Enhanced Photocatalytic Activity of the Bi2O3-NiO Heterojunction for the Degradation of Methyl Orange under Irradiation of Sunlight
Abstract
:1. Introduction
- Direct conversion of organic pollutants molecules into simple inorganic molecules like water and carbon dioxide
- Mineralization of a wide range of organic pollutants irrespective of selectivity
- No production of hazardous side products.
2. Materials and Methods
2.1. Synthesis of Bi2O3-NiO
2.2. Characterization of Bi2O3-NiO
2.3. Photocatalytic Activity
3. Results and Discussion
3.1. Characterization
3.2. Photocatalytic Activity
3.3. Dependance of Photocatalytic Activity on Catalyst Dosage
3.4. Dependance of Photocatalytic Activity on Concentration of Methyl Orange
3.5. Comparison of the Present Photocatalyst with Reported Photocatalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, A.A.; Salama, R.S.; El-Hakam, S.A.; Khder, A.S.; Ahmed, A.I. Synthesis of Sulfated Zirconium Supported MCM-41 Composite with High-Rate Adsorption of Methylene Blue and Excellent Heterogeneous Catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126361. [Google Scholar] [CrossRef]
- Alasri, T.M.; Ali, S.L.; Salama, R.S.; Alshorifi, F.T. Band-Structure Engineering of TiO2 Photocatalyst by AuSe Quantum Dots for Efficient Degradation of Malachite Green and Phenol. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1729–1740. [Google Scholar] [CrossRef]
- Saleh, T.S.; Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Design and Development of Novel Composites Containing Nickel Ferrites Supported on Activated Carbon Derived from Agricultural Wastes and Its Application in Water Remediation. Materials 2023, 16, 2170. [Google Scholar] [CrossRef]
- Leonel, L.V.; de Oliveira Reis, F.; de Assis Figueiredo, F.A.M.M.; Ferraz, T.M.; de Oliveira Maia Júnior, S.; Silva, P.C.; de Andrade, J.R. Light Intensity and Hydrogel Soil Amendment Differentially Affect Growth and Photosynthesis of Successional Tree Species. J. For. Res. 2023, 34, 257–268. [Google Scholar] [CrossRef]
- Wei, S.; Chen, T.; Hou, H.; Xu, Y. Recent Advances in Electrochemical Sterilization. J. Electroanal. Chem. 2023, 937, 117419. [Google Scholar] [CrossRef]
- Feng, X.; Sun, L.; Wang, W.; Zhao, Y.; Shi, J.W. Construction of CdS@ZnO Core–Shell Nanorod Arrays by Atomic Layer Deposition for Efficient Photoelectrochemical H2 Evolution. Sep. Purif. Technol. 2023, 324, 124520. [Google Scholar] [CrossRef]
- Liang, Y.; Li, J.; Xue, Y.; Tan, T.; Jiang, Z.; He, Y.; Shangguan, W.; Yang, J.; Pan, Y. Benzene Decomposition by Non-Thermal Plasma: A Detailed Mechanism Study by Synchrotron Radiation Photoionization Mass Spectrometry and Theoretical Calculations. J. Hazard. Mater. 2021, 420, 126584. [Google Scholar] [CrossRef] [PubMed]
- Nizam, N.U.M.; Hanafiah, M.M.; Mahmoudi, E.; Halim, A.A.; Mohammad, A.W. The Removal of Anionic and Cationic Dyes from an Aqueous Solution Using Biomass-Based Activated Carbon. Sci. Rep. 2021, 11, 8623. [Google Scholar] [CrossRef]
- Kamran, U.; Rhee, K.Y.; Lee, S.Y.; Park, S.J. Innovative Progress in Graphene Derivative-Based Composite Hybrid Membranes for the Removal of Contaminants in Wastewater: A Review. Chemosphere 2022, 306, 135590. [Google Scholar] [CrossRef]
- Rawal, J.; Kamran, U.; Park, M.; Pant, B.; Park, S.J. Nitrogen and Sulfur Co-Doped Graphene Quantum Dots Anchored TiO2 Nanocomposites for Enhanced Photocatalytic Activity. Catalysts 2022, 12, 548. [Google Scholar] [CrossRef]
- Kamran, U.; Lee, S.Y.; Rhee, K.Y.; Park, S.J. Rice Husk Valorization into Sustainable Ni@TiO2/Biochar Nanocomposite for Highly Selective Pb (II) Ions Removal from an Aqueous Media. Chemosphere 2023, 323, 138210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhong, A.; Huang, G.; Yang, M.; Li, D.; Teng, M.; Han, D. Enhanced Efficiency with CDCA Co-Adsorption for Dye-Sensitized Solar Cells Based on Metallosalophen Complexes. Sol. Energy 2020, 209, 316–324. [Google Scholar] [CrossRef]
- Saeed, M.; Al-Saeed, F.A.; Altaf, M.; Alahmari, S.D.; Bokhari, T.H.; Khan, I.; Bajaber, M.A.; Ahmed, A.E. Synthesis of Visible-Light-Driven Ag2O-Co3O4 Z-Scheme Photocatalyst for Enhanced Photodegradation of Reactive Yellow Dye. New J. Chem. 2022, 13, 936. [Google Scholar] [CrossRef]
- Khan, I.; Luo, M.; Khan, S.; Asghar, H.; Saeed, M.; Khan, S.; Khan, A.; Humayun, M.; Guo, L.; Shi, B. Green Synthesis of SrO Bridged LaFeO3/g-C3N4 Nanocomposites for CO2 Conversion and Bisphenol A Degradation with New Insights into Mechanism. Environ. Res. 2022, 207, 112650. [Google Scholar] [CrossRef]
- Khan, I.; Luo, M.; Guo, L.; Khan, S.; Wang, C.; Khan, A.; Saeed, M.; Zaman, S.; Qi, K.; Liu, Q.L. Enhanced Visible-Light Photoactivities of Porous LaFeO3 by Synchronously Doping Ni2+ and Coupling TS-1 for CO2 reduction and 2,4,6-Trinitrophenol Degradation. Catal. Sci. Technol. 2021, 11, 6793–6803. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; ul Haq, A.; Akram, N. Photocatalysis: An Effective Tool for Photodegradation of Dyes—A Review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, J.; Qiao, R.; Zhao, N.; Zhao, M.; Kong, L. Facile Preparation and Controllable Absorption of a Composite Based on PMo12/Ag Nanoparticles: Photodegradation Activity and Mechanism. ChemistrySelect 2022, 7, e202103668. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, X.; Shen, Z.; Gu, Y.; He, L.; Zhang, M.; Lu, N. Single-Atom Fe Nanozymes Coupling with Atomic Clusters as Superior Oxidase Mimics for Ratiometric Fluorescence Detection. Chem. Eng. J. 2023, 469, 143923. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Guo, X.; Chen, Z.; Zhang, W.; Wang, Y.; Tang, X.; Zhang, Y.; Zhao, Y. Sulfur-Doped g-C3N4/RGO Porous Nanosheets for Highly Efficient Photocatalytic Degradation of Refractory Contaminants. J. Mater. Sci. Technol. 2020, 41, 117–126. [Google Scholar] [CrossRef]
- Pang, S.; Zhou, C.; Sun, Y.; Zhang, K.; Ye, W.; Zhao, X.; Cai, L.; Hui, B. Natural Wood-Derived Charcoal Embedded with Bimetallic Iron/Cobalt Sites to Promote Ciprofloxacin Degradation. J. Clean. Prod. 2023, 414, 137569. [Google Scholar] [CrossRef]
- Shimi, A.K.; Parvathiraj, C.; Kumari, S.; Dalal, J.; Kumar, V.; Wabaidur, S.M.; Alothman, Z. Green Synthesis of SrO Nanoparticles Using Leaf Extract of Albizia Julibrissin and Its Recyclable Photocatalytic Activity: An Eco-Friendly Approach for Treatment of Industrial Wastewater. Environ. Sci. Adv. 2022, 1, 849–861. [Google Scholar] [CrossRef]
- Kadian, N.; Kumari, R.; Panchal, A.; Dalal, J.; Padalia, D. Structural and Optical Properties of Gadolinium Doped-Magnetite Nano-Crystal for Photocatalytic Application. J. Alloys Compd. 2023, 960, 170811. [Google Scholar] [CrossRef]
- He, X.; Xie, S.; Xu, J.; Yin, X.-B.; Zhang, M. Reactive Template-Engaged Synthesis of NiSx/MoS2 Nanosheets Decorated on Hollow and Porous Carbon Microtubes with Optimal Electronic Modulation toward High-Performance Enzyme-like Performance. Inorg. Chem. 2023, 62, 8033–8042. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Zheng, Y.; Sun, Z.; Xia, S.; Ni, Z.; Yao, J. Fabrication of ZnAl-LDH Mixed Metal-Oxide Composites for Photocatalytic Degradation of 4-Chlorophenol. Environ. Sci. Pollut. Res. 2022, 29, 39441–39450. [Google Scholar] [CrossRef]
- Lu, C.; Xu, C.; Sun, W.; Ren, R.; Qiao, J.; Wang, Z.; Sun, K.; Pan, G.; Cao, Y. Enhancing Catalytic Activity of CO2 Electrolysis by Building Efficient and Durable Heterostructure for Solid Oxide Electrolysis Cell Cathode. J. Power Sources 2023, 574, 233134. [Google Scholar] [CrossRef]
- Khan, I.; Sun, N.; Zhang, Z.; Li, Z.; Humayun, M.; Ali, S.; Qu, Y.; Jing, L. Improved Visible-Light Photoactivities of Porous LaFeO3 by Coupling with Nanosized Alkaline Earth Metal Oxides and Mechanism Insight. Catal. Sci. Technol. 2019, 9, 3149–3157. [Google Scholar] [CrossRef]
- Li, G.; Huang, S.; Li, K.; Zhu, N.; Zhao, B.; Zhong, Q.; Zhang, Z.; Ge, D.; Wang, D. Near-Infrared Responsive Z-Scheme Heterojunction with Strong Stability and Ultra-High Quantum Efficiency Constructed by Lanthanide-Doped Glass. Appl. Catal. B 2022, 311, 121363. [Google Scholar] [CrossRef]
- Khan, I.; Luo, M.; Guo, L.; Khan, S.; Shah, S.A.; Khan, I.; Khan, A.; Wang, C.; Ai, B.; Zaman, S. Synthesis of Phosphate-Bridged g-C3N4/LaFeO3 Nanosheets Z-Scheme Nanocomposites as Efficient Visible Photocatalysts for CO2 Reduction and Malachite Green Degradation. Appl. Catal. A Gen. 2022, 629, 118418. [Google Scholar] [CrossRef]
- Luo, J.; Han, H.; Wang, X.; Qiu, X.; Liu, B.; Lai, Y.; Chen, X.; Zhong, R.; Wang, L.; Wang, C. Single-Atom Nb Anchored on Graphitic Carbon Nitride for Boosting Electron Transfer towards Improved Photocatalytic Performance. Appl. Catal. B 2023, 328, 122495. [Google Scholar] [CrossRef]
- Adeel, M.; Saeed, M.; Khan, I.; Muneer, M.; Akram, N. Synthesis and Characterization of Co-ZnO and Evaluation of Its Photocatalytic Activity for Photodegradation of Methyl Orange. ACS Omega 2021, 6, 1426–1435. [Google Scholar] [CrossRef]
- Chen, M.; Yao, J.; Huang, Y.; Gong, H.; Chu, W. Enhanced Photocatalytic Degradation of Ciprofloxacin over Bi2O3/(BiO)2CO3 Heterojunctions: Efficiency, Kinetics, Pathways, Mechanisms and Toxicity Evaluation. Chem. Eng. J. 2018, 334, 453–461. [Google Scholar] [CrossRef]
- Song, C.; Wang, L.J.; Sun, S.M.; Wu, Y.; Xu, L.J.; Gan, L. Preparation of Visible-Light Photocatalysts of Bi2O3/Bi Embedded in Porous Carbon from Bi-Based Metal Organic Frameworks for Highly Efficient Rhodamine B Removal from Water. Xinxing Tan. Cailiao/New Carbon. Mater. 2020, 35, 609–618. [Google Scholar] [CrossRef]
- Bhaviya Raj, R.; Umadevi, M.; Parimaladevi, R. Synergy Photodegradation of Basic Dyes by Zno/Bi2o3 Nanocomposites under Visible Light Irradiation. Iran. J. Chem. Chem. Eng. 2021, 40, 1121–1131. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, S.; Bansal, P. Visible Light Responsive Heterostructured α-Bi2O3/Zno Doped β-Bi2O3 Photocatalyst for Remediation of Organic Pollutants. Desalination Water Treat. 2020, 201, 349–355. [Google Scholar] [CrossRef]
- Ansari, S.; Ansari, M.S.; Satsangee, S.P.; Jain, R. Bi2O3/ZnO Nanocomposite: Synthesis, Characterizations and Its Application in Electrochemical Detection of Balofloxacin as an Anti-Biotic Drug. J. Pharm. Anal. 2021, 11, 57–67. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Yuan, S.; Ge, M.; Ren, M.; Sun, C.; Zhou, Z. Nanosheet-Based NiO Microspheres: Controlled Solvothermal Synthesis and Lithium Storage Performances. J. Phys. Chem. C 2009, 114, 251–255. [Google Scholar] [CrossRef]
- Jegatha Christy, A.; Umadevi, M. Novel Combustion Method to Prepare Octahedral NiO Nanoparticles and Its Photocatalytic Activity. Mater. Res. Bull. 2013, 48, 4248–4254. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Sun, L.; Mao, Y.; Wang, M.; Lin, C. An Ultrasound-Assisted Deposition of NiO Nanoparticles on TiO2 Nanotube Arrays for Enhanced Photocatalytic Activity. J. Mater. Chem. A Mater. 2014, 2, 8223–8229. [Google Scholar] [CrossRef]
- Ji, Z.; Natu, G.; Wu, Y. Cyclometalated Ruthenium Sensitizers Bearing a Triphenylamino Group for P-Type NiO Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 8641–8648. [Google Scholar] [CrossRef]
- Wan, X.; Yuan, M.; Tie, S.L.; Lan, S. Effects of Catalyst Characters on the Photocatalytic Activity and Process of NiO Nanoparticles in the Degradation of Methylene Blue. Appl. Surf. Sci. 2013, 277, 40–46. [Google Scholar] [CrossRef]
- Yi, S.; Yue, X.; Xu, D.; Liu, Z.; Zhao, F.; Wang, D.; Lin, Y. Study on Photogenerated Charge Transfer Properties and Enhanced Visible-Light Photocatalytic Activity of p-Type Bi2O3/n-Type ZnO Heterojunctions. New J. Chem. 2015, 39, 2917–2924. [Google Scholar] [CrossRef]
- Saeed, M.; Haq, A.U.; Muneer, M.; Ahmad, A.; Bokhari, T.H.; Sadiq, Q. Synthesis and Characterization of Bi2O3 and Ag-Bi2O3 and Evaluation of Their Photocatalytic Activities towards Photodegradation of Crystal Violet Dye. Phys. Scr. 2021, 96, 125707. [Google Scholar] [CrossRef]
- Balachandran, S.; Swaminathan, M. Facile Fabrication of Heterostructured Bi2O3-ZnO Photocatalyst and Its Enhanced Photocatalytic Activity. J. Phys. Chem. C 2012, 116, 26306–26312. [Google Scholar] [CrossRef]
- Jabeen Fatima, M.J.; Navaneeth, A.; Sindhu, S. Improved Carrier Mobility and Bandgap Tuning of Zinc Doped Bismuth Oxide. RSC Adv. 2015, 5, 2504–2510. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S. A Simple Mechanical Mixing Method for Preparation of Visible-Light-Sensitive NiO–CaO Composite Photocatalysts with High Photocatalytic Activity. J. Hazard. Mater. 2010, 174, 563–566. [Google Scholar] [CrossRef]
- Davar, F.; Fereshteh, Z.; Salavati-Niasari, M. Nanoparticles Ni and NiO: Synthesis, Characterization and Magnetic Properties. J. Alloys Compd. 2009, 476, 797–801. [Google Scholar] [CrossRef]
- Vimal Kumar, M.; Gokul Raja, T.S.; Selvakumar, N.; Jeyasubramanaian, K. Synthesis and Characterization of NiO-ZnO Nanocomposite by a Cost Efficient Self-Combustion Technique. J. Achiev. Mater. Manuf. Eng. 2016, 79, 13–18. [Google Scholar] [CrossRef]
- Saeed, M.; Adeel, S.; Muneer, M.; ul Haq, A. Photo Catalysis: An Effective Tool for Treatment of Dyes Contaminated Wastewater. Bioremediat. Biotechnol. 2020, 2, 175–187. [Google Scholar] [CrossRef]
- Nisar, A.; Saeed, M.; Usman, M.; Muneer, M.; Adeel, M.; Khan, I.; Akhtar, J. Kinetic Modeling of ZnO-RGO Catalyzed Degradation of Methylene Blue. Int. J. Chem. Kinet. 2020, 52, 645–654. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Khosa, M.K.K.; Akram, N.; Khalid, S.; Adeel, M.; Nisar, A.; Sherazi, S. Azadirachta Indica Leaves Extract Assisted Green Synthesis of Ag-TiO2 for Degradation of Methylene Blue and Rhodamine B Dyes in Aqueous Medium. Green. Process. Synth. 2019, 8, 659–666. [Google Scholar] [CrossRef]
- Valian, M.; Beshkar, F.; Salavati-Niasari, M. Urchin-like Dy2CoMnO6 Double Perovskite Nanostructures: New Simple Fabrication and Investigation of Their Photocatalytic Properties. J. Mater. Sci. Mater. Electron. 2017, 28, 12440–12447. [Google Scholar] [CrossRef]
- Beshkar, F.; Zinatloo-Ajabshir, S.; Bagheri, S.; Salavati-Niasari, M. Novel Preparation of Highly Photocatalytically Active Copper Chromite Nanostructured Material via a Simple Hydrothermal Route. PLoS ONE 2017, 12, e0158549. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chen, Y.; Chen, B.; Ma, J. Preparation, Characterization and Photocatalytic Activity of CuBi2O4/NaTaO3 Coupled Photocatalysts. J. Alloys Compd. 2013, 559, 116–122. [Google Scholar] [CrossRef]
- Saeed, M.; Alwadai, N.; ben Farhat, L.; Baig, A.; Nabgan, W.; Iqbal, M. Co3O4-Bi2O3 Heterojunction: An Effective Photocatalyst for Photodegradation of Rhodamine B Dye. Arab. J. Chem. 2022, 15, 103732. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Mannaa, M.A.; Alotaibi, M.T.; El-Bahy, S.M.; Salama, R.S. Facile and Green Synthesis of Silver Quantum Dots Immobilized onto a Polymeric CTS-PEO Blend for the Photocatalytic Degradation of p-Nitrophenol. ACS Omega 2021, 6, 30432–30441. [Google Scholar] [CrossRef]
- Rohilla, S.; Gupta, A.; Kumar, V.; Kumari, S.; Petru, M.; Amor, N.; Noman, M.T.; Dalal, J. Excellent Uv-light Triggered Photocatalytic Performance of Zno.Sio2 Nanocomposite for Water Pollutant Compound Methyl Orange Dye. Nanomaterials 2021, 11, 2548. [Google Scholar] [CrossRef]
- de Moraes, N.P.; Silva, F.N.; da Silva, M.L.C.P.; Campos, T.M.B.; Thim, G.P.; Rodrigues, L.A. Methylene Blue Photodegradation Employing Hexagonal Prism-Shaped Niobium Oxide as Heterogeneous Catalyst: Effect of Catalyst Dosage, Dye Concentration, and Radiation Source. Mater. Chem. Phys. 2018, 214, 95–106. [Google Scholar] [CrossRef]
- Mostafa, E.M.; Amdeha, E. Enhanced Photocatalytic Degradation of Malachite Green Dye by Highly Stable Visible-Light-Responsive Fe-Based Tri-Composite Photocatalysts. Environ. Sci. Pollut. Res. 2022, 29, 69861–69874. [Google Scholar] [CrossRef]
- Patil, S.B.; Ravishankar, T.N.; Lingaraju, K.; Raghu, G.K.; Nagaraju, G. Multiple Applications of Combustion Derived Nickel Oxide Nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 277–287. [Google Scholar] [CrossRef]
- Khairnar, S.D.; Shrivastava, V.S. Facile Synthesis of Nickel Oxide Nanoparticles for the Degradation of Methylene Blue and Rhodamine B Dye: A Comparative Study. J. Taibah Univ. Sci. 2019, 13, 1108–1118. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Ali, S.L.; Salama, R.S. Promotional Synergistic Effect of Cs–Au NPs on the Performance of Cs–Au/MgFe2O4 Catalysts in Catalysis 3,4-Dihydropyrimidin-2(1H)-Ones and Degradation of RhB Dye. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3765–3776. [Google Scholar] [CrossRef]
- El-Hakam, S.A.; ALShorifi, F.T.; Salama, R.S.; Gamal, S.; El-Yazeed, W.S.A.; Ibrahim, A.A.; Ahmed, A.I. Application of Nanostructured Mesoporous Silica/Bismuth Vanadate Composite Catalysts for the Degradation of Methylene Blue and Brilliant Green. J. Mater. Res. Technol. 2022, 18, 1963–1976. [Google Scholar] [CrossRef]
- Dhiman, P.; Sharma, G.; Alodhayb, A.N.; Kumar, A.; Rana, G.; Sithole, T.; ALOthman, Z.A. Constructing a Visible-Active CoFe2O4@Bi2O3/NiO Nanoheterojunction as Magnetically Recoverable Photocatalyst with Boosted Ofloxacin Degradation Efficiency. Molecules 2022, 27, 8234. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Fernandez-Garcia, J.; Khan, M.I.; Shanableh, A.; Khan, N.A.; ur Rehman, A. Formulation of Bismuth (Bi2O3) and Cerium Oxides (CeO2) Nanosheets for Boosted Visible Light Degradation of Methyl Orange and Methylene Blue Dyes in Water. Catalysts 2022, 12, 1197. [Google Scholar] [CrossRef]
- Masula, K.; Sreedhar, P.; Vijay Kumar, P.; Bhongiri, Y.; Pola, S.; Basude, M. Synthesis and Characterization of NiO–Bi2O3 Nanocomposite Material for Effective Photodegradation of the Dyes and Agricultural Soil Pollutants. Mater. Sci. Semicond. Process 2023, 160, 107432. [Google Scholar] [CrossRef]
- Banoth, P.; Narsaiah, B.P.; De Los Santos Valladares, L.; Kargin, J.; Kollu, P. Single-Phase BiFeO3 and BiFeO3-Fe2O3 Nanocomposite Photocatalysts for Photodegradation of Organic Dye Pollutants. Nanoscale Adv. 2023, 5, 2646–2656. [Google Scholar] [CrossRef]
- Poorsajadi, F.; Sayadi, M.H.; Hajiani, M.; Rezaei, M.R. Synthesis of CuO/Bi2O3 Nanocomposite for Efficient and Recycling Photodegradation of Methylene Blue Dye. Int. J. Environ. Anal. Chem. 2022, 102, 7165–7178. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.; Yu, R.; Luo, L.; Li, W.; Cheng, J.; Zhang, Y. Excellent Photocatalytic Degradation of Rhodamine B over Bi2O3 supported on Zn-MOF Nanocomposites under Visible Light. Green. Process. Synth. 2023, 12, 20228123. [Google Scholar] [CrossRef]
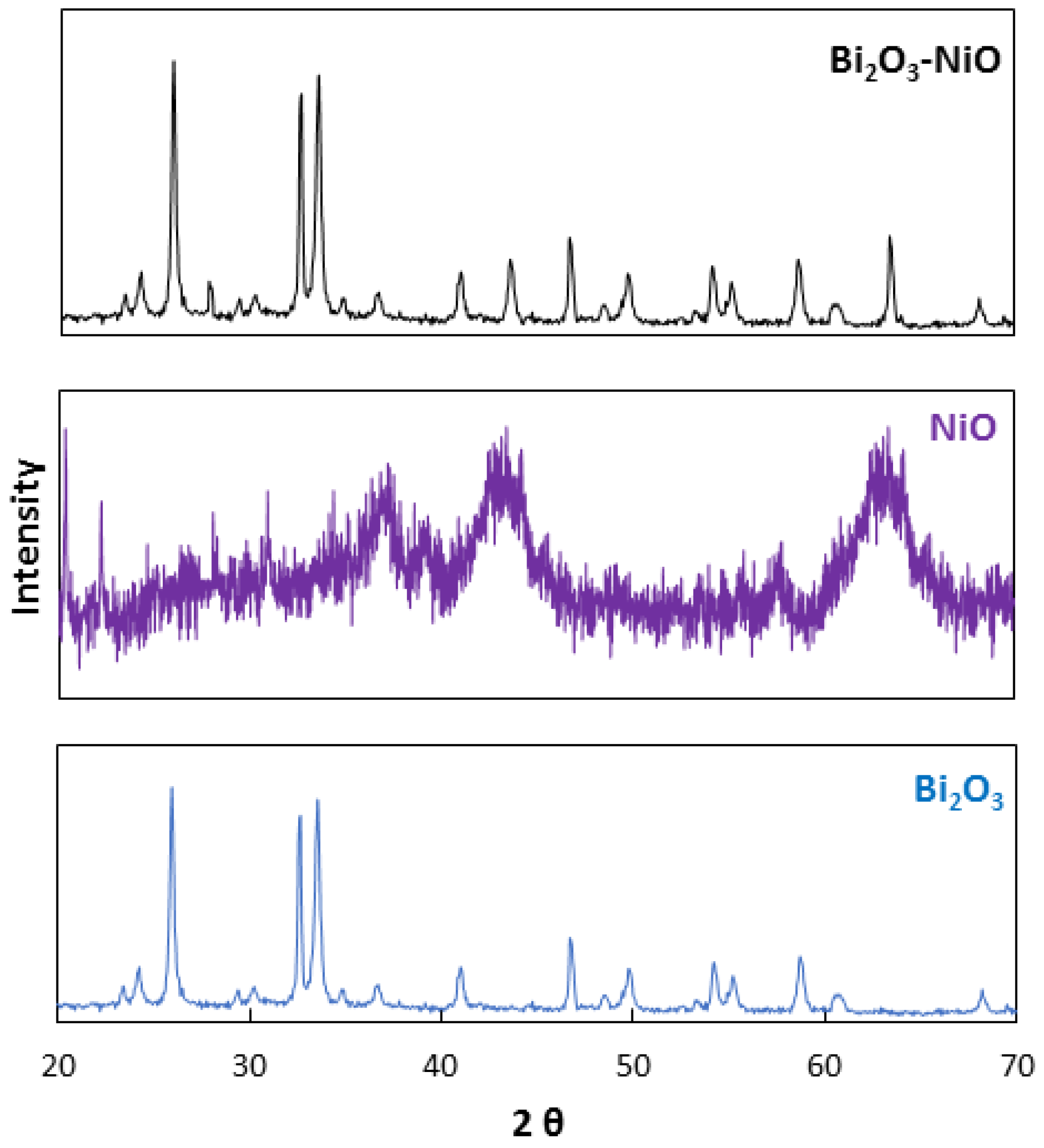
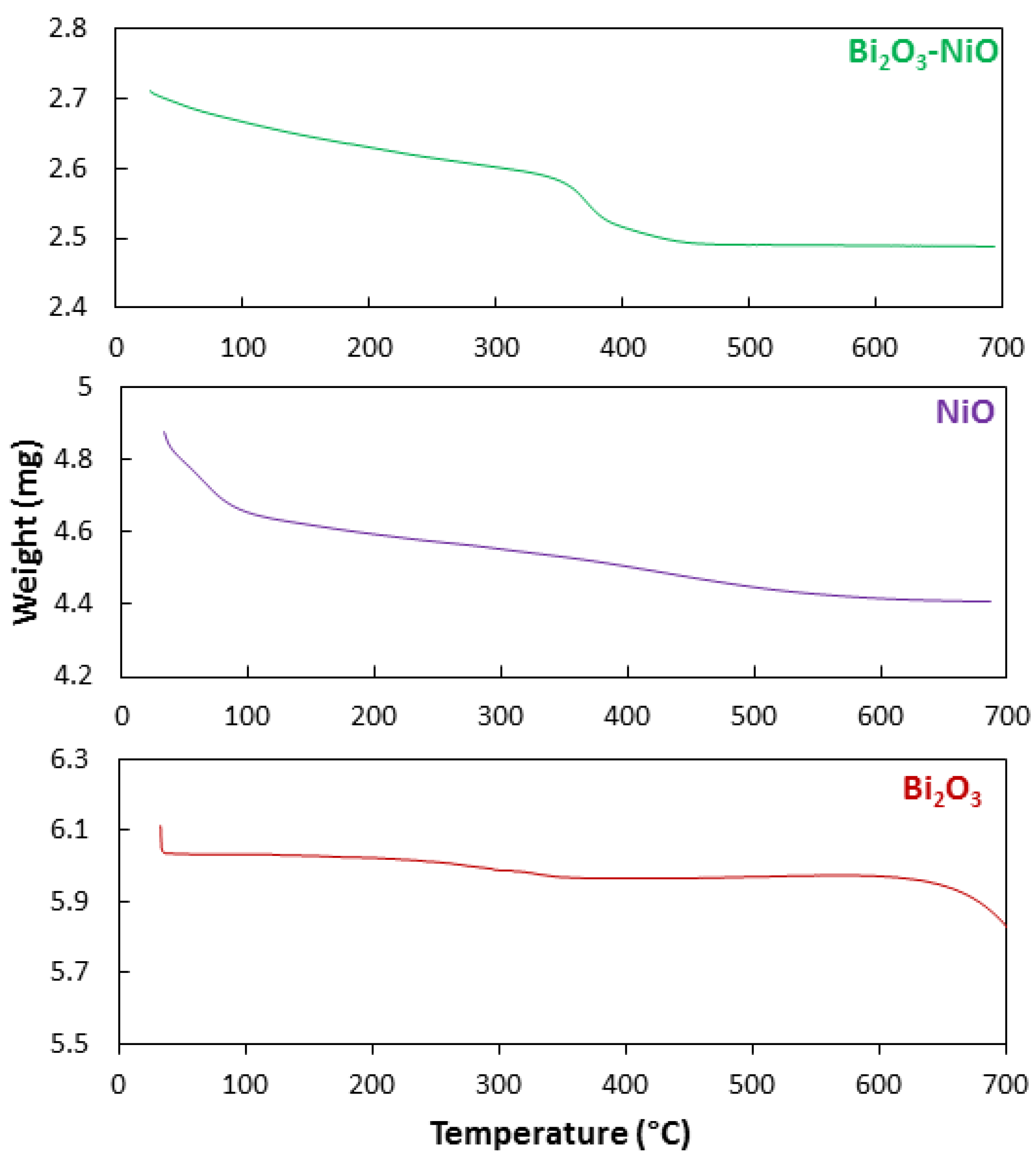
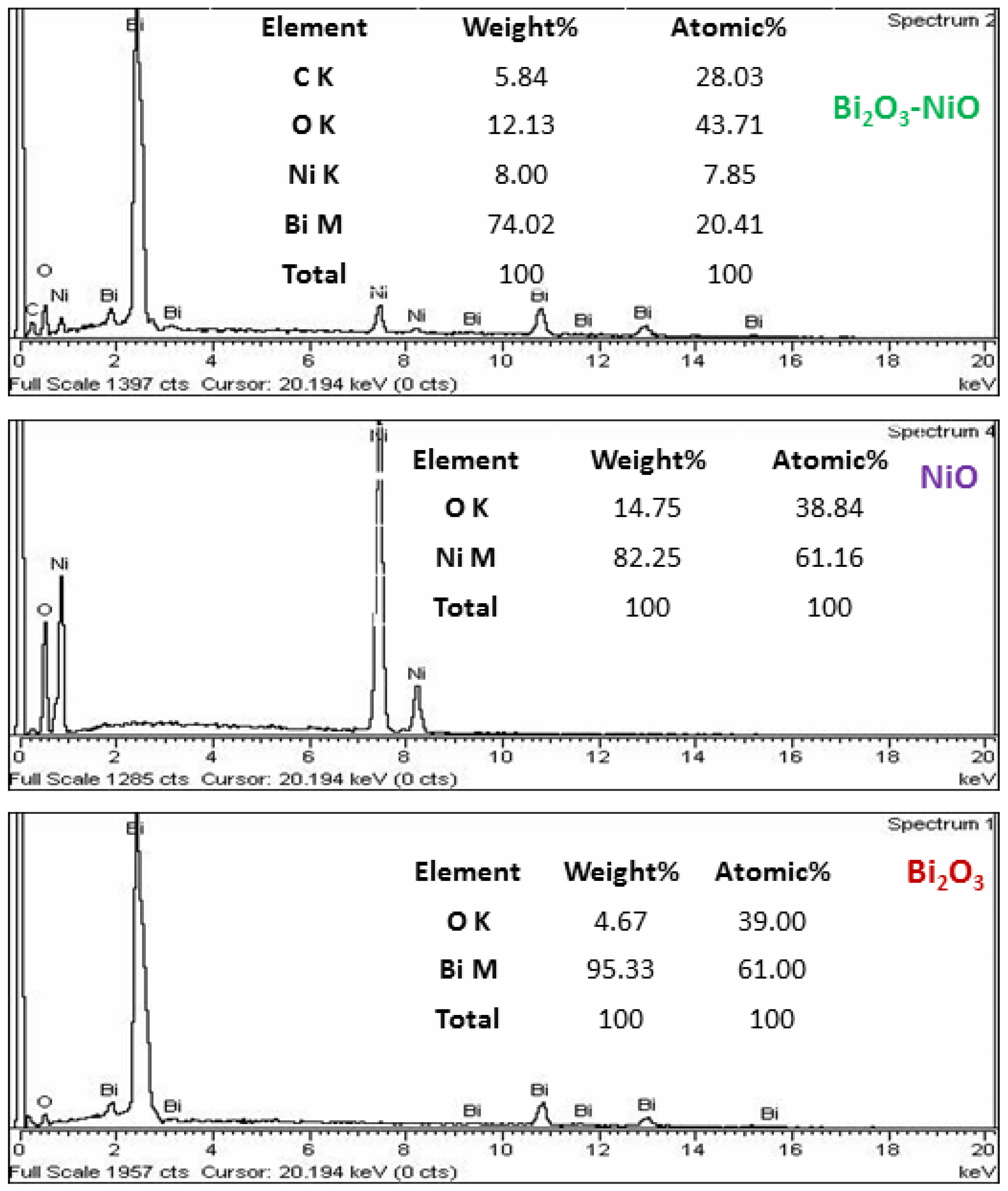

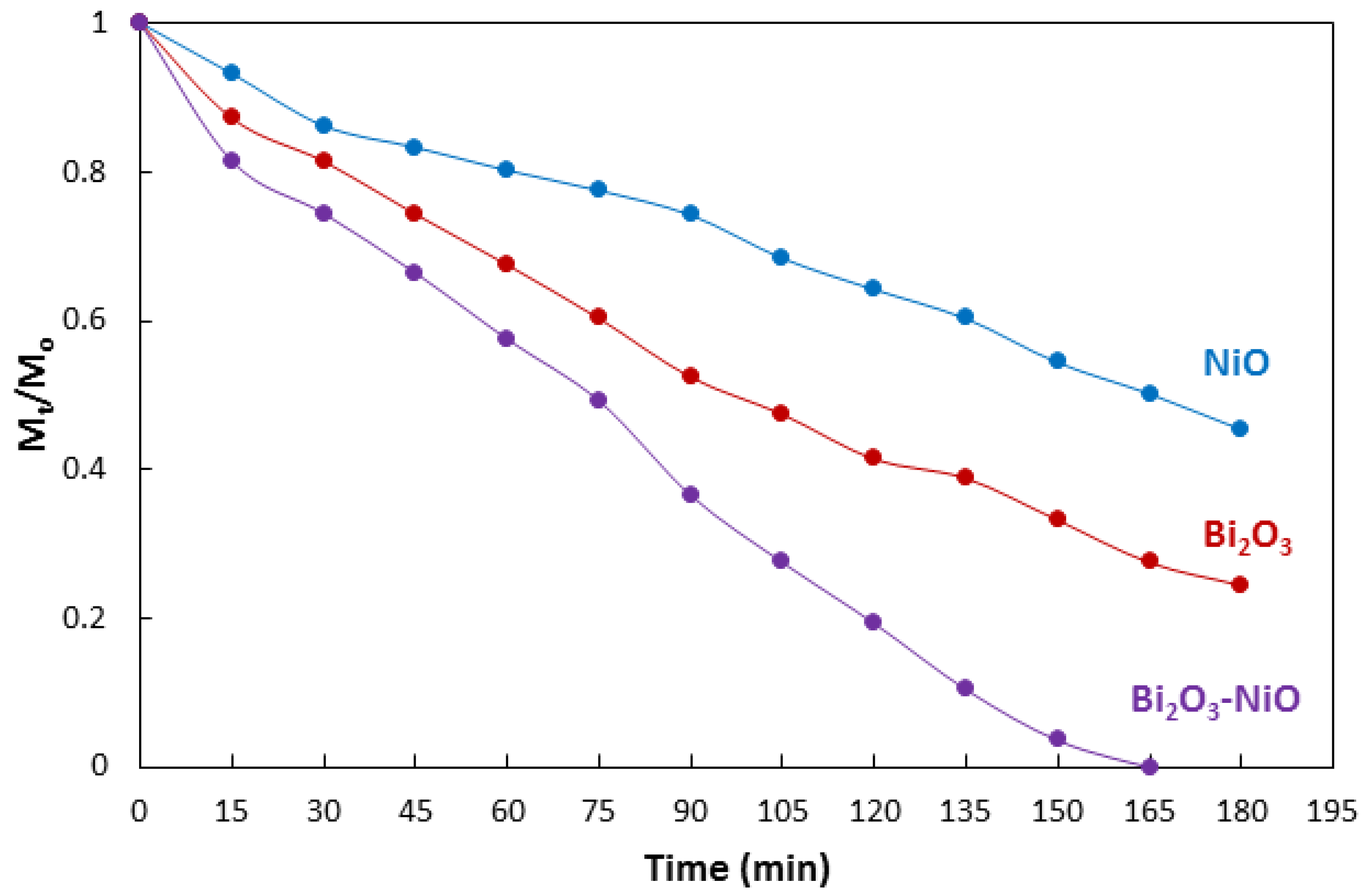
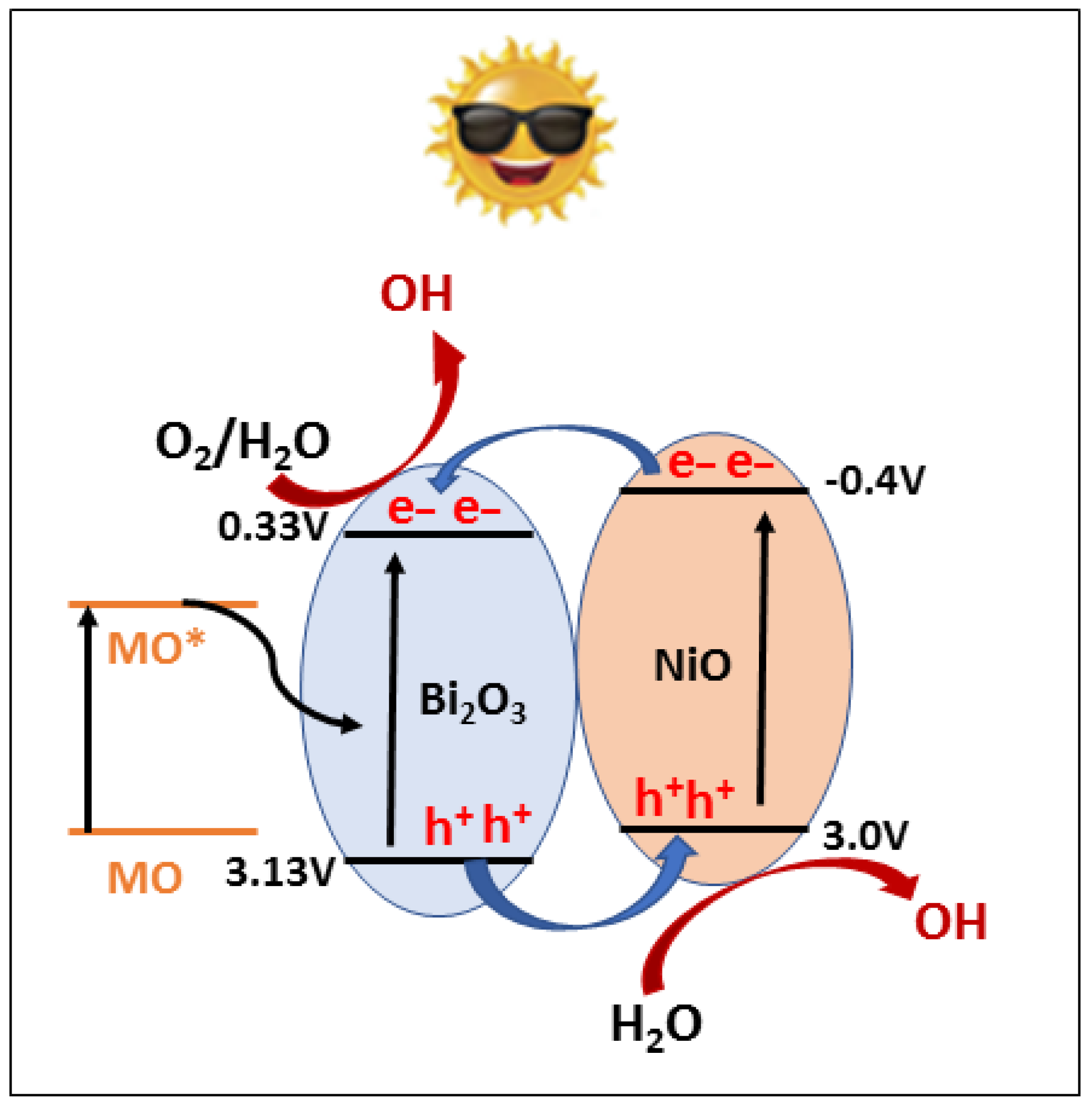
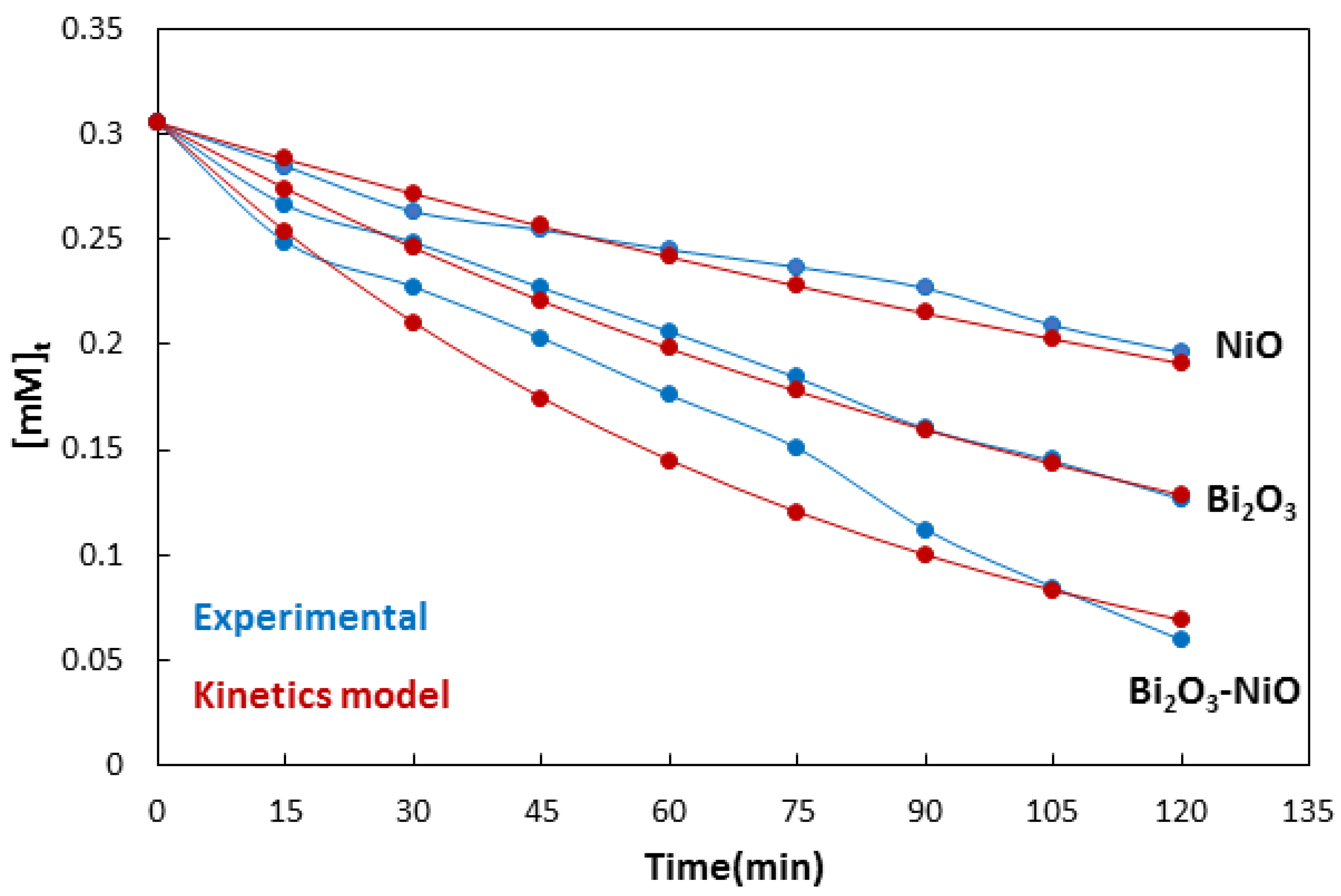
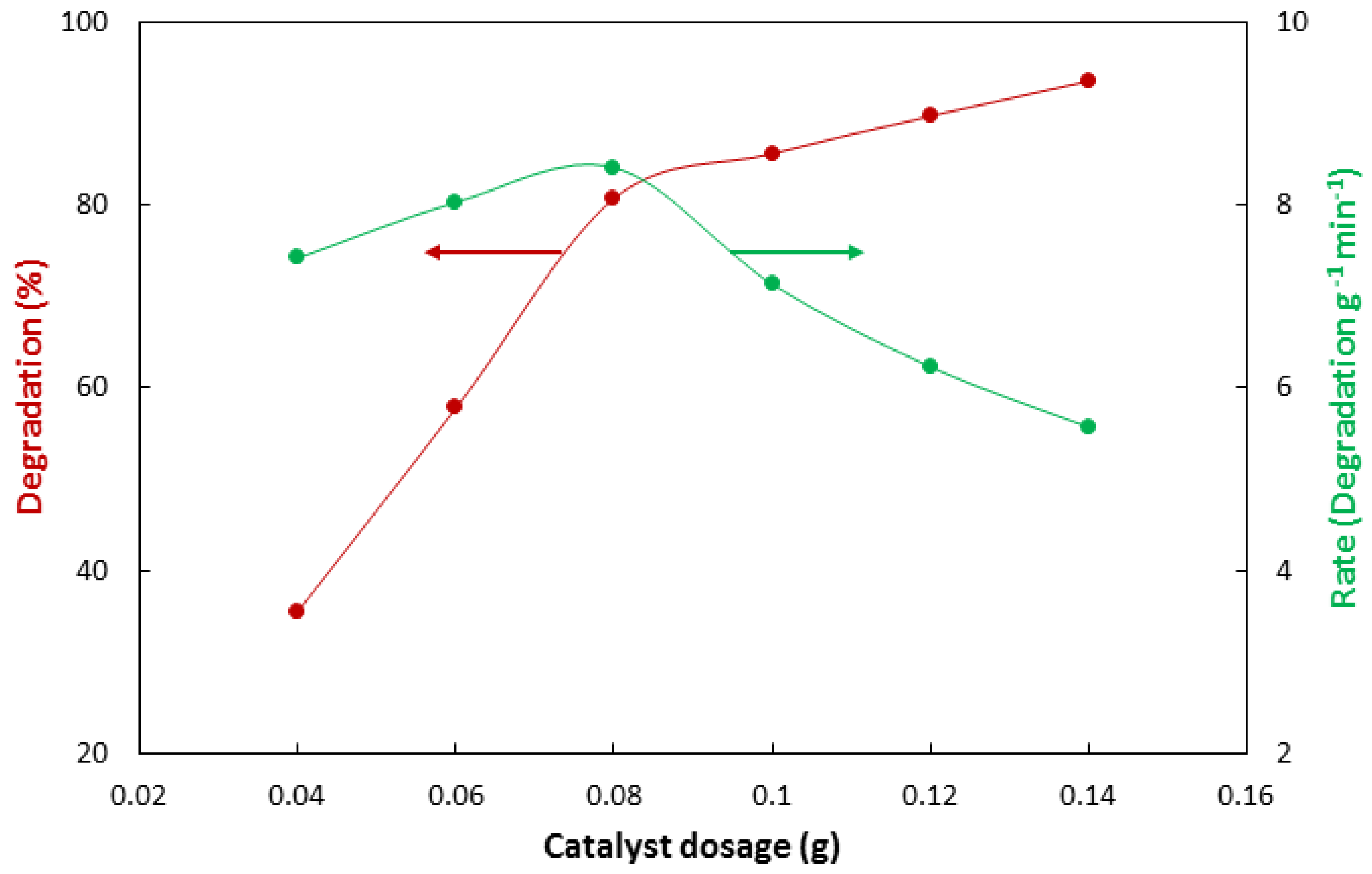
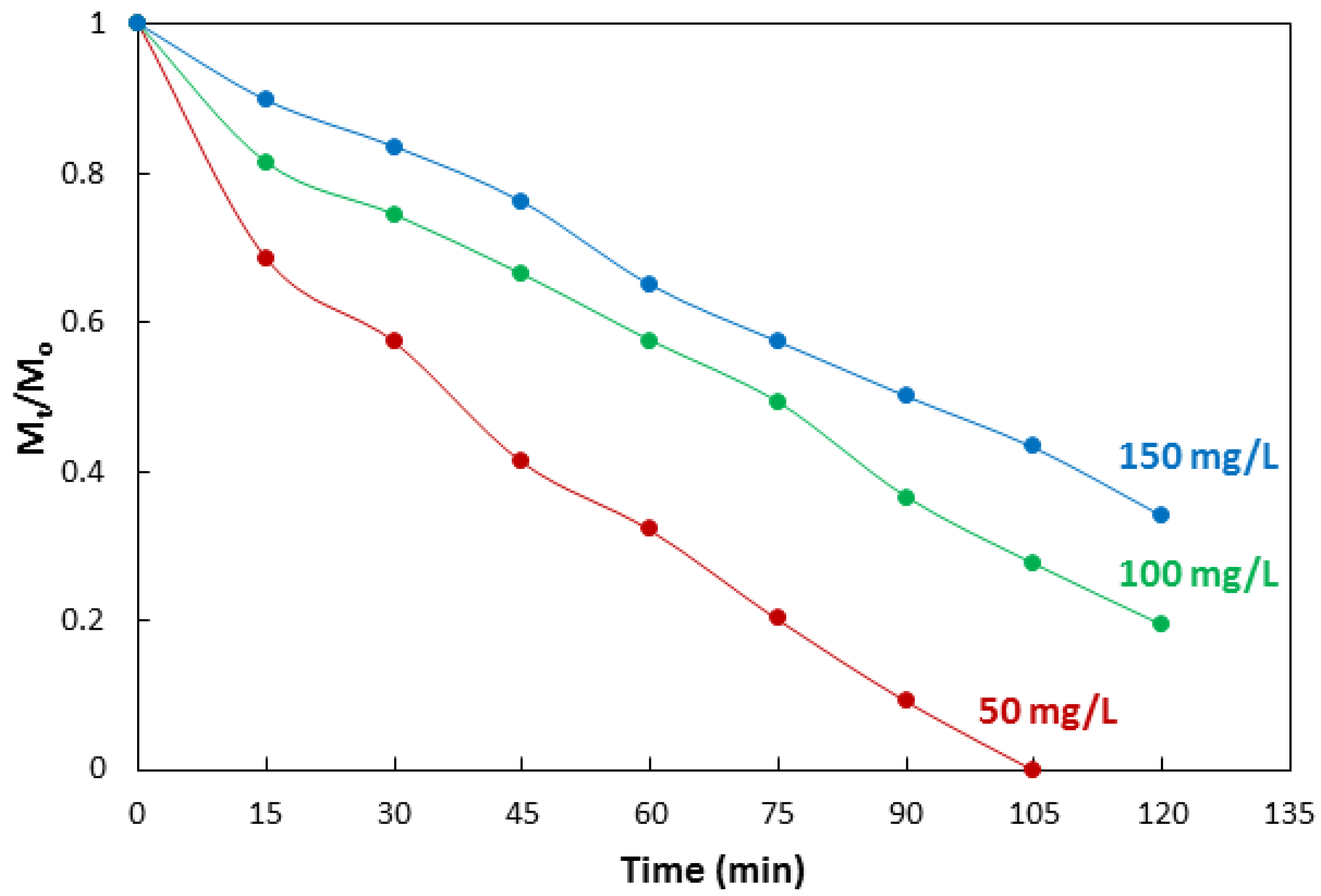
| No | Characterization Technique | Instrument | Model |
|---|---|---|---|
| 1 | XRD analysis | X-ray diffractometer | JOEL-JDX-3532, Japan |
| 2 | EDX analysis | EDX spectrophotometer | JSM5910, UK |
| 3 | SEM analysis | Scanning electron microscope | JEOL-JSM 5910, Japan |
| 4 | FTIR analysis | IR spectrophotometer | Bruker VRTEX70, USA |
| 5 | Thermal gravimetric analysis | TGA analyzer | Perkin Elmer 6300 TGA analyzer, USA |
| 6 | Analysis of dye solution | UV-visible spectrophotometer | Hitachi U-2800, Japan |
| Catalyst | kobs | R2 |
|---|---|---|
| NiO | 0.0039 | 0.99 |
| Bi2O3 | 0.0072 | 0.99 |
| Bi2O3-NiO | 0.0125 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashfaq, M.; Ali, A.; Abbood, N.K.; Panchal, S.; Akram, N.; Saeed, M.; Doshi, O.P.; Ali, F.; Muhammad, S.; Sameeh, M.Y.; et al. Enhanced Photocatalytic Activity of the Bi2O3-NiO Heterojunction for the Degradation of Methyl Orange under Irradiation of Sunlight. Water 2023, 15, 3182. https://doi.org/10.3390/w15183182
Ashfaq M, Ali A, Abbood NK, Panchal S, Akram N, Saeed M, Doshi OP, Ali F, Muhammad S, Sameeh MY, et al. Enhanced Photocatalytic Activity of the Bi2O3-NiO Heterojunction for the Degradation of Methyl Orange under Irradiation of Sunlight. Water. 2023; 15(18):3182. https://doi.org/10.3390/w15183182
Chicago/Turabian StyleAshfaq, Muhammad, Akbar Ali, Nabeel K. Abbood, Sandeep Panchal, Nadia Akram, Muhammad Saeed, Ojas Prakashbhai Doshi, Faiz Ali, Shabbir Muhammad, Manal Y. Sameeh, and et al. 2023. "Enhanced Photocatalytic Activity of the Bi2O3-NiO Heterojunction for the Degradation of Methyl Orange under Irradiation of Sunlight" Water 15, no. 18: 3182. https://doi.org/10.3390/w15183182







