The Identification of Selective Pathogenic Microbial Community Biofilms in Different Distribution Pipeline Materials and Their Disinfection Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Biofilm Annular Reactor Setup
2.2. Sample Collection and Physicochemical Analysis of Inlet Water and Outlet Water
2.3. Biofilm Sampling
2.4. Bacterial Load in Bulk Water and Biofilm
2.5. Bacterial Viability Tests
3. Results
3.1. Physicochemical Analysis of Inlet and Outlet Water Samples of ARs
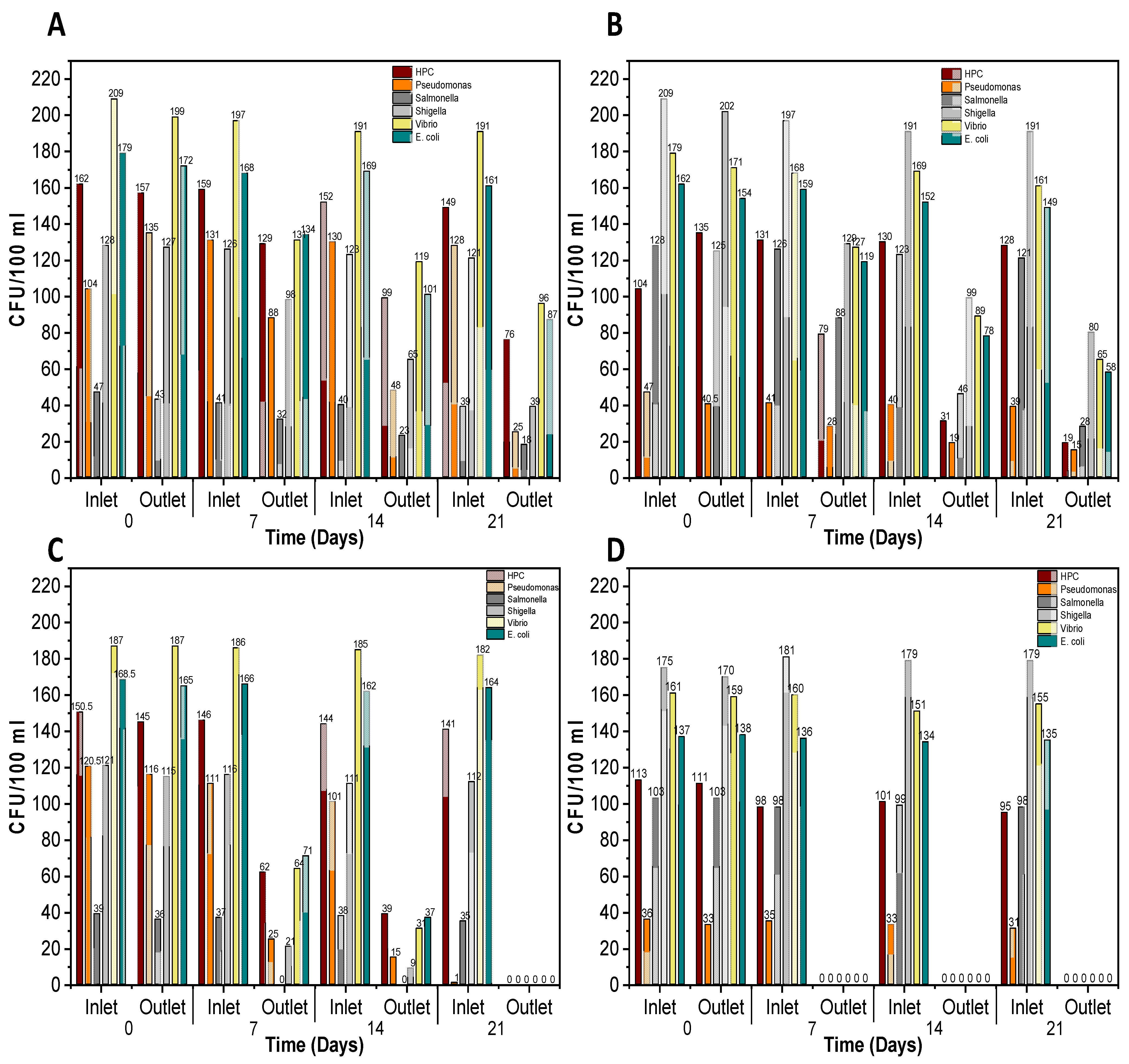
3.2. Bacterial Contamination in Bulk Water Samples
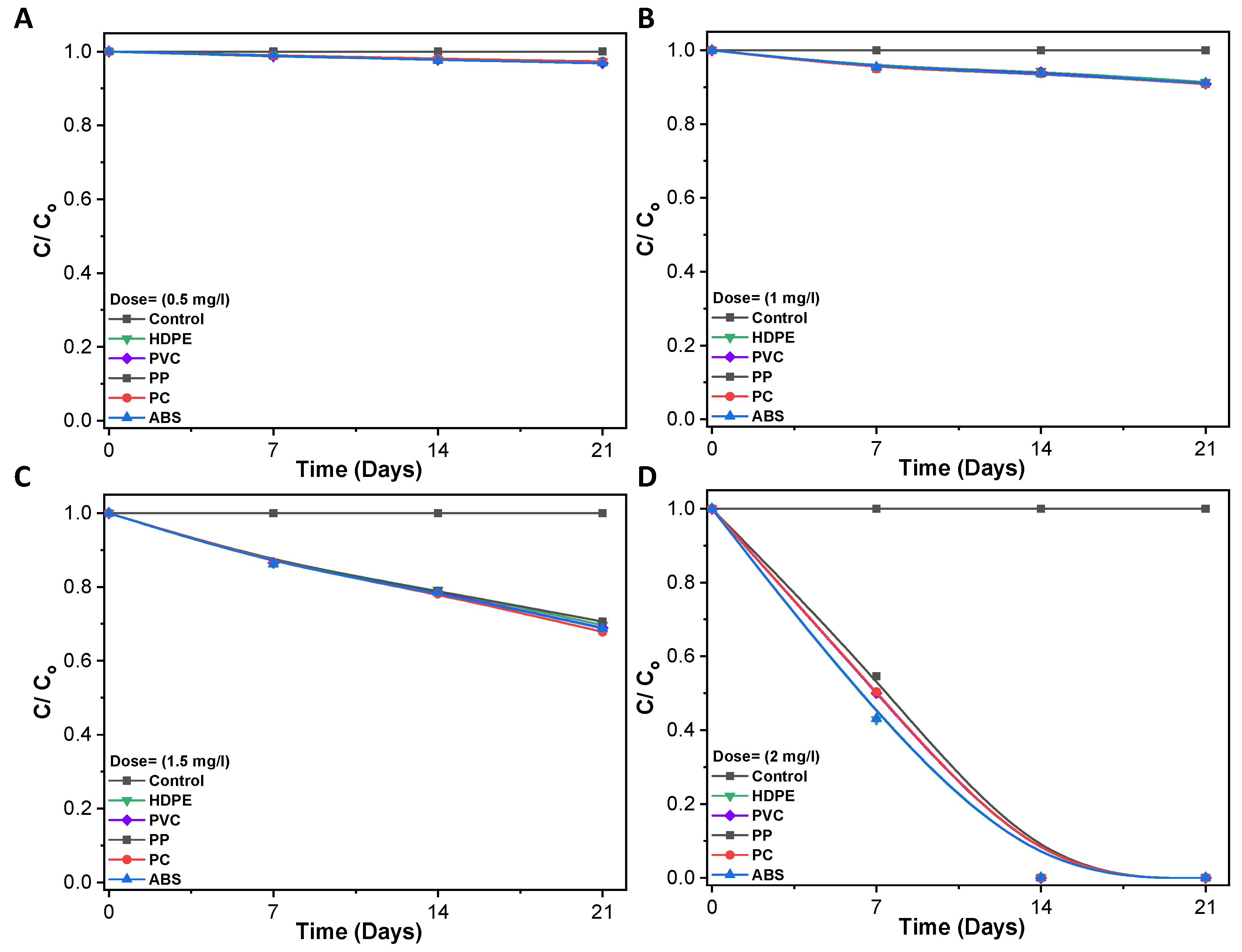
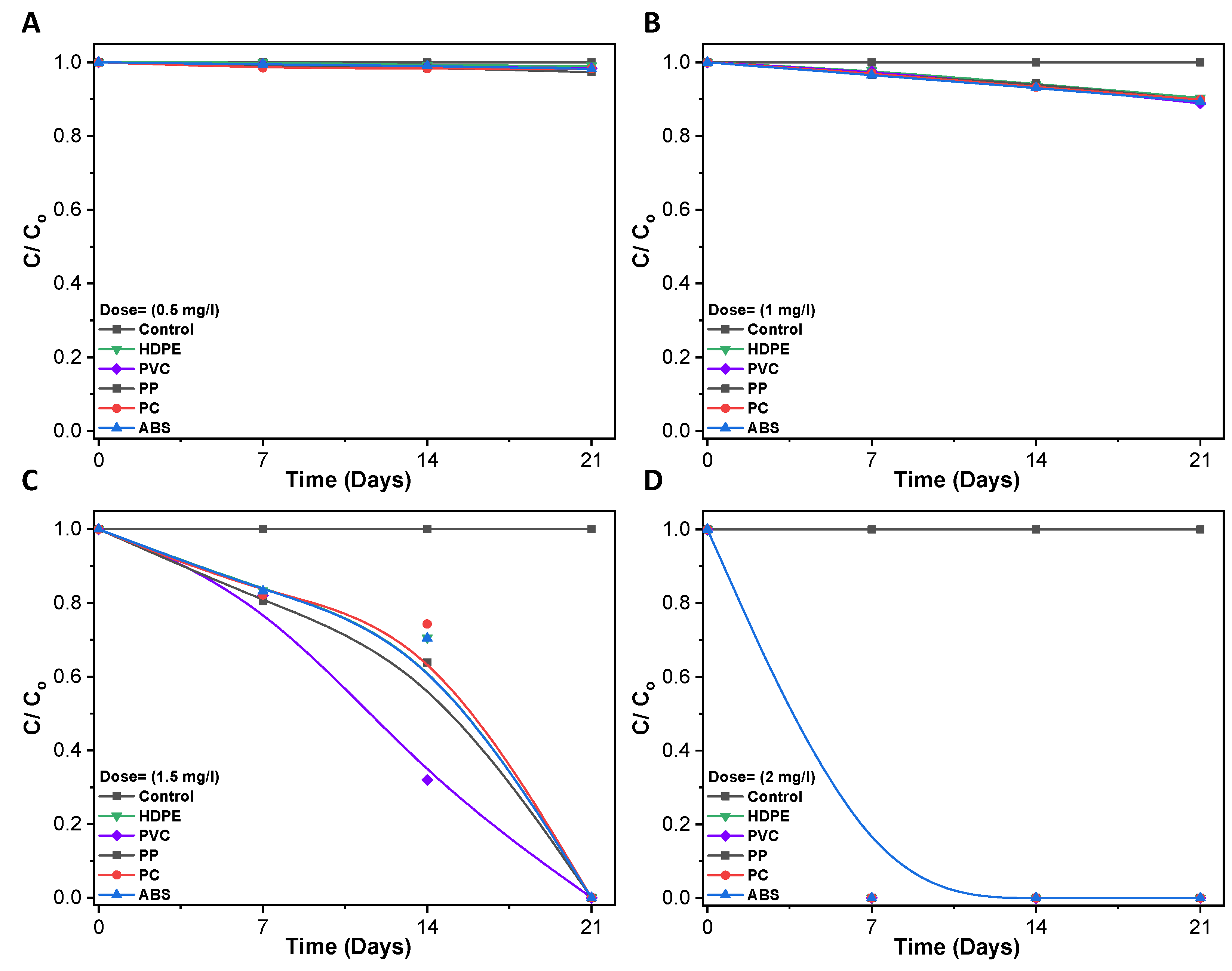
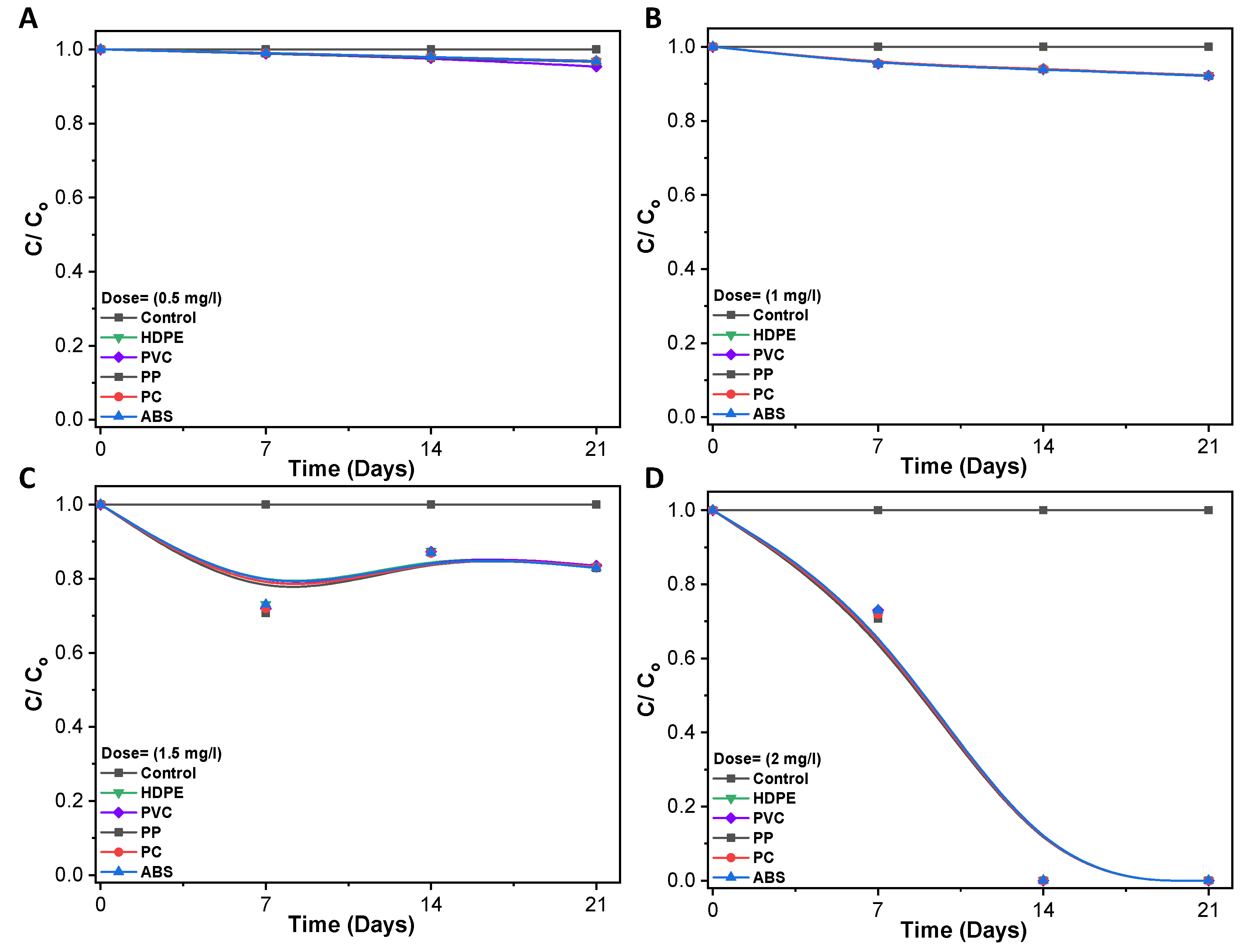
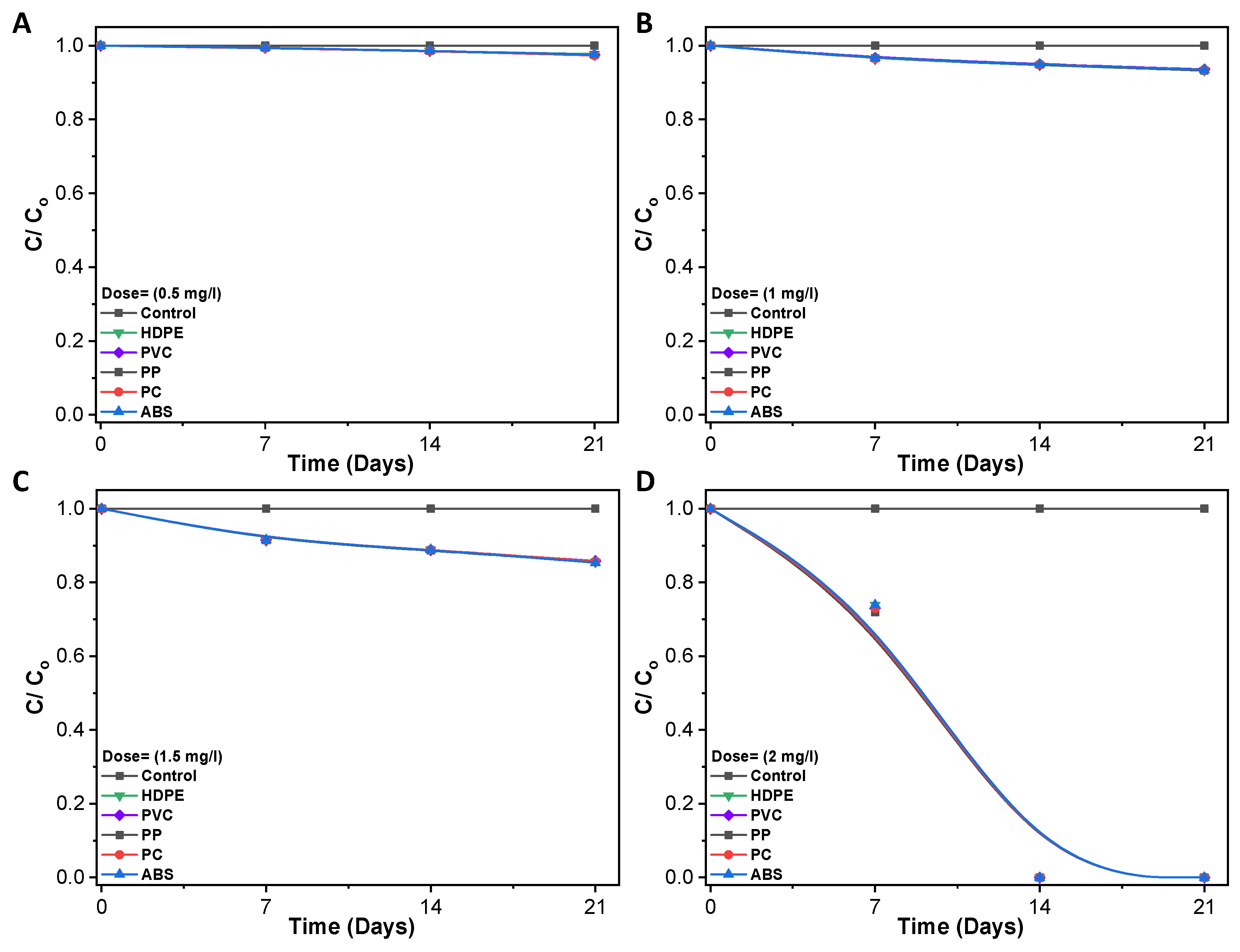
3.3. Disinfection of Pathogenic Bacteria in Biofilm
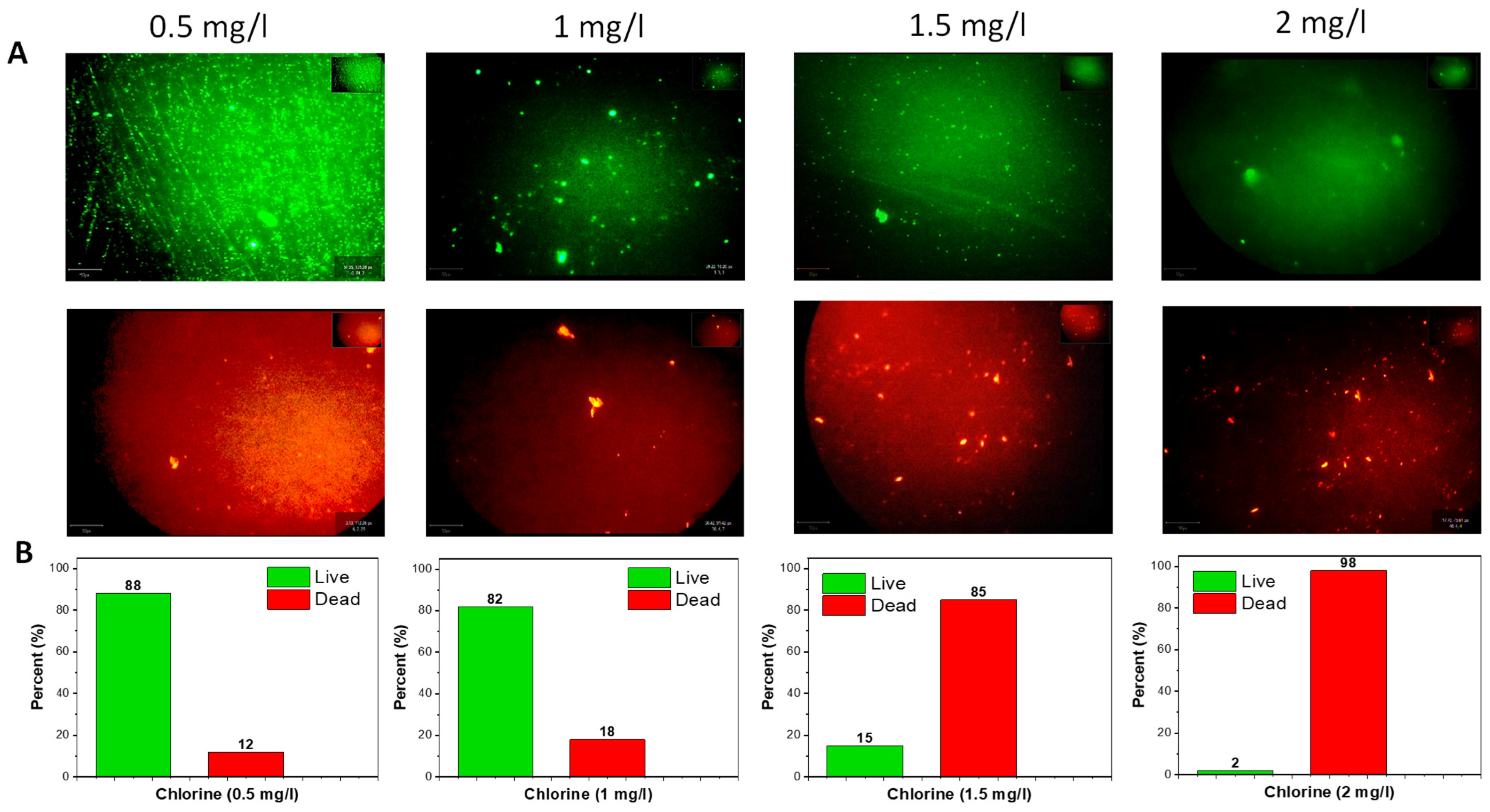
3.4. Bacterial Viability Live/Dead Fluorescence Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, J.; Wong, L.P.; Chua, Y.P.; Channa, N. Drinking Water Quality Mapping Using Water Quality Index and Geospatial Analysis in Primary Schools of Pakistan. Water 2020, 12, 3382. [Google Scholar] [CrossRef]
- Wingender, J.; Flemming, H.C. Biofilms in Drinking Water and Their Role as Reservoir for Pathogens. Int. J. Hyg. Environ. Health 2011, 214, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Masters, S.; Edwards, M.A.; Falkinham, J.O.; Pruden, A. Effect of Disinfectant, Water Age, and Pipe Materials on Bacterial and Eukaryotic Community Structure in Drinking Water Biofilm. Environ. Sci. Technol. 2014, 48, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Kumpel, E.; Nelson, K.L. Intermittent Water Supply: Prevalence, Practice, and Microbial Water Quality. Environ. Sci. Technol. 2016, 50, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, M.J.; Juhna, T.; Miettinen, I.T.; Vartiainen, T.; Martikainen, P.J. Formation of Biofilms in Drinking Water Distribution Networks, a Case Study in Two Cities in Finland and Latvia. J. Ind. Microbiol. Biotechnol. 2004, 31, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, B.A.; El-Taweel, G.E.; Goswami, P.; Pant, D.; Sevda, S. The Role of Biofilm in the Development and Dissemination of Ubiquitous Pathogens in Drinking Water Distribution Systems: An Overview of Surveillance, Outbreaks, and Prevention. World J. Microbiol. Biotechnol. 2021, 37, 36. [Google Scholar] [CrossRef] [PubMed]
- Morvay, A.A.; Decun, M.; Scurtu, M.; Sala, C.; Morar, A.; Sarandan, M. Biofilm Formation on Materials Commonly Used in Household Drinking Water Systems. Water Supply 2011, 11, 252–257. [Google Scholar] [CrossRef]
- Bai, X.; Wu, F.; Zhou, B.; Zhi, X. Biofilm Bacterial Communities and Abundance in a Full-Scale Drinking Water Distribution System in Shanghai. J. Water Health 2010, 8, 593–600. [Google Scholar] [CrossRef]
- Norwood, D.E.; Gilmour, A. The Growth and Resistance to Sodium Hypochlorite of Listeria Monocytogenes in a Steady-State Multispecies Biofilm. J. Appl. Microbiol. 2000, 88, 512–520. [Google Scholar] [CrossRef]
- Cowle, M.W.; Webster, G.; Babatunde, A.O.; Bockelmann-Evans, B.N.; Weightman, A.J. Impact of Flow Hydrodynamics and Pipe Material Properties on Biofilm Development within Drinking Water Systems. Environ. Technol. 2020, 41, 3732–3744. [Google Scholar] [CrossRef]
- Momba, M.N.B.; Makala, N. Comparing the Effect of Various Pipe Materials on Biofilm Formation in Chlorinated and Combined Chlorine-Chloraminated Water Systems. Water SA 2004, 30, 175–182. [Google Scholar] [CrossRef][Green Version]
- Hyun-Jung, J.; Choi, Y.J.; Ka, J.O. Effects of Diverse Water Pipe Materials on Bacterial Communities and Water Quality in the Annular Reactor. J. Microbiol. Biotechnol. 2011, 21, 115–123. [Google Scholar] [CrossRef]
- Yu, J.; Kim, D.; Lee, T. Microbial Diversity in Biofilms on Water Distribution Pipes of Different Materials. Water Sci. Technol. 2010, 61, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Konrat, K.; Schwebke, I.; Laue, M.; Dittmann, C.; Levin, K.; Andrich, R.; Arvand, M.; Schaudinn, C. The Bead Assay for Biofilms: A Quick, Easy and Robust Method for Testing Disinfectants. PLoS ONE 2016, 11, e0157663. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Dai, Y.; Xie, S.; Chen, C.; Zhang, X. Impact of Disinfection on Drinking Water Biofilm Bacterial Community. J. Environ. Sci. 2015, 37, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.E.; Boxall, J.B. Biofilm Microbiome (Re)Growth Dynamics in Drinking Water Distribution Systems Are Impacted by Chlorine Concentration. Front. Microbiol. 2018, 9, 408432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, S.F.; Li, Y.; Xu, H.; Qin, L.; Tay, J.H. The Influence of Cell and Substratum Surface Hydrophobicities on Microbial Attachment. J. Biotechnol. 2004, 110, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Zacheus, O.M.; Iivanainen, E.K.; Nissinen, T.K.; Lehtola, M.J.; Martikainen, P.J. Bacterial Biofilm Formation on Polyvinyl Chloride, Polyethylene and Stainless Steel Exposed to Ozonated Water. Water Res. 2000, 34, 63–70. [Google Scholar] [CrossRef]
- Stefan, D.S.; Bosomoiu, M.; Teodorescu, G. The Behavior of Polymeric Pipes in Drinking Water Distribution System—Comparison with Other Pipe Materials. Polymers 2023, 15, 3872. [Google Scholar] [CrossRef]
- Lu, P.; Chen, C.; Wang, Q.; Wang, Z.; Zhang, X.; Xie, S. Phylogenetic Diversity of Microbial Communities in Real Drinking Water Distribution Systems. Biotechnol. Bioprocess Eng. 2013, 18, 119–124. [Google Scholar] [CrossRef]
- Sun, W.; Jing, Z. Migration of Rare and Abundant Species, Assembly Mechanisms, and Ecological Networks of Microbiomes in Drinking Water Treatment Plants: Effects of Different Treatment Processes. J. Hazard. Mater. 2023, 457, 131726. [Google Scholar] [CrossRef] [PubMed]
- Fayadoglu, M.; Fayadoglu, E.; Er, S.; Koparal, A.T.; Koparal, A.S. Determination of Biological Activities of Nanoparticles Containing Silver and Copper in Water Disinfection with/without Ultrasound Technique. J. Environ. Health Sci. Eng. 2022, 21, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wu, C.; Zhong, D.; Yuan, Y.; Shan, L.; Zhang, J. Effects of Pipe Materials on Chlorine-Resistant Biofilm Formation under Long-Term High Chlorine Level. Appl. Biochem. Biotechnol. 2014, 173, 1564–1578. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, Q.; Le, Y.; Chen, G.; Tong, Z.; Xu, Q.; Wang, G. Chlorination-Mediated EPS Excretion Shapes Early-Stage Biofilm Formation in Drinking Water Systems. Process Biochem. 2017, 55, 41–48. [Google Scholar] [CrossRef]
- Tsagkari, E.; Sloan, W.T. Turbulence Accelerates the Growth of Drinking Water Biofilms. Bioprocess. Biosyst. Eng. 2018, 41, 757–770. [Google Scholar] [CrossRef]
- Butterfield, P.W.; Camper, A.K.; Ellis, B.D.; Jones, W.L. Chlorination of Model Drinking Water Biofilm: Implications for Growth and Organic Carbon Removal. Water Res. 2002, 36, 4391–4405. [Google Scholar] [CrossRef]
- Jia, S.; Jia, R.; Zhang, K.; Sun, S.; Lu, N.; Wang, M.; Zhao, Q. Disinfection Characteristics of Pseudomonas Peli, a Chlorine-Resistant Bacterium Isolated from a Water Supply Network. Environ. Res. 2020, 185, 109417. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Kang, M.; Chen, C.; Song, Y.; Li, H. Effects of Chlorination/Chlorine Dioxide Disinfection on Biofilm Bacterial Community and Corrosion Process in a Reclaimed Water Distribution System. Chemosphere 2019, 215, 62–73. [Google Scholar] [CrossRef]
- Guo, X.; Liu, S.; Zhou, X.; Hu, H.; Zhang, K.; Du, X.; Peng, X.; Ren, B.; Cheng, L.; Li, M. Effect of D-Cysteine on Dual-Species Biofilms of Streptococcus Mutans and Streptococcus Sanguinis. Sci. Rep. 2019, 9, 6689. [Google Scholar] [CrossRef]
- Channa, N.; Gadhi, T.A.; Mahar, R.B.; Chiadò, A.; Bonelli, B.; Tagliaferro, A. Combined Photocatalytic Degradation of Pollutants and Inactivation of Waterborne Pathogens Using Solar Light Active α/β-Bi2O3. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126214. [Google Scholar] [CrossRef]
- Gadhi, T.A.; Qureshi, A.; Channa, N.; Mahar, R.B.; Chiadò, A.; Novara, C.; Tagliaferro, A. Bi2O3/Nylon Multilayered Nanocomposite Membrane for the Photocatalytic Inactivation of Waterborne Pathogens and Degradation of Mixed Organic Pollutants. Environ. Sci. Nano 2021, 8, 342–355. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Chen, C.; Zhang, X.J.; Zheng, Q.; Liu, Y.Y. Inactivation of Resistant Mycobacteria Mucogenicum in Water: Chlorine Resistance and Mechanism Analysis. Biomed. Environ. Sci. 2012, 25, 230–237. [Google Scholar] [CrossRef] [PubMed]
| S. No. | Dosage (mg/L) | Acclimatization (Weeks) | Disinfectant | Dosage Time (Weeks) |
|---|---|---|---|---|
| 1 | 0.5 | 8 | Chlorine | 4 |
| 2 | 1.0 | 8 | Chlorine | 4 |
| 3 | 1.5 | 8 | Chlorine | 4 |
| 4 | 2.0 | 8 | Chlorine | 4 |
| Dose | Water | Collection Time | Temp. (°C) | pH | TDS (mg/L) | EC (µS/cm) | Salinity (mg/L) | Turbidity (NTU) |
|---|---|---|---|---|---|---|---|---|
| 0.5 mg/L | Inlet | BD | 31 ± 1 | 7.9 ± 0.38 | 425.1 ± 137.7 | 654 ± 211.9 | 0.2 ± 0.18 | 11.6 ± 7.4 |
| AD | 30 ± 1 | 7.9 ± 0.05 | 383.7 ± 16.5 | 590.3 ± 25.4 | 0.3 ± 0.1 | 4 ± 0.42 | ||
| Outlet | BD | 30 ± 0 | 8.1 ± 0 | 351 ± 23.9 | 540 ± 36.8 | 0.15 ± 0.07 | 3.1 ± 0.6 | |
| AD | 30 ± 1 | 7.9 ± 0.1 | 370.5 ± 19.2 | 570 ± 29.6 | 0.3 ± 0.1 | 3 ± 0.3 | ||
| 1.0 mg/L | Inlet | BD | 31 ± 1 | 7.9 ± 0.38 | 425.1 ± 137.7 | 654 ± 211.9 | 0.2 ± 0.18 | 11.6 ± 7.4 |
| AD | 30 ± 1 | 7.9 ± 0.05 | 383.7 ± 16.5 | 590.3 ± 25.4 | 0.3 ± 0.1 | 4 ± 0.42 | ||
| Outlet | BD | 30 ± 0 | 7.95 ± 0.2 | 352.95 ± 23.9 | 543 ± 36.8 | 0.15 ± 0.07 | 2.55 ± 0.47 | |
| AD | 30 ± 1 | 7.9 ± 0.1 | 372.0 ± 18 | 572.3 ± 28 | 0.26 ± 0.05 | 2.78 ± 0.4 | ||
| 1.5 mg/L | Inlet | BD | 25 ± 4.2 | 8.1 ± 0.1 | 555.7 ± 61.6 | 855 ± 94.8 | 0.45 ± 0.07 | 4.86 ± 1.27 |
| AD | 22.3 ± 1.53 | 7.9 ± 0.06 | 596.5 ± 61.5 | 917.7 ± 94.7 | 0.46 ± 0.06 | 7.03 ± 1.06 | ||
| Outlet | BD | 22 ± 0 | 8.1 ± 0 | 596.1 ± 0 | 917 ± 0 | 0.5 ± 0 | 3.78 ± 0 | |
| AD | 22.3 ± 1.53 | 7.9 ± 0 | 720.4 ± 1.71.4 | 1108.3 ± 263.7 | 0.47 ± 0.06 | 5.38 ± 1.19 | ||
| 2.0 mg/L | Inlet | BD | 19.8 ± 0.21 | 8 ± 0 | 646.1 ± 16.5 | 994 ± 25.5 | 0.45 ± 0.07 | 5.9 ± 0.81 |
| AD | 19.7 ± 0.7 | 8.07 ± 0.1 | 849.9 ± 192.1 | 1307.7 ± 295.5 | 0.53 ± 0.06 | 3.88 ± 0.87 | ||
| Outlet | BD | 19.8 ± 0 | 8.1 ± 0 | 637 ± 0 | 980 ± 0 | 0.4 ± 0 | 3.92 ± 0 | |
| AD | 19.8 ± 0.8 | 7.9 ± 0.1 | 756.4 ± 139.2 | 1163.7 ± 214.1 | 0.53 ± 0.11 | 3.85 ± 1.14 |
| Disinfection Dose | Free Residual Chlorine | |||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | |
| 0.5 mg/L | 0 | 0 | 0 | 0 |
| 1 mg/L | 0.0 | 0.01 | 0.04 | 0.1 |
| 1.5 mg/L | 0.4 | 0.10 | 0.17 | 0.20 |
| 2 mg/L | 0.22 | 0.37 | 0.48 | 0.69 |
| Dosage | HPC | Pseudomonas | Salmonella | Shigella | Vibrio | E. coli |
|---|---|---|---|---|---|---|
| 0.5 mg/L | 0.10 | 0.19 | 0.07 | 0.13 | 0.06 | 0.08 |
| 1.0 mg/L | 0.16 | 0.05 | 0.12 | 0.07 | 0.09 | 0.09 |
| 1.5 mg/L | 0.06 | 0.16 | 0.04 | 0.05 | 0.06 | 0.07 |
| 2.0 mg/L | 0.05 | 0.04 | 0.06 | 0.05 | 0.06 | 0.06 |
| Time (Days) | 0.5 mg/L | 1.0 mg/L | 1.5 mg/L | 2.0 mg/L |
|---|---|---|---|---|
| 0 | 0.8226 | 0.0028 | 0.0266 | 0.1318 |
| 7 | 0.0183 | 0.0101 | 0.0033 | 0.0091 |
| 14 | 0.0062 | 0.0051 | 0.0066 | 0.0090 |
| 21 | 0.0065 | 0.0054 | 0.0490 | 0.0098 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatti, S.; Gadhi, T.A.; Mahar, R.B.; Ali, I.; Channa, N.; Kori, J.A.; Bonelli, B. The Identification of Selective Pathogenic Microbial Community Biofilms in Different Distribution Pipeline Materials and Their Disinfection Kinetics. Water 2023, 15, 4099. https://doi.org/10.3390/w15234099
Bhatti S, Gadhi TA, Mahar RB, Ali I, Channa N, Kori JA, Bonelli B. The Identification of Selective Pathogenic Microbial Community Biofilms in Different Distribution Pipeline Materials and Their Disinfection Kinetics. Water. 2023; 15(23):4099. https://doi.org/10.3390/w15234099
Chicago/Turabian StyleBhatti, Sanam, Tanveer A. Gadhi, Rasool Bux Mahar, Imran Ali, Najeebullah Channa, Junaid Ahmed Kori, and Barbara Bonelli. 2023. "The Identification of Selective Pathogenic Microbial Community Biofilms in Different Distribution Pipeline Materials and Their Disinfection Kinetics" Water 15, no. 23: 4099. https://doi.org/10.3390/w15234099
APA StyleBhatti, S., Gadhi, T. A., Mahar, R. B., Ali, I., Channa, N., Kori, J. A., & Bonelli, B. (2023). The Identification of Selective Pathogenic Microbial Community Biofilms in Different Distribution Pipeline Materials and Their Disinfection Kinetics. Water, 15(23), 4099. https://doi.org/10.3390/w15234099









