accounting for 50–60% of the annual average rainfall. Because of this seasonal impact, frequent fluctuations occur in the residence time of water in the upstream and downstream areas of artificial lakes [4,5]. In particular, upstream sources easily influence Lake Uiam as it receives inflow from the Hwacheon and Chuncheon dams on the right bank and the Soyanggang Dam on the left bank. This contributes to phytoplankton growth by discharging nutrients into the lakes downstream of Cheongpyeong and Paldang [6]. Therefore, Lake Uiam, located midway through the basin, is expected to experience significant fluctuations in both environmental factors and phytoplankton communities.
Phytoplankton are abundant in natural and artificial environments and are important in material cycling and energy flow in aquatic ecosystems [7]. They are the primary producers in wetlands, lakes, and rivers and are characterized by rapid reproduction and sensitivity to environmental changes. Therefore, the phytoplankton species and community characteristics are important water quality indicators [8,9,10]. Nutrients, temperature, and light affect phytoplankton growth and induce changes in community structure [11,12,13]. Environmental factors favorable for phytoplankton growth vary by taxon and species. Cyanophyceae, which produce toxins and odors, are primarily influenced by phosphorus and water temperature [14].
Nutrient influx from point and non-point pollution sources in watersheds affects phytoplankton growth [9,15]. In Lake Uiam, water quality is influenced by the discharge from a sewage treatment plant located on the left bank and the confluence of the Gongji stream that flows through the city, rendering it an area where cyanobacterial blooms frequently occur in summer [6]. Such cyanobacterial blooms in dam lakes produce odor compounds, such as 2-methylisoborneol (2-MIB) and geosmin, causing inconvenience in the use of water sources [16,17,18,19]. In addition, massive springtime blooms of diatoms, such as Ulnaria spp., can clog water treatment filters, leading to economic issues [20].
The patterns of phytoplankton community succession in most domestic lakes reflect the typical changes in temperate lake communities [21,22]. In spring, diatoms dominate; in summer, cyanobacteria become successors; and in autumn and winter, diatoms regain dominance [23,24]. Studies on these phytoplankton community fluctuations are being conducted for Lake Paldang, located downstream of the watershed, which is influenced by various hydrological, water quality, and seasonal factors due to the Bukhan and Namhan rivers [25,26,27]. However, in the case of Lake Uiam, located at the confluence of the upstream Chuncheon and Soyanggang dams and midway in the Bukhan River watershed, considerable environmental fluctuations were observed. Nevertheless, studies on changes in environmental factors and plankton community fluctuations remain limited.
Therefore, this study investigated the patterns of phytoplankton communities and the dominant species occurrence in Lake Uiam, which displays diverse environmental conditions in a dam lake ecosystem. We statistically analyzed the interrelationships between environmental factors and identified seasonal variations in the phytoplankton community of Lake Uiam.
2. Materials and Methods
2.1. Study Site
Lake Uiam was created in August 1967 by constructing the Uiam Dam, a multipurpose dam with a power generation capacity of 45,000 kW. The height of Uiam is 23 m, the length of the embankment is 273 m, the total storage capacity is 8 million tons, the facility power generation capacity is 45,000 kW, and the basin area is 282.8 km2. The surface area of Uiam Lake is oval, with a width of 5 km and a length of 8 km. The lake’s surface water level is 72 m above sea level. Lake Uiam comprises six administrative districts. The land use area in the Lake Uiam watershed is 339 km2, and the largest area is forestland, covering 211 km2 (Figure 1). The number of livestock was 8408 cattle, 329 cows, and 24 pigs, and the amount of wastewater discharged daily was 2063 m3/day. The total rainfall in Lake Uiam was 758 mm in 2015 and 1329 mm in 2016, and the residence time was 16 days in 2015 and 10 days in 2016.
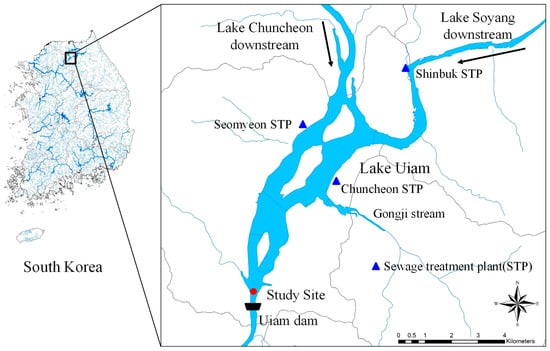
Figure 1.
Study site in Lake Uiam, South Korea.
2.2. Sample Collection and Water Quality Analysis
Water samples were collected for environmental parameters and phytoplankton analysis once a month at one point in front of the Uiam dam from March 2015 to November 2016 (excluding periods (December–February) characterized by freezing). Sampling was conducted using a boat, and amber glass bottles were used to avoid light reactions in the water samples. To prevent contamination, all organic products used in the experiment were washed with detergent, rinsed with deionized water, and heated at 110 °C for at least 2 h. The collected samples were immediately stored in an ice cooler and transported to the laboratory.
Water pH, temperature, dissolved oxygen (DO), and electrical conductivity (EC) were measured in situ using a calibrated digital multiparameter (YSI-EXO, YSI Inc., Yellow Springs, OH, USA). Biological oxygen demand 5 days after incubation (BOD), chemical oxygen demand (COD), total nitrogen (TN), dissolved total nitrogen (DTN), nitrate (NO3-N), ammonia (NH3-N), chlorophyll-a concentration, total phosphorus (TP), dissolved total phosphorus (DTP), phosphate (PO4-P), total suspended solids (TSS), and total organic carbon (TOC) were measured in the laboratory following standard methods described by the Korea Water Quality Standard method [28] (Table S1).
2.3. Phytoplankton Analysis
To analyze the communities and dominant species of phytoplankton, the collected samples were preserved by adding Lugol’s solution to achieve a final concentration of 2%. The preserved samples were examined under a phase-contrast microscope (Eclipse 80i, Nikon, Tokyo, Japan) at magnifications ranging from 100× to 1000×, and both species identification and cell abundance were determined using a Sedgewick–Rafter counting chamber with 1 mL of a sample. Phytoplankton species identification was conducted by referring to John et al. [29], Hirose et al. [30], Krammer [31], and Krammer and Lange-Bertalot [32,33,34].
2.4. Statistical Analysis
To investigate seasonal differences in physicochemical factors and phytoplankton in Lake Uiam, an analysis of variance (ANOVA) was conducted, and post hoc analysis was performed using Duncan’s test. Correlation analysis between environmental factors and phytoplankton was conducted using Pearson’s correlation test, and the overall statistical analysis was performed using SPSS 20 (IBM Corp., Armonk, NY, USA). Statistical significance was set at p < 0.05. To explore the relationship between phytoplankton and environmental variables, canonical correspondence analysis (CCA; PC-ORD5, Lincoln City, OR, USA) was performed. Prior to the analysis, all taxa that appeared in less than 0.5% of the samples were excluded. Cells that could not be assigned to any specific category (unidentified forms <10 μm) were also excluded. All continuous environmental variables were normalized using the formula log(1 + x) [35,36,37]. The water quality concentration and phytoplankton cell number for each season and year were the corresponding averages of two years.
To reduce the dimensions and facilitate easier processing, the large dataset in this study required principal component analysis (PCA; PC-ORD5, Lincoln City, OR, USA) for various purposes ranging from outlier detection to data visualization [38]. These components were uncorrelated and hierarchically arranged. According to Kaiser [39], the first principal component (PC1) retains the largest variance of the original data, whereas the second principal component (PC2) explains the largest remaining variance yet to be accounted for. Before analyzing the data using PCA, outliers and missing data were identified and excluded.
3. Results and Discussion
3.1. Physicochemical Properties of Lake Uiam
A statistical summary of the selected parameters for the water samples is presented in Table 1. Sixteen water quality variables were analyzed for Lake Uiam. Water temperatures showed very different distributions depending on the season (Table 1). BOD, COD, TSS, NH3-N, PO4-P, and Chl-a did not vary significantly throughout the season (p > 0.05). Physicochemical properties typically depend on the source. Therefore, this could be considered a continuous introduction of pollutants from the same source, regardless of the season [40]. As shown in Table 1, organic matter (BOD, COD, and TOC), phosphorus (TP, DTP, and PO4-P), TSS, and Chl-a in Lake Uiam were influenced by summer rainfall. Specifically, these parameters were higher during summer than during spring and autumn. However, the nitrogen (TN, DTN, NH3-N, and NO3-N) concentrations in Lake Uiam were in the following order: spring > summer > autumn, indicating a nitrogen-rich system and dilution of nitrogen during summer. All water quality parameters were relatively high in spring and summer, corresponding to the monsoon and pre-monsoon seasons. These results are similar to those of previous studies [41,42].

Table 1.
Seasonal physicochemical factors exhibited significant differences in the ANOVA results for Lake Uiam.
3.2. Phytoplankton Composition of Taxa
A total of 91 species of phytoplankton were observed in Lake Uiam. Chlorophyceae was the most abundant taxon with 34 species, followed by Bacillariophyceae (31), Cyanophyceae (13), Dinophyceae (4), Cryptophyceae (4), Chrysophyceae (4), and Euglenophyceae (1). The highest average number of phytoplankton species occurred in autumn (September–November), with an average of 24 species, followed by summer (19 species) and spring (17 species), with the latter showing the lowest species count. In lakes in temperate regions, the higher average species count in autumn can be attributed to a decrease in the number of cyanobacterial species as the temperature decreases from summer, when they predominantly dominate, to autumn. This decrease is complemented by a relative increase in the number of diatoms and phytoflagellates, overall increasing the average number of species. The dominant species by taxon were Chlorophyceae (Scenedesmus quadricauda, Monoraphidium contortum, Monoraphidium arcuatum, Micratinium pusillum, and Chlamydomonas angulosa), Bacillariophyceae (Ulnaria acus, Ulnaria delicatissima, Asterionella formosa, Aulacoseira granulata var. angustissima, Fragilaria crotonensis, Urosolenia longiseta, and Stephanocyclus meneghinianus), Cyanophyceae (Pseudanabaena sp., Pseudananbaena limnetica, Microcystis aeruginosa, Dolichospermum circinale, and Leptolyngbya tenuis), Dinophyceae (Peridinium sp. and Gymnodinium sp.), Cryptophyceae (Cryptomonas ovata, Cryptomonas erosa, and Rhodomonas sp.), Chrysophyceae (Dinobryon divergens, Mallomonas sp.), and Euglenophyceae (Euglena sp.). They appeared predominantly and sub-dominantly within their respective taxa. Cyanophyceae (Dolichospermum spp., Pseudanabaena spp., and Leptolyngbya spp.) were dominant in late summer and early autumn (August to September) following the monsoon season, which is consistent with the typical dominance of Cyanophyceae in temperate regions after the monsoon season [16,17,18,23,43] (Table 2 and Table 3).

Table 2.
Dominant phytoplankton taxa in Lake Uiam.

Table 3.
List of phytoplankton species in Lake Uiam.
3.3. Seasonal Variation of Phytoplankton Abundance and Characteristics
During the study period, phytoplankton cell abundance in Lake Uiam ranged from 2112 to 14,351 cells/mL, with an average of 4473 cells/mL (Figure 2). The seasonal average phytoplankton cell abundance was the highest in autumn at 4644 cells/mL, followed by summer (4597 cells/mL) and spring (4178 cells/mL), with the lowest abundance in spring. There was no significant difference in phytoplankton cell abundance between summer and autumn (p > 0.5). This lack of seasonal variation is likely due to the high cell abundance of cyanobacteria from late summer to early autumn (August–September) after the monsoon season, which is influenced by nutrient influx and elevated water temperatures, leading to an increase in Cyanophyceae cell abundance [5,43,44].
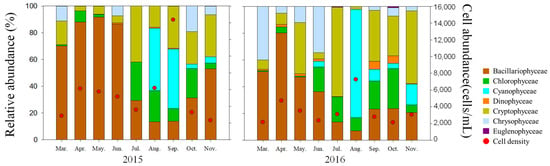
Figure 2.
Monthly variations in phytoplankton relative abundance percentage and cell abundance in Lake Uiam.
The relative abundance percentage of total phytoplankton taxa was highest in Bacillariophyceae (41.4%), followed by Cryptophyceae (21.6%), Cyanophyceae (20.2%), Chlorophyceae (10.8%), Chrysophyceae (5.1%), Dinophyceae (0.9%), and Euglenophyceae (0.05%) (Figure 3).
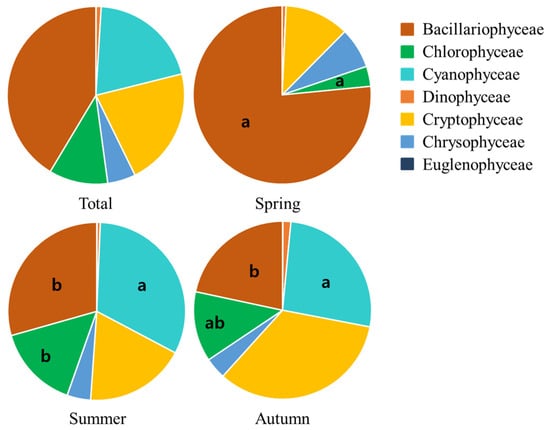
Figure 3.
Seasonal changes in phytoplankton communities and average relative abundance percentage in Lake Uiam from 2015 to 2016 (Spring: March–May; Summer: June–August; Autumn: October–November). Small letters (a and b) indicate the Duncan’s test (ANOVA).
Seasonal relative abundance percentages of phytoplankton taxa were as follows: in spring, Bacillariophyceae (76.5%), Cryptophyceae (11.7%), and Chrysophyceae (7.4%); in summer, Cyanophyceae (32.1%), Bacillariophyceae (29.4%), and Cryptophyceae (18.4%); and in autumn, Cryptophyceae (33.7%), Cyanophyceae (26.5%), and Bacillariophyceae (21.6%) (Figure 3).
The dominant species in spring was Ulnaria acus (average 1630 cells/mL), followed by Ulnaria delicatissima (average 448 cells/mL), Rhodomonas sp. (average 420 cells/mL), and in summer, Pseudanabaena limnetica (average 958 cells/mL) was dominant, followed by Rhodomonas sp. (average 419 cells/mL) and Leptolyngbya tenuis (average 350 cells/mL). In autumn, Dolichospermum circinale (average 747 cells/mL) was dominant, followed by Crytomonas ovata (average 658 cells/mL) and Rhodomonas sp. (average 634 cells/mL) (Table 2).
Seasonal changes in the dominant taxa generally followed the typical temperate–region phytoplankton succession pattern, with diatoms (Bacillariophyceae) predominant in spring and cyanobacteria (Cyanophyceae) predominant in summer. However, unlike typical phytoplankton succession, Cryptophyceae showed high dominance in autumn. Particularly in autumn 2016 (September–November), Cryptophyceae (Rhodomonas sp.) were consistently dominant (Table 2, Figure 2). Chlorophyceae exhibited higher cell densities from July to October than in other seasons, although they did not reach the dominant species. Generally, in temperate lakes, Chlorophyceae tend to increase in late spring to summer following the occurrence of Bacillariophyceae in spring [45,46,47]. However, Chlorophyceae were not dominated in the reservoirs of South Korea, including the upstream Chuncheon and Soyang lakes and the downstream Cheongpyeong and Paldang lakes [17,23,24,48]. The pattern typically shows that Bacillariophyceae and Cryptophyceae appear in spring, followed by Cyanophyceae. Both Chlorophyceae (0.536 *, p < 0.05) and Cyanophyceae (0.687 **, p < 0.01) showed significant positive correlations with water temperature, tending to increase in late spring and summer when water temperatures increased (Table 4). However, in Lake Uiam, a mesotrophic reservoir with ample nutrients, succession to growth-favorable Cyanophyceae occurred more rapidly.

Table 4.
Correlation coefficients of variable factors in Lake Uiam.
3.4. CCA of the Phytoplankton Community and Environmental Factors in Lake Uiam
CCA was conducted at the taxa and species levels to analyze the relationship between environmental factors and the phytoplankton community in Lake Uiam. According to the CCA results from Lake Uiam, the relationship between phytoplankton and environmental factors at the taxa level explained 31.0% for Axis1 and 25.6% for Axis2. At the species level, it explained 15.5% for Axis1 and 13.1% for Axis2 (Figure 4 and Figure 5).
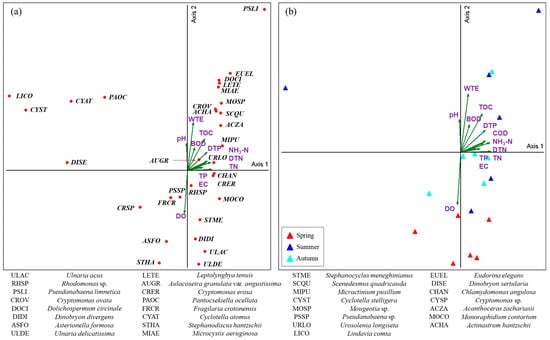
Figure 4.
Canonical correspondence analysis (CCA) ordination of phytoplankton species (a) with environmental variables. Ordination diagram showing the seasonal distribution of Lake Uiam (b).
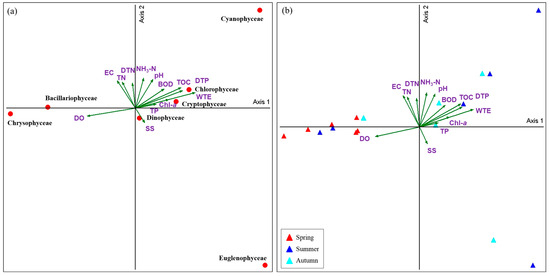
Figure 5.
Canonical correspondence analysis (CCA) ordination of phytoplankton taxa (a) with environmental variables. Ordination diagram showing the seasonal distribution of Lake Uiam (b).
CCA showed that Chlorophyceae, Cryptophyceae, and Cyanophyceae were positively correlated with water temperature (WTE), dissolved total phosphorus (DTP), and total organic carbon (TOC). Cyanophyceae also demonstrated significant positive correlations with water temperature (0.536 *), dissolved total phosphorus (0.678 **), and TOC (0.549 *) in Pearson’s correlation test (Table 4).
Cyanophyceae, such as Pseudanabaena limnetica, Dolichospermum circinale, Leptolyngbya tenuis, and Microcystis aeruginosa, were correlated with nutrients (DTP, NH3-N, and DTN) and water temperature. Pseudanabaena sp. showed a negative correlation with water temperature. This suggests that Pseudanabaena sp. has advantageous growth characteristics, even in environments with lower temperatures and light intensities than other cyanobacteria [49]. Rhodomonas sp. showed a negative correlation with water temperature and pH, indicating its dominance in spring and autumn. In the correlation analysis, Cryptophyceae also showed significant correlations with BOD (0.607 **), Chl-a (0.708 **), and TOC (0.494 *), suggesting its contribution to the Chl-a concentration in Lake Uiam. This finding is consistent with the tendency of Rhodomonas sp. to dominate during periods of low water temperatures in temperate regions [50,51]. This also suggests that Rhodomonas sp. is less affected by low nutrient levels and irradiance, enabling it to grow in this environment [52] (Table 4, Figure 4 and Figure 5).
Bacillariophyceae and Crysophyceae showed positive correlations with dissolved oxygen (DO) and negative correlations with factors such as DTP, WTE, Chl-a, and TP, indicating their occurrence in spring or autumn.
Species such as Ulnaria acus, U. delicatissima, Asterionella formosa, Fragilaria crotonensis, and Stephanodiscus hantzschii were negatively correlated with WTE, DTP, and TOC. Asterionella formosa and Ulnaria acus predominantly appear in temperate lakes and have high biomasses in spring [53,54]. They are resistant to low water temperatures and display advantageous growth characteristics at low water temperatures compared to other algae [55,56,57]. Fragilaria crotonensis has favorable growth characteristics even under relatively low nutrient and silicate conditions compared to other diatoms (Ulnaria spp., A. formosa, and Stephanodiscus hantzschii) [58]. It frequently appears in these low nutrient conditions in May and June, in autumn. Aulacoseira granulata var. angustissima showed a significant positive correlation with DTP. It primarily occurs in September and October, when the water temperatures are high after the rainy season. This aligns with previous reports showing that A. granulate var. angustissima frequently appeared because of the influx of nutrients, indicating a similar trend [59,60] (Table 4, Figure 4 and Figure 5).
Chrysophyceae (Dinobryon spp.) showed a negative correlation with water temperature and nutrients (TP and DTP), suggesting that Dinobryon spp. have advantageous growth characteristics under low-phosphorus conditions compared to other phytoplankton [61]. Dinophyceae exhibited a positive correlation with suspended solids (SS) and negative correlations with TN, DTN, and EC, which is consistent with its tendency to occur primarily under oligotrophic conditions [10,62,63] (Table 4, Figure 4 and Figure 5).
3.5. Seasonal Influence of Water Environmental Factors on Phytoplankton Communities
Based on the results of the one-way ANOVA of the seasonal phytoplankton taxa, Bacillariophyceae and Chlorophyceae showed seasonal differences (Figure 3). Bacillariophyceae showed a high average cell abundance during spring and significant differences in summer and autumn (p < 0.05). Chlorophyceae showed a high average cell abundance in summer, contrasting values in spring and intermediate values in autumn, indicating seasonal variation. This is consistent with the seasonal succession patterns suggested in the PEG model for phytoplankton in temperate lakes, where succession occurs in Chlorophyceae, Cyanophyceae, and Dinophyceae after spring due to the influence of nutrients and water temperature [22]. Cyanophyceae appeared with a high cell abundance in summer and autumn, showing a difference from spring. However, as Cyanophyceae did not appear in spring, no statistically significant seasonal differences were observed.
3.6. PCA of Seasonal Phytoplankton Communities
The PCA results for seasonal phytoplankton taxa and environmental factors showed that Axis 1 and 2 were above 70% in spring, summer, and autumn (Figure 6).
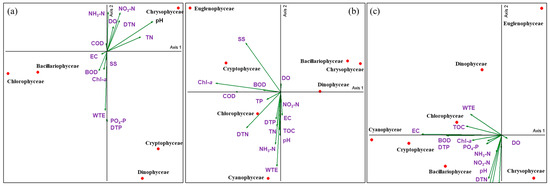
Figure 6.
Principal component analysis (PCA) between phytoplankton taxa and environmental factors in Lake Uiam ((a): spring, (b): summer, (c): autumn).
Bacillariophyceae and Chlorophyceae in Lake Uiam showed a positive correlation with Chl-a, indicating that their contribution increased Chl-a concentration in the lake during spring. Cyanophyceae in Lake Uiam during summer exhibited a positive correlation with water temperature, TOC, and nutrients (NH3-N, DTP, TP, and NO3-N), indicating that an increase in water temperature and nutrients during summer led to Cyanophyceae blooms. Chl-a showed a positive correlation with Cryptophyceae, supporting a previous study that Cryptophyceae contribute more to Chl-a concentrations than other algae [64,65] (Table 4, Figure 6).
In autumn, Cyanophyceae showed a positive correlation with DTP, TN, and WTE, which corresponded to the trends observed for Cyanophyceae in summer. This suggests that the nutrient influx following the monsoon season triggers cyanobacterial blooms in late summer and early autumn. This is consistent with the occurrence of odor problems in South Korea caused by late summer and autumn cyanobacterial blooms [16,17,18]. Such late summer to autumn blooms of Cyanophyceae can occur under advantageous environmental conditions because of their buoyancy-controlling characteristics [66,67,68,69]. They have a competitive advantage in acquiring high temperatures, light, and nutrients from the water surface. Moreover, the buoyancy control feature of cyanobacteria is attributed to the function of gas vesicles inside the cells, which are involved in their vertical movement in water. This vertical movement allows cyanobacterial cells to stay on the surface layer, where they can obtain sufficient light for photosynthesis and a suitable water temperature for growth. They can also avoid exposure to hazardous levels of sunlight and absorb nutrients [70,71,72]. This is advantageous for blooms in stable water bodies after the monsoon season.
Cryptophyceae positively correlated with DTP, Chl-a, and PO4-P. When considering the CCA, seasonal PCA, and occurrence patterns, Cryptophyceae (Rhodomonas sp. and Cryptomonas spp.) are generally more prevalent in spring when the water temperature is low [22,73]. However, in Lake Uiam, they appeared throughout spring and autumn and were dominant in both spring and autumn. They also appeared throughout all seasons in both the upstream and downstream areas of Lake Uiam [17,23,24,48]. This suggests that Cryptophyceae (Rhodomonas sp. and Cryptomonas spp.) can proliferate under conditions of low water temperature, low light levels, and low nutrient concentrations [48,52,74]. They are also more adaptive to various environmental changes, such as upstream dams and summer monsoons, than other algae. This explains their annual occurrence and dominance.
4. Conclusions
Despite its geographical importance in the Han River Basin, which is the largest watershed in South Korea, adequate studies on the changes in the aquatic ecology of Lake Uiam are lacking. This study examined seasonal water quality and phytoplankton characteristics in Lake Uiam. The concentrations of organic matter and nutrients generally increased after the summer rainfall. Phytoplankton was represented by 91 species, with green algae accounting for the largest taxon (34 species). Most species (24) were found during the autumn (September–November). Dominant phytoplankton taxa varied seasonally. In autumn, Cryptophyceae (Rhodomonas sp.) were dominant, contrary to the typical trends observed in temperate regions. However, the dominant taxa in spring (Bacillariophyceae (Ulnaria acus)) and summer (Cyanophyceae (Pseudanabaena limnetica)) were similar to those found in temperate climates. According to the CCA, Rhodomonas sp. appeared to predominate in both spring and fall and was negatively associated with water temperature. Cryptophyceae also showed a strong association with Chl-a (0.708 **) in the correlation analysis, demonstrating that it contributes to spring Chl-a concentrations. Cryptophyceae (Rhodomonas spp. and Cryptomonas spp.) typically appear more frequently in spring when the water temperature is low; however, in the case of Lake Uiam, they are also prevalent in the fall. This is based on the results of CCA and seasonal PCA. This divergence from typical phytoplankton succession patterns in temperate regions is attributed to their ability to proliferate under conditions of low water temperature, low light levels, and low nutrient concentrations, which makes them more adaptive than other algae and leads to their occurrence and dominance throughout all seasons.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/w15234118/s1, Table S1: Analytical methods and instruments of water quality parameters.
Author Contributions
Conceptualization, J.-K.I. and Y.-B.S.; methodology, J.-K.I. and Y.-B.S.; software, J.-K.I. and Y.-B.S.; validation, J.-K.I. and Y.-B.S.; formal analysis, J.-K.I. and Y.-B.S.; investigation, J.-K.I., Y.-B.S., S.-J.H., M.-S.B. and T.-G.K.; resources, T.-G.K.; data curation, Y.-B.S.; writing—original draft preparation, J.-K.I. and Y.-B.S.; writing—review and editing, J.-K.I., Y.-B.S., S.-J.H., M.-S.B. and T.-G.K.; visualization, J.-K.I. and Y.-B.S.; supervision, T.-G.K.; project administration, T.-G.K.; funding acquisition, T.-G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Environment Industry and Technology Institute (KEITI) through The Decision Support System Development Project for Environmental Impact Assessment and the National Institute of Environmental Research (NIER) [grant number NIER-2023-01-01-110], funded by the Korea Ministry of Environment (MOE) (No. 2020002990009) and Environmental Fundamental Data Examination Project of Han River Basin Management Committee of the Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, B.C.; Park, J.H.; Hwang, G.S.; Choi, K.S. Eutrophication of Large Freshwater Ecosystems in Korea. Kor. J. Lim. 1998, 15, 25–30. [Google Scholar]
- Shin, J.-K.; Kang, C.-K.; Kim, H.-S.; Hwang, S.-J. Limnological characteristics of the river-type Paltang Reservoir, Korea: Hydrological and environmental factors. Korean J. Ecol. Environ. 2003, 36, 242–256. [Google Scholar]
- Li, J.; Liao, L.; Dai, X. Economic and Agricultural Impacts of Building a Dam—Evidence from Natural Experience of the Three-Gorges Dam. Agriculture 2022, 12, 1372. [Google Scholar] [CrossRef]
- An, K.-G.; Jones, J.R. Factors regulating bluegreen dominance in a reservoir directly influenced by the Asian monsoon. Hydrobiologia 2000, 432, 37–48. [Google Scholar] [CrossRef]
- Jung, S.; Shin, M.; Kim, J.; Eum, J.; Lee, Y.; Lee, J.; Choi, Y.; You, K.; Owen, J.; Kim, B. The effects of Asian summer monsoons on algal blooms in reservoirs. Inland Waters 2016, 6, 406–413. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Kim, B. Simulation of eutrophication in a reservoir by CE-QUAL-W2 for the evaluation of the importance of point sources and summer monsoon. Lake Reserv. Manag. 2019, 35, 64–76. [Google Scholar] [CrossRef]
- Kocer, M.A.T.; ŞEN, B. Some factors affecting the abundance of phytoplankton in an unproductive alkaline lake (Lake Hazar, Turkey). Turk. J. Bot. 2014, 38, 790–799. [Google Scholar] [CrossRef]
- Bhat, N.A.; Wanganeo, A.; Raina, R. Seasonal dynamics of phytoplankton community in a tropical wetland. Environ. Monit. Assess. 2015, 187, 4136. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, S. Characteristics of phytoplankton distribution for assessment of water quality in the Zhalong Wetland, China. Int. J. Environ. Sci. Technol. 2015, 12, 3657–3664. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Chai, F.; Yu, H.; Sun, X.; Liu, D. Phytoplankton community structure dynamics in relation to water environmental factors in Zhalong Wetland. Int. J. Environ. Res. Public Health 2022, 19, 14996. [Google Scholar] [CrossRef]
- Mesquita, M.C.; Prestes, A.C.C.; Gomes, A.M.; Marinho, M.M. Direct effects of temperature on growth of different tropical phytoplankton species. Microb. Ecol. 2020, 79, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marzetz, V.; Spijkerman, E.; Striebel, M.; Wacker, A. Phytoplankton community responses to interactions between light intensity, light variations, and phosphorus supply. Front. Environ. Sci. 2020, 8, 539733. [Google Scholar] [CrossRef]
- Winder, M.; Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- Jankowiak, J.; Hattenrath-Lehmann, T.; Kramer, B.J.; Ladds, M.; Gobler, C.J. Deciphering the effects of nitrogen, phosphorus, and temperature on cyanobacterial bloom intensification, diversity, and toxicity in western Lake Erie. Limnol. Oceanogr. 2019, 64, 1347–1370. [Google Scholar] [CrossRef]
- Verhamme, E.M.; Redder, T.M.; Schlea, D.A.; Grush, J.; Bratton, J.F.; DePinto, J.V. Development of the Western Lake Erie Ecosystem Model (WLEEM): Application to connect phosphorus loads to cyanobacteria biomass. J. Great Lakes Res. 2016, 42, 1193–1205. [Google Scholar] [CrossRef]
- Youn, S.J.; Im, J.K.; Byeon, M.S.; Yu, S.J. Characteristics of cyanobacteria and odorous compounds production in lake uiam and lower gonji stream. J. Environ. Sci. Int. 2019, 35, 99–104. [Google Scholar]
- Byeon, J.H.; You, M.N.; Lee, E.J.; You, S.J.; Kim, B.H.; Byeon, M.S. Temporal and Spatial Distribution of Microbial Community and Odor Compounds in the Bukhan River System. Korean J. Ecol. Environ. 2018, 51, 299–310. [Google Scholar] [CrossRef]
- Byeon, J.H.; Hwang, S.-J.; Kim, B.H.; Park, J.R.; Lee, J.K.; Lim, B.J. Relationship between a Dense Population of Cyanobacteria and Odorous Compounds in the North Han River System in 2014 and 2015. Korean J. Ecol. Environ. 2015, 48, 263–271. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, Y.; Cho, H.; Hwang, S.-J. Molecular probes to evaluate the synthesis and production potential of an odorous compound (2-methylisoborneol) in cyanobacteria. Int. J. Environ. Res. Public Health 2020, 17, 1933. [Google Scholar] [CrossRef]
- Joh, G.J.; Choi, Y.S.; Shin, J.-K.; Lee, J. Problematic algae in the sedimentation and filtration process of water treatment plants. J. Water Supply Res. Technol. AQUA 2011, 60, 219–230. [Google Scholar] [CrossRef]
- Grover, J.P.; Chrzanowski, T.H. Seasonal dynamics of phytoplankton in two warm temperate reservoirs: Association of taxonomic composition with temperature. J. Plankton. Res. 2006, 28, 1–17. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar] [CrossRef]
- Baek, J.-S.; Youn, S.J.; Kim, H.N.; Sim, Y.; You, S.J.; Im, J.K. Effects of Environmental Factors on Phytoplankton Succession and Community Structure in Lake Chuncheon, South Korea. Korean J. Ecol. Environ. 2019, 52, 71–80. [Google Scholar] [CrossRef]
- Youn, S.J.; Kim, H.N.; Im, J.K.; Kim, Y.-J.; Baek, J.-S.; Lee, S.-W.; Lee, E.J.; Yu, S.J. Effect of environmental factors on phytoplankton communities and dominant species succession in Lake Cheongpyeong. J. Environ. Sci. Int. 2017, 26, 913–925. [Google Scholar] [CrossRef]
- Na, E.H.; Park, S.S. A hydrodynamic and water quality modeling study of spatial and temporal patterns of phytoplankton growth in a stratified lake with buoyant incoming flow. Ecol. Modell. 2006, 199, 298–314. [Google Scholar] [CrossRef]
- Ha, S.-Y.; Lee, Y.; Kim, M.-S.; Kumar, K.S.; Shin, K.-H. Seasonal changes in mycosporine-like amino acid production rate with respect to natural phytoplankton species composition. Mar. Drugs 2015, 13, 6740–6758. [Google Scholar] [CrossRef]
- Sim, Y.; Byeon, M.S.; Kim, K.; Yu, S.J.; Im, J.K. Influence of Zooplankton and Environmental Factors on Clear-Water Phase in Lake Paldang, South Korea. Int. J. Environ. Res. Public Health 2021, 18, 7205. [Google Scholar] [CrossRef]
- MOE. Standard Methods for the Examination Water Quality; The Korean Ministry of Environment: Sejong, Republic of Korea, 2014. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Hirose, H.; Yamagishi, T.; Akiyama, M. Illustrations of the Japanese Freshwater Algae; Uchidarokakuho Publ. Co., Ltd.: Tokyo, Japan, 1977; 927p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Naviculaceae. Süßwasserflora von Mitteleuropa; Gustav Fischer Verlag: Stuttgart, Germany, 1986; Volume 1, p. 876. Available online: http://link.springer.com/book/9783827426154 (accessed on 4 September 2023).
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropa, Bd 2/2. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae; Gustav Fischer: Jena, Germany, 1988. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropa, Bd. 02/3: Bacillariophyceae: Teil 3: Centrales, Fragilariaceae, Eunotiaceae; Springer: Berlin/Heidelberg, Germany, 1991; Volume 2. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropa, Bd. 02/4: Bacillariophyceae: Teil 4: Achnanthaceae, Kritische Ergänzungen zu Achnanthes sl, Navicula s. str., Gomphonema, Gesamtliteraturverzeichnis Teil 1-4, Ergänzter Nachdruck, 2004; Spektrum Akademischer Verlag: Heidelberg, Germany, 1999; Available online: http://link.springer.com/book/9783827408389 (accessed on 4 September 2023).
- Morabito, G.; Oggioni, A.; Caravati, E.; Panzani, P. Seasonal morphological plasticity of phytoplankton in Lago Maggiore (N. Italy). Hydrobiologia 2007, 578, 47–57. [Google Scholar] [CrossRef]
- Hu, R.; Han, B.; Naselli-Flores, L. Comparing biological classifications of freshwater phytoplankton: A case study from South China. Hydrobiologia 2013, 701, 219–233. [Google Scholar] [CrossRef]
- El-Zeiny, A.; Elagami, S.A.; Nour-Eldin, H.; El-Halawany, E.-S.F.; Bonanomi, G.; Abd-ElGawad, A.M.; Soufan, W.; El-Amier, Y.A. Wild plant habitat characterization in the last two decades in the Nile Delta coastal region of Egypt. Agriculture 2022, 12, 108. [Google Scholar] [CrossRef]
- Brown, J.D. Principal components analysis and exploratory factor analysis &ndash Definitions, differences, and choices. Statistics 2009, 13, 26–30. [Google Scholar]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Lokhande, S.; Tare, V. Spatio-temporal trends in the flow and water quality: Response of river Yamuna to urbanization. Environ. Monit. Assess. 2021, 193, 117. [Google Scholar] [CrossRef] [PubMed]
- Giao, N.T.; Nhien, H.T.H.; Anh, P.K.; Thuptimdang, P. Combination of water quality, pollution indices, and multivariate statistical techniques for evaluating the surface water quality variation in Can Tho City, Vietnam. Environ. Monit. Assess. 2022, 194, 844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, H.; Gao, D.; Yu, H. Source identification of surface water pollution using multivariate statistics combined with physicochemical and socioeconomic parameters. Sci. Total Environ. 2022, 806, 151274. [Google Scholar] [CrossRef] [PubMed]
- Jargal, N.; An, K.-G. Seasonal and interannual responses of blue-green algal taxa and chlorophyll to a monsoon climate, flow regimes, and N: P ratios in a temperate drinking-water reservoir. Sci. Total Environ. 2023, 896, 165306. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.; Eum, J.; Jung, S.; Choi, Y.; Owen, J.S.; Kim, B. Export of non-point source suspended sediment, nitrogen, and phosphorus from sloping highland agricultural fields in the East Asian monsoon region. Environ. Monit. Assess. 2016, 188, 692. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology; Kellogg Biological Station-Michigan State University: Hickory Corners, MI, USA, 1983. [Google Scholar]
- Reynolds, C.; Wiseman, S.; Godfrey, B.; Butterwick, C. Some effects of artificial mixing on the dynamics of phytoplankton populations in large limnetic enclosures. J. Plankton. Res. 1983, 5, 203–234. [Google Scholar] [CrossRef]
- Sze, P. A Biology of the Algae; WCB/McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Lee, J.-H.; Park, J.-G.; Kim, E.-J. Trophic states and phytoplankton compositions of Dam Lakes in Korea. Algae 2002, 17, 275–281. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R. Effects of light and temperature on the odor production of 2-methylisoborneol-producing Pseudanabaena sp. and geosmin-producing Anabaena ucrainica (cyanobacteria). Biochem. Syst. Ecol. 2015, 58, 219–226. [Google Scholar] [CrossRef]
- Gilabert, J. Seasonal plankton dynamics in a Mediterranean hypersaline coastal lagoon: The Mar Menor. J. Plankton. Res. 2001, 23, 207–218. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Kim, S.-K. The use of winter water temperature and food composition by the copepod Cyclops vicinus (Uljanin, 1875) to provide a temporal refuge from fish predation. Biology 2021, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Lafarga-De la Cruz, F.; Valenzuela-Espinoza, E.; Millán-Núnez, R.; Trees, C.C.; Santamaría-del-Ángel, E.; Núnez-Cebrero, F. Nutrient uptake, chlorophyll a and carbon fixation by Rhodomonas sp.(Cryptophyceae) cultured at different irradiance and nutrient concentrations. Aquac. Eng. 2006, 35, 51–60. [Google Scholar] [CrossRef]
- Krivtsov, V.; Bellinger, E.; Sigee, D. Changes in the elemental composition of Asterionella formosa during the diatom spring bloom. J. Plankton. Res. 2000, 22, 169–184. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton. Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Gsell, A.S.; de Senerpont Domis, L.N.; Przytulska-Bartosiewicz, A.; Mooij, W.M.; van Donk, E.; Ibelings, B.W. Genotype-by-temperature interactions may help to maintain clonal diversity in Asterionella formosa (Bacillariophyceae). J. Phycol. 2012, 48, 1197–1208. [Google Scholar] [CrossRef]
- Vasconcelos, V.M. Species composition and dynamics of the phytoplankton in a recently-commissioned reservoir (Azibo-Portugal). Arch. Hydrobiol. 1991, 121, 67–78. [Google Scholar] [CrossRef]
- Bondarenko, N.; Guselnikova, N.Y. Studies on Synedra acus Kutz. var. radians (Kutz.) Hust.(Bacillariophyta) in culture. Int. J. Algae 2002, 4, 85–95. [Google Scholar]
- Michel, T.J.; Saros, J.E.; Interlandi, S.J.; Wolfe, A.P. Resource requirements of four freshwater diatom taxa determined by in situ growth bioassays using natural populations from alpine lakes. Hydrobiologia 2006, 568, 235–243. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Lai, Z.; Tan, X.; Pang, S.; Yang, W. Seasonal variations of Aulacoseira granulata population abundance in the Pearl River Estuary. Estuar. Coast. Shelf Sci. 2009, 85, 585–592. [Google Scholar] [CrossRef]
- Tsukada, H.; Tsujimura, S.; Nakahara, H. Seasonal succession of phytoplankton in Lake Yogo over 2 years: Effect of artificial manipulation. Limnology 2006, 7, 3–14. [Google Scholar] [CrossRef]
- Kamjunke, N.; Henrichs, T.; Gaedke, U. Phosphorus gain by bacterivory promotes the mixotrophic flagellate Dinobryon spp. during re-oligotrophication. J. Plankton. Res. 2007, 29, 39–46. [Google Scholar] [CrossRef]
- Vadrucci, M.; Barbone, E.; Ungaro, N.; Romano, A.; Bucci, R. Application of taxonomic and morpho-functional properties of phytoplankton communities to water quality assessment for artificial lakes in the Mediterranean Ecoregion. J. Plankton. Res. 2017, 39, 550–563. [Google Scholar] [CrossRef]
- Sabanci, F.Ç.; Koray, T. The dinoflagellat species distributed in izmir Bay (Aegean Sea) and the seasonal changes of species diversity. Rev. Hydrobiol. 2012, 5, 71–84. [Google Scholar]
- Gieskes, W.; Kraay, G. Dominance of Cryptophyceae during the phytoplankton spring bloom in the central North Sea detected by HPLC analysis of pigments. Mar. Biol. 1983, 75, 179–185. [Google Scholar] [CrossRef]
- Kasprzak, P.; Padisák, J.; Koschel, R.; Krienitz, L.; Gervais, F. Chlorophyll a concentration across a trophic gradient of lakes: An estimator of phytoplankton biomass? Limnologica 2008, 38, 327–338. [Google Scholar] [CrossRef]
- Walsby, A. Gas vesicles. Microbiol. Rev. 1994, 58, 94–144. [Google Scholar] [CrossRef]
- Paerl, H.; Fulton, R. Ecology of harmful cyanobacteria. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 95–109. [Google Scholar]
- Agostoni, M.; Waters, C.M.; Montgomery, B.L. Regulation of biofilm formation and cellular buoyancy through modulating intracellular cyclic di-GMP levels in engineered cyanobacteria. Biotechnol. Bioeng. 2016, 113, 311–319. [Google Scholar] [CrossRef]
- Wang, Z.; Akbar, S.; Sun, Y.; Gu, L.; Zhang, L.; Lyu, K.; Huang, Y.; Yang, Z. Cyanobacterial dominance and succession: Factors, mechanisms, predictions, and managements. J. Environ. Manag. 2021, 297, 113281. [Google Scholar] [CrossRef]
- Kromkamp, J.; Konopka, A.; Mur, L.R. Buoyancy Regulation in a Strain of Aphaniz. omenon flos-aquae (Cyanophyceae): The Importance of Carbohydrate Accumulation and Gas Vesicle Collapse. Microbiology 1986, 132, 2113–2121. [Google Scholar] [CrossRef][Green Version]
- Tashiro, Y.; Monson, R.E.; Ramsay, J.P.; Salmond, G.P. Molecular genetic and physical analysis of gas vesicles in buoyant enterobacteria. Environ. Microbiol. 2016, 18, 1264–1276. [Google Scholar] [CrossRef]
- Wei, K.; Amano, Y.; Machida, M.; Asukabe, H.; Harada, K.-i. Effects of light and potassium ion on buoyancy regulation with gas vesicle in a Cyanobacterium Microcystis aeruginosa NIES-843. Water Air Soil Pollut. 2018, 229, 352. [Google Scholar] [CrossRef]
- Stewart, A.J.; Wetzel, R.G. Cryptophytes and other microflagellates as couplers in planktonic community dynamics. Arch. Hydrobiol. 1986, 106, 1–19. [Google Scholar] [CrossRef]
- Hammer, A.; Schumann, R.; Schubert, H. Light and temperature acclimation of Rhodomonas salina (Cryptophyceae): Photosynthetic performance. Aquat. Microb. Ecol. 2002, 29, 287–296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).