Rapid Antibiotic Adsorption from Water Using MCM-41-Based Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mesoporous MCM-41 Demolding Treatment
2.3. Adsorption Experiments on Three Kinds of Pollutants
3. Results
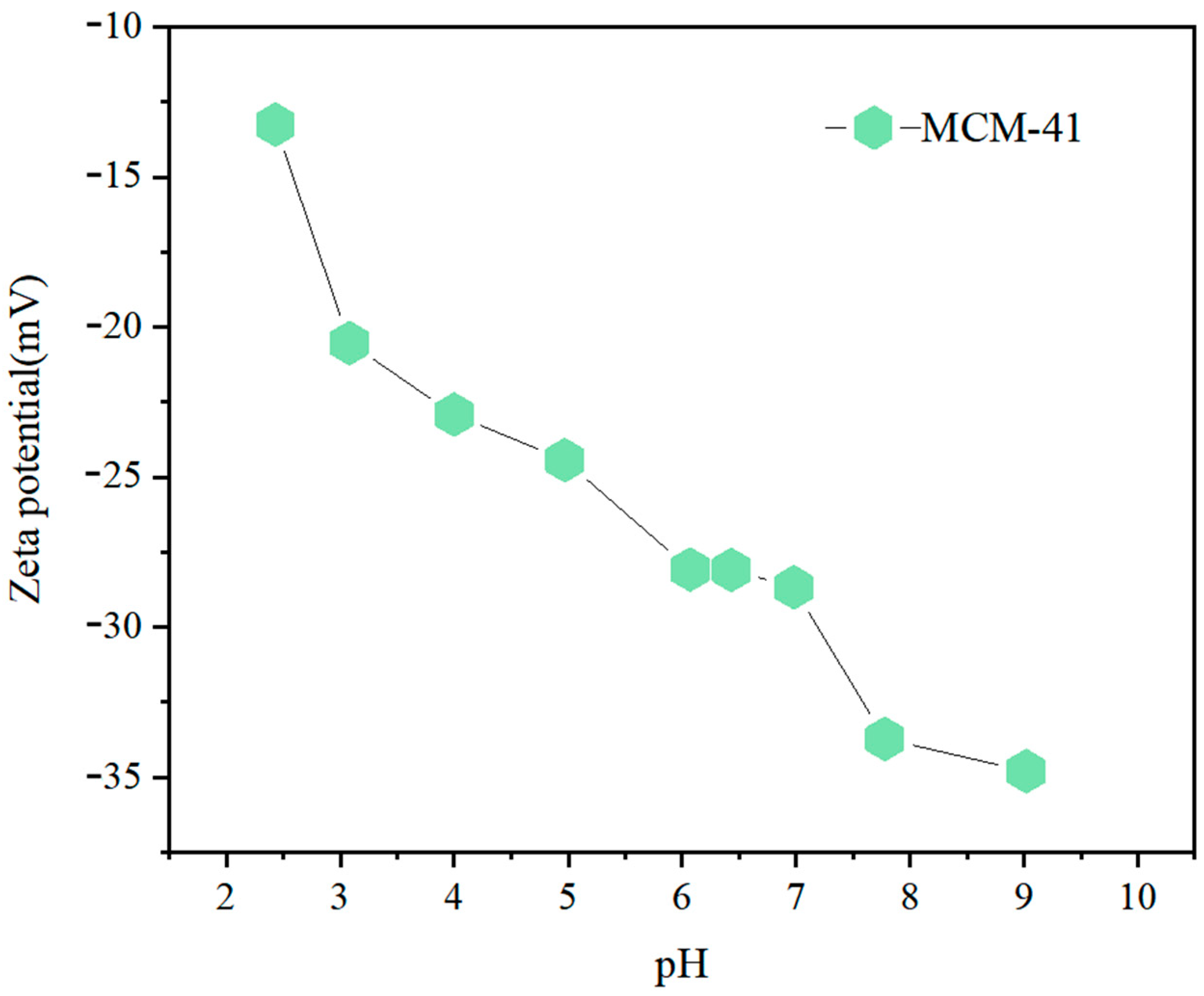
3.1. Physicochemical Properties of MCM-41
3.2. Adsorption of Antibiotics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Xu, S.; Zhao, K.; Song, G.; Zhao, S.; Liu, R. Risk control of antibiotics, antibiotic resistance genes (ARGs) and antibiotic resistant bacteria (ARB) during sewage sludge treatment and disposal: A review. Sci. Total Environ. 2023, 877, 162772. [Google Scholar] [CrossRef]
- Apreja, M.; Sharma, A.; Balda, S.; Kataria, K.; Capalash, N.; Sharma, P. Antibiotic residues in environment: Antimicrobial resistance development, ecological risks, and bioremediation. Environ. Sci. Pollut. Res. 2021, 29, 3355–3371. [Google Scholar] [CrossRef]
- Kalli, M.; Noutsopoulos, C.; Mamais, D. The Fate and Occurrence of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes during Advanced Wastewater Treatment and Disinfection: A Review. Water 2023, 15, 2084. [Google Scholar] [CrossRef]
- McClain, J.B.L.; Ballou, W.R.; Harrison, S.M.; Steinweg, D.L. Doxycycline therapy for leptospirosis. Ann. Intern. Med. 1984, 100, 696–698. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Parmar, P.R.; Basak, S.; Dubey, K.K.; Sutradhar, S.; Bandyopadhyay, D.; Chakrabarti, S. Metal organic framework-derived recyclable magnetic coral Co@Co3O4/C for adsorptive removal of antibiotics from wastewater. Environ. Sci. Pollut. Res. 2023, 30, 50520–50536. [Google Scholar] [CrossRef] [PubMed]
- Mohy-U-Din, N.; Farhan, M.; Wahid, A.; Ciric, L.; Sharif, F. Human health risk estimation of antibiotics transferred from wastewater and soil to crops. Environ. Sci. Pollut. Res. 2023, 30, 20601–20614. [Google Scholar] [CrossRef]
- Zhou, C.-S.; Cao, G.-L.; Wu, X.-K.; Liu, B.-F.; Qi, Q.-Y.; Ma, W.-L. Removal of antibiotic resistant bacteria and genes by nanoscale zero-valent iron activated persulfate: Implication for the contribution of pH decrease. J. Hazard. Mater. 2023, 452, 131343. [Google Scholar] [CrossRef] [PubMed]
- Ajduković, M.; Stevanović, G.; Marinović, S.; Mojović, Z.; Banković, P.; Radulović, K.; Jović-Jovičić, N. Ciprofloxacin Adsorption onto a Smectite-Chitosan-Derived Nanocomposite Obtained by Hydrothermal Synthesis. Water 2023, 15, 2608. [Google Scholar] [CrossRef]
- Raper, E.; Stephenson, T.; Anderson, D.R.; Fisher, R.; Soares, A. Industrial wastewater treatment through bioaugmentation. Process Saf. Environ. Prot. 2018, 118, 178–187. [Google Scholar] [CrossRef]
- Hasan, R.; Chong, C.; Setiabudi, H.; Jusoh, R.; Jalil, A. Process optimization of methylene blue adsorption onto eggshell-treated palm oil fuel ash. Environ. Technol. Innov. 2019, 13, 62–73. [Google Scholar] [CrossRef]
- Gurgenidze, D.; Romanovski, V. The Pharmaceutical Pollution of Water Resources Using the Example of the Kura River (Tbilisi, Georgia). Water 2023, 15, 2574. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G. Mitigation of Diclofenac Pollution in Aqueous Media by Adsorption. ChemBioEng Rev. 2020, 7, 50–64. [Google Scholar] [CrossRef]
- Wang, X.; Yin, R.; Zeng, L.; Zhu, M. A review of graphene-based nanomaterials for removal of antibiotics from aqueous environments. Environ. Pollut. 2019, 253, 100–110. [Google Scholar] [CrossRef]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef]
- Madan, S.; Shaw, R.; Tiwari, S.; Tiwari, S.K. Adsorption dynamics of Congo red dye removal using ZnO functionalized high silica zeolitic particles. Appl. Surf. Sci. 2019, 487, 907–917. [Google Scholar] [CrossRef]
- Zaidi, S.; Chaabane, T.; Sivasankar, V.; Darchen, A.; Maachi, R.; Msagati, T. Electro-coagulation coupled electro-flotation process: Feasible choice in doxycycline removal from pharmaceutical effluents. Arab. J. Chem. 2019, 12, 2798–2809. [Google Scholar] [CrossRef]
- Khan, M.A.; Alothman, Z.A.; Naushad, M.; Khan, M.R.; Luqman, M. Adsorption of methylene blue on strongly basic anion exchange resin (Zerolit DMF): Kinetic, isotherm, and thermodynamic studies. Desalination Water Treat. 2015, 53, 515–523. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; dos Santos, E.V.; Costa, E.C.T.d.A.; Martínez-Huitle, C.A. Electrochemical advanced oxidation processes (EAOPs) as alternative treatment techniques for carwash wastewater reclamation. Chemosphere 2018, 211, 998–1006. [Google Scholar] [CrossRef]
- Tagliavini, M.; Schäfer, A.I. Removal of steroid micropollutants by polymer-based spherical activated carbon (PBSAC) assisted membrane filtration. J. Hazard. Mater. 2018, 353, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Olusegun, S.J.; Freitas, E.T.F.; Lara, L.R.S.; Stumpf, H.O.; Mohallem, N.D.S. Effect of drying process and calcination on the structural and magnetic properties of cobalt ferrite. Ceram. Int. 2019, 45, 8734–8743. [Google Scholar] [CrossRef]
- Ramlow, H.; Machado, R.A.F.; Bierhalz, A.C.K.; Marangoni, C. Dye synthetic solution treatment by direct contact membrane distillation using commercial membranes. Environ. Technol. 2020, 41, 2253–2265. [Google Scholar] [CrossRef]
- Okoli, C.P.; Ofomaja, A.E. Development of sustainable magnetic polyurethane polymer nanocomposite for abatement of tetracycline antibiotics aqueous pollution: Response surface methodology and adsorption dynamics. J. Clean. Prod. 2019, 217, 42–55. [Google Scholar] [CrossRef]
- Li, X.-D.; Zhai, Q.-Z. Use of nanometer mesoporous MCM-41 for the removal of Pb(II) from aqueous solution. Appl. Water Sci. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Qin, X.; Liu, F.; Wang, G.; Weng, L.; Li, L. Adsorption of levofloxacin onto goethite: Effects of pH, calcium and phosphate. Colloids Surf. B Biointerfaces 2014, 116, 591–596. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Jajko, M.; Sobczak, A. Removal of veterinary antibiotics from wastewater by electrocoagulation. Chemosphere 2018, 194, 381–389. [Google Scholar] [CrossRef]
- Sun, J.; Cui, L.; Gao, Y.; He, Y.; Liu, H.; Huang, Z. Environmental application of magnetic cellulose derived from Pennisetum sinese Roxb for efficient tetracycline removal. Carbohydr. Polym. 2021, 251, 117004. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, P.; Dang, Z.; Zhu, N.; Li, P.; Wu, J.; Wang, X. Polymeric Fe/Zr pillared montmorillonite for the removal of Cr(VI) from aqueous solutions. Chem. Eng. J. 2010, 162, 1035–1044. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): Study of adsorption isotherms and kinetic models. Chem. Eng. J. 2011, 168, 722–730. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, J.; Zhao, J.; Zhu, H.; Wang, C. Adsorption and removal of tetracycline from water by petroleum coke-derived highly porous activated carbon. J. Environ. Chem. Eng. 2015, 3, 1504–1512. [Google Scholar] [CrossRef]
- Brigante, M.; Avena, M. Biotemplated synthesis of mesoporous silica for doxycycline removal. Effect of pH, temperature, ionic strength and Ca2+ concentration on the adsorption behaviour. Microporous Mesoporous Mater. 2016, 225, 534–542. [Google Scholar] [CrossRef]
- Lagaly, G.; Ogawa, M.; Dékány, I. Chapter 7.3 Clay Mineral Organic Interactions. Dev. Clay Sci. 2006, 1, 309–377. [Google Scholar]
- Zhao, Y.; Cao, B.; Lin, Z.; Su, X. Synthesis of CoFe2O4/C nano-catalyst with excellent performance by molten salt method and its application in 4-nitrophenol reduction. Environ. Pollut. 2019, 254, 112961. [Google Scholar] [CrossRef] [PubMed]


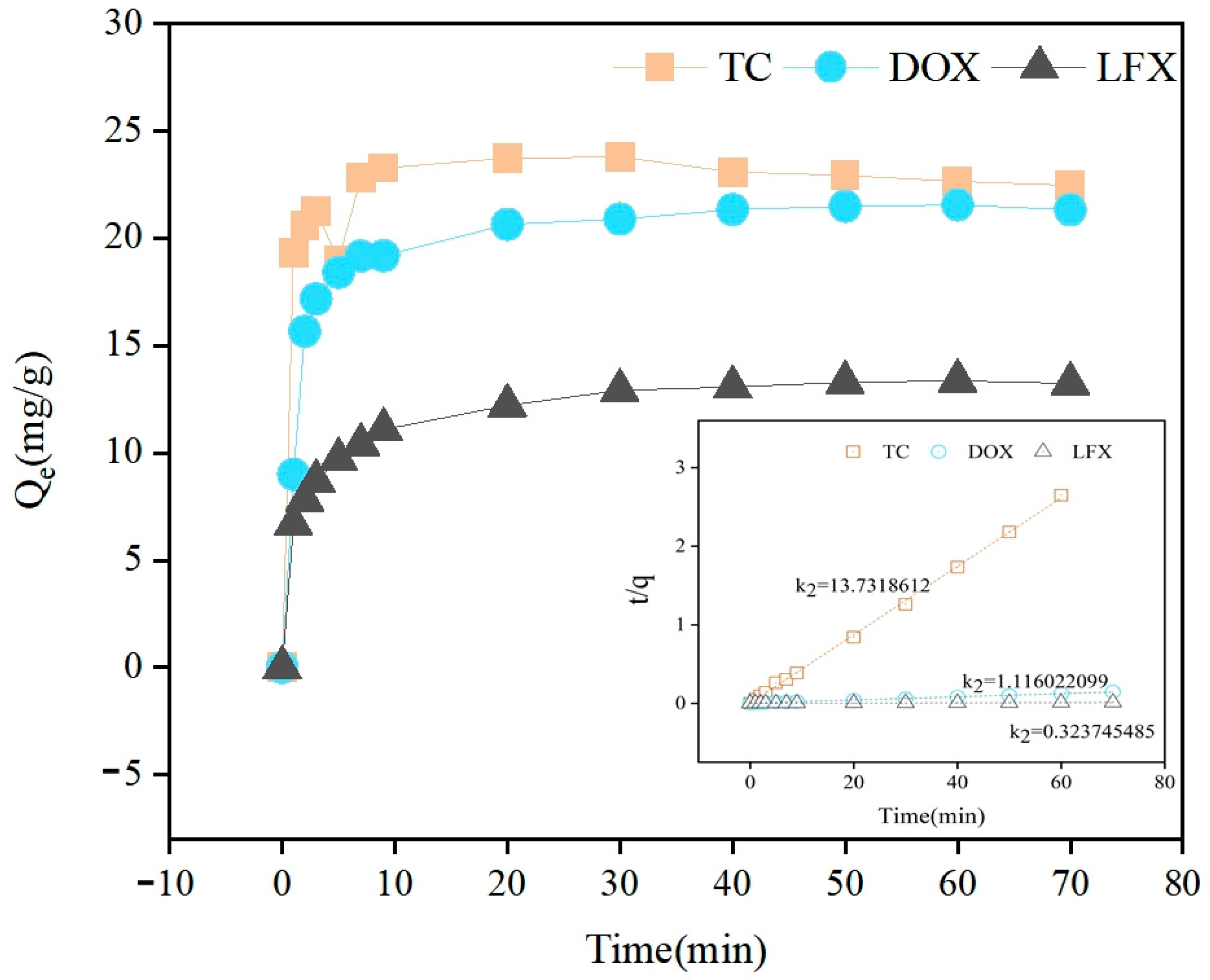
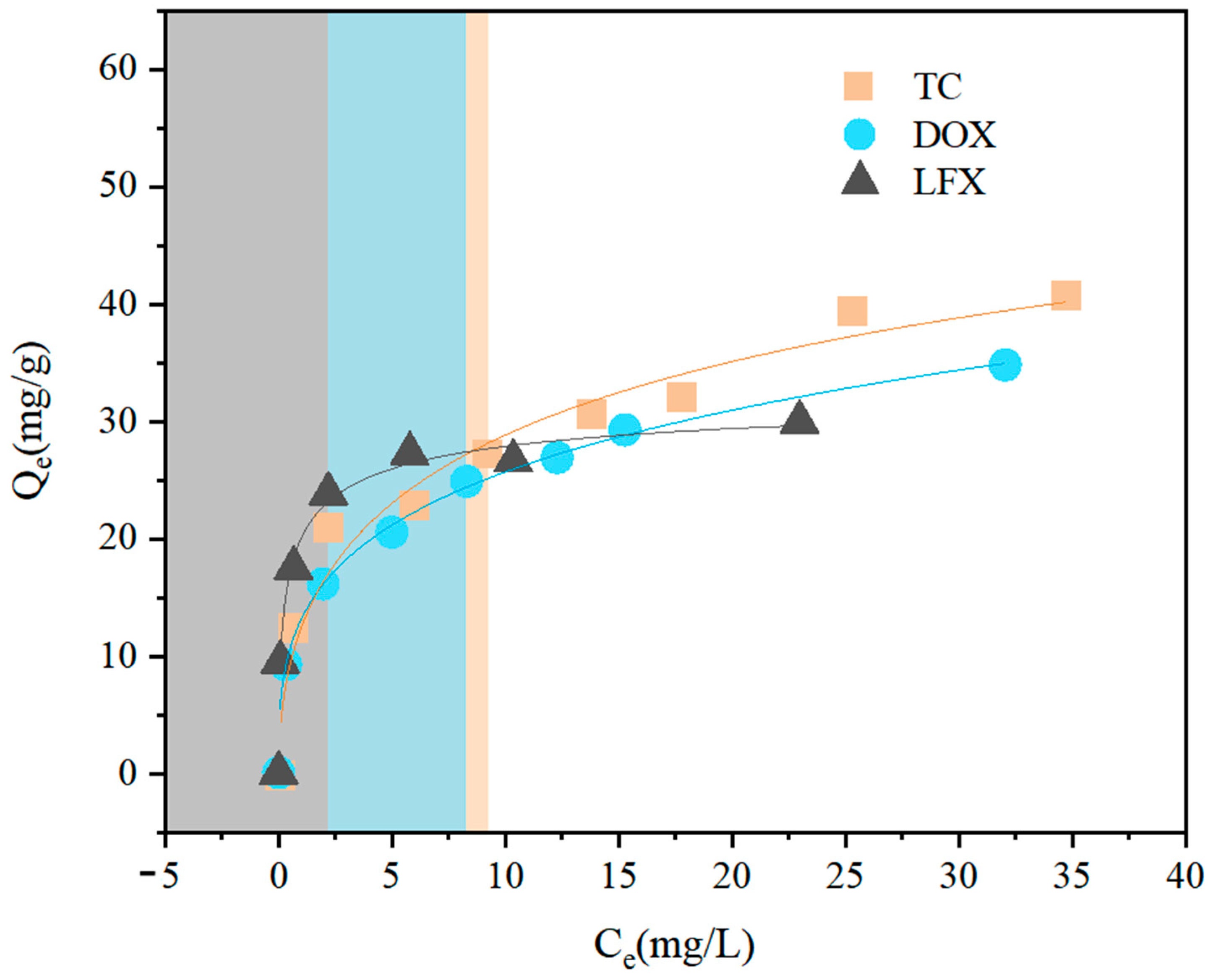

| Pollutants | Adsorption Capacity Determined by Experiment qe, exp (mg/g) | Kinetic Equation of Pseudo-First-Order Adsorption Rate | Kinetic Equation of Pseudo-Second-Order Adsorption Rate | ||||
|---|---|---|---|---|---|---|---|
| TC | 23.77 | 0.02 | 2.30 | 0.126 | 13.73 | 22.97 | 0.999 |
| DOX | 21.54 | 0.06 | 6.39 | 0.843 | 1.12 | 495.04 | 0.998 |
| LFX | 13.35 | 0.06 | 0.93 | 0.941 | 0.32 | 6794.07 | 0.997 |
| Pollutants | Freundlich | Langmuir | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TC | 12.89 | 0.33 | 0.95 | 0.204 | 73.410 | 0.96 | 373.01 | 8.90 | 0.96 |
| DOX | 13.39 | 0.27 | 0.99 | 0.100 | 144.835 | 0.99 | 809.60 | 532.80 | 0.95 |
| LFX | 18.93 | 0.13 | 0.81 | 1.47 | 33.676 | 0.98 | 456.52 | 12.89 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yang, Y.; Yao, Y.; Huang, Z.; Xu, Q.; He, L.; Gong, B. Rapid Antibiotic Adsorption from Water Using MCM-41-Based Material. Water 2023, 15, 4027. https://doi.org/10.3390/w15224027
Chen J, Yang Y, Yao Y, Huang Z, Xu Q, He L, Gong B. Rapid Antibiotic Adsorption from Water Using MCM-41-Based Material. Water. 2023; 15(22):4027. https://doi.org/10.3390/w15224027
Chicago/Turabian StyleChen, Jie, Yao Yang, Yuanyuan Yao, Zhujian Huang, Qiaoling Xu, Liping He, and Beini Gong. 2023. "Rapid Antibiotic Adsorption from Water Using MCM-41-Based Material" Water 15, no. 22: 4027. https://doi.org/10.3390/w15224027
APA StyleChen, J., Yang, Y., Yao, Y., Huang, Z., Xu, Q., He, L., & Gong, B. (2023). Rapid Antibiotic Adsorption from Water Using MCM-41-Based Material. Water, 15(22), 4027. https://doi.org/10.3390/w15224027









