A Feasibility Study of Vortex Tube-Powered Membrane Distillation (MD) for Desalination
Abstract
:1. Introduction
2. Process Description
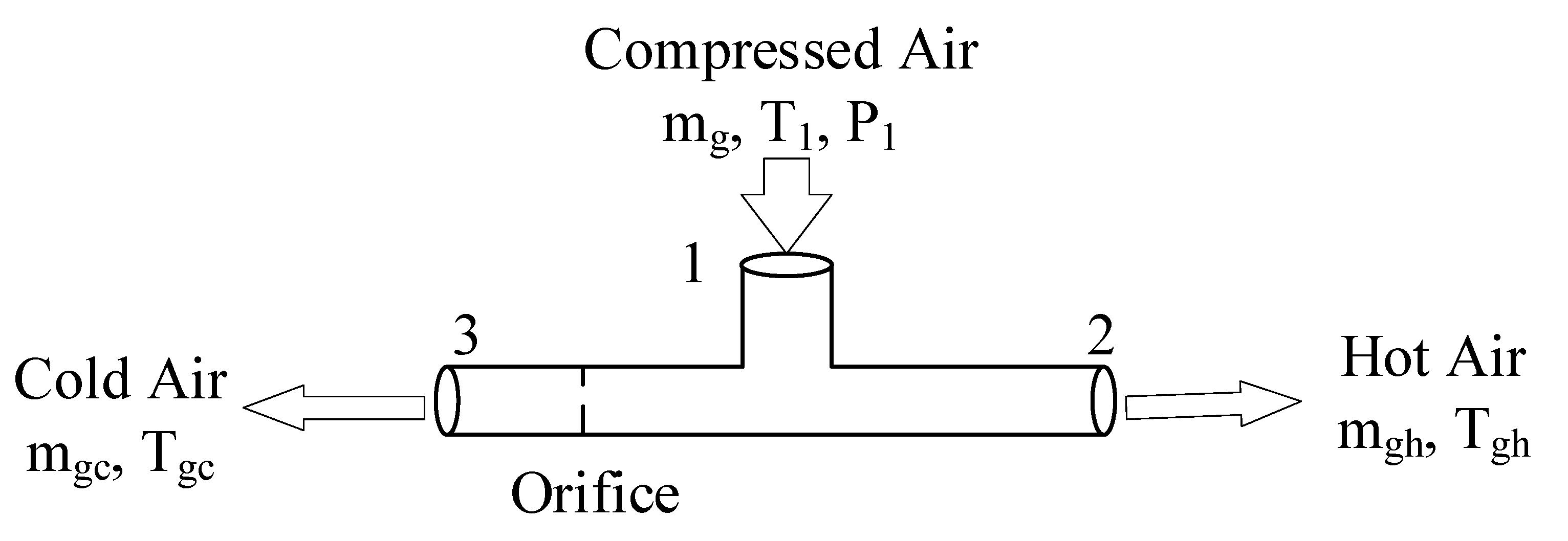
2.1. Vortex Tube
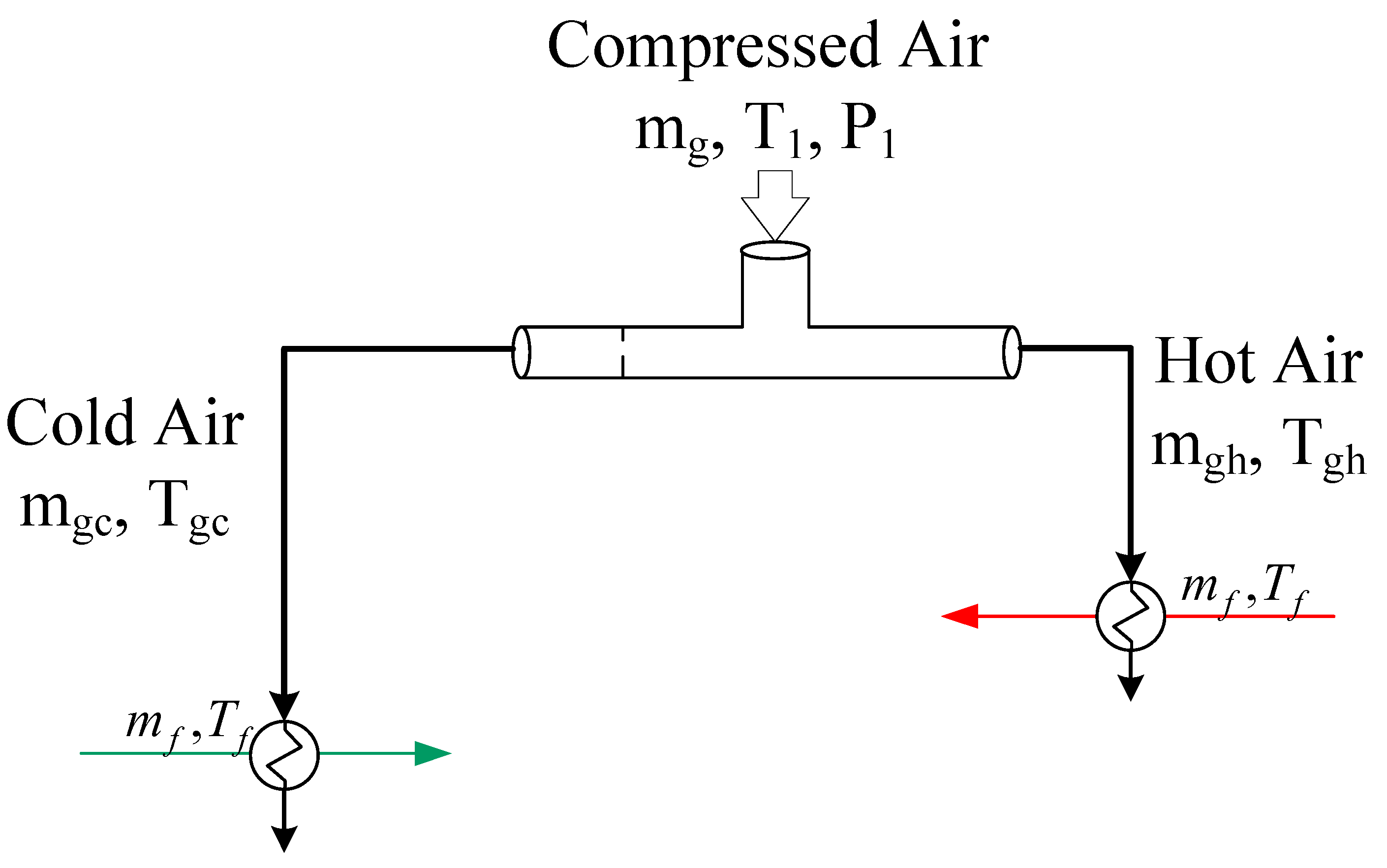
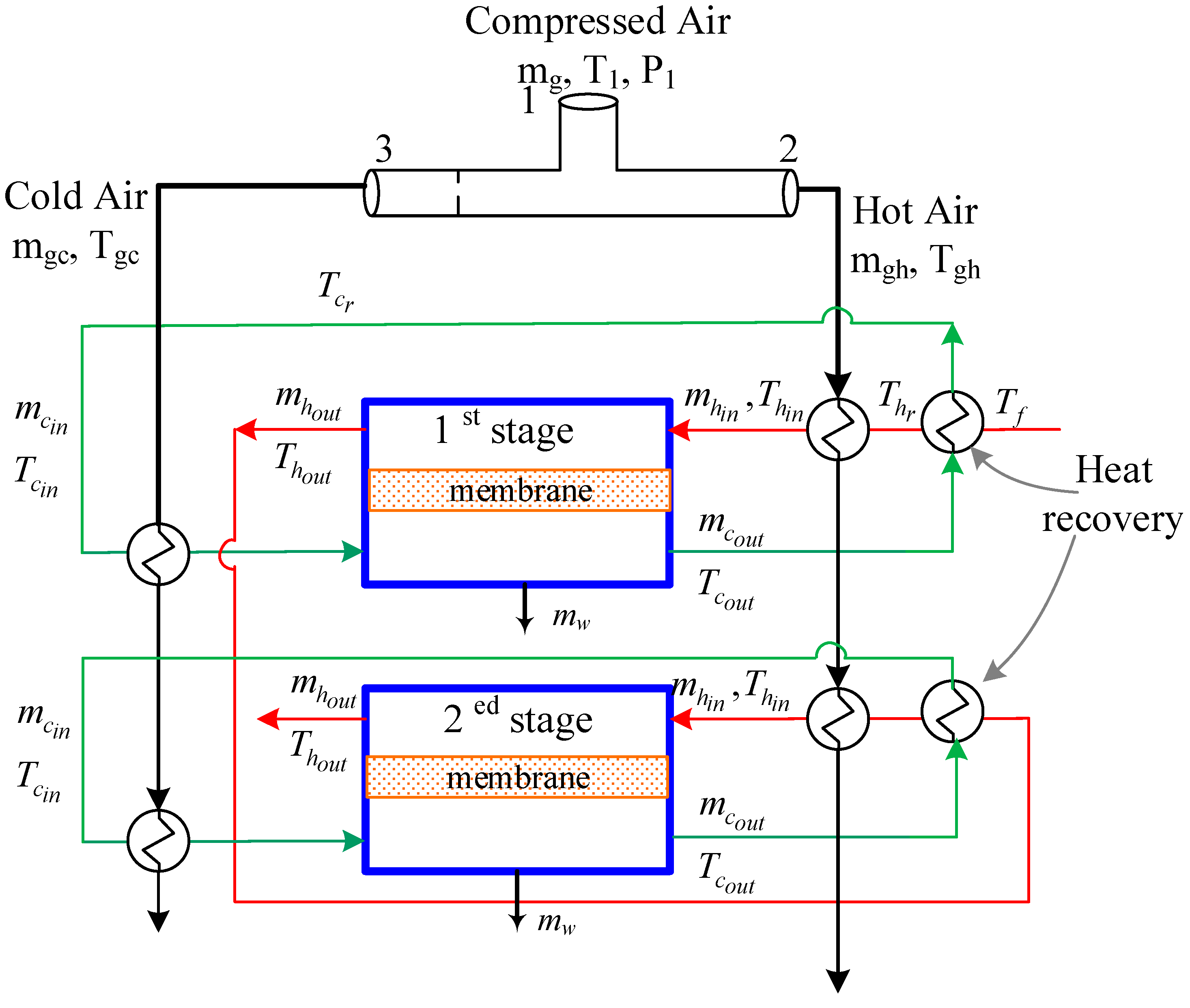
2.2. Integrated Vortex MD System
- Given the bulk temperature at both sides of the MD membrane (), the local heat transfer coefficients () are calculated from the Nusselt number as follows [15]:where Re = dρu/μ is the Reynolds number and Pr = Cpμ/k is the Prandtl number.
- Set .
- Knowing the membrane characteristics and the average membrane temperature, i.e., , the membrane coefficient Cm can be estimated utilizing the correlation in [32] according to the designated mechanism:
- Knudsen flow mechanism, kn > 1:
- Molecular diffusion mechanism, kn < 0.01:
- Knudsen–molecular diffusion transition mechanism, 0.01 < kn < 1:where the Knudsen number is defined as and λ is the mean free path of water molecules, expressed as (15):
- 5.
- Calculate the latent heat of vaporization at the average membrane temperature using [33]:
- 6.
- Calculate the mass flux using:
- 7.
- Compute the overall heat transfer coefficient using [13]:
- 8.
- At equilibrium, the heat convection from the hot side to the membrane interface and heat convection from the membrane interface to the cold side are equal. Hence, the following conditions hold [32]:
- 9.
- If , then stop the iteration, otherwise set and go back to step 3.
3. Results and Discussion
3.1. Vortex Model Validation
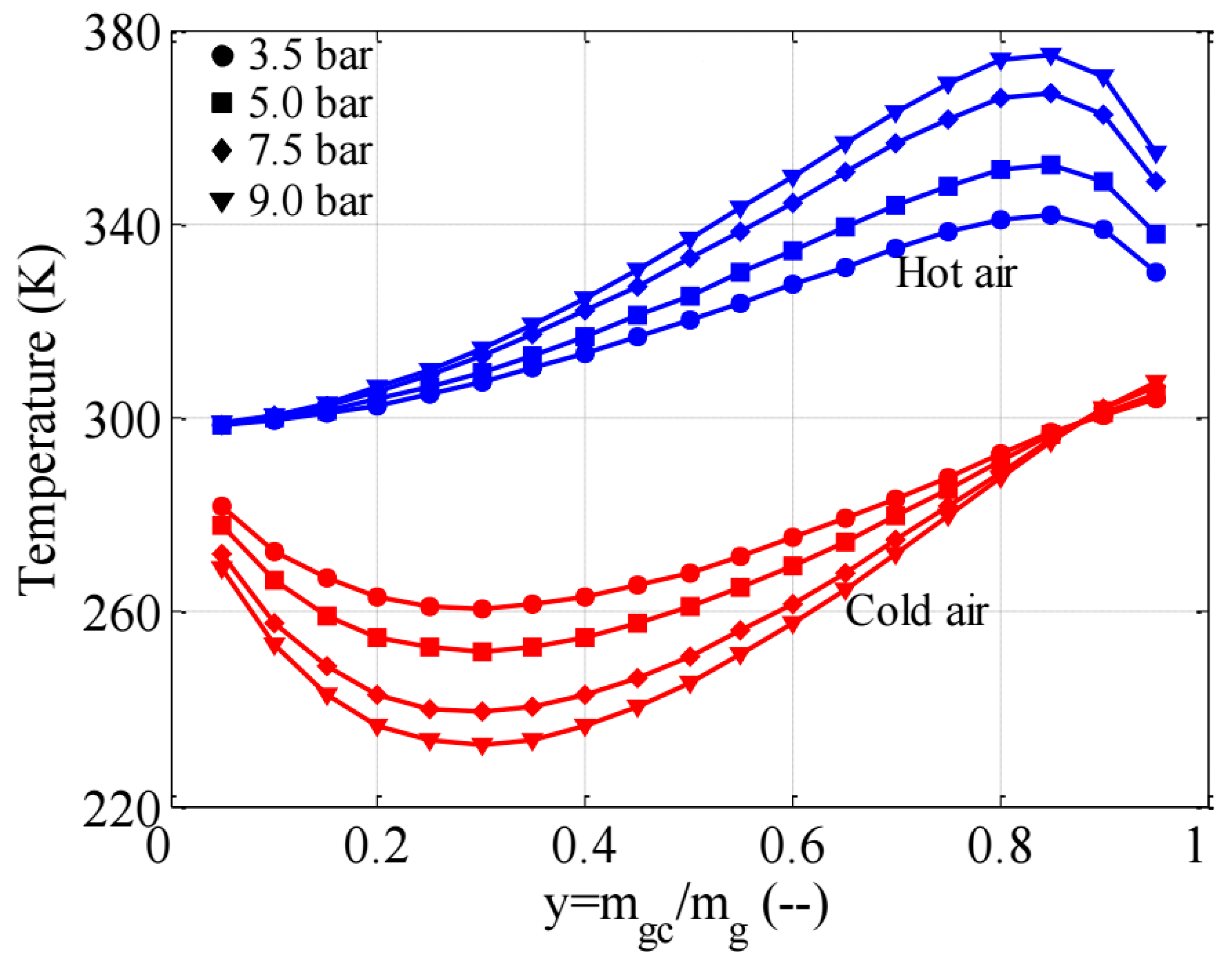
3.2. Heating and Cooling Capability of the Vortex Tube
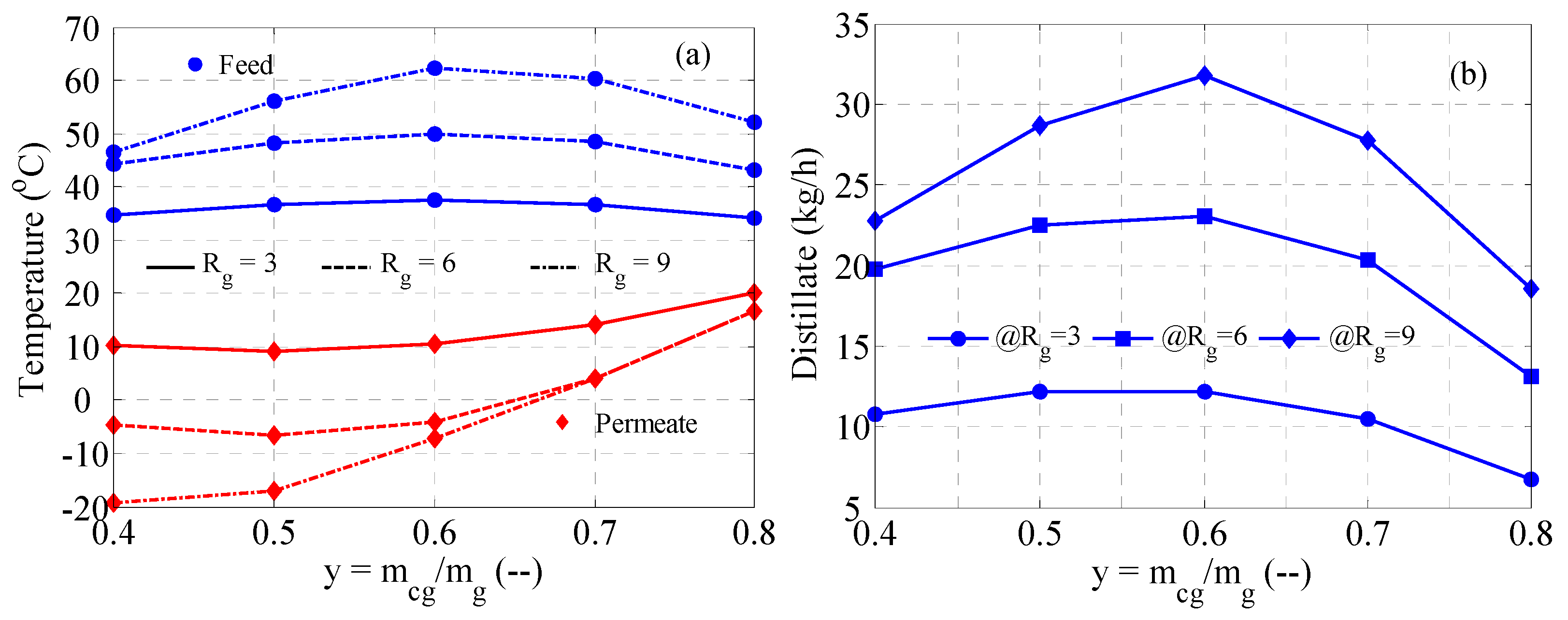
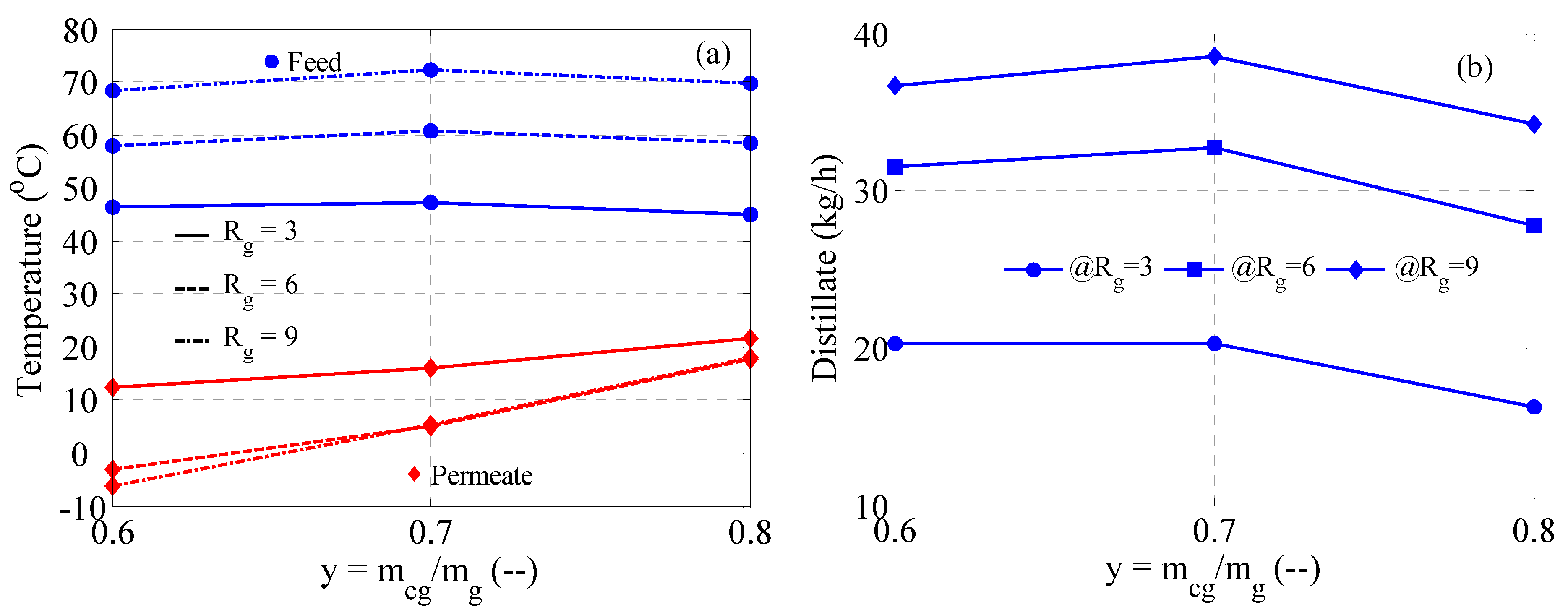
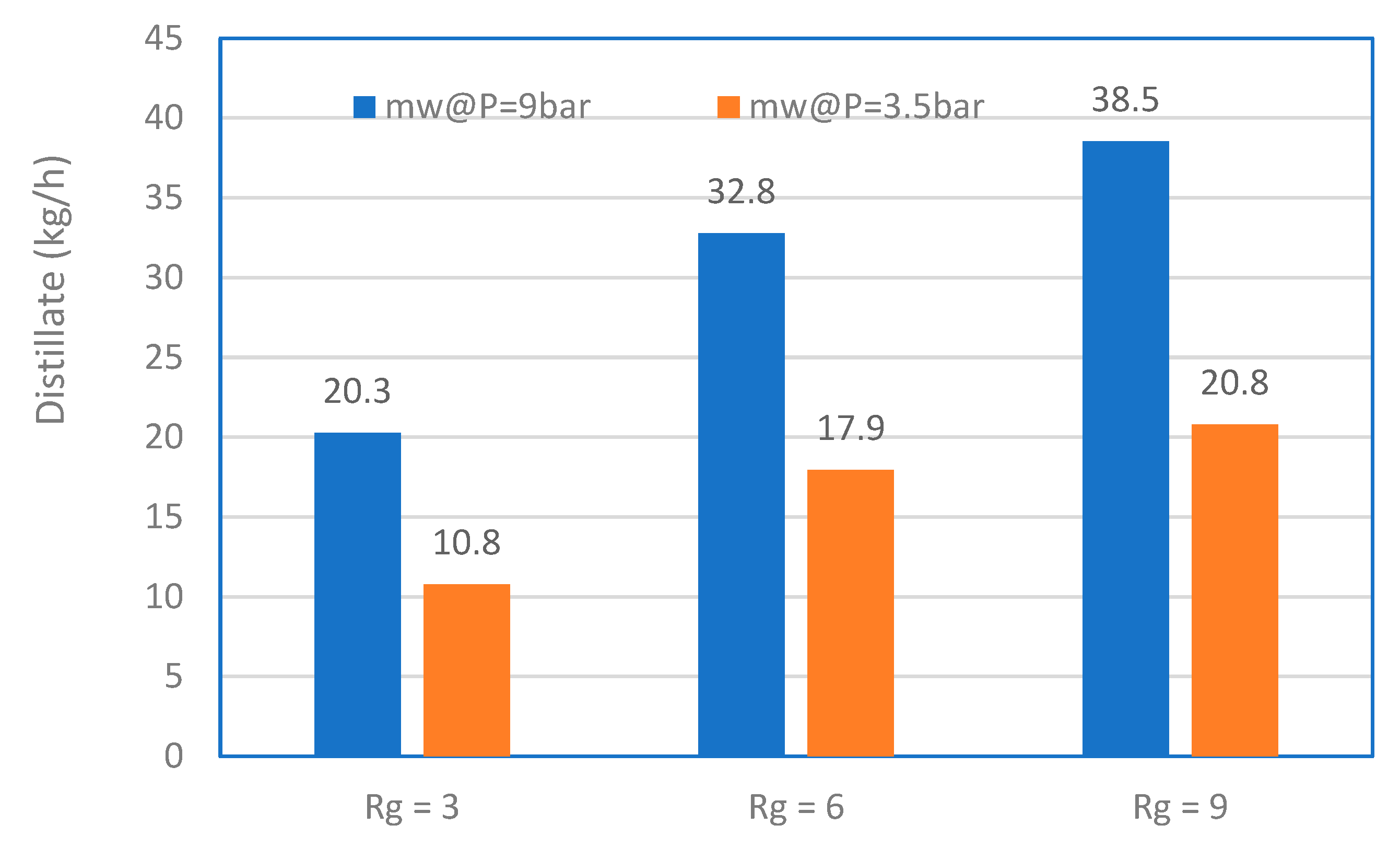
3.3. MD Performance
3.4. MD with Heat Recovery Performance
3.5. Energy Consumption
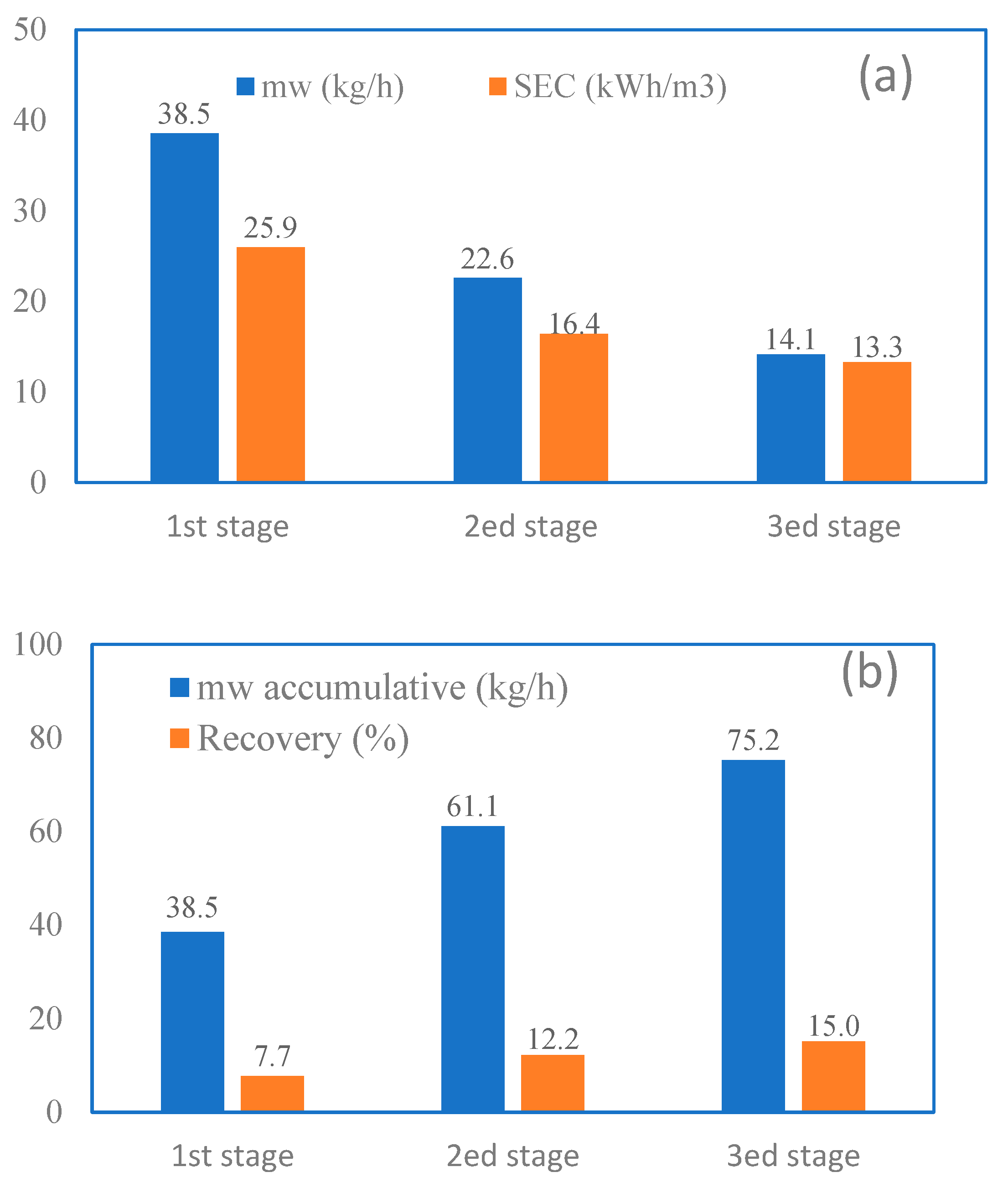
3.6. Cascaded MD in Series
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Amd | MD surface area, m2 |
| Cm | Permeability coefficient, kg/m2sPa |
| Knudsen mass flux coefficient, kg/m2sPa | |
| Molecular diffusion mass flux coefficient, kg/m2sPa | |
| Transition mass flux coefficient, kg/m2sPa | |
| Cp | Specific heat capacity, J/kg K |
| de | Collision diameter of the water vapor and air, m2 |
| hv | Latent heat of vaporization for MD, J/kg |
| hf, hp, hm | Feed, permeate, and membrane heat transfer coefficient, W/m2 K |
| Jw | Mass flux, kg/m2 h |
| kB | Boltzmann constant |
| km | Membrane conductivity, W/m K |
| ks | Solid phase thermal conductivity, W/m.K |
| kg | Gas phase thermal conductivity, W/m.K |
| kn | Knudsen number |
| l | Channel height, m |
| Air mass rate, kg/h | |
| Hot air mass rate, kg/h | |
| Inlet and outlet MD feed flow rate, kg/h | |
| Inlet and outlet MD permeate flow rate, kg/s. | |
| Cold air rate, kg/s | |
| Distillate mass rate for MD, kg/s | |
| Mw | Molecular weight |
| Nu | Nusselt number |
| P1, P2 | Vapor pressure at feed and permeate membrane surface, Pa |
| P | Average membrane interface pressure, Pa |
| Pa | Entrapped air pressure, Pa |
| PD | Membrane pressure multiplied by diffusivity, Pa.m2/s |
| Pr | Prandtl number |
| Inlet air pressure, bar | |
| Vapor pressure at , Pa | |
| r | Membrane pore size, m |
| R | Ideal gas constant, also flow rate ratio |
| Rc | Recovery ratio |
| Re | Reynolds number |
| Rg | Air-to-water mass ratio |
| SEC | Specific energy consumption, kWh/m3 |
| Tgh, Tgc | Air hot and cold temperature, K |
| Thb, Tcb | Feed (hot) and permeate (cold) bulk temperature, K |
| Thm, Tcm | Feed and permeate membrane temperature, K |
| Inlet and outlet MD feed temperature, °C | |
| Inlet and outlet permeate (cold) temperature, °C | |
| Feed temperature leaving heat recovery, °C | |
| Permeate temperature leaving heat recovery, °C | |
| T | The average temperature at the membrane interface, K |
| Intake temperature, °C | |
| T1 | Air inlet temperature, K |
| U | Overall heat transfer coefficient, W/m2K |
| Feed, ppm | |
| y | Cold air to inlet air mass ratio |
| Greek letters | |
| τ | Tortuosity |
| ρ | Water density, kg/m3 |
| δ | Membrane thickness |
| ε | Porosity |
| λ | Mean free path, m |
References
- Abid, M.B.; Wahab, R.A.; Abdelsalam, M.; Gzara, L.; Moujdin, I.A. Desalination technologies, membrane distillation, and electrospinning, an overview. Heliyon 2023, 9, e12810. [Google Scholar] [CrossRef]
- Ravi, J.; Othman, M.H.D.; Matsuura, T.; Bilad, M.R.I.; El-Badawy, T.; Aziz, F.; Ismail, A.; Rahman, M.A.; Jaafar, J. Polymeric membranes for desalination using membrane distillation: A review. Desalination 2020, 490, 114530. [Google Scholar] [CrossRef]
- Stillwell, A.S.; Webber, M.E. Predicting the specific energy consumption of reverse osmosis desalination. Water 2016, 8, 601. [Google Scholar] [CrossRef]
- Gude, V.G. Energy storage for desalination processes powered by renewable energy and waste heat sources. Appl. Energy 2015, 137, 877–898. [Google Scholar] [CrossRef]
- Najib, A.; Orfi, J.; Ali, E.; Saleh, J. Thermodynamic analysis of a direct contact membrane distillation system with and without heat recovery based on experimental data. Desalination 2019, 466, 52–67. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.D.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Nafey, A.; Fath, H.; Mabrouk, A. Thermo-economic investigation of multi effect evaporation (MEE) and hybrid multi effect evaporation—Multi stage flash (MEE-MSF) systems. Desalination 2006, 201, 241–254. [Google Scholar] [CrossRef]
- Farsi, A.; Dincer, I. Development and evaluation of an integrated MED/membrane desalination system. Desalination 2019, 463, 55–68. [Google Scholar] [CrossRef]
- Manesh, M.K.; Ghalami, H.; Amidpour, M.; Hamedi, M. Optimal coupling of site utility steam network with MED-RO desalination through total site analysis and exergoeconomic optimization. Desalination 2013, 316, 42–52. [Google Scholar] [CrossRef]
- Son, H.S.; Shahzad, M.W.; Ghaffour, N.; Ng, K.C. Pilot studies on synergetic impacts of energy utilization in hybrid desalination system: Multi-effect distillation and adsorption cycle (MED-AD). Desalination 2020, 477, 114266. [Google Scholar] [CrossRef]
- Goosen, M.F.; Mahmoudi, H.; Ghaffour, N. Today’s and future challenges in applications of renewable energy technologies for desalination. Crit. Rev. Environ. Sci. Technol. 2014, 44, 929–999. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Assad, M.; Sayed, E.T.; Soudan, B. Recent progress in the use of renewable energy sources to power water desalination plants. Desalination 2018, 435, 97–113. [Google Scholar]
- Alkaisi, A.; Mossad, R.; Sharifian-Barforoush, A. A review of the water desalination systems integrated with renewable energy. Energy Procedia 2017, 110, 268–274. [Google Scholar] [CrossRef]
- Lawal, D.U.; Khalifa, A.E. Flux prediction in direct contact membrane distillation. Int. J. Mater. Mech. Manuf. 2014, 2, 302–308. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Zhang, Z.; Lokare, O.R.; Gusa, A.V.; Vidic, R.D. Pretreatment of brackish water reverse osmosis (BWRO) concentrate to enhance water recovery in inland desalination plants by direct contact membrane distillation (DCMD). Desalination 2021, 508, 115050. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J. Minimal Liquid Discharge (MLD) and Zero Liquid Discharge (ZLD) strategies for wastewater management and resource recovery–Analysis, challenges and prospects. J. Environ. Chem. Eng. 2020, 8, 104418. [Google Scholar] [CrossRef]
- Guan, G.; Yang, X.; Wang, R.; Fane, A.G. Evaluation of heat utilization in membrane distillation desalination system integrated with heat recovery. Desalination 2015, 366, 80–93. [Google Scholar] [CrossRef]
- Lokare, O.R.; Tavakkoli, S.; Khanna, V.; Vidic, R.D. Importance of feed recirculation for the overall energy consumption in membrane distillation systems. Desalination 2018, 428, 250–254. [Google Scholar] [CrossRef]
- Geng, H.; Wang, J.; Zhang, C.; Li, P.; Chang, H. High water recovery of RO brine using multi-stage air gap membrane distillation. Desalination 2015, 355, 178–185. [Google Scholar] [CrossRef]
- Lienhard, J.H.; Antar, M.A.; Bilton, A.; Blanco, J.; Zaragoza, G. Solar desalination. Annu. Rev. Heat Transf. 2012, 15, 277–347. [Google Scholar]
- Shannak, B.A. Temperature separation and friction losses in vortex tube. Heat Mass Transf. 2004, 40, 779–785. [Google Scholar] [CrossRef]
- Nollau, A.; Gerling, D. A new cooling approach for traction motors in hybrid drives. In Proceedings of the 2013 International Electric Machines & Drives Conference, Chigaco, IL, USA, 12–15 May 2013; pp. 456–461. [Google Scholar]
- Ahlborn, B.; Keller, J.; Rebhan, E. The heat pump in a vortex tube. J. Non-Equilib. Thermodyn. 1998, 23, 159–165. [Google Scholar] [CrossRef]
- Khodorkov, I.; Poshernev, N.; Zhidkov, M. The vortex tube—A universal device for heating, cooling, cleaning, and drying gases and separating gas mixtures. Chem. Pet. Eng. 2003, 39, 409–415. [Google Scholar] [CrossRef]
- Balepin, V.; Rosholt, D. Progress in air separation with the vortex tube. In Proceedings of the 9th International Space Planes and Hypersonic Systems and Technolgies Conference, Norfolk, VA, USA, 1–4 November 1999. [Google Scholar]
- Ebmeier, R.; Whitney, S.; Alugupally, S.; Nelson, M.; Padhye, N.; Gogos, G.; Viljoen, H. Ranque–Hilsch vortex tube thermocycler for fast DNA amplification and real time optical detection. Rev. Sci. Instrum. 2004, 75, 5356–5359. [Google Scholar] [CrossRef]
- Aljuwayhel, N.; Nellis, G.; Klein, S. Parametric and internal study of the vortex tube using a CFD model. Int. J. Refrig. 2005, 28, 442–450. [Google Scholar] [CrossRef]
- Ali, E.; Orfi, J. An experimentally calibrated model for heat and mass transfer in full-scale direct contact membrane distillation. Desalin. Water Treat. 2018, 116, 1–18. [Google Scholar] [CrossRef]
- Orfi, J.; Najib, A.; Ali Emad Ajbar, A.; Almatrafi, M.; Boumaza, M.; Alhumaizi, K. Membrane distillation and reverse osmosis based desalination driven by geothermal energy sources. Desalin. Water Treat. 2017, 76, 40–52. [Google Scholar] [CrossRef]
- Najib, A.; Orfi, J.; Ali, E.; Ajbar, A.; Boumaaza, M.; Alhumaizi, K. Performance analysis of cascaded membrane distillation arrangements for desalination of brackish water. Desalin. Water Treat. 2017, 76, 19–29. [Google Scholar] [CrossRef]
- Nakoa, K.; Date, A.; Akbarzadeh, A. A research on water desalination using membrane distillation. Desalin. Water Treat. 2015, 56, 2618–2630. [Google Scholar] [CrossRef]
- Fard, A.K.; Manawi, Y.M.; Rhadfi, T.; Mahmoud, K.A.; Khraisheh, M.; Benyahia, F. Synoptic analysis of direct contact membrane distillation performance in Qatar: A case study. Desalination 2015, 360, 97–107. [Google Scholar] [CrossRef]
- Winter, D.; Koschikowski, J.; Wieghaus, M. Desalination using membrane distillation: Experimental studies on full scale spiral wound modules. J. Membr. Sci. 2011, 375, 104–112. [Google Scholar] [CrossRef]
- He, F.; Gilron, J.; Sirkar, K.K. High water recovery in direct contact membrane distillation using a series of cascades. Desalination 2013, 323, 48–54. [Google Scholar] [CrossRef]
- Summers, E.K.; Arafat, H.A. Energy efficiency comparison of single-stage membrane distillation (MD) desalination cycles in different configurations. Desalination 2012, 290, 54–66. [Google Scholar] [CrossRef]
- Ali, A.; Tsai, J.-H.; Tung, K.-L.; Drioli, E.; Macedonio, F. Designing and optimization of continuous direct contact membrane distillation process. Desalination 2018, 426, 97–107. [Google Scholar] [CrossRef]
- Swaminathan, J.; Chung, H.W.; Warsinger, D.M. Simple method for balancing direct contact membrane distillation. Desalination 2016, 383, 53–59. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| Effective surface area | 10 m2 |
| Membrane thickness | 230 mm |
| Channel length | 14 m |
| Channel height | 0.7 m |
| Pore diameter | 0.2 mm |
| Channel gap | 0.2 mm |
| Porosity | 0.8 |
| Entry pressure | 4.1 bar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orfi, J.; Ali, E. A Feasibility Study of Vortex Tube-Powered Membrane Distillation (MD) for Desalination. Water 2023, 15, 3767. https://doi.org/10.3390/w15213767
Orfi J, Ali E. A Feasibility Study of Vortex Tube-Powered Membrane Distillation (MD) for Desalination. Water. 2023; 15(21):3767. https://doi.org/10.3390/w15213767
Chicago/Turabian StyleOrfi, Jamel, and Emad Ali. 2023. "A Feasibility Study of Vortex Tube-Powered Membrane Distillation (MD) for Desalination" Water 15, no. 21: 3767. https://doi.org/10.3390/w15213767







