Assessing the Impact of Hg-Contaminated Sediments Washing through Sentinel Species: A Mesocosm Approach
Abstract
:1. Introduction
2. Materials and Methods
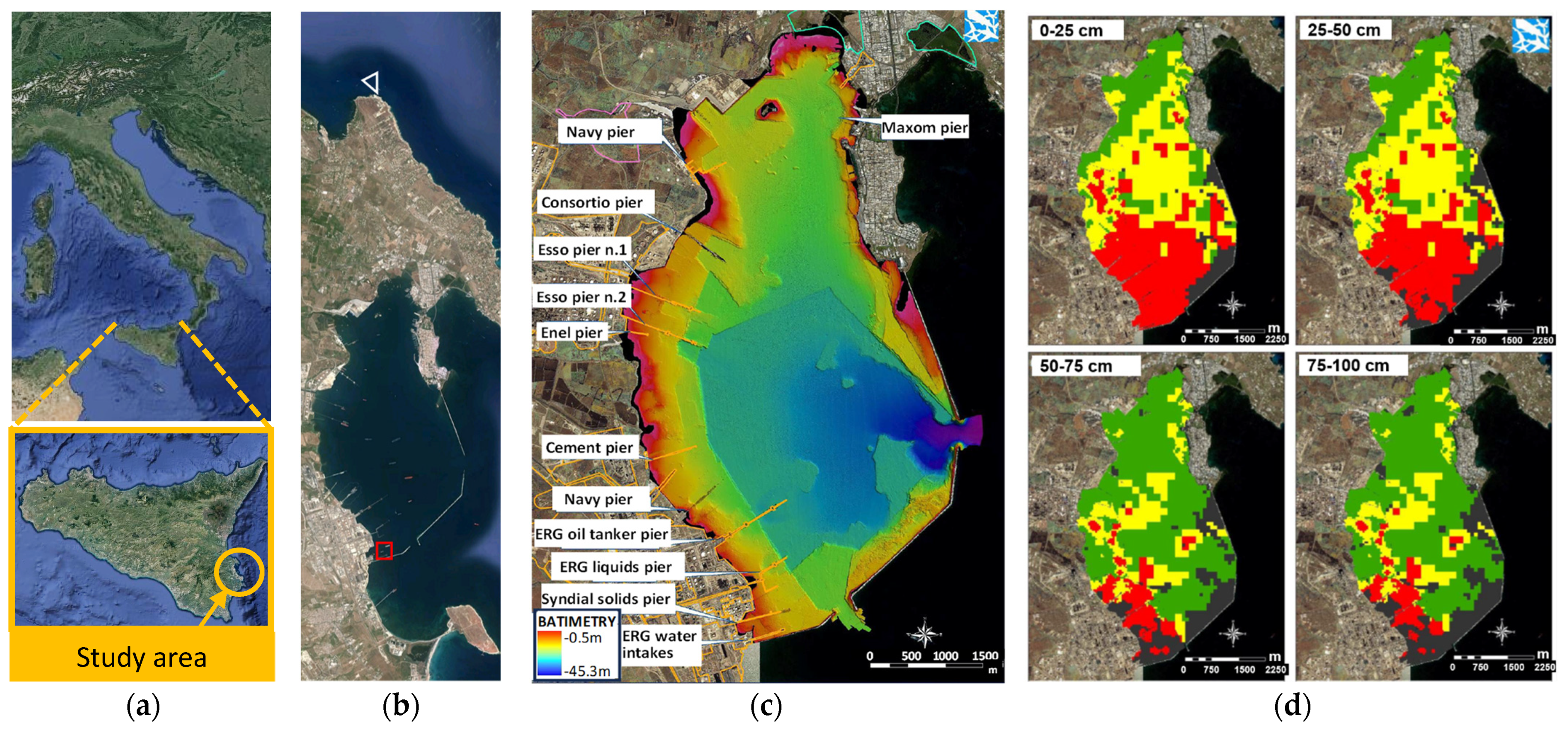
2.1. Sediments
2.2. Mesocosm Setup
- A white (W) mesocosm, with a bottom layer made of uncontaminated sediment (18 kg, with a thickness of ~6 mm), which was used as a control.
- A black (B) mesocosm, with a bottom layer made of contaminated sediment (18 kg, with a thickness of ~6 mm) from Augusta, which was used to measure the effects of contamination on the M. galloprovincialis chosen as a sentinel.
- A gray (G) mesocosm, with a bottom layer made of Augusta sediments previously treated by a soil-washing process, which was conceived to test the efficiency of the treatment process considered on M. galloprovincialis, as well as any undesired side-effects. The sediment-washing treatment was designed according to studies in the published literature [45] and previous experiments by the authors. In mesocosm G, 30 kg of the polluted sediments were previously treated with three cycles of a KI/I2 soil-washing solution, which had been selected during bench-scale experiments and is explained in more detail later on in this paper. A total of 18 kg of treated sediments, with a thickness of ~6 mm, were disposed at the bottom of the gray mesocosm.
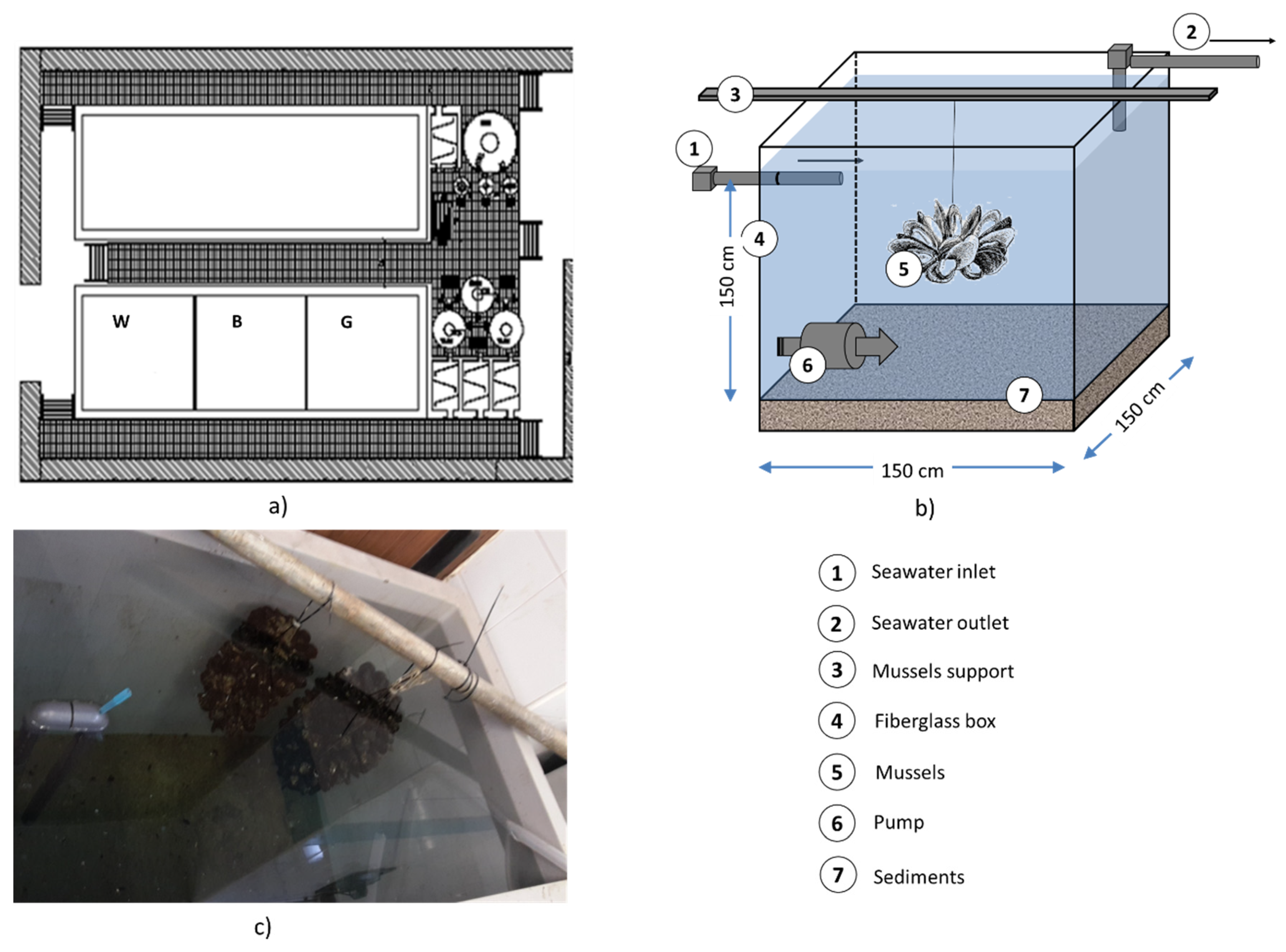
2.3. Chemical–Physical Parameters and Metal Measurements in Sediments and Water
2.4. Quality Control Hg Analysis
2.5. Sequential Extraction Procedure
2.6. Soil-Washing Tests
- EDTA disodium salt (Ethylenedinitrilotetraacetic acid, disodium salt dihydrate, C10H12N2Na2O8·2H2O) 0.2 M solution;
- EDDS 1 M ([S,S]-ethylenediaminedisuccinic acid,C10H13N2Na3O8) 1 M;
- Na2S2O3 Sodium Thiosulfate 1 M;
- KI (potassium iodide) 0.2 M + I2 (Iodine) 0.2 M.
- (1)
- a single-step batch process (using the 4 leaching agents separately). This set was repeated twice in triplicate;
- (2)
- a three-step batch process, performed in triplicate, with the best-performing leaching agents as determined in the previous set of experiments.
2.7. Histological Analysis
2.8. Immunohistochemical Analysis
2.9. Enzymatic Analysis
2.10. Statistical Analysis
3. Results
3.1. Water and Sediment Characterization in the Mesocosms
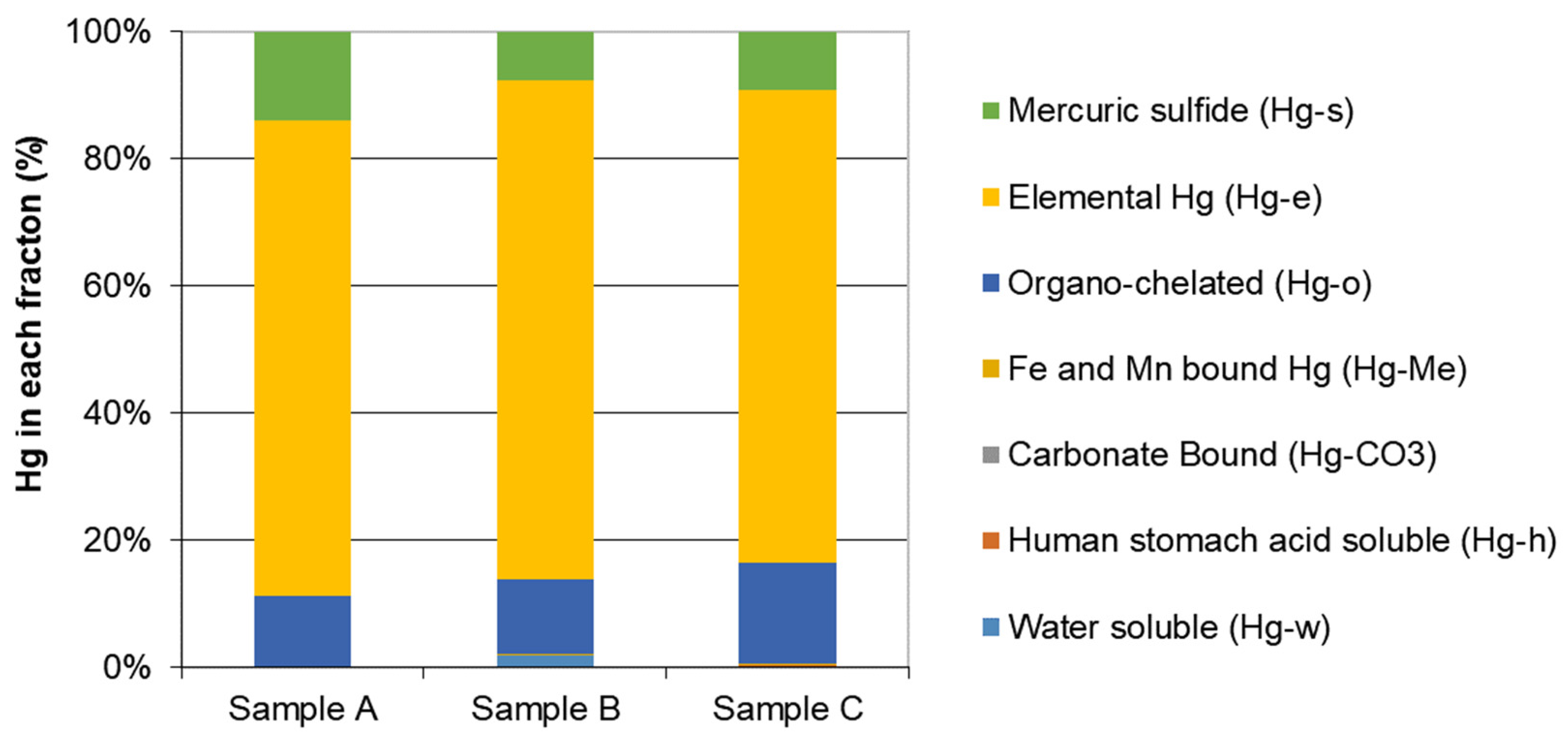
3.2. Hg Sequential Extraction
3.2.1. Sample A
3.2.2. Sample B
3.2.3. Sample C
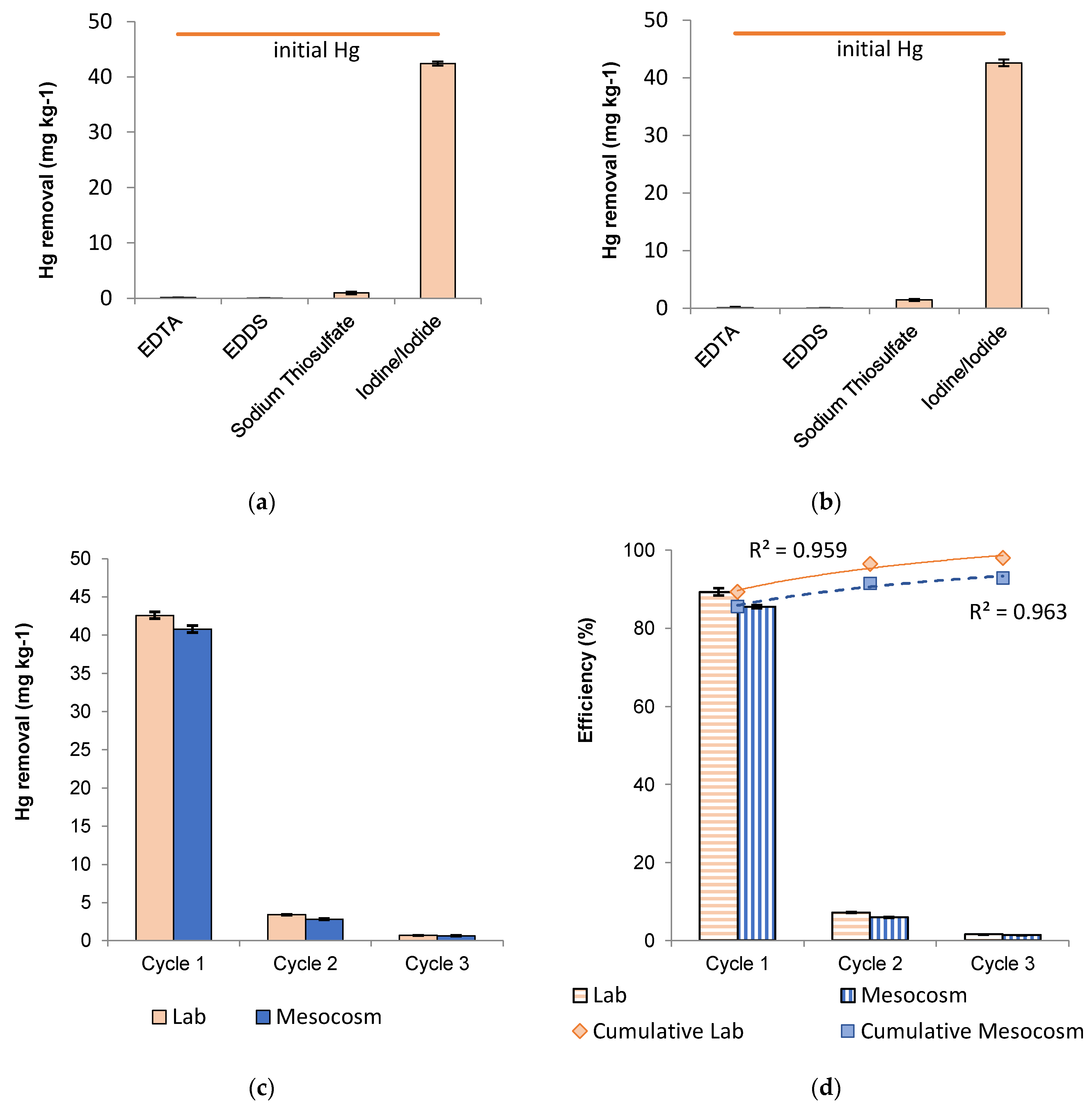
3.3. Soil Washing
3.3.1. Single-Step Batch Process: First Set
3.3.2. Single-Step Batch Process: Second Set
3.3.3. Multi-Step Batch Process
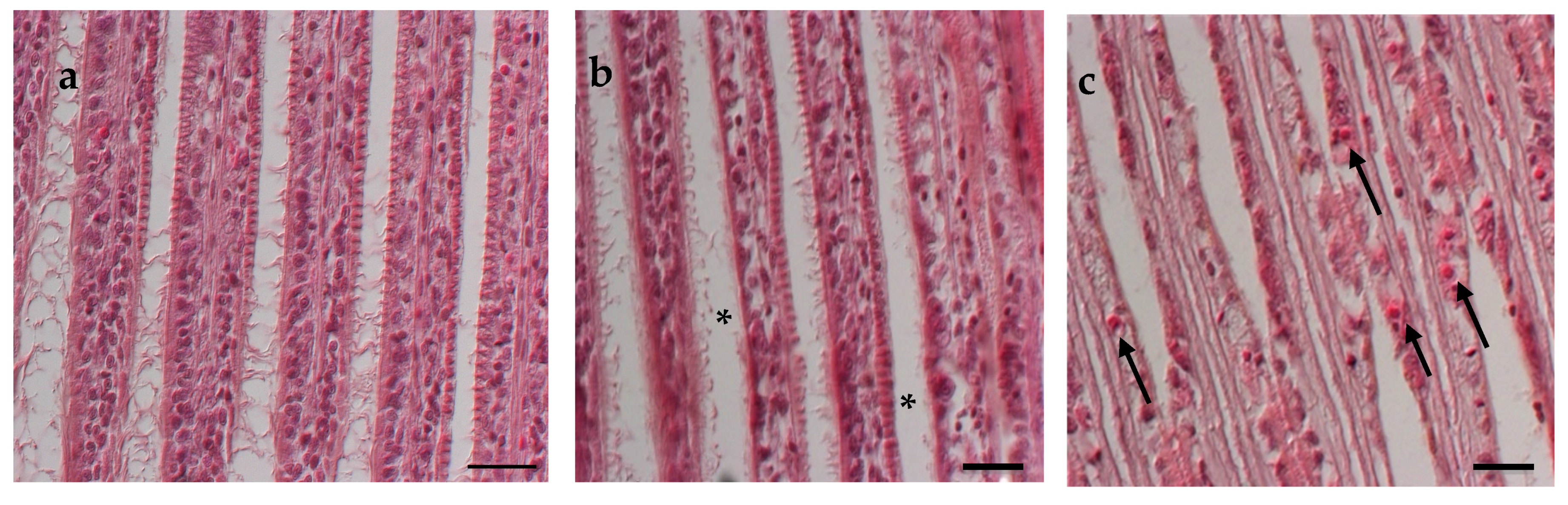
3.4. Histological Analysis
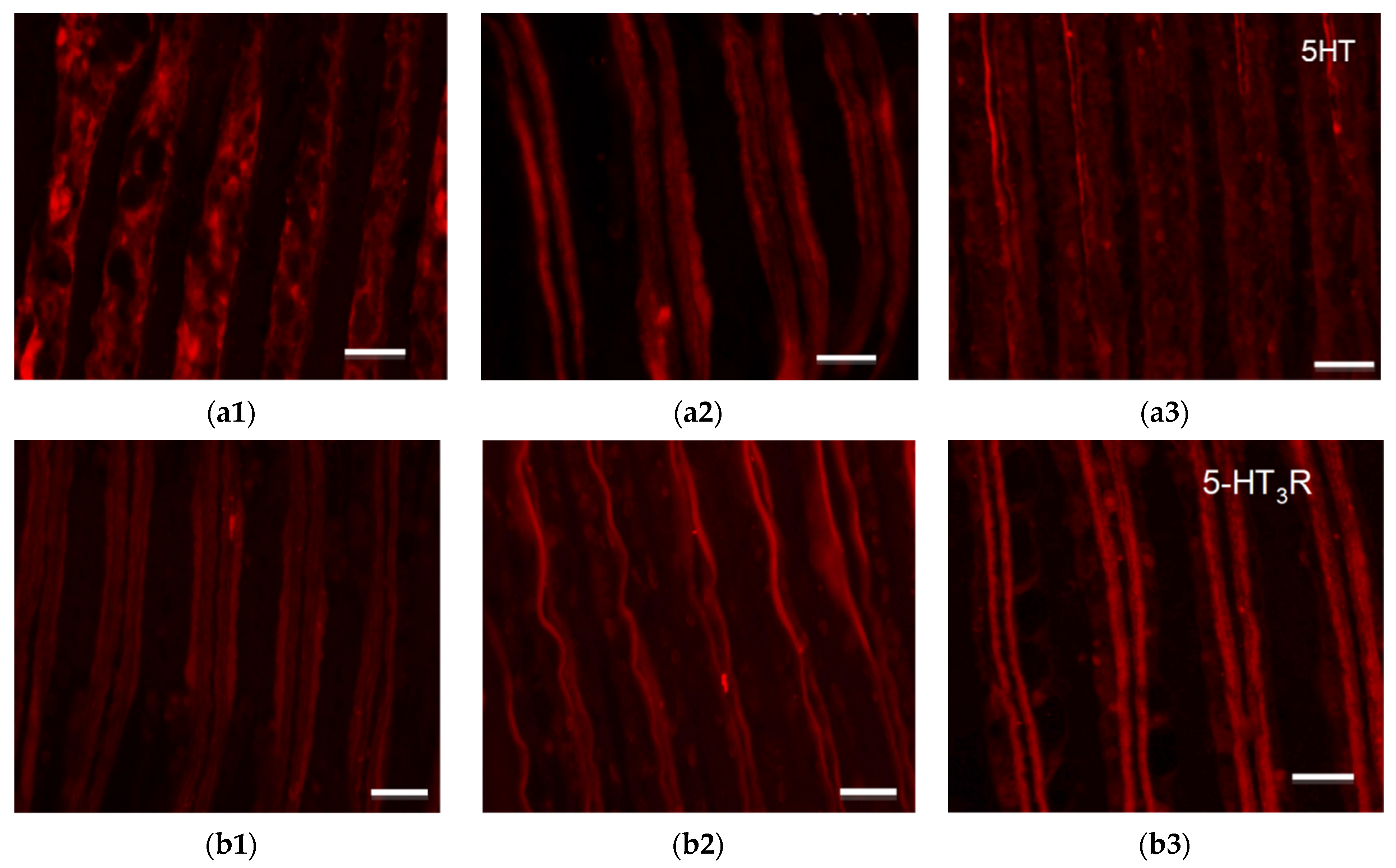
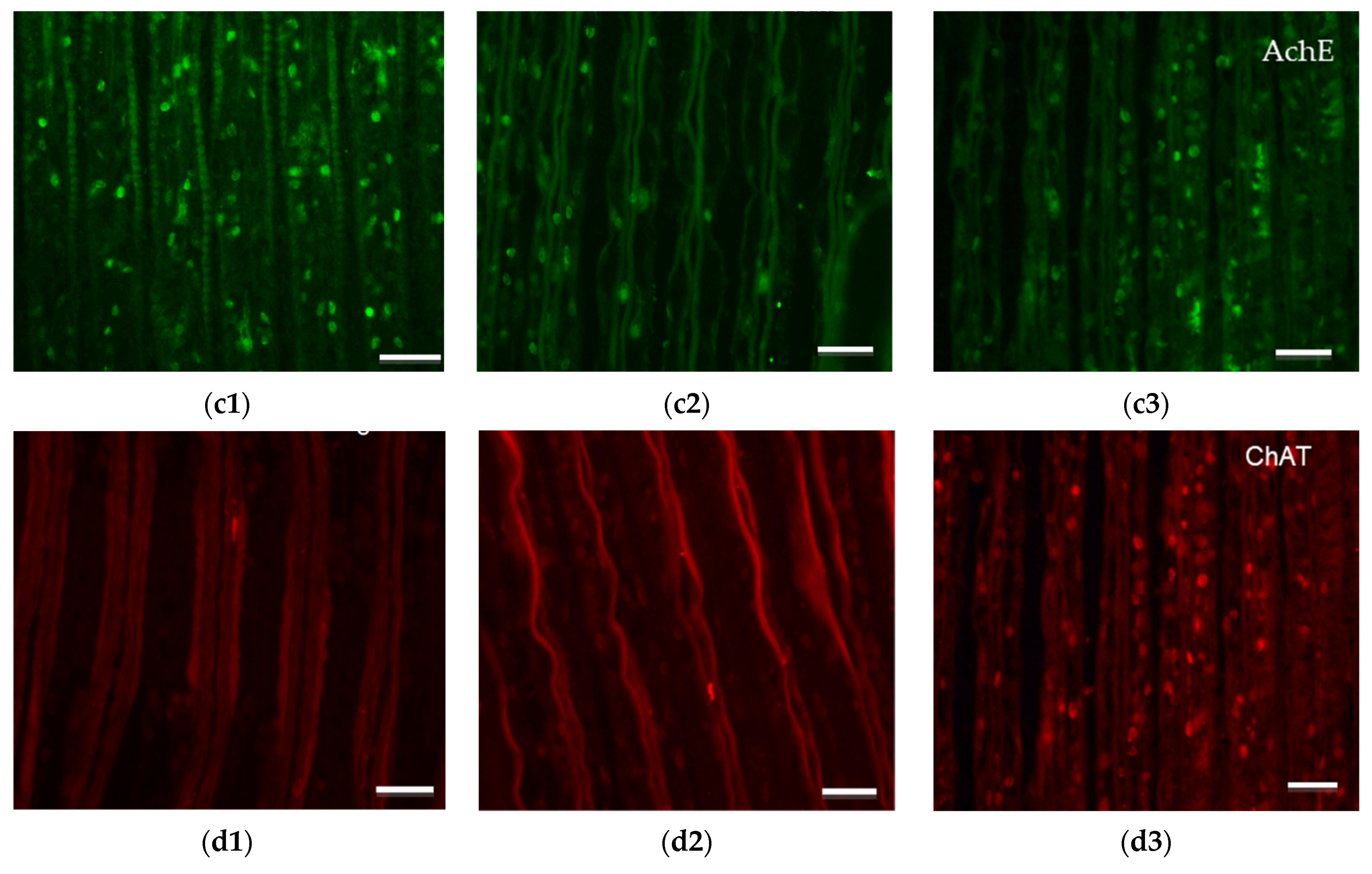
3.5. Immunohistochemical Analysis
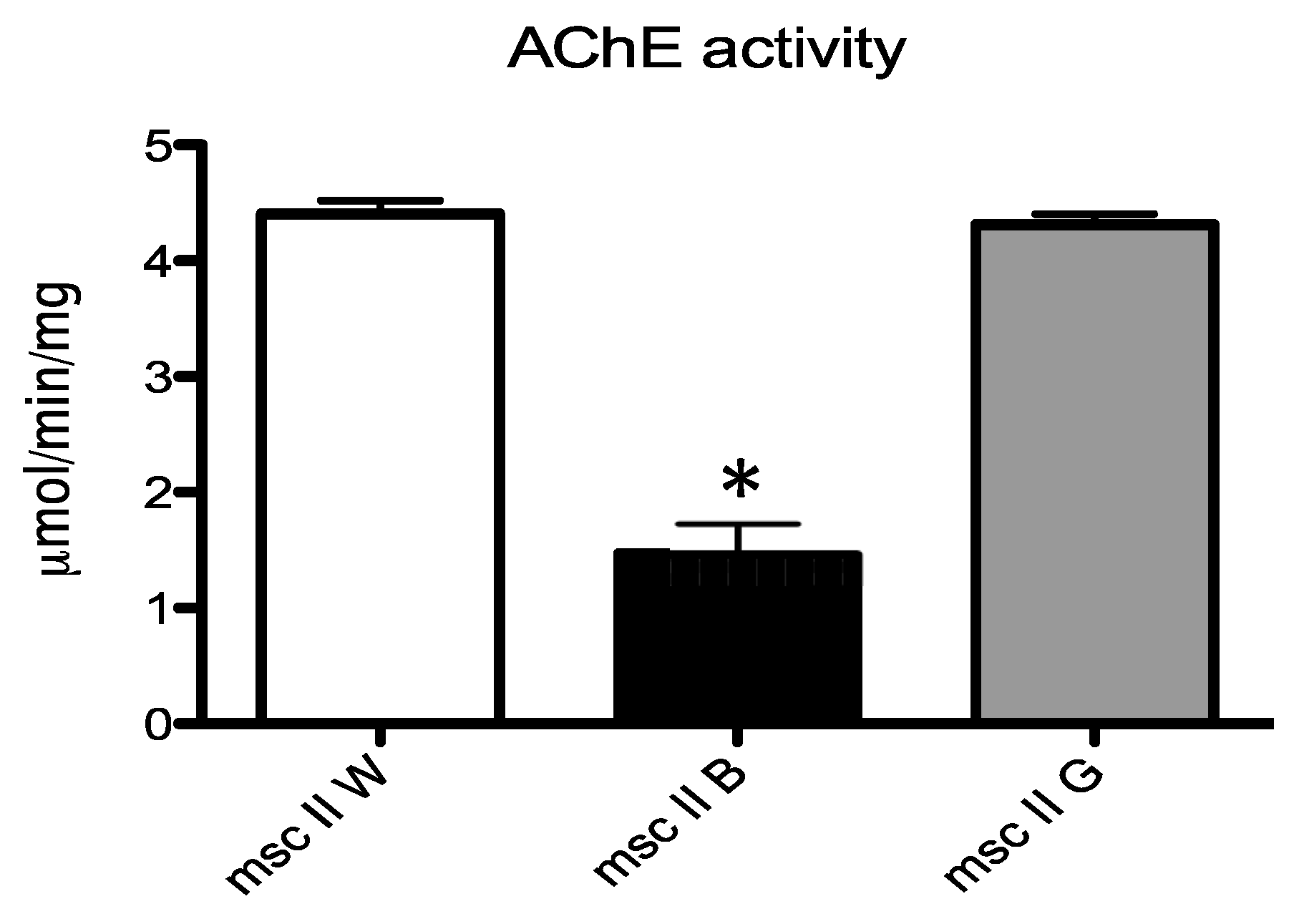
3.6. Enzymatic Analysis
3.7. Hg Removal Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sprovieri, M.; Oliveri, E.; Di Leonardo, R.; Romano, E.; Ausili, A.; Gabellini, M.; Barra, M.; Tranchida, G.; Bellanca, A.; Neri, R.; et al. The key role played by the Augusta basin (southern Italy) in the mercury contamination of the Mediterranean Sea. J. Environ. Monit. 2011, 13, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Law No. 319 of 10th May 1976 ‘Water Protection against Pollution’ (Merli Law). Available online: https://www.gazzettaufficiale.it/eli/id/1976/05/29/076U0319/sg (accessed on 21 November 2021).
- Ausili, A.; Gabellini, M.; Cammarata, G.; Fattorini, D.; Benedetti, M.; Pisanelli, B.; Gorbi, S.; Regoli, F. Ecotoxicological and Human Health Risk in a Petrochemical District of Southern Italy. Mar. Environ. Res. 2008, 66, 215–217. [Google Scholar] [CrossRef]
- Feng, C.; Pedrero, Z.; Lima, L.; Olivares, S.; de La Rosa, D.; Berail, S.; Tessier, E.; Pannier, F.; Amouroux, D. Assessment of Hg contamination by a Chlor-Alkali Plant in riverine and coastal sites combining Hg speciation and isotopic signature (Sagua la Grande River, Cuba). J. Hazard. Mater. 2019, 371, 558–565. [Google Scholar] [CrossRef]
- Wang, C.; Song, Z.; Li, Z.; Zhu, W.; Li, P.; Feng, X. Mercury speciation and mobility in salt slurry and soils from an abandoned chlor-alkali plant, Southwest China. Sci. Total Environ. 2019, 652, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Guney, M.; Akimzhanova, Z.; Kumisbek, A.; Beisova, K.; Kismelyeva, S.; Satayeva, A.; Inglezakis, V.; Karaca, F. Mercury (Hg) Contaminated Sites in Kazakhstan: Review of Current Cases and Site Remediation Responses. Int. J. Environ. Res. Public Health 2020, 17, 8936. [Google Scholar] [CrossRef]
- Salvagio Manta, D.; Bonsignore, M.; Oliveri, E.; Barra, M.; Tranchida, G.; Giaramita, L.; Mazzola, S.; Sprovieri, M. Fluxes and the Mass Balance of Mercury in Augusta Bay (Sicily, Southern Italy). Estuar. Coast. Shelf Sci. 2016, 181, 134–143. [Google Scholar] [CrossRef]
- Di Leonardo, R.; Adelfio, G.; Bellanca, A.; Chiodi, M.; Mazzola, S. Analysis and Assessment of Trace Element Contamination in Offshore Sediments of the Augusta Bay (SE Sicily): A Multivariate Statistical Approach Based on Canonical Correlation Analysis and Mixture Density Estimation Approach. J. Sea Res. 2014, 85, 428–442. [Google Scholar] [CrossRef]
- Harding, G.; Dalziel, J.; Vass, P. Bioaccumulation of methylmercury within the marine food web of the outer Bay of Fundy, Gulf of Maine. PLoS ONE 2018, 13, 0197220. [Google Scholar] [CrossRef]
- Al-Sulaiti, M.M.; Soubra, L.; Al-Ghouti, M.A. The Causes and Effects of Mercury and Methylmercury Contamination in the Marine Environment: A Review. Curr. Pollut. Rep. 2022, 8, 249–272. [Google Scholar] [CrossRef]
- Eckley, C.S.; Gilmour, C.C.; Janssen, S.; Luxton, T.P.; Randall, P.M.; Whalin, L.; Austin, C. The assessment and remediation of mercury contaminated sites: A review of current approaches. Sci. Total Environ. 2020, 707, 136031. [Google Scholar] [CrossRef]
- Randall, P.M.; Chattopadhyay, S. Mercury Contaminated Sediment Sites-An Evaluation of Remedial Options. Environ. Res. 2013, 125, 131–149. [Google Scholar] [CrossRef]
- Schultz, T.; Korhonen, P.; Virtanen, M. A mercury model used for assessment of dredging impacts. Water Air Soil Pollut. 1995, 80, 1171–1180. [Google Scholar] [CrossRef]
- Garbaciak, S.; Spadaro, P.; Thornburg, T.; Fox, R. Sequential risk mitigationand the role of natural recovery in contaminated sediment projects. Water Sci. Technol. 1998, 37, 331–336. [Google Scholar] [CrossRef]
- Ebinghaus, R.; Turner, R.R.; de Lacerda, L.D.; Vasiliev, O.; Salomons, W. Mercury Contaminated Sites: Characterization, Risk Assessment and Remediation; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Yan, F.; Reible, D. Electro-bioremediation of contaminated sediment by electrode enhanced capping. J. Environ. Manag. 2015, 155, 154–161. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate Assisted Phytoextraction of Heavy Metals from Soil. Effect, Mechanism, Toxicity, and Fate of Chelating Agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Lesa, B.; Aneggi, E.; Rossi, G.; Comuzzi, C.; Goi, D. Bench-scale tests on ultrasound-assisted acid washing and thermal desorption of mercury from dredging sludge and other solid matrices. J. Hazard. Mater. 2009, 171, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Luciano, A.; Viotti, P.; Torretta, V.; Mancini, G. Numerical approach to modelling pulse-mode soil flushing on a Pb-contaminated soil. J. Soils Sediments 2013, 13, 43–55. [Google Scholar] [CrossRef]
- Satyro, S.; Race, M.; Marotta, R.; Dezotti, M.; Spasiano, D.; Mancini, G.; Fabbricino, M. Simulated Solar Photocatalytic Processes for the Simultaneous Removal of EDDS, Cu(II), Fe(III) and Zn(II) in Synthetic and Real Contaminated Soil Washing Solutions. J. Environ. Chem. Eng. 2014, 2, 1969–1979. [Google Scholar] [CrossRef]
- Xu, J.; Bravo, A.G.; Lagerkvist, A.; Bertilsson, S.; Sjöblom, R.; Kumpiene, J. Sources and remediation techniques for mercury contaminated soil. Environ. Int. 2015, 74, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wang, H.; Xie, F.; Wang, W.; Zhang, T.; Dreisinger, D. Feasibility study on the use of thiosulfate to remediate mercury-contaminated soil. Env. Environ. Technol. 2019, 40, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Taube, F.; Pommer, L.; Larsson, T.; Shchukarev, A.; Nordin, A. Soil Remediation—Mercury Speciation in Soil and Vapor Phase During Thermal Treatment. Water Air Soil. Pollut. 2008, 193, 155–163. [Google Scholar] [CrossRef]
- Issaro, N.; Abi-Ghanem, C.; Bermond, A. Fractionation studies of mercury in soils and sediments: A review of the chemical reagents used for mercury extraction. Anal. Chim. Acta 2009, 631, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cappello, S.; Genovese, M.; Denaro, R.; Santisi, S.; Volta, A.; Bonsignore, M.; Mancini, G.; Giuliano, L.; Genovese, L.; Yakimov, M.M. Quick Stimulation of Alcanivorax Sp. By Bioemulsificant EPS2003 on Microcosm Oil Spill Simulation. Braz. J. Microbiol. 2015, 45, 1317–1323. [Google Scholar] [CrossRef]
- Mancini, G.; Cappello, S.; Yakimov, M.M.; Polizzi, A.; Torregrossa, M. Biological approaches to the treatment of saline oily waste(waters) originated from marine transportation. Chem. Eng. Trans. 2012, 27, 37–42. [Google Scholar]
- Mancini, G.; Lanciotti, E.; Bruno, M. Chemical-physical and biological treatment of high salinity wastewaters contaminated by oily xenobiotic compounds. Chem. Eng. Trans. 2010, 20, 271–278. [Google Scholar]
- Adel, M.; Copat, C.; Oliveri Conti, G.; Sakhaie, F.; Hashemi, Z.; Mancini, G.; Cristaldi, A.; Ferrante, M. Trace elements in the muscle tissue of Hemiculter leucisculus and Abramis brama orientalis from the Anzali International wetland, south-west of Caspian Sea: An exposure risk assessment. Mar. Pollut. Bull. 2022, 180, 113756. [Google Scholar] [CrossRef]
- Copat, C.; Rizzo, M.; Zuccaro AGrasso, A.; Zuccarello, P.; Fiore, M.; Mancini, G.; Ferrante, M. Metals/Metalloids and Oxidative Status Markers in Saltwater Fish from the Ionic Coast of Sicily, Mediterranean Sea. Int. J. Environ. Res. 2020, 14, 15–27. [Google Scholar] [CrossRef]
- Scalici, M.; Traversetti, L.; Spani, F.; Malafoglia, V.; Colamartino, M.; Persichini, T.; Cappello, S.; Mancini, G.; Guerriero, G.; Colasanti, M. Shell fluctuating asymmetry in the sea-dwelling benthic bivalve Mytilus galloprovincialis (Lamarck, 1819) as morphological markers to detect environmental chemical contamination. Ecotoxicology 2017, 26, 396–404. [Google Scholar] [CrossRef]
- Santisi, S.; Cappello, S.; Catalfamo, M.; Mancini, G.; Hassanshahian, M.; Genovese, L.; Giuliano, L.; Yakimov, M.M. Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium. Braz. J. Microbiol. 2015, 46, 377–387. [Google Scholar] [CrossRef]
- Santisi, S.; Catalfamo, M.; Bonsignore, M.; Gentile, G.; Di Salvo, E.; Genovese, M.; Mahjoubi, M.; Cherif, A.; Mancini, G.; Hassanshahian, M.; et al. Biodegradation ability of two selected microbial autochthonous consortia from a chronically polluted marine coastal area (Priolo Gargallo, Italy). J. Appl. Microbiol. 2019, 127, 618–629. [Google Scholar] [CrossRef]
- Giacoletti, A.; Cappello, S.; Mancini, G.; Mangano, M.C.; Sarà, G. Predicting the effectiveness of oil recovery strategies in the marine polluted environment. J. Environ. Manag. 2018, 223, 749–757. [Google Scholar] [CrossRef]
- Mancini, G.; Panzica, M.; Fino, D.; Cappello, S.; Yakimov, M.M.; Luciano, A. Feasibility of treating emulsified oily and salty wastewaters through coagulation and bio-regenerated GAC filtration. J. Environ. Manag. 2017, 203, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Cappello, S.; Volta, A.; Santisi, S.; Morici, C.; Mancini, G.; Quatrini, P.; Genovese, M.; Yakimov, M.M.; Torregrossa, M. Oil-degrading bacteria from a membrane bioreactor (BF-MBR) system for treatment of saline oily waste: Isolation, identification and characterization of the biotechnological potential. Int. Biodeterior. Biodegrad. 2016, 110, 235–244. [Google Scholar] [CrossRef]
- Cappello, S.; Calogero, R.; Santisi, S.; Genovese, M.; Denaro, R.; Genovese, L.; Giuliano, L.; Mancini, G.; Yakimov, M.M. Bioremediation of oil polluted marine sediments: A bio-engineering treatment. Int. Microbiol. 2015, 18, 127–134. [Google Scholar] [PubMed]
- Fasulo, S.; Guerriero, G.; Cappello, S.; Colasanti, M.; Schettino, T.; Leonzio, C.; Mancini, G.; Gornati, R. The “SYSTEMS BIOLOGY” in the study of xenobiotic effects on marine organisms for evaluation of the environmental health status: Biotechnological applications for potential recovery strategies. Rev. Environ. Sci. Biotechnol. 2015, 14, 339–345. [Google Scholar] [CrossRef]
- Caricato, R.; Giordano, M.E.; Schettino, T.; Maisano, M.; Mauceri, A.; Giannetto, A.; Cappello, T.; Parrino, V.; Ancora, S.; Caliani, I.; et al. Carbonic anhydrase integrated into a multimarker approach for the detection of the stress status induced by pollution exposure in Mytilus galloprovincialis: A field case study. Sci. Total Environ. 2019, 690, 140–150. [Google Scholar] [CrossRef]
- Rossi, F.; Palombella, S.; Pirrone, C.; Mancini, G.; Bernardini, G.; Gornati, R. Evaluation of Tissue Morphology and Gene Expression as Biomarkers of Pollution in Mussel Mytilus Galloprovincialis Caging Experiment. Aquat. Toxicol. 2016, 181, 57–66. [Google Scholar] [CrossRef]
- Caliani, I.; De Marco, G.; Cappello, T.; Giannetto, A.; Mancini, G.; Ancora, S.; Maisano, M.; Parrino, V.; Cappello, S.; Bianchi, N.; et al. Assessment of the effectiveness of a novel BioFilm-Membrane BioReactor oil-polluted wastewater treatment technology by applying biomarkers in the mussel Mytilus galloprovincialis. Aquat. Toxicol. 2022, 243, 106059. [Google Scholar] [CrossRef]
- Ancora, S.; Rossi, F.; Borgese, M.; Pirrone, C.; Caliani, I.; Cappello, S.; Mancini, G.; Bianchi, N.; Leonzio, C.; Bernardini, G.; et al. Assessing the Effect of Contaminated and Restored Marine Sediments in Different Experimental Mesocosms Using an Integrated Approach and Mytilus galloprovincialis as a Model. Mar. Biotechnol. 2020, 22, 411–422. [Google Scholar] [CrossRef]
- Gornati, R.; Maisano, M.; Pirrone, C.; Cappello, T.; Rossi, F.; Borgese, M.; Giannetto, A.; Cappello, S.; Mancini, G.; Bernardini, G.; et al. Mesocosm System to Evaluate BF-MBR Efficacy in Mitigating Oily Wastewater Discharges: An Integrated Study on Mytilus galloprovincialis. Mar. Biotechnol. 2019, 21, 773–790. [Google Scholar] [CrossRef]
- ICRAM. Progetto Preliminare di Bonifica dei Fondali Della Rada di Augusta nel Sito di Interesse Nazionale di Priolo—Elaborazione Definitiva; BoI-Pr-SI-PR-Rada di Augusta-03.22 (182p); Istituto Centrale Per La Ricerca Scientifica E Tecnologica Applicata Al Mare: Rome, Italy, 2008. [Google Scholar]
- Pirrone, C.; Rossi, F.; Cappello, S.; Borgese, M.; Mancini, G.; Bernardini, G.; Gornati, R. Evaluation of biomarkers in Mytilus galloprovincialis as an integrated measure of biofilm-membrane bioreactor (BF-MBR) system efficiency in mitigating the impact of oily wastewater discharge to marine environment: A microcosm approach. Aquat. Toxicol. 2018, 198, 49–62. [Google Scholar] [CrossRef]
- Subirés-Muñoz, J.D.; García-Rubio, A.; Vereda-Alonso, C.; Gómez-Lahoz, C.; Rodríguez-Maroto, J.M.; García-Herruzo, F.; Paz-García, J.M. Feasibility Study of the Use of Different Extractant Agents in the Remediation of a Mercury Contaminated Soil from Almaden. Sep. Purif. Technol. 2011, 79, 151–156. [Google Scholar] [CrossRef]
- Orecchio, S.; Polizzotto, G. Fractionation of mercury in sediments during draining of Augusta (Italy) coastal area by modified Tessier method. Microchem. J. 2013, 110, 452–457. [Google Scholar] [CrossRef]
- Wasay, S.A.; Arnfalk, P.; Tokunaga, S. Remediation of a Soil Polluted by Mercury with Acidic Potassium Iodide. J. Hazard. Mater. 1995, 44, 93–102. [Google Scholar] [CrossRef]
- Klasson, K.T.; Koran, L.J., Jr.; Gates, D.D.; Cameron, P.A. Removal of Mercury from Solids Using the Potassium Iodide/Iodine Leaching Process; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 1997. [Google Scholar]
- Ray, A.B.; Selvakumar, A. Laboratory studies on the remediation of mercury-contaminated soils. Remediat. J. 2000, 10, 49–56. [Google Scholar] [CrossRef]
- Mauceri, A.; Fossi, M.C.; Leonzio, C.; Ancora, S.; Minniti, F.; Maisano, M.; Lo Cascio, P.; Ferrando, S.; Fasulo, S. Stress factors in the gills of Liza aurata (Perciformes, Mugilidae) living in polluted environments. Ital. J. Zool. 2005, 72, 285–292. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Benedetti, M.; Romano, E.; Ausili, A.; Fattorini, D.; Gorbi, S.; Maggi, C.; Salmeri, A.; Salvagio Manta, D.; Sesta, G.; Sprovieri, M.; et al. 10-year time course of Hg and organic compounds in Augusta Bay: Bioavailability and biological effects in marine organisms. Front. Public Health 2022, 10, 968296. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, C.C.; Henry, E.A.; Mitchell, R. Sulfate stimulation of mercury methylation in freshwater sediments. Environ. Sci. Technol. 1992, 26, 2281–2287. [Google Scholar] [CrossRef]
- van den Berg, G.A.; Buykx, S.E.J.; van den Hoop, M.A.G.T.; van der Heijdt, L.M.; Zwolsman, J.J.G. Vertical profiles of trace metals and acid-volatile sulphide in a dynamic sedimentary environment: Lake Ketel, The Netherlands. Appl. Geochem. 2001, 16, 781–791. [Google Scholar] [CrossRef]
- García-Ordiales, E.; Covelli, S.; Braidotti, G.; Petranich, E.; Pavoni, E.; Acquavita, A.; Sanz-Prada, L.; Roqueñí, N.; Loredo, J. Mercury and arsenic mobility in resuspended contaminated estuarine sediments (Asturias, Spain): A laboratory-based study. Sci. Tot. Env. 2020, 744, 140870. [Google Scholar] [CrossRef]
- Bonsignore, M.; Tamburrino, S.; Oliveri, E.; Marchetti, A.; Durante, C.; Berni, A.; Quinci, E.; Sprovieri, M. Tracing Mercury Pathways in Augusta Bay (Southern Italy) by Total Concentration and Isotope Determination. Environ. Pollut. 2015, 205, 178–185. [Google Scholar] [CrossRef]
- Cappello, T.; Maisano, M.; Giannetto, A.; Parrino, V.; Mauceri, A.; Fasulo, S. Neurotoxicological Effects on Marine Mussel Mytilus Galloprovincialis Caged at Petrochemical Contaminated Areas (Eastern Sicily, Italy): 1H NMR and Immunohistochemical Assays. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 169, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Maisano, M.; Cappello, T.; Natalotto, A.; Vitale, V.; Parrino, V.; Giannetto, A.; Oliva, S.; Mancini, G.; Cappello, S.; Mauceri, A.; et al. Effects of Petrochemical Contamination on Caged Marine Mussels Using a Multi-Biomarker Approach: Histological Changes, Neurotoxicity and Hypoxic Stress. Mar. Environ. Res. 2017, 128, 114–123. [Google Scholar] [CrossRef]
- Cappello, T.; Mauceri, A.; Corsaro, C.; Maisano, M.; Parrino, V.; Lo Paro, G.; Messina, G.; Fasulo, S. Impact of environmental pollution on caged mussels Mytilus galloprovincialis using NMR-based metabolomics. Mar. Pollut. Bull. 2013, 77, 132–139. [Google Scholar] [CrossRef]
- Humphries, J.E.; Yoshino, T.P. Cellular receptors and signal transduction in molluscan hemocytes: Connections with the innate immune system of vertebrates. Integr. Comp. Biol. 2003, 43, 305–312. [Google Scholar] [CrossRef]
- Franzellitti, S.; Fabbri, E. Cyclic-AMP mediated regulation of ABCB mRNA expression in mussel haemocytes. PLoS ONE 2013, 8, e61634. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.E. The cilioexcitatory activity of serotonin. J. Cell. Comp. Physiol. 1961, 58, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.A.; Catapane, E.J. The nervous system control of lateral ciliary activity of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 445–450. [Google Scholar] [CrossRef]
- Matozzo, V.; Tomei, A.; Marin, M. Acetylcholinesterase as a biomarker of exposure to neurotoxic compounds in the clam Tapes philippinarum from the Lagoon of Venice. Mar. Pollut. Bull. 2005, 50, 1686–1693. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Schettino, T. Acetylcholinesterase as a biomarker in environmental and occupational medicine: New insights and future perspectives. Biomed. Res. Int. 2013, 2013, 321213. [Google Scholar] [CrossRef] [PubMed]
| Step | Fraction | Extraction |
|---|---|---|
| I | Water-soluble (Hg-w) | Solution: Distilled water Stirred for 20 min (250 rpm) Treated for 1 h at 95 °C |
| II | Soluble (Hg-h) or exchangeable human stomach acid | Solution: 1M of sodium acetate + 0.1 M di HCl Stirred for 1 h (250 rpm) |
| III | Carbonate Bound (Hg-CO3) | Solution: 1M of sodium acetate + acetic acid (pH = 5) Stirred for 4 h (250 rpm) |
| IV | Fe and Mn bound Hg (Hg-Me) | Solution: 0.04 M Hydroxylammonium chloride on 25% (v/v) acetic acid/water. Stirred for 20 min (250 rpm) Treated for 6 h at 95 °C |
| V | Organo-chelated (Hg-o) | Solution: 1 M potassium hydroxide Stirred for 18 h (250 rpm) |
| VI | Elemental Hg (Hg-e) | Solution: 12 M nitric acid Stirred for 18 h (250 rpm) |
| VII | Mercuric sulfide (Hg-s) | Acid digestion with aqua regia |
| Element | Black Sediment (mg kg−1) | White Sediment (mg kg−1) | Intervention Values [43] (mg kg−1) |
|---|---|---|---|
| Harmful heavy Metals | |||
| As (mg/kg) | 12.4 ± 1.9 | 10.09 ± 6.7 | 32 |
| Cd (mg/kg) | 0.197 ± 0.03 | 0.30 ± 0.16 | 1 |
| Cr total (mg/kg) | 27.7 ± 4.2 | 7.0 ± 0.3 | 150 |
| Hg (mg/kg) | 47.7 ± 7.2 | 0.04 ± 0.00 | 1 |
| Ni (mg/kg) | 12.6 ± 1.9 | 3.9 ± 0.1 | 63 |
| Pb (mg/kg) | 23 ± 3.5 | 5.55 ± 0.6 | 80 |
| Cu (mg/kg) | 60.3 ± 9.1 | 2.35 ± 0.06 | 75 |
| Zn (mg/kg) | 86 ± 13 | 10.1 ± 1.0 | 165 |
| Hydrocarbons | |||
| Light Hydrocarbons (C6-C12) | BDL (0.5) | BDL (0.5) | |
| Heavy hydrocarbons (C12-C20) | BDL (0.5) | BDL (0.5) | |
| Heavy hydrocarbons (C20-C30) | BDL (0.5) | BDL (0.5) | |
| Heavy hydrocarbons (C30-C40) | BDL (0.5) | BDL (0.5) | |
| Heavy hydrocarbons (C40-C50) | BDL (0.5) | BDL (0.5) | |
| Σ Heavy hydrocarbons (C12-C50) | BDL (0.5) | BDL (0.5) |
| Ref. | Process | Reagent | Hg Initial Concentration (mg/g) | Hg Removal Efficiency (%) |
|---|---|---|---|---|
| [47] | Column | 0.1 M KI + 0.5 M HCl | 113.5 | 76 |
| Batch | 0.1 M KI + 0.5 M HCl | 47.1 | 99 | |
| [48] | Batch | 0.2 M I2 + 0.4 M KI | 35.0 | 98 |
| [45] | Batch | 0.1 M KI | 6.1 | 28 |
| Batch | 1 M Na2S2O3 | 6.1 | 37 | |
| Column | 0.1 M KI | 6.1 | 35 | |
| [49] | Three-step batch | H2O2, Na2S2O3, Na2S | 2.1 | 87 |
| This study | Single-step batch | EDTA 0.2 M | 47.7 | 0.2 |
| This study | Single-step batch | EDDS 0.1 M | 47.7 | 0.7 |
| This study | Single-step batch | Na2S2O3 | 47.7 | 2.5 |
| This study | Single-step batch | KI 0.2 M + I2 0.2 M. | 47.7 | 89.3–85.5 * |
| This study | Double-step batch | KI 0.2 M + I2 0.2 M. | 47.7 | 96.4–91.5 * |
| This study | Three-step batch | KI 0.2 M + I2 0.2 M. | 47.7 | 98.0–93.0 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, G.; Cappello, S.; De Marco, G.; Cappello, T.; Maisano, M.; Gornati, R.; Scalici, M.; Luciano, A.; Viotti, P.; Fino, D. Assessing the Impact of Hg-Contaminated Sediments Washing through Sentinel Species: A Mesocosm Approach. Water 2023, 15, 3258. https://doi.org/10.3390/w15183258
Mancini G, Cappello S, De Marco G, Cappello T, Maisano M, Gornati R, Scalici M, Luciano A, Viotti P, Fino D. Assessing the Impact of Hg-Contaminated Sediments Washing through Sentinel Species: A Mesocosm Approach. Water. 2023; 15(18):3258. https://doi.org/10.3390/w15183258
Chicago/Turabian StyleMancini, Giuseppe, Simone Cappello, Giuseppe De Marco, Tiziana Cappello, Maria Maisano, Rosalba Gornati, Massimiliano Scalici, Antonella Luciano, Paolo Viotti, and Debora Fino. 2023. "Assessing the Impact of Hg-Contaminated Sediments Washing through Sentinel Species: A Mesocosm Approach" Water 15, no. 18: 3258. https://doi.org/10.3390/w15183258
APA StyleMancini, G., Cappello, S., De Marco, G., Cappello, T., Maisano, M., Gornati, R., Scalici, M., Luciano, A., Viotti, P., & Fino, D. (2023). Assessing the Impact of Hg-Contaminated Sediments Washing through Sentinel Species: A Mesocosm Approach. Water, 15(18), 3258. https://doi.org/10.3390/w15183258












