Abatement of Nitrophenol in Aqueous Solution by HOCl and UV/HOCl Processes: Kinetics, Mechanisms, and Formation of Chlorinated Nitrogenous Byproducts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Chlorination Experiments
2.3. UV/HOCl Experiments
2.4. HPLC Analyses
2.5. Quantification of TCNM
2.6. Identification of Intermediate Products
2.7. Evaluate the Influence of Wastewater
3. Results and Discussions
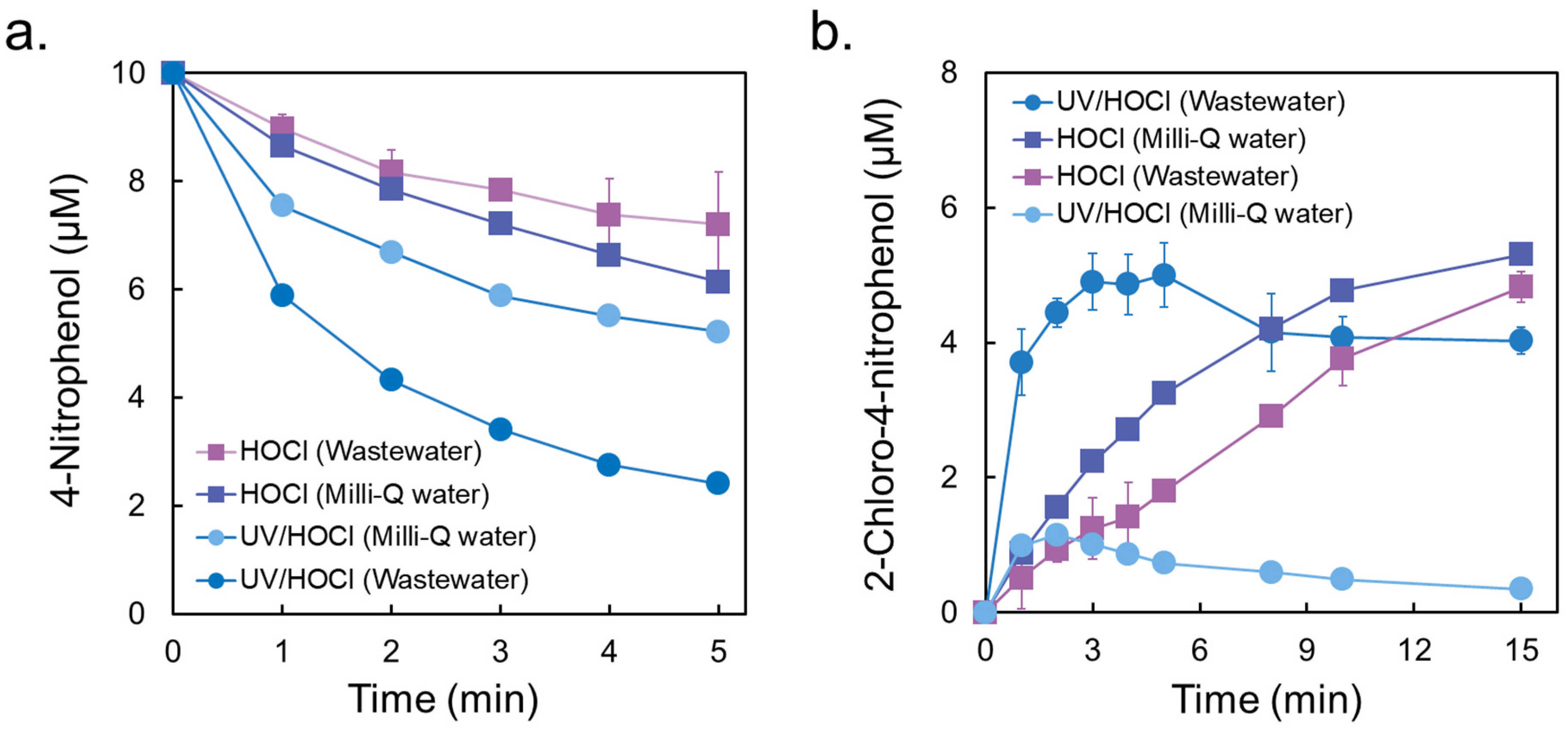
3.1. Degradation Kinetics
3.2. Formation of Chlorinated Nitrophenols
3.3. Formation Potential of TCNM
3.4. Intermediate Products
3.5. Transformation Mechanisms and Pathways
3.6. Effects of Wastewater Matrix
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, F.; Zhu, L.Z.; Chen, H.R. Effects of pH on the chlorination process of phenols in drinking water. J. Hazard. Mater. 2006, 133, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Abou Mehrez, O.; Dossier-Berne, F.; Legube, B. Chlorination and chloramination of aminophenols in aqueous solution: Oxidant demand and by-product formation. Environ. Technol. 2015, 36, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Hu, X.X.; Wang, H.B.; Hu, C. Synergistic effect of the sequential use of UV irradiation and chlorine to disinfect reclaimed water. Water Res. 2012, 46, 1225–1232. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Wu, Y.T.; Zhu, S.M.; Sun, J.L.; Bu, L.J.; Dionysiou, D.D. Nitrogen conversion from ammonia to trichloronitromethane: Potential risk during UV/chlorine process. Water Res. 2020, 172, 115508. [Google Scholar] [CrossRef]
- Deng, L.; Luo, W.; Chi, X.; Huang, T.T.; Wen, L.J.; Dong, H.Y.; Wu, M.X.; Hu, J. Formation of halonitromethanes from methylamine in the presence of bromide during UV/Cl2 disinfection. J. Environ. Sci. 2022, 117, 28–36. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef]
- Li, G.Q.; Huo, Z.Y.; Wu, Q.Y.; Lu, Y.; Hu, H.Y. Synergistic effect of combined UV-LED and chlorine treatment on Bacillus subtilis spore inactivation. Sci. Total Environ. 2018, 639, 1233–1240. [Google Scholar] [CrossRef]
- Remucal, C.K.; Manley, D. Emerging investigators series: The efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 565–579. [Google Scholar] [CrossRef]
- Cai, W.W.; Peng, T.; Yang, B.; Xu, C.; Liu, Y.S.; Zhao, J.L.; Gu, F.L.; Ying, G.G. Kinetics and mechanism of reactive radical mediated fluconazole degradation by the UV/chlorine process: Experimental and theoretical studies. Chem. Eng. J. 2020, 402, 126224. [Google Scholar] [CrossRef]
- Watts, M.J.; Linden, K.G. Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Water Res. 2007, 41, 2871–2878. [Google Scholar] [CrossRef]
- Wu, Z.H.; Fang, J.Y.; Xiang, Y.Y.; Shang, C.; Li, X.C.; Meng, F.G.; Yang, X. Roles of reactive chlorine species in trimethoprim degradation in the UV/chlorine process: Kinetics and transformation pathways. Water Res. 2016, 104, 272–282. [Google Scholar] [CrossRef]
- Mártire, D.O.; Rosso, J.A.; Bertolotti, S.; Le Roux, G.C.; Braun, A.M.; Gonzalez, M.C. Kinetic study of the reactions of chlorine atoms and Cl2•− radical anions in aqueous solutions. II. Toluene, benzoic acid, and chlorobenzene. J. Phys. Chem. A 2001, 105, 5385–5392. [Google Scholar] [CrossRef]
- Lei, Y.; Cheng, S.S.; Luo, N.; Yang, X.; An, T.C. Rate Constants and Mechanisms of the Reactions of Cl• and Cl•− with Trace Organic Contaminants. Environ. Sci. Technol. 2019, 53, 11170–11182. [Google Scholar] [CrossRef]
- Fang, J.Y.; Fu, Y.; Shang, C. The Roles of Reactive Species in Micropollutant Degradation in the UV/Free Chlorine System. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.H.; Lu, Z.J.; Tang, Q.; Huang, X.L.; Ruan, H.Z.; Jiang, W.; Chen, Y.Q.; Liu, Z.Z.; Kang, J.X.; Liu, D.Q. UV-LED/chlorine degradation of propranolol in water: Degradation pathway and product toxicity. Chemosphere 2020, 248, 125957. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hou, Z.C.; Li, Y.J.; Lei, Y.J.; Xu, Z.H.; Gu, J.J.; Tian, S.L. Modeling degradation kinetics of gemfibrozil and naproxen in the UV/chlorine system: Roles of reactive species and effects of water matrix. Water Res. 2021, 202, 117445. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.J.; Jiang, J.; Ma, J.; Yang, Y.; Liu, W.L.; Liu, Y.L. Degradation of atrazine by UV/chlorine: Efficiency, influencing factors, and products. Water Res. 2016, 90, 15–23. [Google Scholar] [CrossRef]
- Yeom, Y.J.; Han, J.R.; Zhang, X.R.; Shang, C.; Zhang, T.; Li, X.Y.; Duan, X.D.; Dionysiou, D.D. A review on the degradation efficiency, DBP formation, and toxicity variation in the UV/chlorine treatment of micropollutants. Chem. Eng. J. 2021, 424, 130053. [Google Scholar] [CrossRef]
- Yin, R.; Ling, L.; Shang, C. Wavelength-dependent chlorine photolysis and subsequent radical production using UV-LEDs as light sources. Water Res. 2018, 142, 452–458. [Google Scholar] [CrossRef]
- Feng, Y.G.; Smith, D.W.; Bolton, J.R. Photolysis of aqueous free chlorine species (NOCl and OCl−) with 254 nm ultraviolet light. J. Environ. Eng. Sci. 2007, 6, 277–284. [Google Scholar] [CrossRef]
- Cooper, W.J.; Jones, A.C.; Whitehead, R.F.; Zika, R.G. Sunlight-induced photochemical decay of oxidants in natural waters: Implications in ballast water treatment. Environ. Sci. Technol. 2007, 41, 3728–3733. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jain, T.; Ishida, K.; Liu, H.Z. A mechanistic understanding of the degradation of trace organic contaminants by UV/hydrogen peroxide, UV/persulfate and UV/free chlorine for water reuse. Environ. Sci. Water Res. Technol. 2017, 3, 128–138. [Google Scholar] [CrossRef]
- Magara, Y.; Aizawa, T.; Matumoto, N.; Souna, F. Degradation of pesticides by chlorination during water-purification. Water Sci. Technol. 1994, 30, 119–128. [Google Scholar] [CrossRef]
- Deborde, M.; von Gunten, U. Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanisms: A critical review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef] [PubMed]
- Duirk, S.E.; Bridenstine, D.R.; Leslie, D.C. Reaction of benzophenone UV filters in the presence of aqueous chlorine: Kinetics and chloroform formation. Water Res. 2013, 47, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Li, J.; Xu, L. Aqueous chlorination of fenamic acids: Kinetic study, transformation products identification and toxicity prediction. Chemosphere 2017, 175, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Criquet, J.; Rodriguez, E.M.; Allard, S.; Wellauer, S.; Salhi, E.; Joll, C.A.; von Gunten, U. Reaction of bromine and chlorine with phenolic compounds and natural organic matter extracts—Electrophilic aromatic substitution and oxidation. Water Res. 2015, 85, 476–486. [Google Scholar] [CrossRef]
- Wang, D.; Hua, Z.C.; Cui, Y.L.; Dong, Z.J.; Li, C.; Fang, J.Y. Probing into the mechanisms of disinfection by-product formation from natural organic matter and model compounds after UV/chlorine treatment. Environ. Sci. Water Res. Technol. 2023, 9, 1587–1598. [Google Scholar] [CrossRef]
- Vione, D.; Maurino, V.; Minero, C.; Calza, P.; Pelizzetti, E. Phenol chlorination and photochlorination in the presence of chloride ions in homogeneous aqueous solution. Environ. Sci. Technol. 2005, 39, 5066–5075. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Igbinosa, E.O. Chlorophenols and other related derivatives of environmental concern: Properties, distribution and microbial degradation processes. Chemosphere 2011, 83, 1297–1306. [Google Scholar] [CrossRef]
- Wennrich, L.; Popp, P.; Möder, M. Determination of chlorophenols in soils using accelerated solvent extraction combined with solid-phase microextraction. Anal. Chem. 2000, 72, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Bellar, T.A.; Lichtenberg, J.J.; Kroner, R.C. The Occurrence of Organohalides in Chlorinated Drinking Waters. J. Am. Water Work. Assoc. 1974, 66, 703–706. [Google Scholar] [CrossRef]
- Singer, P.C. Control of Disinfection by-Products in Drinking-Water. J. Environ. Eng. 1994, 120, 727–744. [Google Scholar] [CrossRef]
- Postigo, C.; Richardson, S.D. Transformation of pharmaceuticals during oxidation/disinfection processes in drinking water treatment. J. Hazard. Mater. 2014, 279, 461–475. [Google Scholar] [CrossRef]
- Ma, X.Y.; Deng, J.; Feng, J.; Shanaiah, N.; Smiley, E.; Dietrich, A.M. Identification and characterization of phenylacetonitrile as a nitrogenous disinfection byproduct derived from chlorination of phenylalanine in drinking water. Water Res. 2016, 102, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Qu, D.X.; Bu, L.J.; Zhu, S.M.; Zhou, S.Q. Enhanced trichloronitromethane formation during chlorine-UV treatment of nitrite-containing water by organic amines. Sci. Total Environ. 2022, 853, 158304. [Google Scholar] [CrossRef]
- Plewa, M.J.; Wagner, E.D.; Jazwierska, P.; Richardson, S.D.; Chen, P.H.; McKague, A.B. Halonitromethane drinking water disinfection byproducts: Chemical characterization and mammalian cell cytotoxicity and genotoxicity. Environ. Sci. Technol. 2004, 38, 62–68. [Google Scholar] [CrossRef]
- Yin, J.B.; Wu, B.; Zhang, X.X.; Xian, Q.M. Comparative toxicity of chloro- and bromo-nitromethanes in mice based on a metabolomic method. Chemosphere 2017, 185, 20–28. [Google Scholar] [CrossRef]
- Chen, H.F.; Yin, J.B.; Zhu, M.J.; Cao, C.; Gong, T.T.; Xian, Q.M. Cold on-column injection coupled with gas chromatography/mass spectrometry for determining halonitromethanes in drinking water. Anal. Methods 2016, 8, 362–370. [Google Scholar] [CrossRef]
- Wang, J.J.; Li, Z.G.; Hu, S.Y.; Ma, J.; Gong, T.T.; Xian, Q.M. Formation and influence factors of halonitromethanes in chlorination of nitro-aromatic compounds. Chemosphere 2021, 278, 130497. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Huang, C.H.; Wang, Y.L. Effects of Combined UV and Chlorine Treatment on the Formation of Trichloronitromethane from Amine Precursors. Environ. Sci. Technol. 2014, 48, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shen, Q.Q.; Guo, W.H.; Peng, J.F.; Liang, Y.M. Precursors and nitrogen origins of trichloronitromethane and dichloroacetonitrile during chlorination/chloramination. Chemosphere 2012, 88, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Han, J.R.; Zhang, X.R.; Jiang, J.Y.; Li, W.X. How Much of the Total Organic Halogen and Developmental Toxicity of Chlorinated Drinking Water Might Be Attributed to Aromatic Halogenated DBPs? Environ. Sci. Technol. 2021, 55, 5906–5916. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.T.; Zhang, X.R. Comparative Developmental Toxicity of New Aromatic Halogenated DBPs in a Chlorinated Saline Sewage Effluent to the Marine Polychaete Platynereis dumerilii. Environ. Sci. Technol. 2013, 47, 10868–10876. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.A.; Richardson, S.D.; Zhang, X.R.; Gonsior, M. High-molecular-weight by-products of chlorine disinfection. Nat. Water 2023, 1, 336–347. [Google Scholar] [CrossRef]
- Ye, J.; Singh, A.; Ward, O.P. Biodegradation of nitroaromatics and other nitrogen-containing xenobiotics. World J. Microbiol. Biotechnol. 2004, 20, 117–135. [Google Scholar] [CrossRef]
- Podeh, M.R.H.; Bhattacharya, S.K.; Qu, M.B. Effects of nitrophenols on acetate utilizing methanogenic systems. Water Res. 1995, 29, 391–399. [Google Scholar] [CrossRef]
- Ahmed, E.; Nagaoka, K.; Fayez, M.; Abdel-Daim, M.M.; Samir, H.; Watanabe, G. Suppressive effects of long-term exposure to P-nitrophenol on gonadal development, hormonal profile with disruption of tissue integrity, and activation of caspase-3 in male Japanese quail (Coturnix japonica). Environ. Sci. Pollut. Res. 2015, 22, 10930–10942. [Google Scholar] [CrossRef]
- Di Paola, A.; Augugliaro, V.; Palmisano, L.; Pantaleo, G.; Savinov, E. Heterogeneous photocatalytic degradation of nitrophenols. J. Photochem. Photobiol. A 2003, 155, 207–214. [Google Scholar] [CrossRef]
- Daneshvar, N.; Behnajady, M.A.; Asghar, Y.Z. Photooxidative degradation of 4-nitrophenol (4-NP) in UV/H2O2 process: Influence of operational parameters and reaction mechanism. J. Hazard. Mater. 2007, 139, 275–279. [Google Scholar] [CrossRef]
- Chen, C.Y.; Du, Y.; Zhou, Y.J.; Wu, Q.Y.; Zheng, S.S.; Fang, J.Y. Formation of nitro(so) and chlorinated products and toxicity alteration during the UV/monochloramine treatment of phenol. Water Res. 2021, 194, 116914. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Guo, K.H.; Fang, J.Y.; Yang, X.Q.; Xiao, H.; Hou, S.D.; Kong, X.J.; Shang, C.; Yang, X.; Meng, F.A.; et al. Factors affecting the roles of reactive species in the degradation of micropollutants by the UV/chlorine process. Water Res. 2017, 126, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.Y.; Fang, J.Y.; Shang, C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water Res. 2016, 90, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Q.; Gao, N.Y.; Chen, J.X.; Zhang, J.; Yin, D.Q. Oxidation of β-blocker atenolol by a combination of UV light and chlorine: Kinetics, degradation pathways and toxicity assessment. Sep. Purif. Technol. 2020, 231, 115927. [Google Scholar] [CrossRef]
- Canonica, S.; Meunier, L.; Von Gunten, U. Phototransformation of selected pharmaceuticals during UV treatment of drinking water. Water Res. 2008, 42, 121–128. [Google Scholar] [CrossRef]
- USEPA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998.
- Bordwell, F.G.; Cheng, J.P. Substituent effects on the stabilities of phenoxyl radicals and the acidities of phenoxyl radical cations. J. Am. Chem. Soc. 1991, 113, 1736–1743. [Google Scholar] [CrossRef]
- Guo, K.H.; Wu, Z.H.; Shang, C.; Yao, B.; Hou, S.D.; Yang, X.; Song, W.H.; Fang, J.Y. Radical Chemistry and Structural Relationships of PPCP Degradation by UV/Chlorine Treatment in Simulated Drinking Water. Environ. Sci. Technol. 2017, 51, 10431–10439. [Google Scholar] [CrossRef]
- Gilbert, B.C.; Stell, J.K.; Peet, W.J.; Radford, K.J. Generation and Reactions of the Chlorine Atom in Aqueous Solution. J. Chem. Soc. Faraday Trans. 1 1988, 84, 3319–3330. [Google Scholar] [CrossRef]
- Negreira, N.; Regueiro, J.; de Alda, M.L.; Barceló, D. Transformation of tamoxifen and its major metabolites during water chlorination: Identification and in silico toxicity assessment of their disinfection byproducts. Water Res. 2015, 85, 199–207. [Google Scholar] [CrossRef]
- Nika, M.C.; Bletsou, A.A.; Koumaki, E.; Noutsopoulos, C.; Mamais, D.; Stasinakis, A.S.; Thomaidis, N.S. Chlorination of benzothiazoles and benzotriazoles and transformation products identification by LC-HR-MS/MS. J. Hazard. Mater. 2017, 323, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Merlet, N.; Thibaud, H.; Dore, M. Chloropicrin formation during oxidative treatments in the preparation of drinking-water. Sci. Total Environ. 1985, 47, 223–228. [Google Scholar] [CrossRef]
- Yuan, R.X.; Ramjaun, S.N.; Wang, Z.H.; Liu, J.S. Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process: Implications for formation of chlorinated aromatic compounds. J. Hazard. Mater. 2011, 196, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, J.; Manasfi, T.; von Gunten, U. Chlorination and bromination of olefins: Kinetic and mechanistic aspects. Water Res. 2020, 187, 116424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Li, Y.Y.; Lu, J.H.; Zhou, L.; Chovelon, J.M.; Zhou, Q.S.; Ji, Y.F. UV/H2O2 oxidation of chloronitrobenzenes in waters revisited: Hydroxyl radical induced self-nitration. J. Photochem. Photobiol. A 2021, 410, 113162. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Dzengel, J.; Theurich, J.; Bahnemann, D.W. Formation of nitroaromatic compounds in advanced oxidation processes: Photolysis versus photocatalysis. Environ. Sci. Technol. 1999, 33, 294–300. [Google Scholar] [CrossRef]
- Wu, Y.T.; Bu, L.J.; Duan, X.D.; Zhu, S.M.; Kong, M.H.; Zhu, N.Y.; Zhou, S.Q. Mini review on the roles of nitrate/nitrite in advanced oxidation processes: Radicals transformation and products formation. J. Clean. Prod. 2020, 273, 123065. [Google Scholar] [CrossRef]
- Zhao, X.L.; Zhang, T.; Lu, J.H.; Zhou, L.; Chovelon, J.M.; Ji, Y.F. Formation of chloronitrophenols upon sulfate radical-based oxidation of 2-chlorophenol in the presence of nitrite. Environ. Pollut. 2020, 261, 114242. [Google Scholar] [CrossRef]
- Augusto, O.; Bonini, M.G.; Amanso, A.M.; Linares, E.; Santos, C.C.X.; De Menezes, S.L. Nitrogen dioxide and carbonate radical anion: Two emerging radicals in biology. Free Radic. Biol. Med. 2002, 32, 841–859. [Google Scholar] [CrossRef]
- Ji, Y.F.; Shi, Y.Y.; Yang, Y.; Yang, P.Z.; Wang, L.; Lu, J.H.; Li, J.H.; Zhou, L.; Ferronato, C.; Chovelon, J.M. Rethinking sulfate radical-based oxidation of nitrophenols: Formation of toxic polynitrophenols, nitrated biphenyls and diphenyl ethers. J. Hazard. Mater. 2019, 361, 152–161. [Google Scholar] [CrossRef]
- Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E. Aqueous atmospheric chemistry: Formation of 2,4-dinitrophenol upon nitration of 2-nitrophenol and 4-nitrophenol in solution. Environ. Sci. Technol. 2005, 39, 7921–7931. [Google Scholar] [CrossRef] [PubMed]
- Keen, O.S.; Love, N.G.; Linden, K.G. The role of effluent nitrate in trace organic chemical oxidation during UV disinfection. Water Res. 2012, 46, 5224–5234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Roddick, F.A.; Fan, L.H. Direct and indirect photolysis of seven micropollutants in secondary effluent from a wastewater lagoon. Chemosphere 2017, 185, 297–308. [Google Scholar] [CrossRef]
- Guo, K.H.; Wu, Z.H.; Yan, S.W.; Yao, B.; Song, W.H.; Hua, Z.C.; Zhang, X.W.; Kong, X.J.; Li, X.C.; Fang, J.Y. Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements. Water Res. 2018, 147, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; dos Santos, M.M.; Ding, X.X.; Labanowski, J.; Gombert, B.; Snyder, S.A.; Croué, J.P. Impact of EfOM in the elimination of PPCPs by UV/chlorine: Radical chemistry and toxicity bioassays. Water Res. 2021, 204, 117634. [Google Scholar] [CrossRef]
- Wang, W.L.; Wu, Q.Y.; Huang, N.; Wang, T.; Hu, H.Y. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species. Water Res. 2016, 98, 190–198. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
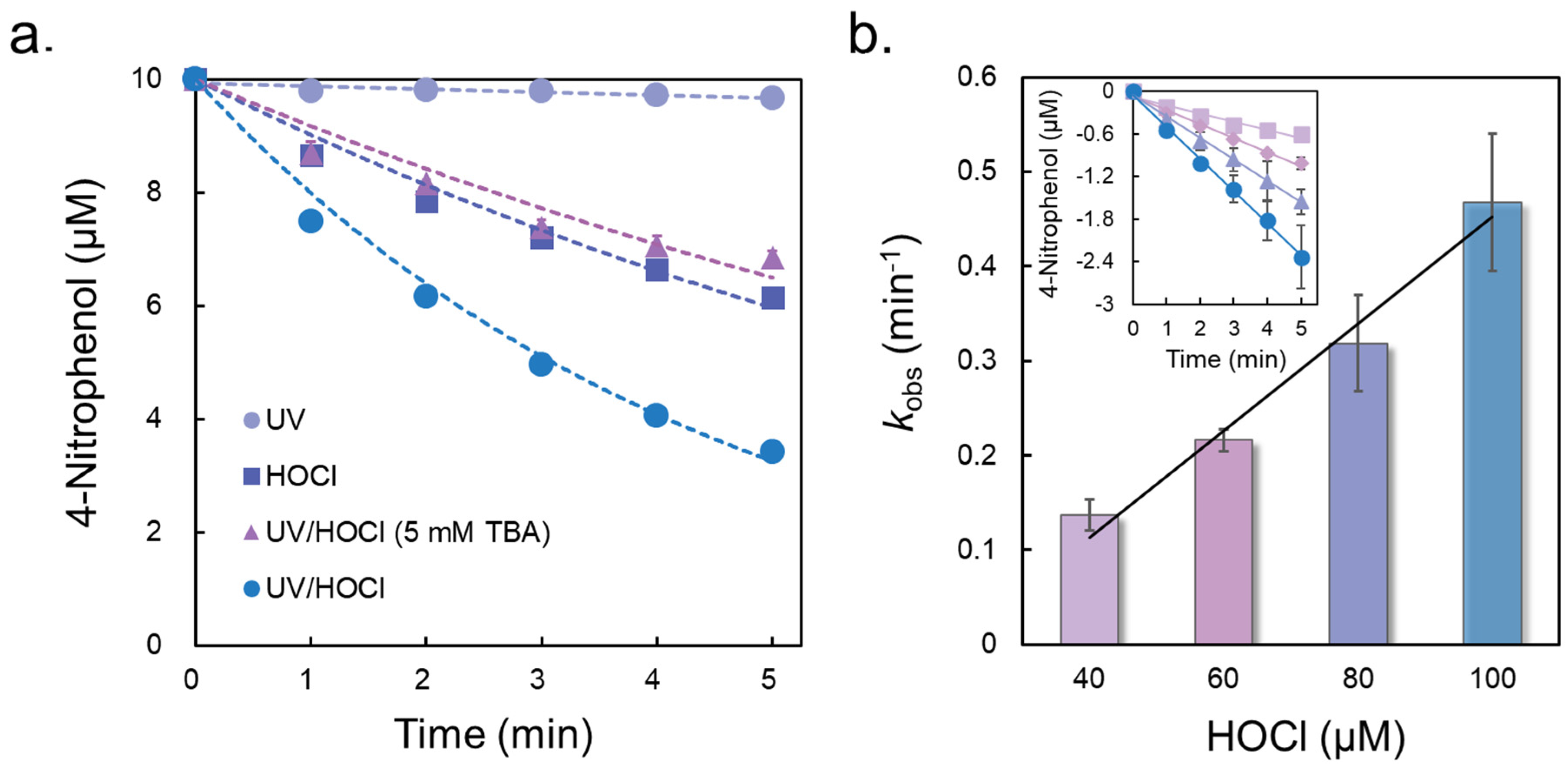
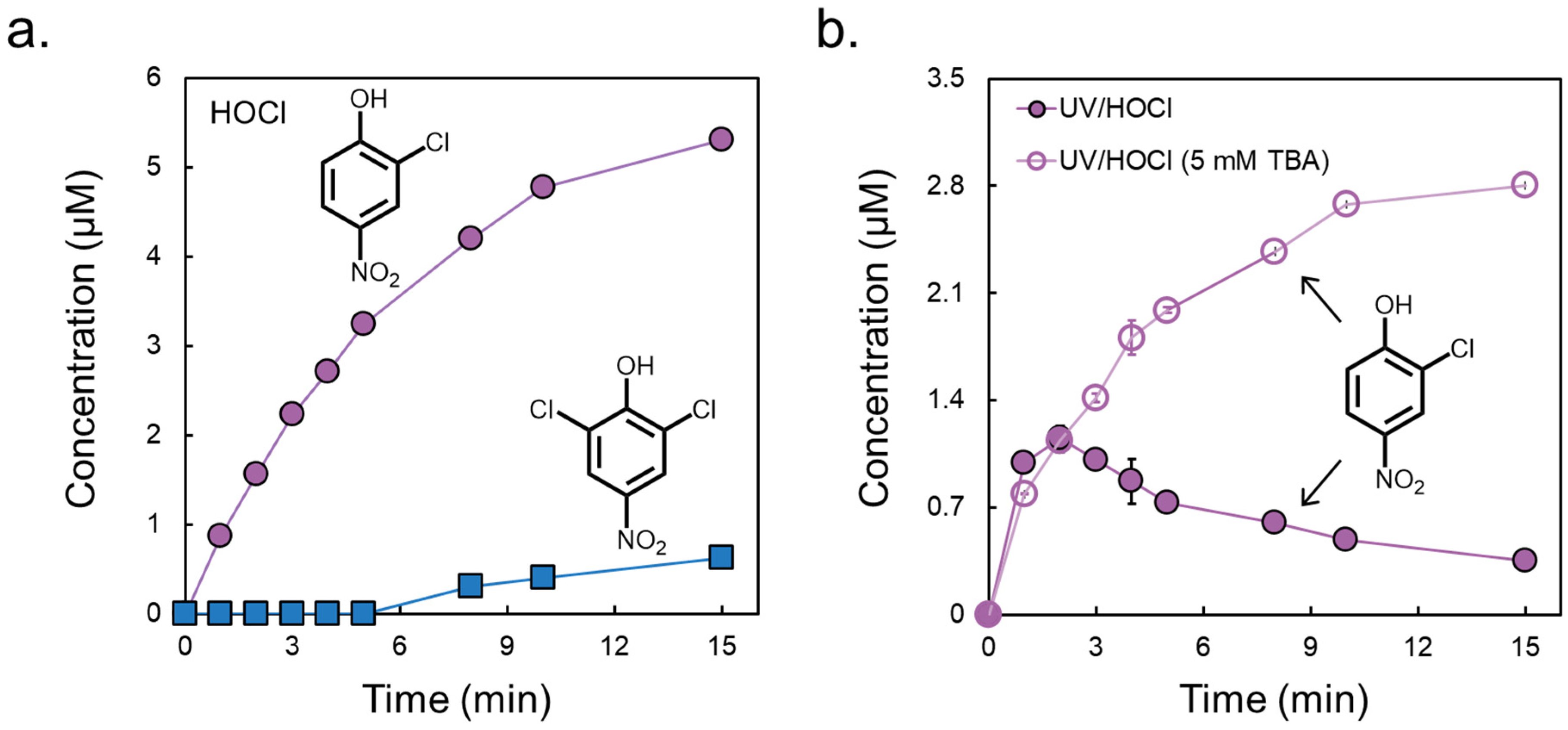
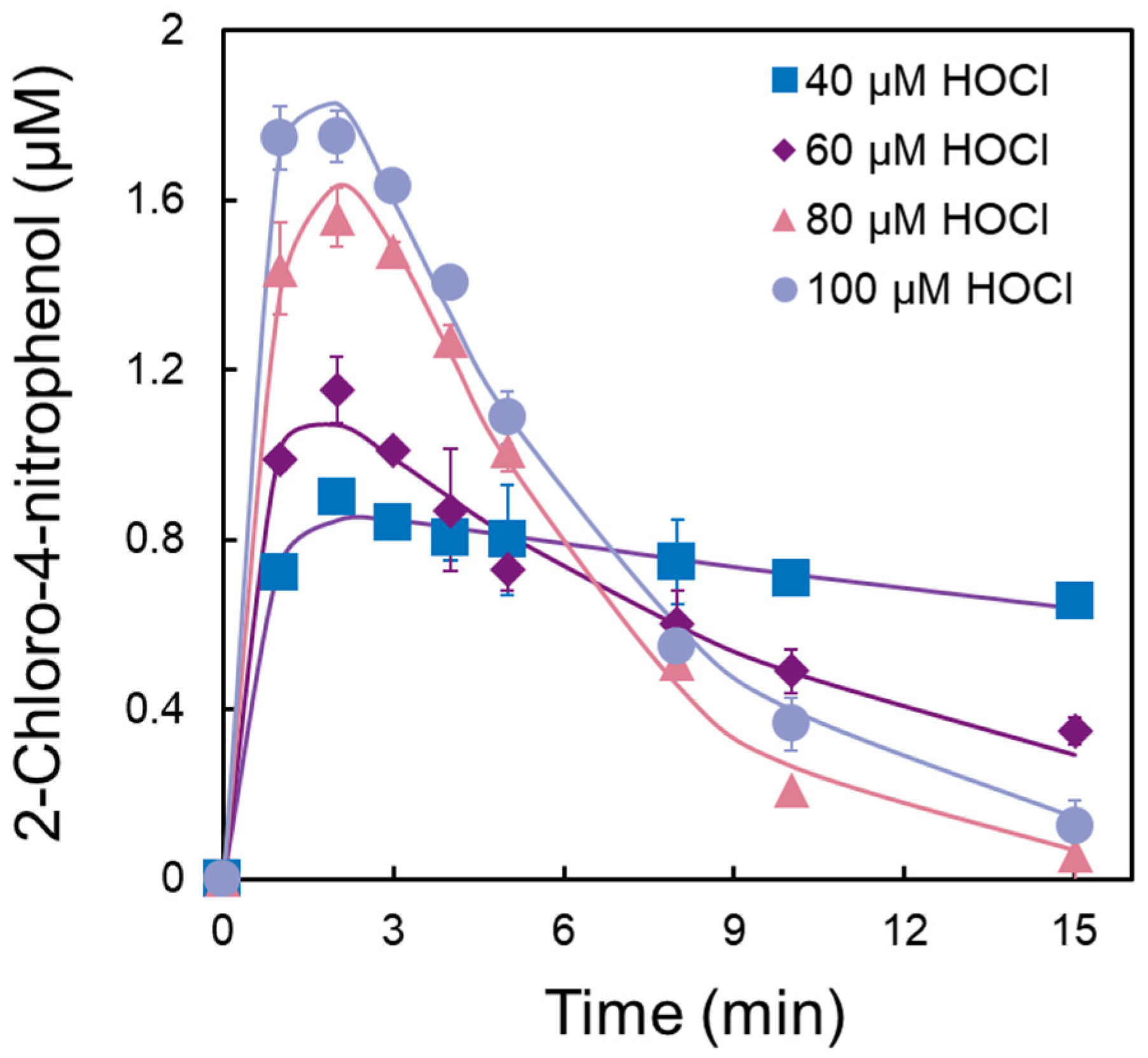

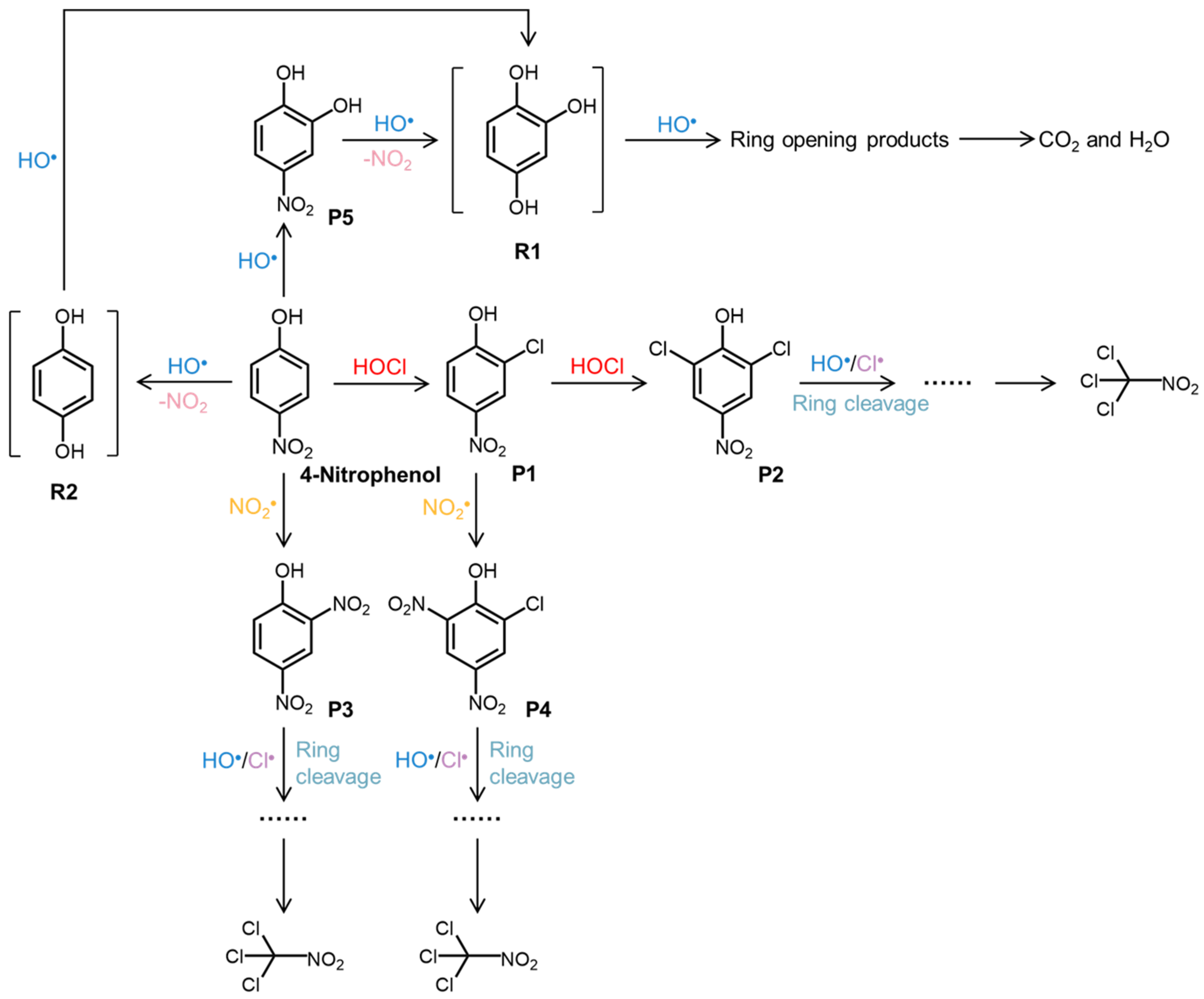
| Concentration of HOCl (μM) | k1 (min−1) | k2 (min−1) |
|---|---|---|
| 40 | 1.801 | 0.024 |
| 60 | 1.872 | 0.102 |
| 80 | 0.868 | 0.271 |
| 100 | 1.409 | 0.201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Cai, Y.; Lu, J.; Chovelon, J.-M.; Chen, J.; Jiang, C.; Ji, Y. Abatement of Nitrophenol in Aqueous Solution by HOCl and UV/HOCl Processes: Kinetics, Mechanisms, and Formation of Chlorinated Nitrogenous Byproducts. Water 2023, 15, 4038. https://doi.org/10.3390/w15234038
Li X, Cai Y, Lu J, Chovelon J-M, Chen J, Jiang C, Ji Y. Abatement of Nitrophenol in Aqueous Solution by HOCl and UV/HOCl Processes: Kinetics, Mechanisms, and Formation of Chlorinated Nitrogenous Byproducts. Water. 2023; 15(23):4038. https://doi.org/10.3390/w15234038
Chicago/Turabian StyleLi, Xiaoci, Yan Cai, Junhe Lu, Jean-Marc Chovelon, Jing Chen, Canlan Jiang, and Yuefei Ji. 2023. "Abatement of Nitrophenol in Aqueous Solution by HOCl and UV/HOCl Processes: Kinetics, Mechanisms, and Formation of Chlorinated Nitrogenous Byproducts" Water 15, no. 23: 4038. https://doi.org/10.3390/w15234038
APA StyleLi, X., Cai, Y., Lu, J., Chovelon, J.-M., Chen, J., Jiang, C., & Ji, Y. (2023). Abatement of Nitrophenol in Aqueous Solution by HOCl and UV/HOCl Processes: Kinetics, Mechanisms, and Formation of Chlorinated Nitrogenous Byproducts. Water, 15(23), 4038. https://doi.org/10.3390/w15234038










