Excess Sludge Disintegration by Discharge Plasma Coupled with Thiosulfate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sludge Collection and Chemicals
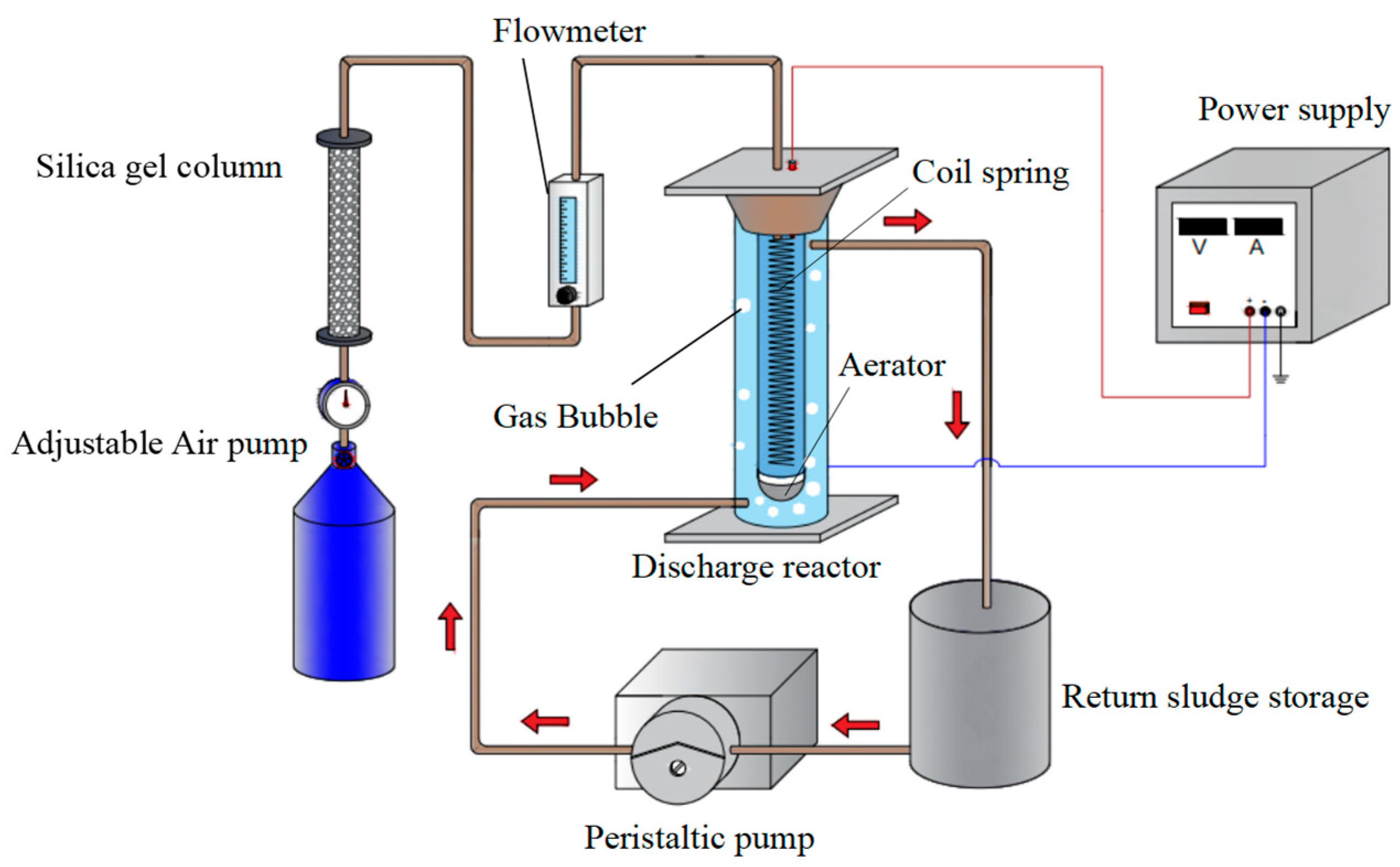
2.2. Experimental Section
2.3. Analytical Methods
3. Results and Discussion
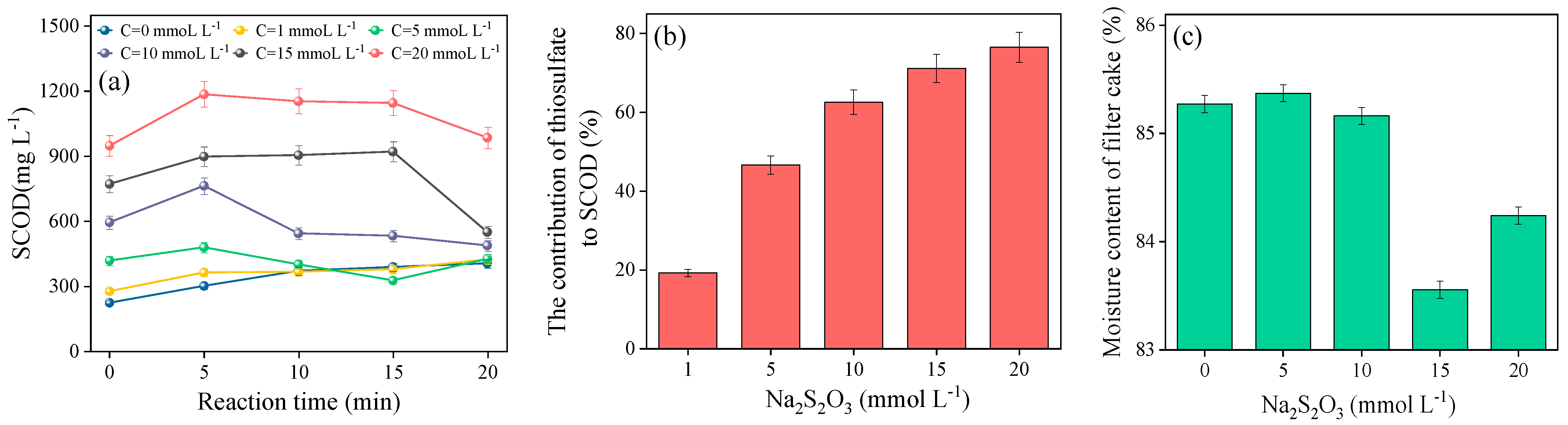
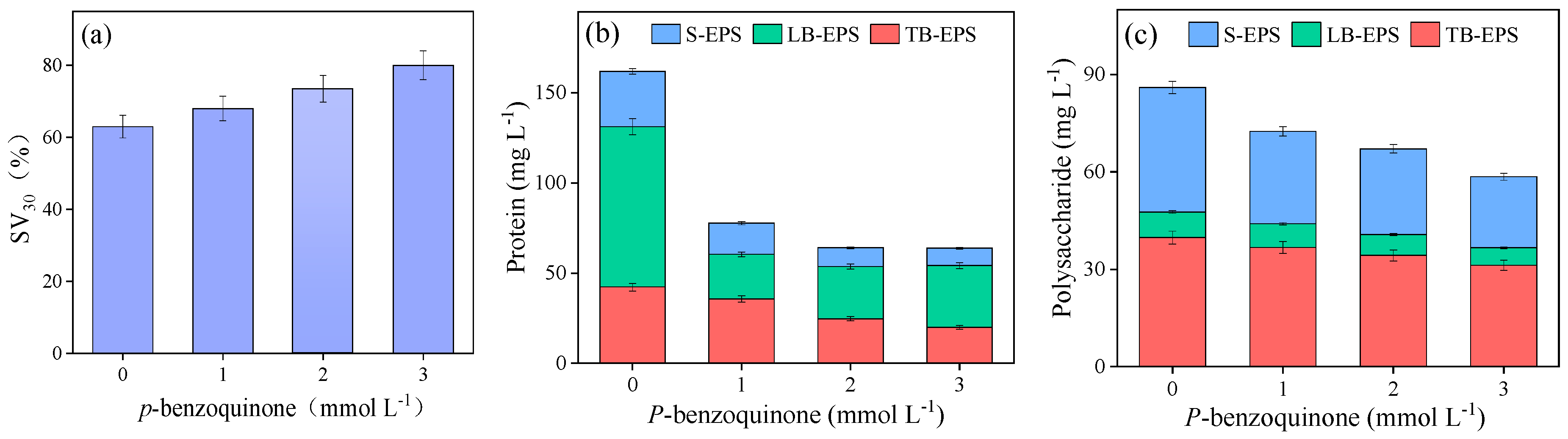
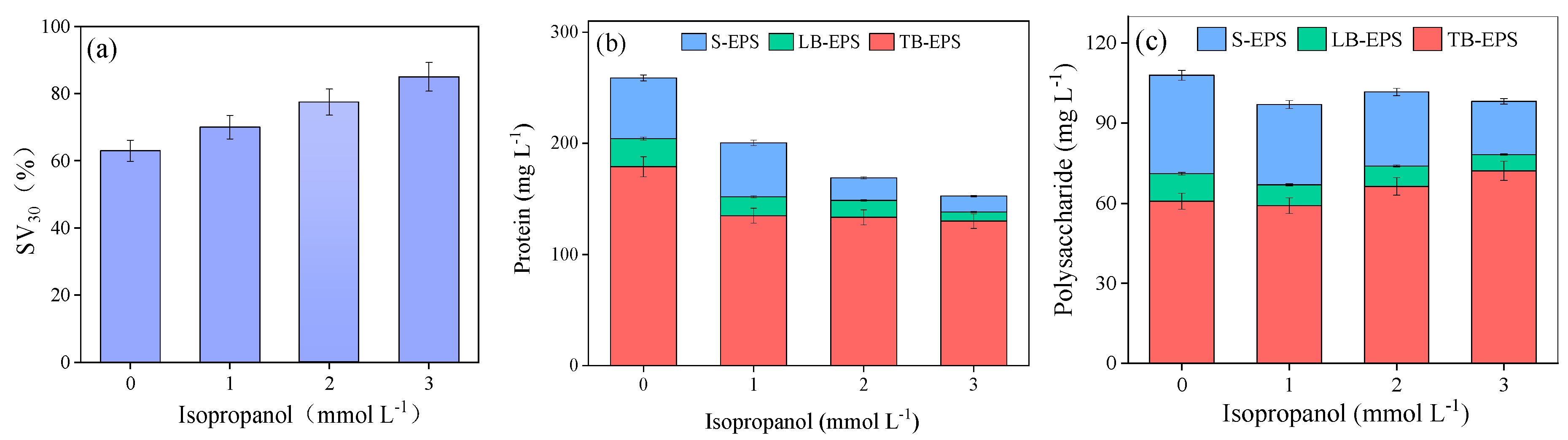
3.1. Sewage Sludge Disintegration Performance
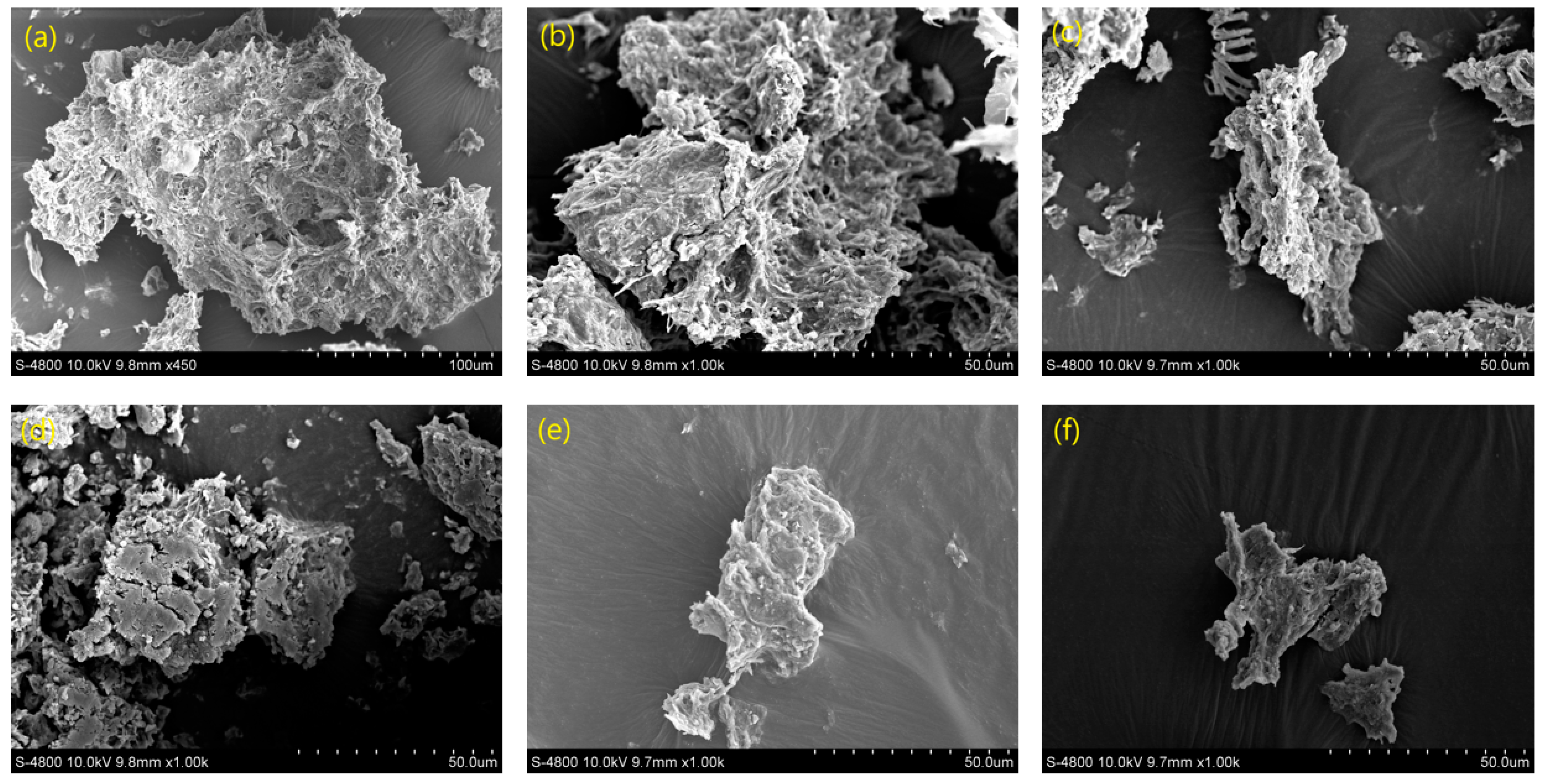
3.2. Changes in the Sludge Particle Size and Morphology during the Dewatering Process
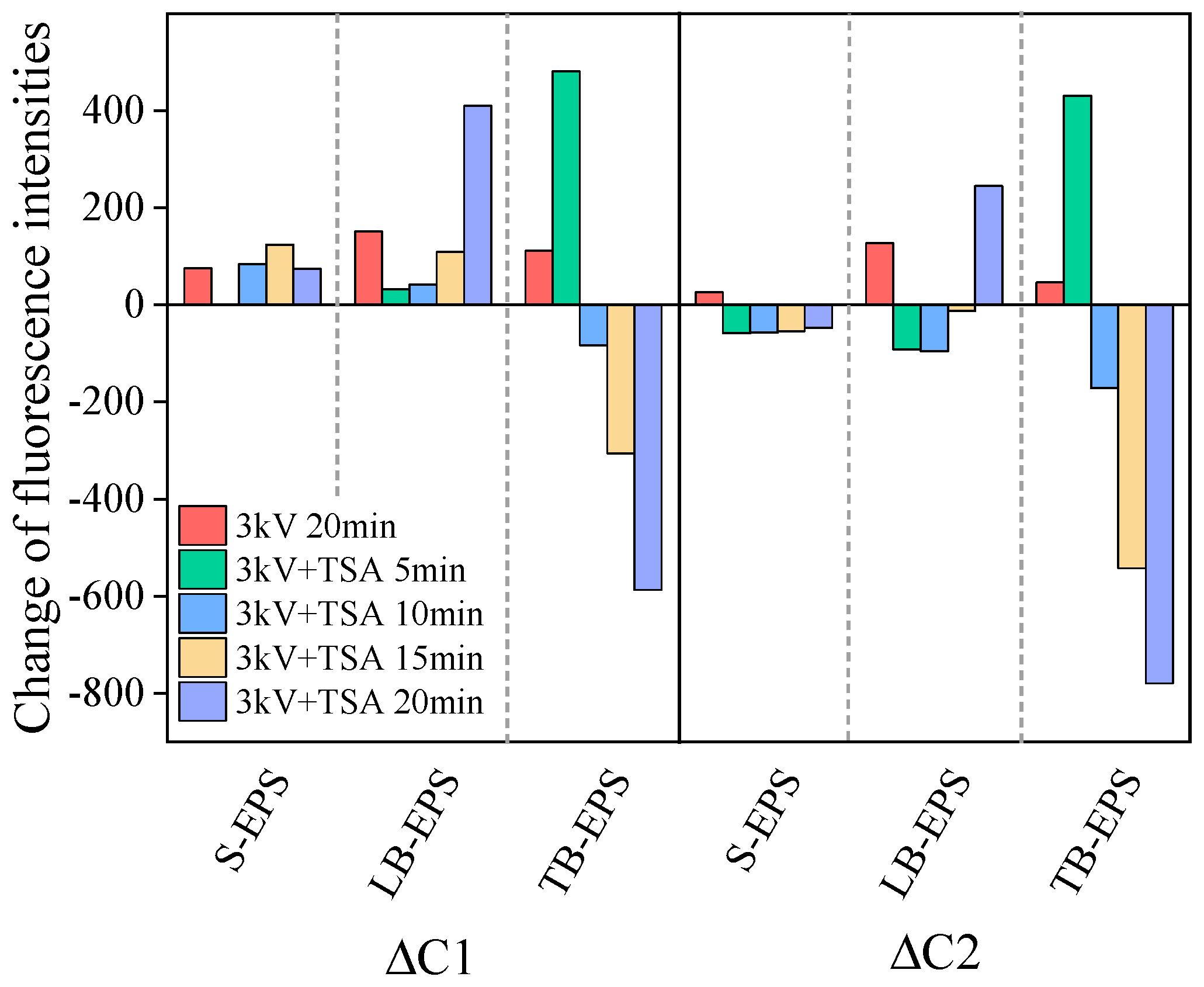
3.3. Changes in the EPS Distribution
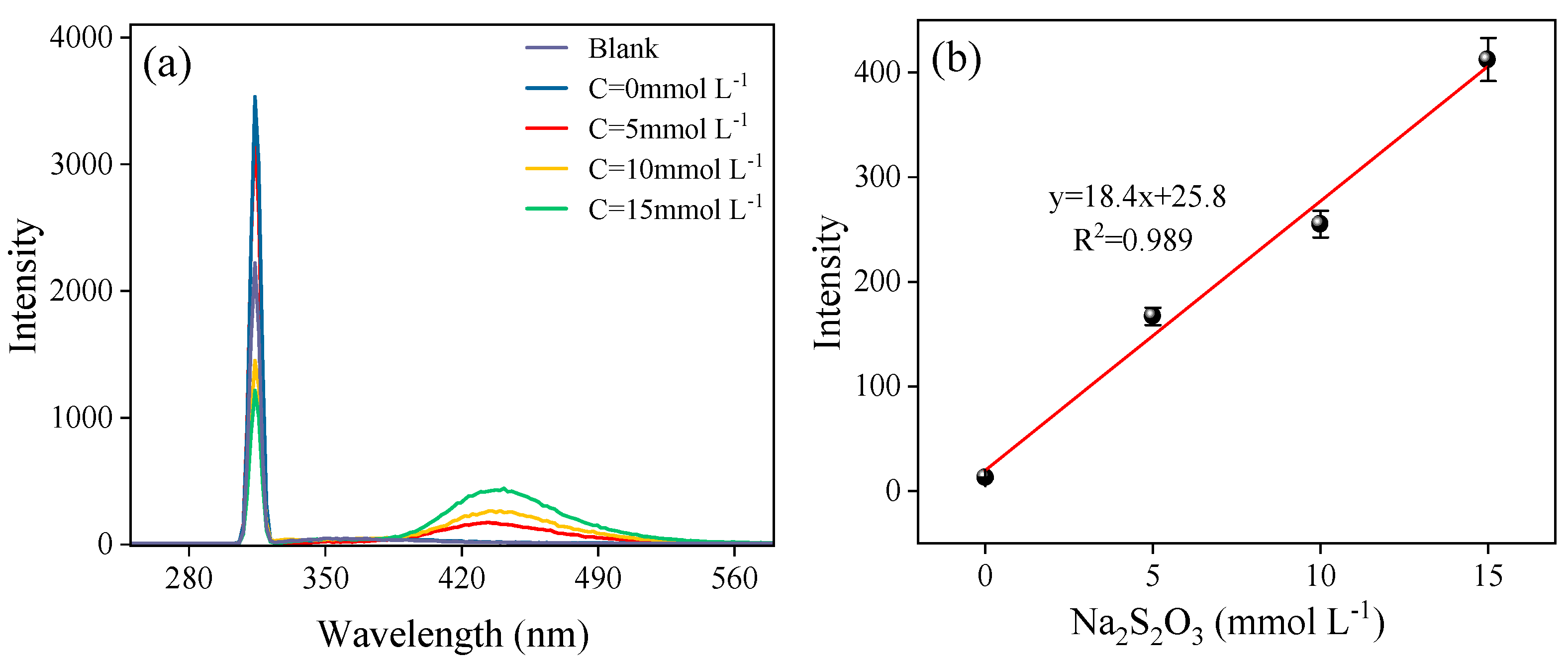
3.4. Mechanisms of Sludge Cracking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Q.; Li, X.; Ding, R.; Wang, D.; Liu, Y.; Wang, Q.; Zhao, J.; Chen, F.; Zeng, G.; Yang, Q.; et al. Understanding and mitigating the toxicity of cadmium to the anaerobic fermentation of waste activated sludge. Water Res. 2017, 124, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.C.; Serrano, A.; Siles, J.A.; Chica, A.F.; Martin, M.A. Centralized management of sewage sludge and agro-industrial waste through co-composting. J. Environ. Manag. 2017, 196, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, W.; Wang, D.; Ma, T.; Bai, R. Enhancement of activated sludge dewatering performance by combined composite enzymatic lysis and chemical re-flocculation with inorganic coagulants: Kinetics of enzymatic reaction and re-flocculation morphology. Water Res. 2015, 83, 367–376. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Wang, B.; Zhao, Y.; Chai, X.; Niu, D.; Zhao, T. Enhanced dewatering characteristics of waste activated sludge with Fenton pretreatment: Effectiveness and statistical optimization. Fron. Environ. Sci. Eng. 2014, 8, 267–276. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J.; Dewil, R.; De heyder, B. Advanced sludge treatment affects extracellular polymeric substances to improve activated sludge dewatering. J. Hazard. Mater. 2004, 106, 83–92. [Google Scholar] [CrossRef]
- Zubrowska-Sudol, M.; Walczak, J. Enhancing combined biological nitrogen and phosphorus removal from wastewater by applying mechanically disintegrated excess sludge. Water Res. 2015, 76, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Deng, J.; Lei, H.; Bai, T.; Fan, Q.; Li, Z. Dewaterability of waste activated sludge with ultrasound conditioning. Bioresour. Technol. 2009, 100, 1074–1081. [Google Scholar] [CrossRef]
- Ye, F.; Liu, X.; Li, Y. Effects of potassium ferrate on extracellular polymeric substances (EPS) and physicochemical properties of excess activated sludge. J. Hazard. Mater. 2012, 199, 158–163. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, W.; Wang, Q.; Huang, Y.; Meng, C.; Wang, D. Wastewater sludge dewaterability enhancement using hydroxyl aluminum conditioning: Role of aluminum speciation. Water Res. 2016, 105, 615–624. [Google Scholar] [CrossRef]
- Zahedi, S.; Icaran, P.; Yuan, Z.; Pijuan, M. Effect of free nitrous acid pre-treatment on primary sludge at low exposure times. Bioresour. Technol. 2017, 228, 272–278. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, C.; Zheng, G.; Zhou, L. Improving the compression dewatering of sewage sludge through bioacidification conditioning driven by Acidithiobacillus ferrooxidans: Dewatering rate vs. dewatering extent. Environ. Technol. 2019, 40, 3176–3189. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.-A.; Chen, H.; Wu, J.; Wang, Y.; Liu, J.; Lin, M. Effects of ultrasound assisted Fenton treatment on textile dyeing sludge structure and dewaterability. Chem. Eng. J. 2014, 242, 102–108. [Google Scholar] [CrossRef]
- Anjum, M.; Al-Talhi, H.A.; Mohamed, S.A.; Kumar, R.; Barakat, M.A. Visible light photocatalytic disintegration of waste activated sludge for enhancing biogas production. J. Environ. Manag. 2018, 216, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Feki, E.; Khoufi, S.; Loukil, S.; Sayadi, S. Improvement of anaerobic digestion of waste-activated sludge by using H2O2 oxidation, electrolysis, electro-oxidation and thermo-alkaline pretreatments. Environ. Sci. Pollut. Res. 2015, 22, 14717–14726. [Google Scholar] [CrossRef]
- Shi, P.; Su, R.; Zhu, S.; Zhu, M.; Li, D.; Xu, S. Supported cobalt oxide on graphene oxide: Highly efficient catalysts for the removal of Orange II from water. J. Hazard. Mater. 2012, 229, 331–339. [Google Scholar] [CrossRef]
- Yuan, H.-P.; Yan, X.-F.; Yang, C.-F.; Zhu, N.-W. Enhancement of waste activated sludge dewaterability by electro-chemical pretreatment. J. Hazard. Mater. 2011, 187, 82–88. [Google Scholar] [CrossRef]
- Divyapriya, G.; Nambi, I.M.; Senthilnathan, J. An innate quinone functionalized electrochemically exfoliated graphene/Fe3O4 composite electrode for the continuous generation of reactive oxygen species. Chem. Eng. J. 2017, 316, 964–977. [Google Scholar] [CrossRef]
- Ching, W.K.; Colussi, A.J.; Sun, H.J.; Nealson, K.H.; Hoffmann, M.R. Escherichia coli disinfection by electrohydraulic discharges. Environ. Sci. Technol. 2001, 35, 4139–4144. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.; Lv, L.; Wang, J. Degradation of caffeic acid by dielectric barrier discharge plasma combined with Ce doped CoOOH catalyst. J. Hazard. Mater. 2021, 402, 123772. [Google Scholar] [CrossRef]
- Mok, Y.S.; Nam, C.M.; Cho, M.H.; Nam, I.S. Decomposition of volatile organic compounds and nitric oxide by nonthermal plasma discharge processes. IEEE Trans. Plasma Sci. 2002, 30, 408–416. [Google Scholar]
- Larouss, M. The biomedical applications of plasma: A brief history of the development of a new field of research. IEEE Trans. Plasma Sci. 2008, 36, 1612–1614. [Google Scholar] [CrossRef]
- He, Y.; Sang, W.; Lu, W.; Zhang, W.; Zhan, C.; Jia, D. Recent Advances of Emerging Organic Pollutants Degradation in Environment by Non-Thermal Plasma Technology: A Review. Water 2022, 14, 1351. [Google Scholar] [CrossRef]
- Ye, Q.; Wan, H.; Lei, Y.; Zhang, J.; Li, J. Study on the technique of water-treatment by discharge plasma. High Voltage Eng. 2003, 29, 32. [Google Scholar]
- Nilsson, F.; Davidsson, A.; Falas, P.; Bengtsson, S.; Bester, K.; Jonsson, K. Impact of activated sludge ozonation on filamentous bacteria viability and possible added benefits. Environ. Technol. 2019, 40, 2601–2607. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Gan, J.Y.; Papiernik, S.K.; Yates, S.R. Isomeric effects on thiosulfate transformation and detoxification of 1,3-dichloropropene. Environ. Toxicol. Chem. 2001, 20, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Hemdal, J.F. Reduction of Ozone Oxidants in Synthetic Seawater by Use of Sodium Thiosulfate. Progress. Fish-Cult. 1992, 54, 54–56. [Google Scholar] [CrossRef]
- Pollmann, J.; Ortega, J.; Helmig, D. Analysis of atmospheric sesquiterpenes: Sampling losses and mitigation of ozone interferences. Environ. Sci. Technol. 2005, 39, 9620–9629. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, C.; Zhu, Y.; Lin, F.; Ma, Q.; Wang, Z.; Cen, K. Simultaneous removal of SO2 and NOx by combination of ozone oxidation and Na2S2O3 solution spray. CIESC J. 2016, 67, 2041–2047. [Google Scholar]
- Yang, J.; Luo, C.; Li, T.; Cao, J.; Dong, W.; Li, J.; Ma, J. Superfast degradation of refractory organic contaminants by ozone activated with thiosulfate: Efficiency and mechanisms. Water Res. 2020, 176, 115751. [Google Scholar] [CrossRef]
- Ge, L.; Wang, H.; Ma, L.; Li, X. Optimization of extraction process of extracellular substances by physic method. Environ. Chem. 2006, 25, 722–725. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Frolund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Bureau, N.E. Methods for Monitoring and Analysis of Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002; ISBN 9787801634009. [Google Scholar]
- Zhang, J.; Tian, Y.; Li, N.; Kong, L.C.; Sun, L.; Yu, M.; Zuo, W. Changes of physicochemical properties of sewage sludge during ozonation treatment: Correlation to sludge dewaterability. Chem. Eng. J. 2016, 301, 238–248. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; He, S.; Zhou, J.; Wang, T.; Zhu, L. Efficient degradation of antibiotics by non-thermal discharge plasma: Highlight the impacts of molecular structures and degradation pathways. Chem. Eng. J. 2020, 395, 125091. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Li, J.; Xiao, L. Rapid and efficient activated sludge treatment by electro-Fenton oxidation. Water Res. 2019, 152, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.Y.; Wang, J.H.; Lu, X.Q.; Su, L.H.; Zhu, X.F.; Zhou, T.; Zhao, Y.C. Effective gel-like floc matrix destruction and water seepage for enhancing waste activated sludge dewaterability under hybrid microwave-initiated Fe(II)-persulfate oxidation process. Chemosphere 2019, 221, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ni, B.J.; Horvat, K.; Song, L.; Chai, X.; Dai, X.; Mahajan, D. Occurrence State and Molecular Structure Analysis of Extracellular Proteins with Implications on the Dewaterability of Waste-Activated Sludge. Environ. Sci. Technol. 2017, 51, 9235–9243. [Google Scholar] [CrossRef]
- You, G.; Wang, P.; Hou, J.; Wang, C.; Xu, Y.; Miao, L.; Lv, B.; Yang, Y.; Liu, Z.; Zhang, F. Insights into the short-term effects of CeO2 nanoparticles on sludge dewatering and related mechanism. Water Res. 2017, 118, 93–103. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Yang, Q.; Xu, Q.; Tao, Z.; Huang, X.; Wu, Y.; Tao, L.; Pi, Z.; Chen, Z.; et al. Effect of citric acid on extracellular polymeric substances disruption and cell lysis in the waste activated sludge by pH regulation. Bioresource Technol. 2020, 302, 122859. [Google Scholar] [CrossRef]
- Peipei, H.; Guanghui, Y.; Liming, S.; Pinjing, H. Effects of protein and polysaccharide distribution in sludge on dewatering performance. Environ. Sci. 2008, 29, 3457–3461. [Google Scholar]
- Niu, M.; Zhang, W.; Wang, D.; Chen, Y.; Chen, R. Correlation of physicochemical properties and sludge dewaterability under chemical conditioning using inorganic coagulants. Bioresour. Technol. 2013, 144, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Zhou, Z.; Ren, W.; Jiang, L.-M.; Li, B.; Wei, H.; Li, J.; Wang, L. Effects of potassium peroxymonosulfate on disintegration of waste sludge and properties of extracellular polymeric substances. Int. Biod. Biodegrad. 2016, 106, 170–177. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, A.; Jiang, G.; Li, Q. Transformation and degradation mechanism of landfill leachates in a combined process of SAARB and ozonation. Waste Manag. 2019, 85, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Gholikandi, G.B.; Zakizadeh, N.; Masihi, H. Application of peroxymonosulfate-ozone advanced oxidation process for simultaneous waste-activated sludge stabilization and dewatering purposes: A comparative study. J. Environ. Manag. 2018, 206, 523–531. [Google Scholar] [CrossRef]
- Li, L.X.; Abe, Y.; Nagasawa, Y.; Kudo, R.; Usui, N.; Imai, K.; Mashino, T.; Mochizuki, M.; Miyata, N. An HPLC assay of hydroxyl radicals by the hydroxylation reaction of terephthalic acid. Biomed. Chrom. 2004, 18, 470–474. [Google Scholar]
- Ding, Q.; Wang, J.; Wang, Y. Detection of hydroxyl radicals in MIL-88A photoFenton catalytic system. Anal. Inst. 2021, 4, 132–138. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Lin, S.; Yang, Y.; Liang, Z.; Chen, Y.; Hua, L.; Zhang, Y.; Jia, H.; Zhang, G.; Wang, T. Excess Sludge Disintegration by Discharge Plasma Coupled with Thiosulfate. Water 2023, 15, 3313. https://doi.org/10.3390/w15183313
Jin H, Lin S, Yang Y, Liang Z, Chen Y, Hua L, Zhang Y, Jia H, Zhang G, Wang T. Excess Sludge Disintegration by Discharge Plasma Coupled with Thiosulfate. Water. 2023; 15(18):3313. https://doi.org/10.3390/w15183313
Chicago/Turabian StyleJin, Hekai, Song Lin, Yueyun Yang, Zhixin Liang, Yiqi Chen, Lei Hua, Ying Zhang, Hongtao Jia, Guodong Zhang, and Tiecheng Wang. 2023. "Excess Sludge Disintegration by Discharge Plasma Coupled with Thiosulfate" Water 15, no. 18: 3313. https://doi.org/10.3390/w15183313
APA StyleJin, H., Lin, S., Yang, Y., Liang, Z., Chen, Y., Hua, L., Zhang, Y., Jia, H., Zhang, G., & Wang, T. (2023). Excess Sludge Disintegration by Discharge Plasma Coupled with Thiosulfate. Water, 15(18), 3313. https://doi.org/10.3390/w15183313





