Abstract
The present work focuses on a comprehensive hydrochemical assessment of groundwater within a shallow aquifer located in the central region of Saudi Arabia. This aquifer serves as the principal source of groundwater supply for agricultural irrigation purposes. A total of twelve groundwater samples were systematically collected and subjected to thorough analysis to determine various physicochemical parameters. These parameters encompassed electrical conductivity (EC), pH, temperature, total dissolved solids (TDS), as well as concentrations of major ions. Analysis of major ion analysis was employed to elucidate the chemical attributes of groundwater within the research area. This approach facilitated an understanding of the geochemical evolution and the potential suitability of the groundwater for agriculture irrigation. The recorded range of electrical conductivity (EC) for the groundwater in this region falls between 3283 and 11,000 µS/cm, with an average value of 6709.4 µS/cm. The pH levels of the groundwater exhibited a slightly alkaline nature, ranging from 7.8 to 8.6. All sampled wells exhibited brackish water conditions (TDS > 1000 mg/L) based on TDS concentrations. The findings of this investigation demonstrated that the average ion concentration followed the order: Na+ > Ca+ > Mg2+ > K+ and Cl− > SO42− > HCO3− > NO32−. Applying Piper’s classification system, the groundwater samples were classified into two predominant categories: Ca-Cl and mix-Ca-Mg-Cl. The predominance of Ca2+ and Mg2+ over alkalis indicated mixed groundwater facies influenced by processes of reverse ion exchange and extensive interactions between water and rock formations. The distinct chemical characteristics of the groundwater were attributed to a combination of factors, including the percolation of irrigation-returned water, chemical transformations within the vadose zone, and extensive interactions with subsurface lithology. Despite major ion concentrations surpassing the permissible limits outlined by the World Health Organization (WHO) for drinking water, the assessment of quality indices was conducted to ascertain the suitability of the groundwater for irrigation purposes. These quality indices encompassed the permeability index (PI), magnesium hazard (MH), Kelly’s ratio (KR), sodium-adsorption ratio (SAR), residual sodium carbonate (RSC), sodium percentage (Na%), and magnesium ratio (MR). A meticulous evaluation of groundwater quality for agricultural utilization indicated that all sampled groundwater sources were deemed suitable for irrigation purposes.
1. Introduction
The quality of water has deteriorated due to the escalating utilization of groundwater reserves globally, leading to an increase in pollutant levels [1].
Geochemical processes arising from interactions between aquifers and water play a pivotal role in governing groundwater quality, especially in regions with limited recharge from rainfall, where it serves as a vital resource for domestic and agricultural needs [2,3,4,5].
The Kingdom of Saudi Arabia (KSA) faces a scarcity of renewable water resources due to its arid climate [6]. Groundwater is the primary source of agricultural and potable water within the KSA [7,8]. Over the past three decades, groundwater extraction in the KSA has surged, reaching a volume of 17 billion m3/year [6], meeting 80% of the nation’s water demand [9].
Numerous studies indicated degradation of groundwater in the KSA, with notable sources of pollution including irrigation runoff [10], landfill sites [11,12], disposal facilities [13], cesspools [14], wastewater systems [15,16,17], local, and industrial waste [18]. Anthropogenic activities have led to a notable rise in nitrate and trace metal concentrations in certain KSA groundwater sites [19].
Recent years have witnessed a surge in agricultural projects reliant on groundwater resources, contributing to groundwater stress and degradation [6]. Coupled with anthropogenic activities and rapidly changing climatic conditions, water shortages are becoming a looming concern [20]. Groundwater recharge lags behind pumping rates [21], and excessive exploitation has led to a decline in groundwater levels and compromised quality, with substantial socio-economic consequences [22,23].
The principal source of groundwater in the region is the fractured Precambrian bedrock, overlain by deep aquifers in the sedimentary formations of the Arabian Shield, encompassing a significant sequence of Paleozoic to modern sedimentary deposits [24]. Additionally, the shallow aquifers found mainly in valleys play a vital role in the Arabian Shield and coastal zones [15]. In the central region of Saudi Arabia, thick sedimentary layers from the Mesozoic and Cenozoic periods serve as an abundant groundwater reservoirs [4]. The Arabian Shield, western and southwestern coastal plains, and shallow alluvial aquifers (wadies) constitute significant water sources. High evaporation rates, reduced soil moisture due to the high temperatures, and unpredictable rainfall collectively undermine Saudi Arabia’s water accessibility [24].
Assessment of water quality is imperative for its comprehensive management and effective utilization, especially amid growing climate impacts and community health concerns. Groundwater quality hinges on complex interactions involving water–soil–sediment dynamics, the flow system, rock types, and prevailing geochemical conditions encompassing dissolution, redox state, precipitation, leaching, ion exchange, etc. [25]. Hydrochemistry in arid aquifers is influenced by several variables such as topography, soil chemistry, water–mineral interactions, and subsurface mixing of chemically diverse groundwater [26,27]. Primary natural processes impacting groundwater chemistry encompass rock–water interactions [25], evaporation/crystallization, and precipitation-induced dilution. Scarce rainfall and high evaporation rates in arid settings influence groundwater chemistry [28].
Water quality investigations have evolved from traditional approaches such as scatter plots to modern methodologies such as geographic information systems (GIS), fuzzy modeling, and machine learning approaches [29,30,31,32]. The classical graphical approach involves ionic ratios and scatter plots to comprehend the geochemical processes [33]. Contemporary methods encompass geostatistical simulation, multivariate statistical methods, geographic information systems, analytical hierarchical process (AHP), and algorithmic methods for machine learning (e.g., random forest modeling, artificial neural networks, etc.) enabling effective groundwater quality characterization and visualization.
Leveraging GIS, groundwater quality assessments, and spatial mapping aids in identifying contaminated areas, and assessing contamination levels and sources [34,35,36,37,38].
Geostatistical interpolation techniques, particularly ordinary kriging, are often used for spatial mapping [35,39,40,41].
This study focuses on the North of Al-Quwayiyah region in central Saudi Arabia. Despite limited prior investigations, this study aims to comprehensively assess groundwater chemical composition for potable and irrigation suitability using irrigation quality indicators. The irrigation water quality index identifies suitable groundwater extraction zones for irrigation and potential resource management. Spatial distribution maps further facilitate the assessment of groundwater suitability for varied applications.
2. The Study Area
The research area is situated within the province of Al-Riyadh in central Saudi Arabia, approximately 150 Km from the country’s capital Riyadh. The study area is specifically located in the northern part of Al-Quwayiyah Governate (Figure 1). It has an approximate area of 695 km2 and the topography exhibits varying altitudes, ranging from 740 m above sea level downstream of the area (the eastern part) to approximately 1270 m at the highest point of the catchment (the western part) (Figure 1).
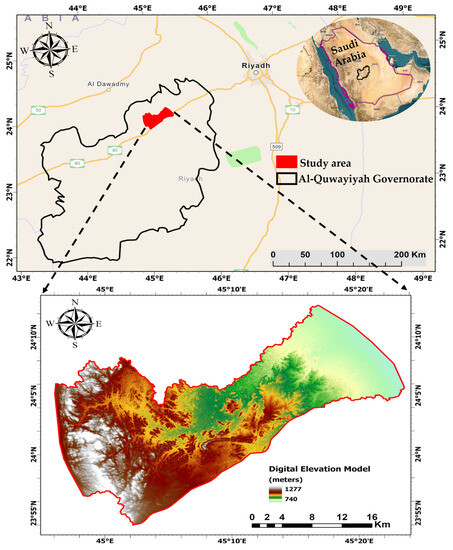
Figure 1.
Location and digital elevation model of the study area.
The predominant landscape of Saudi Arabia consists of arid desert conditions characterized by high temperatures. The study area demonstrates an exceedingly hot climate, particularly from May to September of each year when the average monthly temperature might exceed 30 °C (Figure 2a).
KSA is characterized by annual average rainfall ranging from 50 to 100 mm [42]. Greater rainfall can be found north of Riyadh and along the Red Sea escarpment, where annual average maximum monthly rainfall rates in January, March, and April (Figure 2b). The average annual evaporation at Riyadh is 2900 mm, with daily peaks that can approach 18.5 mm/day in the summer [42].
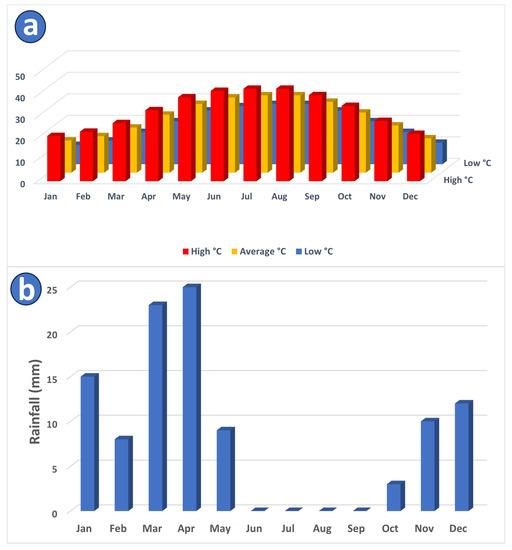
Figure 2.
(a) Monthly high, average, and low temperatures within the study area [43]. (b) Average monthly rainfall amount (mm) in the study area [43].
Geological and Hydrogeological Setting
KSA is tectonically situated on the Arabian Plate. It is made up of volcanic and volcano-sedimentary rocks that have undergone Late Precambrian metamorphism [42]. The Arabian Shield and Arabian Platform are the two geological regimes that make up the plate’s regional division (Figure 3a). These strata have an eastern dip towards the Arabian Gulf as a consequence of the Arabian Shield basements tilted [42].
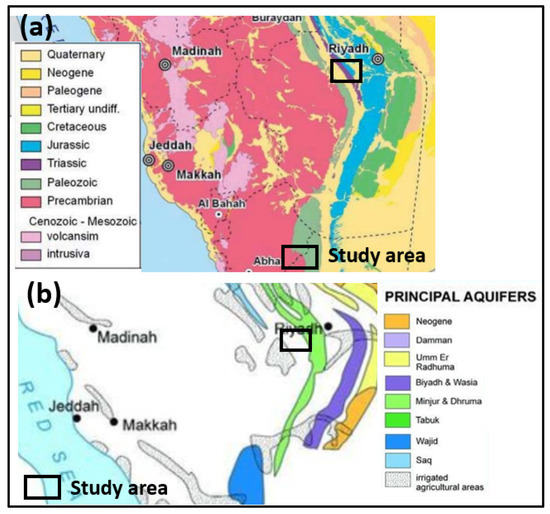
Figure 3.
(a) Geological map of Saudi Arabia [44], (b) the main aquifers of Saudi Arabia [45].
The subsurface geology of the research area is composed of the following [42]:
- Surficial, aeolian, alluvial, and colluvial rocks. Quaternary sediments constitute an unconfined aquifer in the western region and a confined aquifer in the eastern region.
- The Permian Khuff Formation’s Ash Shiqqah is composed of huge conglomerates and shale. Marl, dolomite, sandstone, and gypsum with fossils are all found in the top section. The Khuff Formation is a discontinuous aquifer of lesser significance.
- Saq Sandstone, which dates from the Ordovician to the Cambrian. It represents a regional aquifer system (Figure 3b). The Saq Sandstone often dips to the east and is above.
- The Arabian shield complex from the Precambrian basement.
Except in areas where a reversal of the groundwater hydraulic gradient has occurred because of significant water-level declines, natural groundwater flow is towards the northeast within the study area [42].
3. Methodology
3.1. Groundwater Sampling and Analysis
Twelve wells in North Al-Quwayiyah Governorate (Figure 4) were used to collect groundwater samples for irrigation. Groundwater samples were named starting with GW01 (located east of the study region) and ending with GW12 (located west of the study area). The distribution of these wells is a result of the numerous farming activities near the sample locations. During the collection of groundwater samples in the eastern portion of the research region, the average distance between the wells was found to be approximately 2 km, while the remaining samples had an average distance between the wells of 5–6 km.
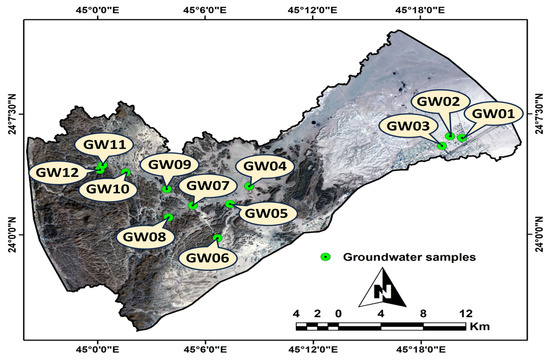
Figure 4.
The Satellite Landsat-9 map shows the locations of twelve groundwater samples within the study area.
In March 2023, groundwater samples were collected using acid-washed plastic bottles. Each sample, which had a volume of 1000 mL, was taken immediately from the well after it had been pumped. The bottles were acidified, closed, and kept at room temperature for analysis the next day. The chemical analysis was conducted using the recommended standard procedures [46]. Based on the APHA standard method, volumetric titration procedures were employed to measure Ca2+, Mg2+, Cl−, and HCO3− [46]. Ca2+ and Mg2+ were quantified using the EDTA titrimetric method, Cl using the argentometric titrimetric method, and HCO3 using the phenolphthalein (P). A flame photometer (AFP 100, Hamburg, Germany) was used to measure K+ and Na+, while a spectrophotometer (UV 1600 PC) was used to quantify SO42− and NO3−. These analyses were conducted at the Al-Jazzar Engineering Consulting Company in Riyadh, Saudi Arabia. Groundwater samples were examined in situ using a HANNA portable multi-parameter device to determine pH, electric conductivity (EC), and total dissolved solids (TDS).
The Piper triangular diagram and the Gibbs diagram were used to represent the chemical analysis findings and describe the chemical composition of groundwater in the study area. Identifying the hydrogeochemical facies of groundwater was carried out using Piper’s trilinear diagram [47]. The two base triangles of the Piper diagram display milliequivalent percentages of cation and anion to express the main ions. The total of the cations and anions is determined to be 100%. The diagonal field, which indicates the whole ionic association, is projected with the suitable positions of the cations and anions for examination [48]. The interaction between the chemical composition of water and aquifer geology was investigated using the Gibbs diagram. To represent hydrogeochemical processes in the geochemistry of groundwater, such as water–rock interaction, precipitation, and evaporation, Gibbs (1970) [49] developed two plots. For this reason, the weight proportions of Na+/(Na+ + Ca2+) for the cations and Cl−/(Cl− + HCO3−) for the anions were used for illustrating the diagrams based on the concentrations of TDS.
3.2. Groundwater Quality Indices
The following indices are all necessary in this study to evaluate the quality of groundwater for irrigation [50]. These indices include sodium adsorption ratio (SAR), sodium percentage (Na%), residual sodium carbonate (RSC), Kelly’s ratio (KR), magnesium hazard (MH), and the permeability index (PI).
3.2.1. Sodium Adsorption Ratio (SAR)
The SAR value is calculated using the concentration of primary alkaline and earth alkaline cations, which is determined in units of meq/L in the water. Increased sodium levels in the soil due to the high levels of salt in irrigation water may change the soil’s permeability and cause infiltration problems. It may be possible for the soil to spread out, making it challenging to cultivate. The following equation was used to calculate SAR Keesari et al., 2016 [51]:
3.2.2. Sodium Percentage (Na%)
The estimation of Na% is crucial for managing the supply of water for agricultural applications since high sodium levels in soil and water impede plant growth by decreasing soil permeability. The following equation [52] is used to compute it:
3.2.3. Residual Sodium Carbonate (RSC)
All cations and anions are measured in meq/L. The RSC ratio is yet another sign of the water’s efficacy for irrigation. When Ca2+ and Mg2+ ions dissolve out of groundwater with a higher concentration of HCO3− and CO32−, the excess NaHCO3 and CaCO3 that results could be harmful to the soil mechanism. The following equation [53] can be used to determine residual sodium carbonate:
RSC = (CO32− + HCO3−) − (Ca+2 + Mg+2)
3.2.4. Kelly’s Ratio (KR)
The Kelly’s ratio (KR), which can be determined using the following formula [54], can be used to determine whether water has excessive levels of sodium.
3.2.5. Magnesium Hazard (MH)
Generally, Ca2+ and Mg2+ exist in equilibrium. High Mg2+ concentrations occasionally affect the equilibrium, and excessive Mg may prevent plants from growing by altering the water to more alkaline. The following formula is used to calculate the MR content [55]:
3.2.6. Permeability Index (PI)
In another way, crop yield is impacted when soil permeability is decreased by using mineral-rich water over a longer time. According to the accepted standards, the PI was calculated using the following formula [56]:
3.3. Spatial Distribution Maps
Regular monitoring and assessment of a wide range of physical and chemical properties are necessary for groundwater investigations. The collection, preservation, analysis, and visualization of both spatial and non-spatial data can be improved using geographical information systems (GIS). It is frequently used in the evaluation of hazards, spatial mapping, and water quality assessments [57,58,59]. In this work, ArcGIS was used to construct spatial distribution maps of the groundwater quality variables using the inverse distance weighted (IDW) approach.
3.4. Statistical Approaches
To determine the interrelationships between the hydrochemical factors, two correlation techniques were used: Spearman’s rank-order index and Pearson’s index, which assess monotonic correlations and measure linear linkages between variables, respectively. In addition, certain ionic ratios between elements illustrate the hydrogeochemical process that may occur.
4. Results and Discussion
4.1. Major Ion Distribution
The pH ranges from 7.8 to 8.6 and the groundwater is slightly alkaline. The average electrical conductivity (EC) is 6709 µS/cm, with a range of 3283 to 11,000 µS/cm. Each sample exceeds the EC’s maximum allowable limit of 1500 µS/cm. The substantial increase in EC values is related to anthropogenic effects and geochemical development of groundwater through rock–water interaction over an extended time. The total dissolved solids (TDS), which have an average value of 4025 mg/L, varied from 1970 to 6600 mg/L. According to the TDS characterization, all of the wells have brackish water (TDS > 1000) [60] (Table 1). There is not much variety in the research area. According to spatial distribution maps, pH, EC, and TDS values all show a similar pattern of trending toward groundwater flow (Figure 5). At samples GW08, GW09, and GW12, relatively high values of EC and TDS were found in the western regions of the basin while low values were found in the center part.

Table 1.
The physiochemical analyses of groundwater samples.
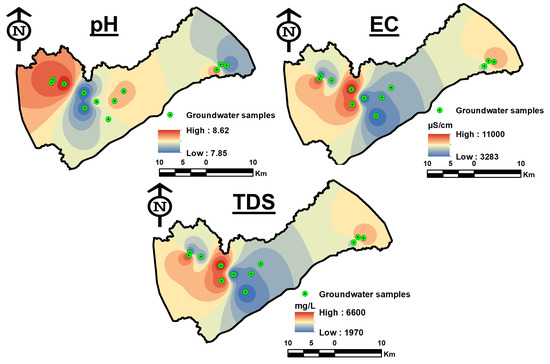
Figure 5.
Spatial distribution maps of physical parameters (pH, EC, and TDS).
The major ions in the groundwater are distributed as follows: Na+ > Ca+ > Mg2+ > K+ and Cl− > SO42− > HCO3− > NO32−. Na+ concentrations range widely from 297 to 1478 mg/L, with an average of 804.6 mg/L. Na levels in all samples were higher than the 200 mg/L acceptable limit for drinking water. Ca2+ levels in the study area range from 320 to 1240 mg/L, with an average of 628.3 mg/L. All samples exceeded the permitted limit of 200 mg/L according to WHO guidelines. The ion exchange of minerals from these region’s rocks is the main cause of Ca2+ in groundwater. Additionally, this might be brought on by the minerals CaCO3 and CaSO4 found in the soil, as well as gypsum anhydrides, dolomite, etc. The average Mg2+ concentration was 200 mg/L, but the range was 36 to 300 mg/L. All samples had Mg2+ values that are higher than the desired 100 mg/L threshold except for two samples. K+ concentrations range from 1.8 to 24.6 mg/L, with an average of 12.7 mg/L. In the study location, the spatial distribution patterns of Ca2+, Mg2+, Na+, and K+ are shown in Figure 6. Except for sodium and magnesium, major cation concentrations all decrease towards the east. The highest sodium concentrations were found in the east (GW01) and west (GW12). Regarding magnesium, the eastern direction had the maximum concentration (GW01).
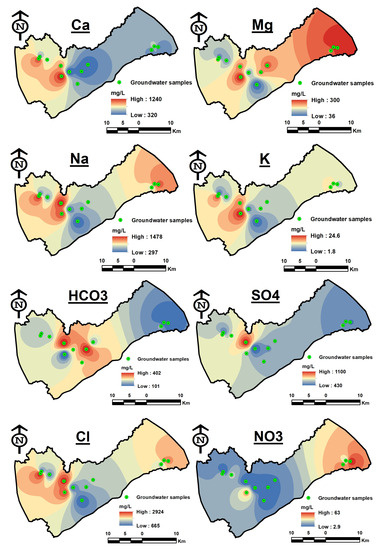
Figure 6.
Spatial distribution maps of major cations (Ca, Mg, Na, and K) and major anions (HCO3, SO4, Cl, and NO3).
The content of Cl varies between 665 and 2924 mg/L, with an average of 1579.7 mg/L. Cl concentrations in all samples were higher than the permitted limit of 250 mg/L. The average SO4 content was 630.83 mg/L, with a range of 430 to 1100 mg/L. SO4 concentration in all samples was higher than the allowed limit of 250 mg/L. The average HCO3 content was 222.5 mg/L, while the range was 101 to 401 mg/L. Figure 6 demonstrates a decrease in element concentrations (HCO3, SO4, and Cl) as groundwater flows eastward. While the western or middle regions of the basin have the highest concentrations.
The average NO32− concentration was 21.6 mg/L, with a range of 2.9 to 63 mg/L. NO32− is often low in deep and confined aquifers because of the generally decreasing situations at deeper depths. According to Trojan et al. [61] and White et al. [62], the concentration of NO32− can be utilized as an effective indicator of how land use is interacting with the groundwater ecosystem. This concentration might point to sewage pollution and the usage of fertilizers (gypsum, potassium fertilizers, etc.). It can be challenging to distinguish between municipal and/or agricultural contamination sources [63]. In many instances, the land use pattern can be used to identify the real source of NO3 pollution. Even if they have a common ancestor, there is not always a strong association between them because the percentage might be diluted by a variety of reactions in the soil horizon and vadose zone.
In contrast to the other ions, the NO32− has a different geographical distribution pattern where high levels were observed toward groundwater flow in the eastern portions of the basin (Figure 6) and comparatively low concentrations were found in wells located in the western and central regions. Figure 7 demonstrates the evidence of human activity, such as urban areas close to certain wells that recorded the highest values. These activities might be responsible for the increases in nitrate levels in the research region.
Figure 7.
The recent map displays the urban region near the wells that recorded the high nitrate concentrations.
Generally, the water quality may be suitable for other purposes, such as irrigation and agriculture, even while the element concentrations are higher than the permitted level for drinking. However, recent investigations, whether conducted within the Kingdom of Saudi Arabia [64,65,66] or other places such as Egypt [67,68], India [69], Morocco [70], and Tunisia [71] demonstrated agreement with this issue. Therefore, groundwater indices are calculated to assess the quality of groundwater for irrigation.
4.2. Scatter Matrix Plot and Correlation Analysis
Using a scatter matrix (Figure 8a), it was possible to examine the relationships and distribution of different hydrochemical parameters in the study area. The scatter matrix revealed that some factors, such as TDS and some other parameters, especially EC, have linear correlations, whereas the remaining elements either show irregular or no relationships. Most of the parameters have irregular distributions, according to the matrix’s presented histograms. The relationships between several factors that influence groundwater quality were studied and presented using two distinct correlation types: the Pearson correlation, which relies on normally distributed data for analysis, and the Spearman correlation, which does not.
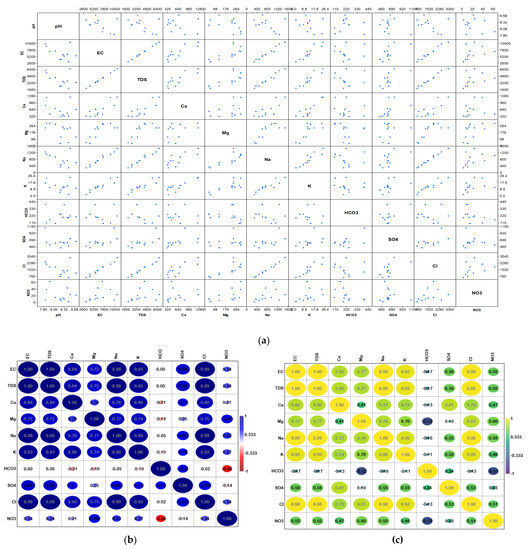
Figure 8.
(a) Scatter matric plot, (b) Pearson, and (c) Spearman matrices of correlations between the hydrochemical parameters.
Figure 8b,c shows the two correlation coefficients (r) that the two types of correlations yielded as results. Significant correlations were between 0.7 and 1 for both kinds of correlations, moderate relationships were between 0.5 and 0.7, weak correlations were from 0.5 to 0.3, while values less than 0.3 represent no correlations. Negative numbers signify negative correlations, whereas positive ones signify positive correlations. An important positive correlation suggested that there was a shared cause, whether it was artificial or natural.
According to the findings of the two-correlation analysis, TDS exhibits substantial positive correlations with EC, Na, Cl, Ca, Mg, Cl, and SO4, demonstrating that these ions regulate the aquifer’s groundwater salinity and electric conductivity, and that EC and TDS are strongly reliant. Na and Cl, Na and Ca, Na and SO4, Ca and SO4, and Ca and Cl. All show a highly positive association, with these relationships primarily resulting from natural events such as rock–water interaction and dissolution. The dissolution of rocks including gypsum, halite, and silicates.
4.3. Water Type
Using Piper’s schematic [47], groundwater facies were categorized. The ternary diagrams (Figure 9) display the enrichment trends of the Cl anion, Na+, Ca2+, and mix cation types. The plots of acceptable data on the diamond-shaped field divide the different types of groundwater into two categories: Ca-Mg-Cl and Ca-Cl water types.
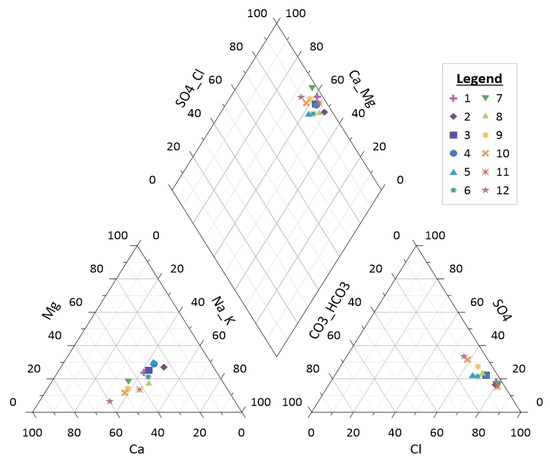
Figure 9.
Piper diagram for determining the types of water.
The majority of the sample belongs to the Ca-Cl water type, which demonstrates groundwater chemistry is accomplished by reactions in unsaturated zones, rock–water interaction, ion exchange, reverse ion exchange, and anthropogenic impacts.
4.4. Hydrochemical Process
To define groundwater properties and understand chemical modification, the findings of groundwater chemistry analyses were examined. To reveal numerous relationships that are useful in pointing to potential sources of changes in groundwater chemistry, it was necessary to convert ions to equivalent weights before plotting. The main mechanisms governing the chemistry of groundwater were identified using the Gibbs plot. The findings on the Gibbs diagram (Figure 10) reveal that the evaporation process, which is the main cause of increased salinity, results in poor groundwater quality [72,73], and controls groundwater chemistry primarily. This is generally expected because raised salinity results from evaporation considerably increasing the ion concentrations created by chemical weathering of the rock.
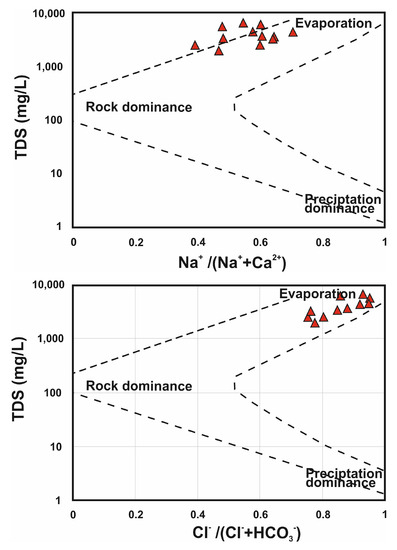
Figure 10.
Gibbs diagram illustrates the main mechanism affecting the chemistry of groundwater.
The proportions and ionic relations of the main elements found in groundwater were determined from their ionic concentrations. High correlation (r = 0.82) in the alkalis with Ca + Mg ionic abundance plot suggests mix-type patterns in concentrations. Alkalis were abundant in most of the samples, and Ca + Mg concentrations were higher in the remaining samples than they were in the alkalis (Figure 11a). According to Subba Rao et al. [74], ion-exchange processes are shown by an abundance of Na with Ca concentrations (Figure 11b) and the dominance of Ca and Mg over Na and K.
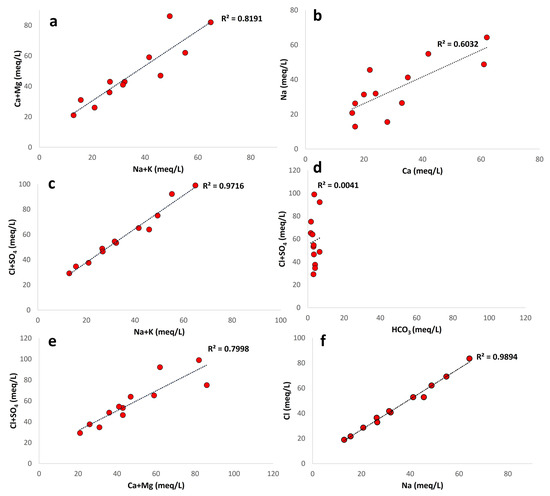
Figure 11.
Major ion ratio of the shallow aquifer: (a) Alkalis’ relationship with Ca and Mg. (b) Na versus Ca. (c) (C1 + SO4) versus alkalis. (d) (C1 + SO4) versus HCO3. (e) (C1 + SO4) versus (Ca + Mg). (f) C1 versus Na.
Alkalis have a strong preference for bonding with Cl and SO4 (Figure 11c); the current analysis, which has a positive correlation with Cl + SO4 (r = 0.97) and reveals that nearly all alkalis are consumed when bonding with Cl and SO4, excludes the origin of Na+ from anthropogenic sources. In addition, a strong association between alkalis with Cl and SO4 suggests that rocks rather than anthropogenic activity are the source of this development [75,76]. Additionally, a plot of Na and Cl demonstrates a strong correlation (r = 0.98), suggesting that the dissolution of halite may be the cause of groundwater origin (Figure 11f).
Figure 11d shows the relative abundance of the anionic facies, where Cl + SO4 is significantly more prevalent than HCO3. First, the main affinities between ionic pairs were evaluated to determine ionic affinity correlations.
The plots of Ca + Mg with Cl + SO4 have a strong association (r = 0.79), which may indicate that they came from source rocks including dolomites and gypsum (Figure 11e). In addition to geogenic processes, human activity such as sewage contamination, waste from industry, specialized fertilizers, etc., may result in SO4 enrichment into groundwater systems [77,78]. Regardless of lithology or depth, the spatial distribution of NO3 supports the prevalent anthropogenic impact on groundwater.
4.5. Compatibility for Irrigation
The impacts of the water’s mineral components on both plants and the soil determine whether groundwater is suitable for agriculture [79,80]. Estimates of sodium adsorption ratio (SAR), relative quantities of Na+ indicated by Na%, and residual sodium carbonate (RSC), Kelly’s ratio (KR), magnesium hazard (MH), and the permeability index (PI) are among the general parameters for evaluating the irrigation water quality (Table 2).

Table 2.
Summary of statical indices of groundwater quality.
4.5.1. Sodium Adsorption Ratio (SAR)
Groundwater for irrigation is classified depending on its SAR value. SAR values less than 10 (SAR < 10) are classified as “Excellent”, while those between 10 and 18 are considered “Good”. When the SAR value is between 18 and 26, it is identified “Doubtful” When the SAR value of groundwater exceeds 26, it is called “unsuitable” (SAR > 26) [81,82].
When the SAR value is greater than 10, it is regarded as unsuitable for irrigation purposes according to the World Health Organization (WHO 2011) [83] and the Food and Agriculture Organization (FAO 2017) [84]. Higher SAR values increase the danger of sodium salinity, which subsequently in turn impacts crop growth by decreasing the amount of soil water that is accessible and the proportion of the two main minerals, calcium, and magnesium. Our results showed that all groundwater samples, except for GW12 (SAR = 10.04), were appropriate for irrigation (Table 3). The SAR ranged from 3.95 to 10, averaging 6.98 (Table 2). Therefore, 91.6 of groundwater samples in the research area have SAR values in the “Excellent” range, making them acceptable for irrigation. In fact, there are certain crops that have already been cultivated. The farms there primarily produce dates and leaves, with the existence of other fruit trees such as lemon and orange.

Table 3.
Groundwater quality for irrigation according to SAR.
The spatial distribution map (Figure 12) displays areas that are characterized by high values in the east (GW01) and in the center of the research region (GW09), which recorded values close to 10, as well as GW12 located in the western region.
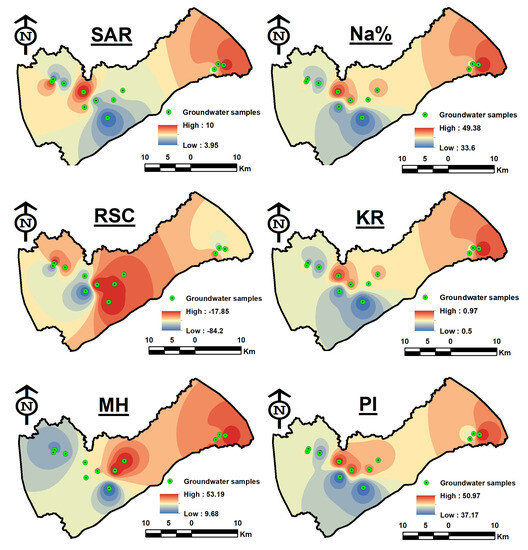
Figure 12.
Spatial distribution maps of irrigation water quality indices.
4.5.2. Sodium Percentage (Na%)
The Na% in the groundwater samples varied from 33.6 to 49.3%, with an average of 41.84% (Table 2). Na% values within ranges 20–40 and 40–60 were recognized as being good and permissible for irrigation [66]. Four groundwater samples were found to be good for irrigation, while eight groundwater samples were permissible for irrigation uses (Table 4). Excessive salt concentrations in soil harm aeration, infiltration, and soil composition [85]. As seen in Figure 12, there is a similarity between Na% and SAR, where the direction of increase is towards the east (GW01), as well as GW09 in the middle of the study region.

Table 4.
Groundwater quality for irrigation according to Na%.
4.5.3. Residual Sodium Carbonate (RSC)
Agriculture suffers when water exceeds acceptable limits for carbonate and bicarbonate over alkaline earth, especially calcium and magnesium [79,86]. Organic matter is essential for agricultural productivity; however, high HCO3 concentrations cause an increase in pH levels, which dissolve organic matter. To discriminate between the various water classes for irrigation, residual sodium carbonate is used. RSC is considered to be a helpful method for figuring out whether groundwater is acceptable for irrigation through the determination of the carbonate and bicarbonate ratio [87]. By precipitating calcium as CO2, the extra sodium ion opposes the excesses of calcium and magnesium and produces a negative RSC value in the process. Additionally, the positive value of RSC indicates the increased concentration of calcium and magnesium, which is caused by the interaction with HCO3 to produce calcium bicarbonate and magnesium bicarbonate [88,89]. RSC levels were categorized as excellent for irrigation purposes if they were less than 1.25, dubious if they were between 1.25 and 2.5, and unsuitable if they were more than 2.5 [66]. RSC levels in the groundwater samples ranged from −84.21 to −17.85, and an average value of −44.44. The findings revealed that the groundwater samples were appropriate for irrigation (Table 5). In contrast to the spatial distribution maps of the other ratios, the highest readings for this index were found at GW05, GW06, and GW07 in the center of the research region (Figure 12).

Table 5.
Groundwater quality for irrigation according to RSC.
4.5.4. Kelley’s Ratio (KR)
According to Kelley et al. [90], the values of Kelley’s ratio might be used to effectively solve the sodium issue with irrigational water. When Kelley’s index is greater than one, there is excessive sodium in the water. As a result, water with Kelley’s index below one is appropriate for irrigation, whereas water with a ratio above one is not. The results showed that groundwater samples within the study area varied from 0.5 to 0.97 with an average of 0.72. This means 100% of KR values are less than one (Table 6), indicating that the groundwater was acceptable for irrigation. According to Figure 12, GW01 in the east appears to have recorded the highest value, followed by GW09 in the center of the research region.

Table 6.
Groundwater quality for irrigation according to KR.
4.5.5. Magnesium Hazard (MH)
In most fluids, Ca2+ and Mg2+ usually keep an equilibrium state; however, in soil systems, Ca2+ and Mg2+ react differently, and Mg2+ deteriorates soil structure, especially in waters that are salt predominant and extremely saline [91]. High levels of Mg2+ are typically caused by exchangeable Na+ in irrigation soils. In a state of equilibrium increased Mg2+ concentrations in water will have a negative impact on soil quality, converting it to alkaline and negatively affecting agricultural yields. Magnesium has the potential to be more harmful to soil than calcium. Magnesium concentrations range from 9.68 to 53.19, as shown in Table 2, with an average of 34.85. Nine groundwater samples were thought to be below 50%, making them acceptable for irrigation while three groundwater samples were above 50% and therefore unsuitable for irrigation (Table 7). The greatest values were found in GW01 in the east, followed by GW05 and GW04 in the research area’s center (Figure 12). The most probable causes of the excessive levels of sodium in the water are the utilization of agrochemicals in agricultural practices and lithological origins resulting in the dissolution of these minerals in the water.

Table 7.
Groundwater quality for irrigation according to MH.
4.5.6. Permeability Index (PI)
Findings varied from 37.17 to 50.97, with 44.37 on average (Table 2). PI can be divided into three categories, according to Doneen (1964) [92]: class I (>75%, appropriate), class II (25–75%, good), and class III (25%, unsuitable). It is advised to use water under classes I and II for irrigation. These results showed that the collected groundwater samples had PI levels between 25 and 75, which be regarded as good for irrigation (Table 8) and are consistent with previous research [69,70]. Figure 12 illustrates the spatial distribution, particularly GW07 and GW09 in the middle and GW01 in the east had the greatest values.

Table 8.
Groundwater quality for irrigation according to PI.
Although integrated methodologies were used to assess groundwater quality and its suitability for irrigation in the study region, there are still certain limitations that need to be considered in future research. For instance, the distribution of groundwater samples in the research area is not perfect, despite the use of geospatial modeling and other statistical methodologies to solve this issue. Temporal changes will also be considered in future studies, which may give more accurate results. The use of the extra methodologies explains the variances in the study area’s hydrogeochemistry. To identify the degree to which the hydrogeochemical processes and the origin of groundwater have affected the research region, additional hydrochemical analyses such as heavy metals and isotopic analysis should be performed.
5. Conclusions
In arid regions, groundwater stands as one of the most reliable water sources. Saudi Arabia exemplifies such an area, reliant on this enduring and consistent supply, particularly within ongoing agricultural initiatives. This study aimed to ascertain the chemical composition of this groundwater and its viability for irrigation purposes. A total of twelve groundwater samples were procured for subsequent chemical analysis.
The outcomes unveiled discernible ion patterns, with Na+ > Ca+ > Mg2+ > K+ and Cl− > SO42− > HCO3− > NO32− prevailing among the major ions. The groundwater exhibited characteristics of Ca-Cl and Ca-Mg-Cl types, demonstrating an average salinity of 4025 mg/L, potentially attributed to the evaporation process. The question of whether this groundwater is suitable for irrigation necessitates further investigation. To ascertain irrigation feasibility, various contamination indices were employed, including the permeability index (PI), magnesium hazard (MH), Kelly’s ratio (KR), sodium adsorption ratio (SAR), residual sodium carbonate (RSC), and sodium percentage (Na%).
Based on SAR analysis, 91.6% of the samples fall within the low-sodium category, while the remaining samples are categorized as medium sodium, indicating excellent and good suitability for irrigation water. Employing the Na% criterion, only 33.3% of the evaluated water samples exhibit a favorable irrigation classification, with 66.7% being deemed permissible. The entirety of the investigated groundwater samples demonstrates suitability for irrigation, as per the residual sodium carbonate (RSC) parameter. In terms of Kelly’s ratio (KR), all samples exhibit values below unity, signifying their suitability for irrigation. In consideration of the magnesium hazard (MH), 75% of the analyzed water samples are deemed suitable, whereas 25% are determined unsuitable for irrigation. All assessed samples belong to the favorable permeability index (PI) classes, thus indicating their viability for use in irrigation practices.
Overall, the results of this study indicate that a variety of factors, natural and human activity, affect groundwater quality in the studied area. Additionally, groundwater samples are suitable for irrigation purposes. But to maintain the long-term quantitative and qualitative purity of this resource, it is essential to use groundwater carefully, supported by a thorough, long-term investigation.
Author Contributions
Conceptualization, H.M.A., E.M.M.E.-B. and M.A.; methodology, H.M.A., E.M.M.E.-B. and M.A.; funding acquisition, H.M.A. and E.M.M.E.-B.; validation, H.M.A. and E.M.M.E.-B.; statical analysis, M.A.; writing—original draft preparation M.A., writing—review and editing, H.M.A., E.M.M.E.-B. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R243), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aly, A.A.; Al-Omran, A.M.; Alharby, M.M. The Water Quality Index and Hydrochemical Characterization of Groundwater Resources in Hafar Albatin, Saudi Arabia. Arab. J. Geosci. 2015, 8, 4177–4190. [Google Scholar] [CrossRef]
- Gamal, G.; Hassan, T.M.; Gaber, A.; Abdelfattah, M. Groundwater Quality Assessment along the West of New Damietta Coastal City of Egypt Using an Integrated Geophysical and Hydrochemical Approaches. Environ. Earth Sci. 2023, 82, 107. [Google Scholar] [CrossRef]
- Abdelfattah, M.; Abu-Bakr, H.A.-A.; Mewafy, F.M.; Hassan, T.M.; Geriesh, M.H.; Saber, M.; Gaber, A. Hydrogeophysical and Hydrochemical Assessment of the Northeastern Coastal Aquifer of Egypt for Desalination Suitability. Water 2023, 15, 423. [Google Scholar] [CrossRef]
- Alharbi, T.G. Identification of Hydrogeochemical Processes and Their Influence on Groundwater Quality for Drinking and Agricultural Usage in Wadi Nisah, Central Saudi Arabia. Arab. J. Geosci. 2018, 11, 359. [Google Scholar] [CrossRef]
- Alshehri, F.; Almadani, S.; El-Sorogy, A.S.; Alwaqdani, E.; Alfaifi, H.J.; Alharbi, T. Influence of Seawater Intrusion and Heavy Metals Contamination on Groundwater Quality, Red Sea Coast, Saudi Arabia. Mar. Pollut. Bull. 2021, 165, 112094. [Google Scholar] [CrossRef] [PubMed]
- Al-Omran, A.M.; Aly, A.A.; Al-Wabel, M.I.; Sallam, A.S.; Al-Shayaa, M.S. Hydrochemical Characterization of Groundwater under Agricultural Land in Arid Environment: A Case Study of Al-Kharj, Saudi Arabia. Arab. J. Geosci. 2016, 9, 68. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Aly, A.A.; Aldhumri, S.A.; Al-Barakaha, F.N. Hydrochemical and Quality Assessment of Groundwater Resources in Al-Madinah City, Western Saudi Arabia. Sustainability 2020, 12, 3106. [Google Scholar] [CrossRef]
- Saud, A.G.; Abdullah, S.A. Water Resources and Reuse in Al-Madinah. In Proceedings of the International Conference on Water Conservation in Arid Regions (ICWCAR’09), Water Research Center-King Abdulaziz University, Jeddah, Saudi Arabia, 12–14 October 2009; pp. 12–14. [Google Scholar]
- Ali, I.; Hasan, M.A.; Alharbi, O.M.L. Toxic Metal Ions Contamination in the Groundwater, Kingdom of Saudi Arabia. J. Taibah Univ. Sci. 2020, 14, 1571–1579. [Google Scholar] [CrossRef]
- Subyani, A.M. Hydrochemical Identification and Salinity Problem of Ground-Water in Wadi Yalamlam Basin, Western Saudi Arabia. J. Arid Environ. 2005, 60, 53–66. [Google Scholar] [CrossRef]
- Hejazi, R.F. Investigation of Leachate from a Sanitary Landfill in Saudi Arabia. Ph.D Thesis, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia, 1989. [Google Scholar]
- Al-Arifi, S.N.; Al-Agha, R.M.; El-Nahhal, Z.Y. Environmental Impact of Landfill on Groundwater, South East of Riyadh, Saudi Arabia. J. Nat. Sci. Res. 2013, 3, 222–242. [Google Scholar]
- Rehman, F.; Cheema, T. Effects of Sewage Waste Disposal on the Groundwater Quality and Agricultural Potential of a Floodplain near Jeddah, Saudi Arabia. Arab. J. Geosci. 2016, 9, 307. [Google Scholar] [CrossRef]
- Alyamani, M.S. Effects of Cesspool Systems on Groundwater Quality of Shallow Bedrock Aquifer in the Recharge Area of Wadi Fatimah, Western Arabian Shield, Saudi Arabia. J. Environ. Hydrol. 2007, 15, 1. [Google Scholar]
- Al-Shaibani, A.M. Hydrogeology and Hydrochemistry of a Shallow Alluvial Aquifer, Western Saudi Arabia. Hydrogeol. J. 2008, 16, 155–165. [Google Scholar] [CrossRef]
- Alabdula’aly, A.I.; Al-Rehaili, A.M.; Al-Zarah, A.I.; Khan, M.A. Assessment of Nitrate Concentration in Groundwater in Saudi Arabia. Environ. Monit. Assess. 2010, 161, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.; Cheema, T. Boron Contamination in Groundwater at a Sewage Waste Disposal Facility near Jeddah, Saudi Arabia. Environ. Earth Sci. 2017, 76, 218. [Google Scholar] [CrossRef]
- Al-Oud, S.S. Impact of Municipal and Industrial Waste on the Distribution and Accumulation of Some Heavy Metals in Sandy Soils of Al-Qassim Region at Central of Saudi Arabia. J. Environ. Sci. Technol. 2008, 1, 135–142. [Google Scholar] [CrossRef]
- Bamousa, A.O.; El Maghraby, M. Groundwater Characterization and Quality Assessment, and Sources of Pollution in Madinah, Saudi Arabia. Arab. J. Geosci. 2016, 9, 536. [Google Scholar] [CrossRef]
- Fallatah, O.A. Groundwater Quality Patterns and Spatiotemporal Change in Depletion in the Regions of the Arabian Shield and Arabian Shelf. Arab. J. Sci. Eng. 2020, 45, 341–350. [Google Scholar] [CrossRef]
- Abderrahman, W.A.; Rasheeduddin, M.; Al-Harazin, I.M.; Esuflebbe, M.; Eqnaibi, B.S. Impacts of Management Practices on Groundwater Conditions in the Eastern Province, Saudi Arabia. Hydrogeol. J. 1995, 3, 32–41. [Google Scholar] [CrossRef]
- Al-Ibrahim, A.A. Excessive Use of Groundwater Resources in Saudi Arabia: Impacts and Policy Options. Ambio 1991, 20, 34–37. [Google Scholar]
- Ben Brahim, F.; Khanfir, H.; Bouri, S. Groundwater Vulnerability and Risk Mapping of the Northern Sfax Aquifer, Tunisia. Arab. J. Sci. Eng. 2012, 37, 1405–1421. [Google Scholar] [CrossRef]
- Alkolibi, F.M. Possible Effects of Global Warming on Agriculture and Water Resources in Saudi Arabia: Impacts and Responses. Clim. Chang. 2002, 54, 225–245. [Google Scholar] [CrossRef]
- Corteel, C.; Dini, A.; Deyhle, A. Element and Isotope Mobility during Water–Rock Interaction Processes. Phys. Chem. Earth Parts A/B/C 2005, 30, 993–996. [Google Scholar] [CrossRef]
- Reghunath, R.; Murthy, T.R.S.; Raghavan, B.R. The Utility of Multivariate Statistical Techniques in Hydrogeochemical Studies: An Example from Karnataka, India. Water Res. 2002, 36, 2437–2442. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Rina, K.; Singh, R.P.; Shashtri, S.; Kamal, V.; Mukherjee, S. Geochemical Modeling of High Fluoride Concentration in Groundwater of Pokhran Area of Rajasthan, India. Bull. Environ. Contam. Toxicol. 2011, 86, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Al-Bassam, A.M. Evaluation of Ground Water Quality in Al-Qassim Area, Saudi Arabia, Using Cluster and Factor Analyses. Kuwait J. Sci. Eng. 2006, 33, 101. [Google Scholar]
- Haider, H.; Al-Salamah, I.S.; Ghumman, A.R. Development of Groundwater Quality Index Using Fuzzy-Based Multicriteria Analysis for Buraydah, Qassim, Saudi Arabia. Arab. J. Sci. Eng. 2017, 42, 4033–4051. [Google Scholar] [CrossRef]
- Alfaifi, H.; El-Sorogy, A.S.; Qaysi, S.; Kahal, A.; Almadani, S.; Alshehri, F.; Zaidi, F.K. Evaluation of Heavy Metal Contamination and Groundwater Quality along the Red Sea Coast, Southern Saudi Arabia. Mar. Pollut. Bull. 2021, 163, 111975. [Google Scholar] [CrossRef]
- Mallick, J. Hydrogeochemical Characteristics and Assessment of Water Quality in the Al-Saad Lake, Abha Saudi Arabia. Appl. Water Sci. 2017, 7, 2869–2882. [Google Scholar] [CrossRef]
- Al-Omran, A.M.; Aly, A.A.; Al-Wabel, M.I.; Al-Shayaa, M.S.; Sallam, A.S.; Nadeem, M.E. Geostatistical Methods in Evaluating Spatial Variability of Groundwater Quality in Al-Kharj Region, Saudi Arabia. Appl. Water Sci. 2017, 7, 4013–4023. [Google Scholar] [CrossRef]
- Mallick, J.; Singh, C.K.; AlMesfer, M.K.; Kumar, A.; Khan, R.A.; Islam, S.; Rahman, A. Hydro-Geochemical Assessment of Groundwater Quality in Aseer Region, Saudi Arabia. Water 2018, 10, 1847. [Google Scholar] [CrossRef]
- Dixon, B. Groundwater Vulnerability Mapping: A GIS and Fuzzy Rule Based Integrated Tool. Appl. Geogr. 2005, 25, 327–347. [Google Scholar] [CrossRef]
- Marko, K.; Al-Amri, N.S.; Elfeki, A.M.M. Geostatistical Analysis Using GIS for Mapping Groundwater Quality: Case Study in the Recharge Area of Wadi Usfan, Western Saudi Arabia. Arab. J. Geosci. 2014, 7, 5239–5252. [Google Scholar] [CrossRef]
- Dhanasekarapandian, M.; Chandran, S.; Devi, D.S.; Kumar, V. Spatial and Temporal Variation of Groundwater Quality and Its Suitability for Irrigation and Drinking Purpose Using GIS and WQI in an Urban Fringe. J. Afr. Earth Sci. 2016, 124, 270–288. [Google Scholar] [CrossRef]
- Elumalai, V.; Brindha, K.; Sithole, B.; Lakshmanan, E. Spatial Interpolation Methods and Geostatistics for Mapping Groundwater Contamination in a Coastal Area. Environ. Sci. Pollut. Res. 2017, 24, 11601–11617. [Google Scholar] [CrossRef]
- Manoj, S.; Thirumurugan, M.; Elango, L. An Integrated Approach for Assessment of Groundwater Quality in and around Uranium Mineralized Zone, Gogi Region, Karnataka, India. Arab. J. Geosci. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Goovaerts, P. Geostatistics for Natural Resources Evaluation; Applied Geostatistics; Oxford University Press: New York, NY, USA, 1997; ISBN 0195115384. [Google Scholar]
- Mirzaei, R.; Sakizadeh, M. Comparison of Interpolation Methods for the Estimation of Groundwater Contamination in Andimeshk-Shush Plain, Southwest of Iran. Environ. Sci. Pollut. Res. 2016, 23, 2758–2769. [Google Scholar] [CrossRef]
- Masoud, M.H.Z.; Basahi, J.M.; Rajmohan, N. Impact of Flash Flood Recharge on Groundwater Quality and Its Suitability in the Wadi Baysh Basin, Western Saudi Arabia: An Integrated Approach. Environ. Earth Sci. 2018, 77, 395. [Google Scholar] [CrossRef]
- Yihdego, Y.; Drury, L. Mine Water Supply Assessment and Evaluation of the System Response to the Designed Demand in a Desert Region, Central Saudi Arabia. Environ. Monit. Assess. 2016, 188, 1–21. [Google Scholar] [CrossRef]
- Riyadh, Saudi Arabia: Annual Weather Averages. Available online: https://www.holiday-weather.com/riyadh/averages/#chart-head-temperature (accessed on 10 July 2023).
- Al Saud, M.; Rausch, R. Integrated Groundwater Management in the Kingdom of Saudi Arabia. Proc. Hydrogeol. Arid Environ. 2012, 1–6. [Google Scholar]
- Abderrahman, W.A. Saudi Arabia Aquifers. In Non-Renewable Groundwater Resources: A Guidebook on Socially-Sustainable Management for Water-Policy Makers; UNESCO: Paris, France, 2006; pp. 63–67. [Google Scholar]
- Association, A.P.H. APHA Standard Methods for the Examination of Water and Wastewater. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Piper, A.M. A Graphic Procedure in the Geochemical Interpretation of Water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Chadha, D.K. A Proposed New Diagram for Geochemical Classification of Natural Waters and Interpretation of Chemical Data. Hydrogeol. J. 1999, 7, 431–439. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Shetty, B.K.; Guddattu, V.; Chourasia, M.K.; Pundir, P. High Prevalence of Dental Fluorosis among Adolescents Is a Growing Concern: A School Based Cross-Sectional Study from Southern India. Environ. Health Prev. Med. 2017, 22, 17. [Google Scholar] [CrossRef]
- Keesari, T.; Ramakumar, K.L.; Chidambaram, S.; Pethperumal, S.; Thilagavathi, R. Understanding the Hydrochemical Behavior of Groundwater and Its Suitability for Drinking and Agricultural Purposes in Pondicherry Area, South India–a Step towards Sustainable Development. Groundw. Sustain. Dev. 2016, 2, 143–153. [Google Scholar] [CrossRef]
- Kopittke, P.M.; So, H.B.; Menzies, N.W. Effect of Ionic Strength and Clay Mineralogy on Na–Ca Exchange and the SAR–ESP Relationship. Eur. J. Soil Sci. 2006, 57, 626–633. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gupta, I.C. Management of Saline Soils and Waters; Oxford & IBH Publishing Co.: New Delhi, India, 1987; ISBN 0120402068. [Google Scholar]
- Hao, C.; Huang, Y.; Ma, D.; Fan, X. Hydro-Geochemistry Evolution in Ordovician Limestone Water Induced by Mountainous Coal Mining: A Case Study from North China. J. Mt. Sci. 2020, 17, 614–623. [Google Scholar] [CrossRef]
- Abdulhussein, F.M. Hydrochemical Assessment of Groundwater of Dibdibba Aquifer in Al-Zubair Area, Basra, South of Iraq and Its Suitability for IrrigationPurposes. Iraqi J. Sci. 2018, 59, 135–143. [Google Scholar]
- Falowo, O.O.; Akindureni, Y.; Ojo, O. Irrigation and Drinking Water Quality Index Determination for Groundwater Quality Evaluation in Akoko Northwest and Northeast Areas of Ondo State, Southwestern Nigeria. Am. J. Water Sci. Eng. 2017, 3, 50–60. [Google Scholar] [CrossRef]
- Tiwari, K.; Goyal, R.; Sarkar, A. GIS-Based Spatial Distribution of Groundwater Quality and Regional Suitability Evaluation for Drinking Water. Environ. Process. 2017, 4, 645–662. [Google Scholar] [CrossRef]
- Zolekar, R.B.; Bhagat, V.S. Multi-Criteria Land Suitability Analysis for Agriculture in Hilly Zone: Remote Sensing and GIS Approach. Comput. Electron. Agric. 2015, 118, 300–321. [Google Scholar]
- Duarte, L.B.; Pereira, J.A. Open Source GIS Tools: Two Environmental Applications. GIS-Overv. Appl. 2018, 1, 102–123. [Google Scholar]
- Freeze, R.; Cherry, J.A. Groundwater; Prentice-Hall: Englewood Cliffs, NJ, USA, 1979. [Google Scholar]
- Trojan, M.D.; Maloney, J.S.; Stockinger, J.M.; Eid, E.P.; Lahtinen, M.J. Effects of Land Use on Ground Water Quality in the Anoka Sand Plain Aquifer of Minnesota. Groundwater 2003, 41, 482–492. [Google Scholar] [CrossRef] [PubMed]
- White, P.; Ruble, C.L.; Lane, M.E. The Effect of Changes in Land Use on Nitrate Concentration in Water Supply Wells in Southern Chester County, Pennsylvania. Environ. Monit. Assess. 2013, 185, 643–651. [Google Scholar] [CrossRef]
- Gold, A.J.; DeRagon, W.R.; Sullivan, W.M.; Lemunyon, J.L. Nitrate-Nitrogen Losses to Groundwater from Rural and Suburban Land Uses. J. Soil Water Conserv. 1990, 45, 305–310. [Google Scholar]
- Alshehri, F.; El-Sorogy, A.S.; Almadani, S.; Aldossari, M. Groundwater Quality Assessment in Western Saudi Arabia Using GIS and Multivariate Analysis. J. King Saud Univ. 2023, 35, 102586. [Google Scholar] [CrossRef]
- Alrowais, R.; Abdel Daiem, M.M.; Li, R.; Maklad, M.A.; Helmi, A.M.; Nasef, B.M.; Said, N. Groundwater Quality Assessment for Drinking and Irrigation Purposes at Al-Jouf Area in KSA Using Artificial Neural Network, GIS, and Multivariate Statistical Techniques. Water 2023, 15, 2982. [Google Scholar] [CrossRef]
- Fallatah, O.; Khattab, M.R. Evaluation of Groundwater Quality and Suitability for Irrigation Purposes and Human Consumption in Saudi Arabia. Water 2023, 15, 2352. [Google Scholar] [CrossRef]
- Ismail, E.; Snousy, M.G.; Alexakis, D.E.; Gamvroula, D.E.; Howard, G.; El Sayed, E.; Ahmed, M.S.; Ali, A.; Abdelhalim, A. Multivariate Statistical Analysis and Geospatial Mapping for Assessing Groundwater Quality in West El Minia District, Egypt. Water 2023, 15, 2909. [Google Scholar] [CrossRef]
- Gad, M.; Saleh, A.H.; Hussein, H.; Elsayed, S.; Farouk, M. Water Quality Evaluation and Prediction Using Irrigation Indices, Artificial Neural Networks, and Partial Least Square Regression Models for the Nile River, Egypt. Water 2023, 15, 2244. [Google Scholar] [CrossRef]
- Rawat, K.S.; Singh, S.K.; Gautam, S.K. Assessment of Groundwater Quality for Irrigation Use: A Peninsular Case Study. Appl. Water Sci. 2018, 8, 233. [Google Scholar] [CrossRef]
- Amrani, S.; Hinaje, S.; El Fartati, M.; Gharmane, Y.; Yaagoub, D. Assessment of Groundwater Quality for Drinking and Irrigation in the Timahdite–Almis Guigou Area (Middle Atlas, Morocco). Appl. Water Sci. 2022, 12, 82. [Google Scholar] [CrossRef]
- Ali, Z.I.; Gharbi, A.; Zairi, M. Evaluation of Groundwater Quality in Intensive Irrigated Zone of Northeastern Tunisia. Groundw. Sustain. Dev. 2020, 11, 100482. [Google Scholar]
- Subba Rao, N. Geochemistry of Groundwater in Parts of Guntur District, Andhra Pradesh, India. Environ. Geol. 2002, 41, 552–562. [Google Scholar] [CrossRef]
- Srinivasamoorthy, K.; Chidambaram, S.; Prasanna, M.V.; Vasanthavihar, M.; Peter, J.; Anandhan, P. Identification of Major Sources Controlling Groundwater Chemistry from a Hard Rock Terrain—A Case Study from Mettur Taluk, Salem District, Tamil Nadu, India. J. Earth Syst. Sci. 2008, 117, 49–58. [Google Scholar] [CrossRef]
- Rao, N.S.; Rao, P.S.; Reddy, G.V.; Nagamani, M.; Vidyasagar, G.; Satyanarayana, N. Chemical Characteristics of Groundwater and Assessment of Groundwater Quality in Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ. Monit. Assess. 2012, 184, 5189–5214. [Google Scholar] [CrossRef]
- Sarin, M.M.; Krishnaswami, S.; Dilli, K.; Somayajulu, B.L.K.; Moore, W.S. Major Ion Chemistry of the Ganga-Brahmaputra River System: Weathering Processes and Fluxes to the Bay of Bengal. Geochim. Cosmochim. Acta 1989, 53, 997–1009. [Google Scholar] [CrossRef]
- Datta, P.S.; Bhattacharya, S.K.; Tyagi, S.K. 18O Studies on Recharge of Phreatic Aquifers and Groundwater Flow-Paths of Mixing in the Delhi Area. J. Hydrol. 1996, 176, 25–36. [Google Scholar] [CrossRef]
- Umar, R.; Ahmed, I. Hydrochemical Characteristics of Groundwater in Parts of Krishni-Yamuna Basin, Muzaffarnagar District, UP. Geol. Soc. India 2007, 69, 989–995. [Google Scholar]
- Umar, R.; Ahmed, I.; Alam, F.; Khan, M.M. Hydrochemical Characteristics and Seasonal Variations in Groundwater Quality of an Alluvial Aquifer in Parts of Central Ganga Plain, Western Uttar Pradesh, India. Environ. Geol. 2009, 58, 1295–1300. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; US Government Printing Office: Washington, DC, USA, 1954.
- Wilcox, L. Classification and Use of Irrigation Waters; US Department of Agriculture: Washington, DC, USA, 1955.
- Todd, D.K.; Mays, L.W. Groundwater Hydrology; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0471059374. [Google Scholar]
- Sadashivaiah, C.; Ramakrishnaiah, C.R.; Ranganna, G. Hydrochemical Analysis and Evaluation of Groundwater Quality in Tumkur Taluk, Karnataka State, India. Int. J. Environ. Res. Public Health 2008, 5, 158–164. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinkingwater Quality, 4th ed.; WHO Press: Geneva, Switzerland, 2011. [Google Scholar]
- FAO. Water for Sustainable Food and Agriculture: A Report Produced for the G20 Presidency of Germany; FAO: Rome, Italy, 2017. [Google Scholar]
- Chidambaram, S.; Anandhan, P.; Prasanna, M.V.; Thivya, C.; Thilagavathi, R.; Sarathidasan, J. Hydrochemistry of Groundwater in a Coastal Region and Its Repercussion on Quality, a Case Study—Thoothukudi District, Tamil Nadu, India. Arab. J. Geosci. 2014, 7, 939–950. [Google Scholar]
- Eaton, F.M. Significant of Carbonate in Irrigation Waters. Soil Sci. 1950, 59, 123–133. [Google Scholar] [CrossRef]
- Subba Rao, N.; Marghade, D.; Dinakar, A.; Chandana, I.; Sunitha, B.; Ravindra, B.; Balaji, T. Geochemical Characteristics and Controlling Factors of Chemical Composition of Groundwater in a Part of Guntur District, Andhra Pradesh, India. Environ. Earth Sci. 2017, 76, 747. [Google Scholar] [CrossRef]
- Chitsazan, M.; Aghazadeh, N.; Mirzaee, Y.; Golestan, Y.; Mosavi, S. Hydrochemical Characteristics and Quality Assessment of Urban Groundwater in Urmia City, NW Iran. Water Sci. Technol. Water Supply 2017, 17, 1410–1425. [Google Scholar] [CrossRef]
- Obiefuna, G.I.; Sheriff, A. Assessment of Shallow Ground Water Quality of Pindiga Gombe Area, Yola Area, NE, Nigeria for Irrigation and Domestic Purposes. Res. J. Environ. Earth Sci. 2011, 3, 131–141. [Google Scholar]
- Kelley, W.P.; Brown, S.M.; Liebig, G.F., Jr. Chemical Effects of Saline Irrigation Water on Soils. Soil Sci. 1940, 49, 95–108. [Google Scholar] [CrossRef]
- Ravikumar, P.; Venkatesharaju, K.; Prakash, K.L.; Somashekar, R.K. Geochemistry of Groundwater and Groundwater Prospects Evaluation, Anekal Taluk, Bangalore Urban District, Karnataka, India. Environ. Monit. Assess. 2011, 179, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Doneen, L.D. Notes on Water Quality in Agriculture, Water Science and Engineering Paper 4001; Department of Water Science and Engineering, University of California: Davis, CA, USA, 1964. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).