Synthesis of Ag-OMS Catalyst for Sunlight-Assisted Photodegradation of Crystal Violet Dye
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Manganese Oxide OMS
2.2. Synthesis of Ag-Decorated Manganese Oxide OMS
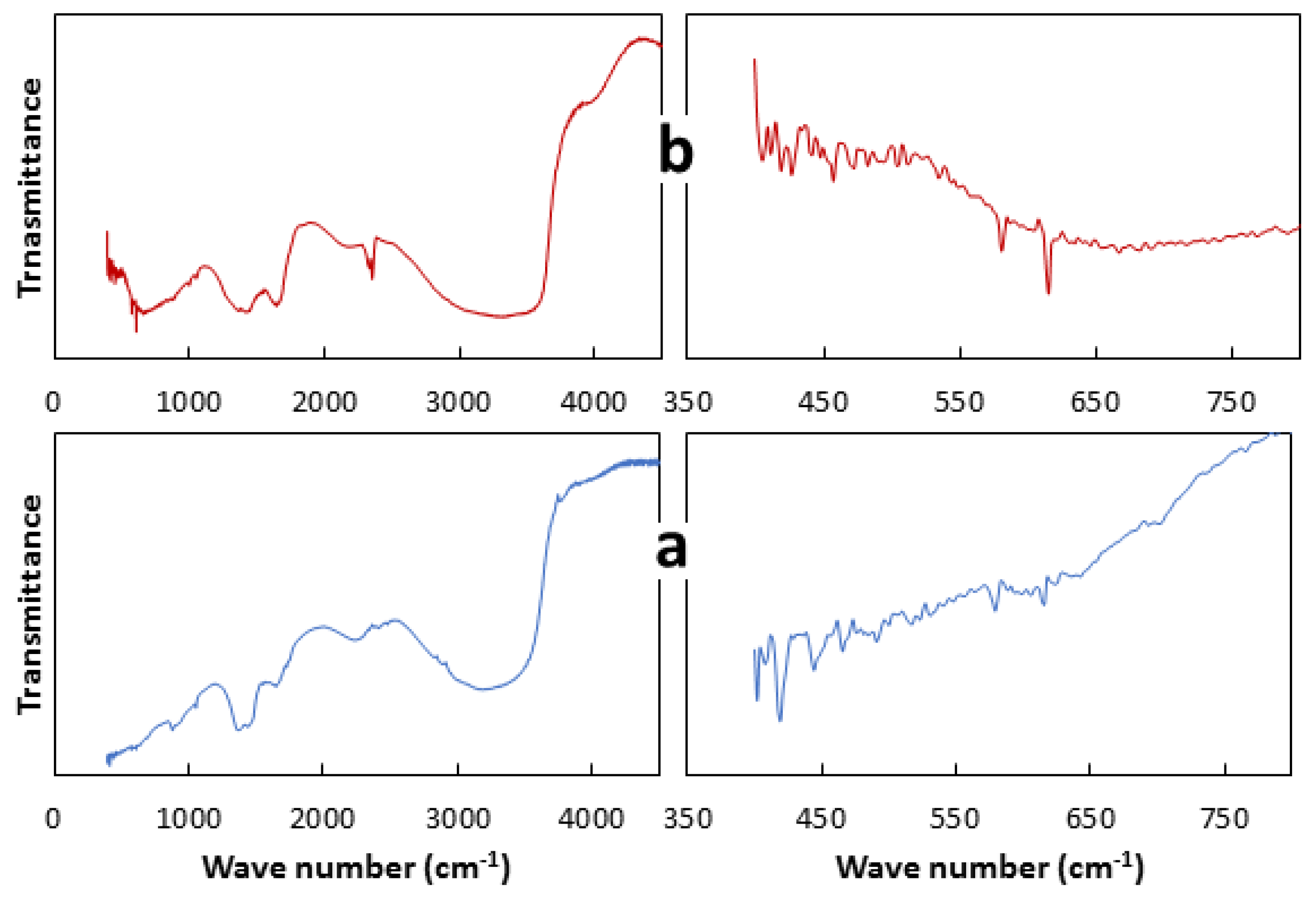
2.3. Characterization
2.4. Photocatalysis
3. Results and Discussion
3.1. Synthesis
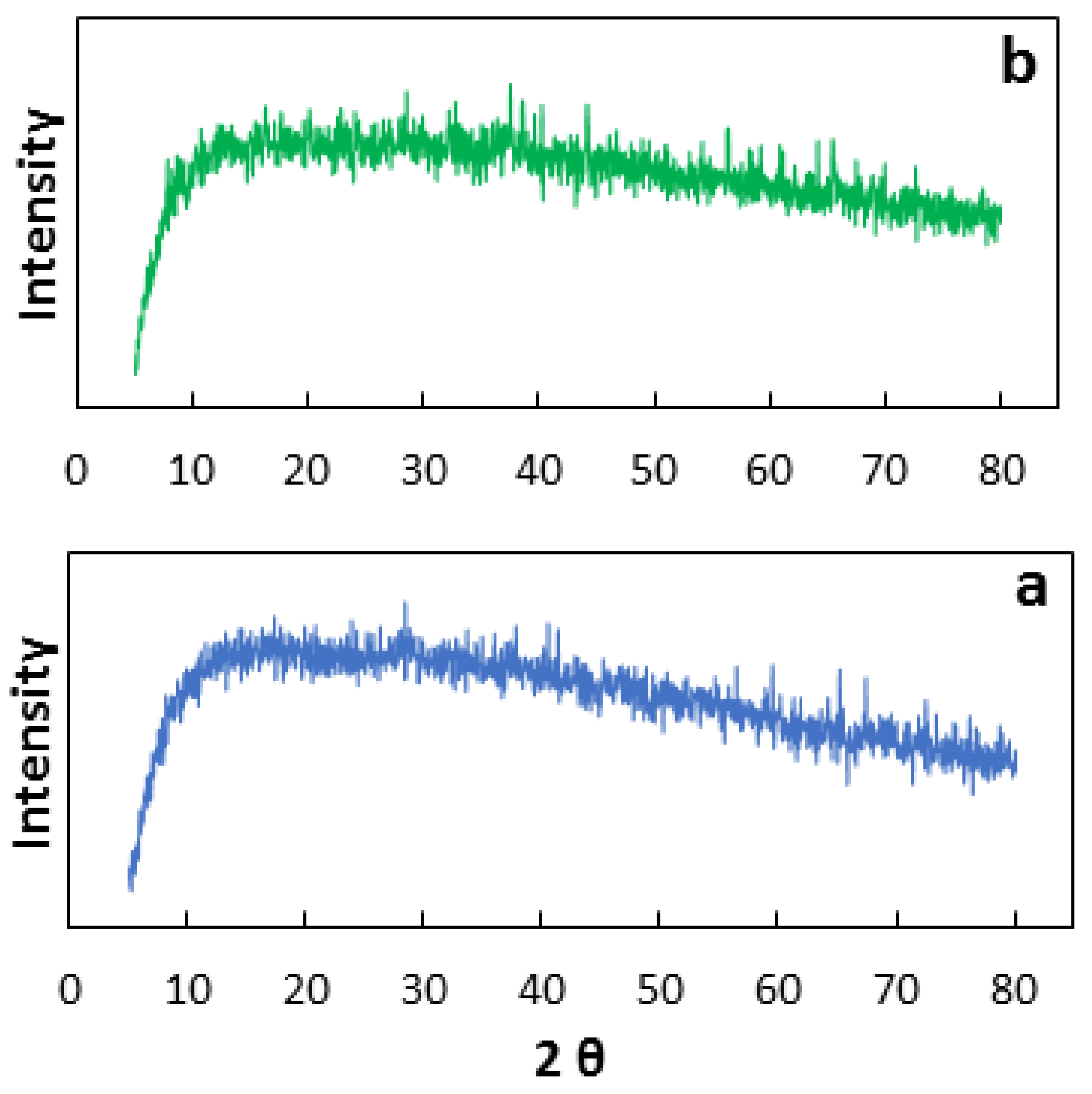
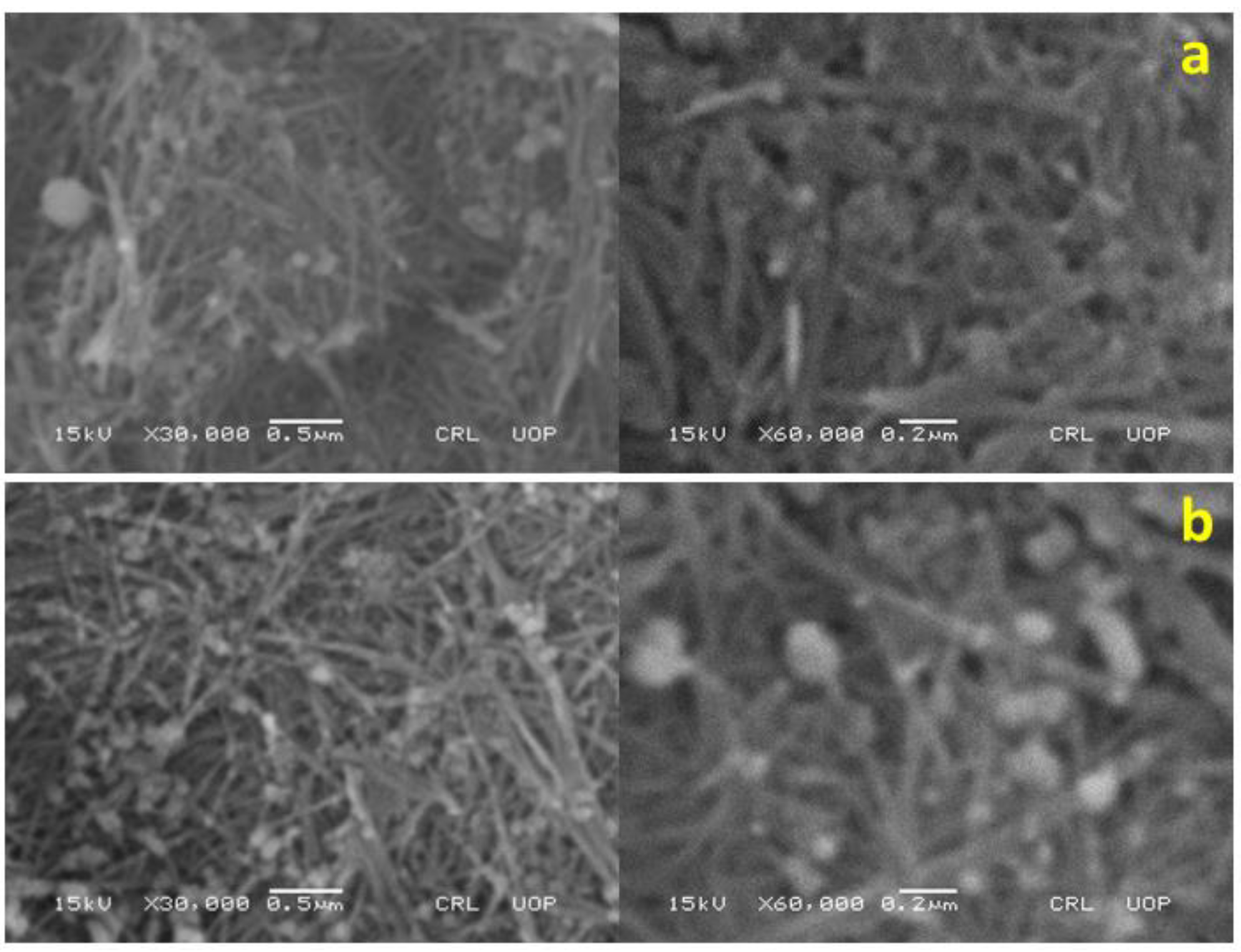
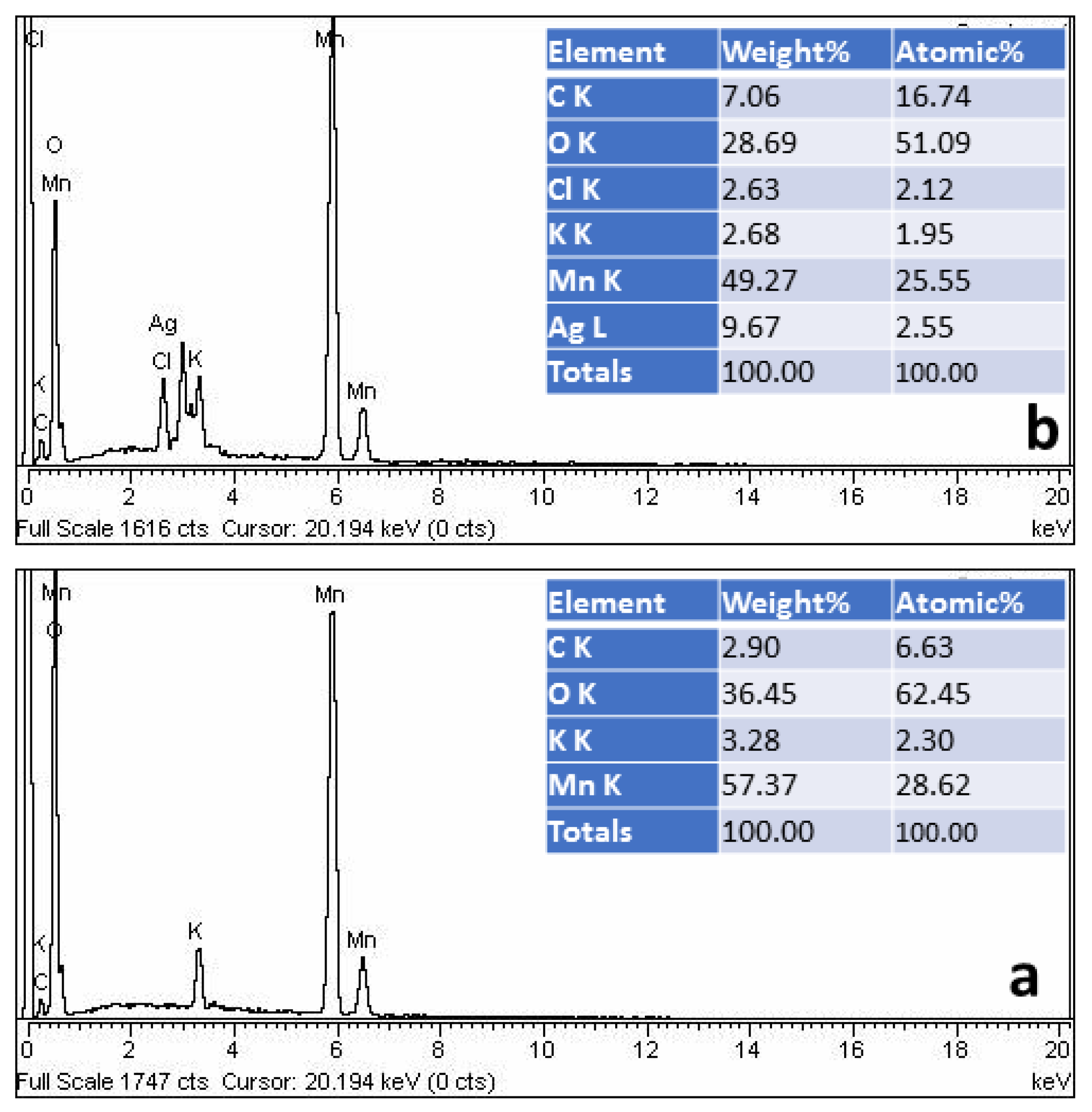
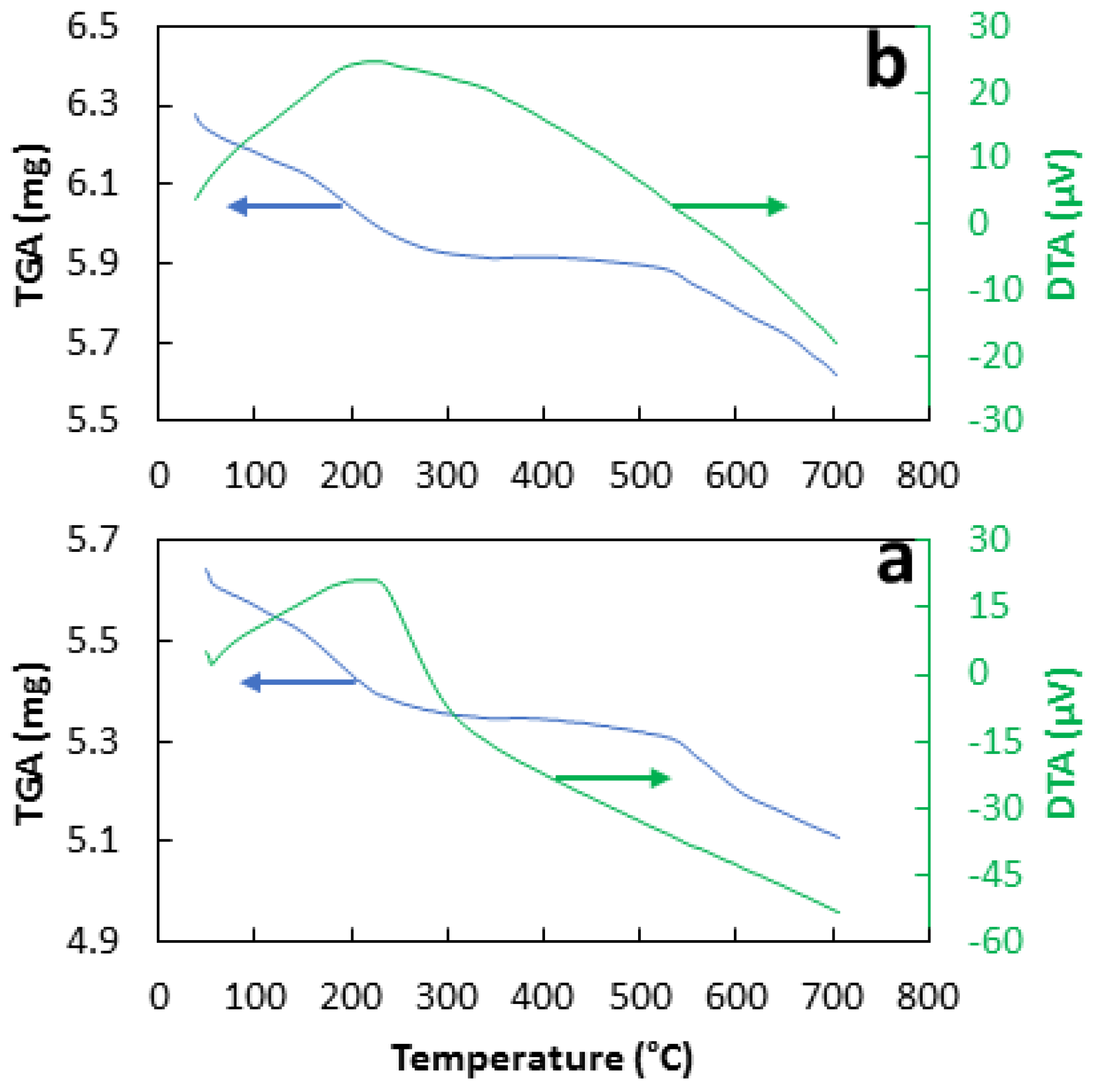
3.2. Characterization
3.3. Photocatalysis
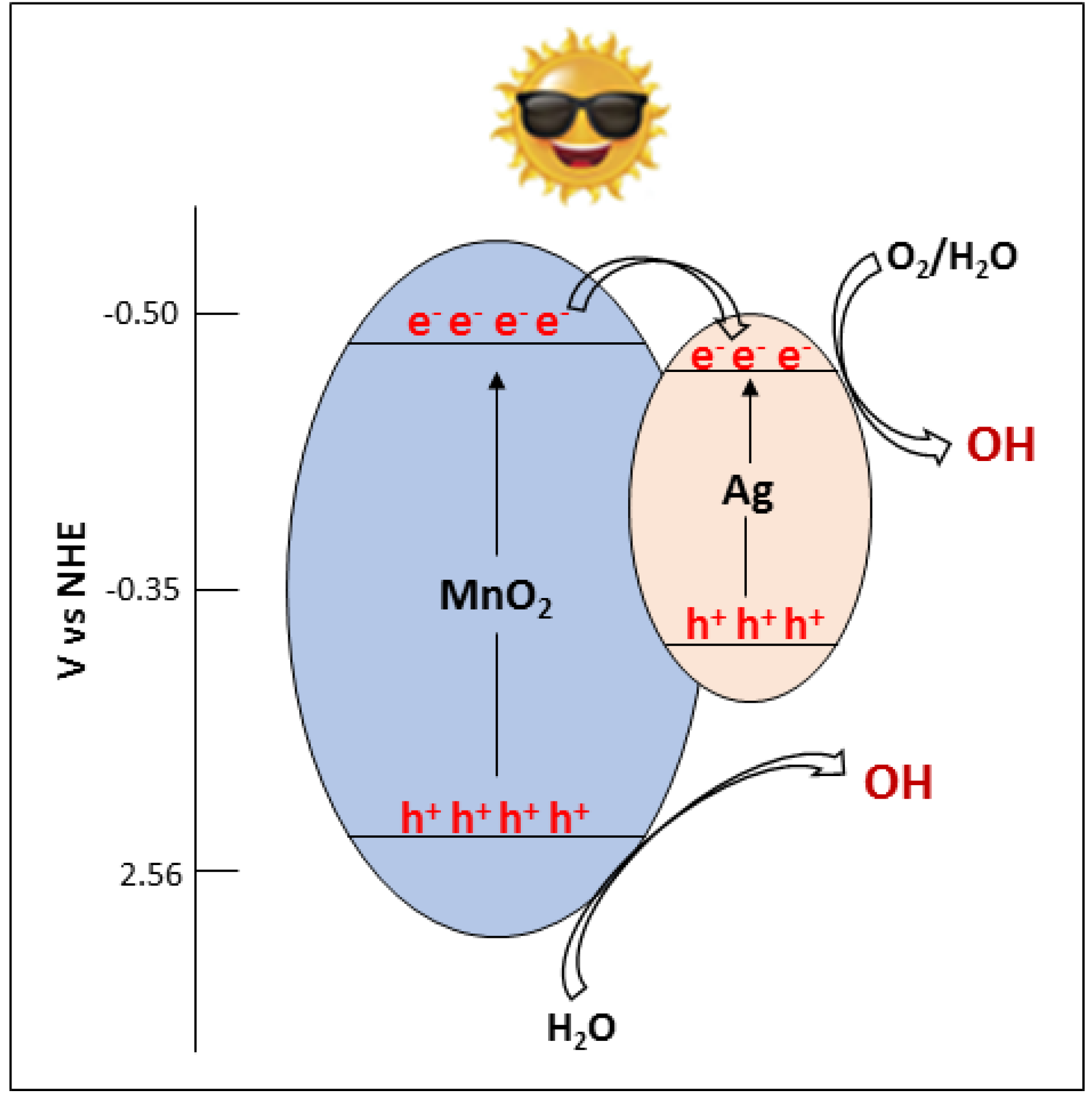
3.4. Mechanism of the Photocatalytic Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miao, C.; Li, P.; Huang, Y.; Sun, Y.; Chen, W.; Yu, S. Coupling Outdoor Air Quality with Thermal Comfort in the Presence of Street Trees: A Pilot Investigation in Shenyang, Northeast China. J. For. Res. 2022, 34, 831–839. [Google Scholar] [CrossRef]
- Agathokleous, S.; Saitanis, C.J.; Savvides, C.; Sicard, P.; Agathokleous, E.; De Marco, A. Spatiotemporal Variations of Ozone Exposure and Its Risks to Vegetation and Human Health in Cyprus: An Analysis across a Gradient of Altitudes. J. For. Res. 2022, 34, 579–594. [Google Scholar] [CrossRef]
- Arshad, M.; Qayyum, A.; Abbas, G.; Haider, R.; Iqbal, M.; Nazir, A. Influence of Different Solvents on Portrayal and Photocatalytic Activity of Tin-Doped Zinc Oxide Nanoparticles. J. Mol. Liq. 2018, 260, 272–278. [Google Scholar] [CrossRef]
- Thao, L.T.S.; Dang, T.T.T.; Khanitchaidecha, W.; Channei, D.; Nakaruk, A. Photocatalytic Degradation of Organic Dye under UV-A Irradiation Using TiO2-Vetiver Multifunctional Nano Particles. Materials 2017, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A.; Naeem, K.; Tariq, M.; Nazli, Z.I.H.; Bhatti, H.N.; Jubeen, F.; Nazir, A.; Iqbal, M. Preparation and Characterization of Chitosan/Clay Composite for Direct Rose FRN Dye Removal from Aqueous Media: Comparison of Linear and Non-Linear Regression Methods. J. Mater. Res. Technol. 2019, 8, 1161–1174. [Google Scholar] [CrossRef]
- Kamran, U.; Rhee, K.Y.; Lee, S.Y.; Park, S.J. Innovative Progress in Graphene Derivative-Based Composite Hybrid Membranes for the Removal of Contaminants in Wastewater: A Review. Chemosphere 2022, 306, 135590. [Google Scholar] [CrossRef] [PubMed]
- Kamran, U.; Lee, S.Y.; Rhee, K.Y.; Park, S.J. Rice Husk Valorization into Sustainable Ni@TiO2/Biochar Nanocomposite for Highly Selective Pb (II) Ions Removal from an Aqueous Media. Chemosphere 2023, 323, 138210. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Chen, T.; Hou, H.; Xu, Y. Recent Advances in Electrochemical Sterilization. J. Electroanal. Chem. 2023, 937, 117419. [Google Scholar] [CrossRef]
- Li, M.; Xia, Q.; Lv, S.; Tong, J.; Wang, Z.; Nie, Q.; Yang, J. Enhanced CO2 Capture for Photosynthetic Lycopene Production in Engineered Rhodopseudomonas Palustris, a Purple Nonsulfur Bacterium. Green Chem. 2022, 24, 7500–7518. [Google Scholar] [CrossRef]
- Padervand, M.; Heidarpour, H.; Bargahi, A. A Mechanistic Study and In-Vivo Toxicity Bioassay on Acetamiprid Photodegradation over the Zeolite Supported Cerium-Based Photocatalyst. J. Photochem. Photobiol. A Chem. 2020, 395, 112526. [Google Scholar] [CrossRef]
- Salari, H. Efficient Photocatalytic Degradation of Environmental Pollutant with Enhanced Photocarrier Separation in Novel Z-Scheme a-MnO2 Nanorod/a-MoO3 Nanocomposites. J. Photochem. Photobiol. A Chem. 2020, 401, 112787. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Ni, S.Q.; Zhuang, X.; Lee, T. Nano Zero-Valent Iron Improves Anammox Activity by Promoting the Activity of Quorum Sensing System. Water Res. 2021, 202, 117491. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Pang, H.; Huang, S.; Tang, H.; Wang, S.; Qiu, M.; Chen, Z.; Yang, H.; Song, G.; Fu, D.; et al. Recent Advances in Metal-Organic Framework Membranes for Water Treatment: A Review. Sci. Total Environ. 2021, 800, 149662. [Google Scholar] [CrossRef]
- Hao, M.; Qiu, M.; Yang, H.; Hu, B.; Wang, X. Recent Advances on Preparation and Environmental Applications of MOF-Derived Carbons in Catalysis. Sci. Total Environ. 2021, 760, 143333. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the Removal of Contaminants from Soil and Water: A Review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Kamran, U.; Bhatti, H.N.; Iqbal, M.; Jamil, S.; Zahid, M. Biogenic Synthesis, Characterization and Investigation of Photocatalytic and Antimicrobial Activity of Manganese Nanoparticles Synthesized from Cinnamomum Verum Bark Extract. J. Mol. Struct. 2019, 1179, 532–539. [Google Scholar] [CrossRef]
- Li, G.; Huang, S.; Li, K.; Zhu, N.; Zhao, B.; Zhong, Q.; Zhang, Z.; Ge, D.; Wang, D. Near-Infrared Responsive Z-Scheme Heterojunction with Strong Stability and Ultra-High Quantum Efficiency Constructed by Lanthanide-Doped Glass. Appl. Catal. B 2022, 311, 121363. [Google Scholar] [CrossRef]
- Khan, S.B.; Irfan, S.; Lam, S.S.; Sun, X.; Chen, S. 3D Printed Nanofiltration Membrane Technology for Waste Water Distillation. J. Water Process Eng. 2022, 49, 102958. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, J.; Qiao, R.; Zhao, N.; Zhao, M.; Kong, L. Facile Preparation and Controllable Absorption of a Composite Based on PMo12/Ag Nanoparticles: Photodegradation Activity and Mechanism”. ChemistrySelect 2022, 7, e202103668. [Google Scholar] [CrossRef]
- Mo, X.; Liu, X.; Chen, J.; Zhu, S.; Xu, W.; Tan, K.; Wang, Q.; Lin, Z.; Liu, W. Separation of Lattice-Incorporated Cr(vi) from Calcium Carbonate by Converting Microcrystals into Nanocrystals via the Carbonation Pathway Based on the Density Functional Theory Study of Incorporation Energy. Environ. Sci. Nano 2022, 9, 1617–1626. [Google Scholar] [CrossRef]
- Kamran, U.; Bhatti, H.N.; Noreen, S.; Tahir, M.A.; Park, S.J. Chemically Modified Sugarcane Bagasse-Based Biocomposites for Efficient Removal of Acid Red 1 Dye: Kinetics, Isotherms, Thermodynamics, and Desorption Studies. Chemosphere 2022, 291, 132796. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ding, C.; Li, Z.; Yu, R.; Cui, H.; Gao, S. Photocatalytic Degradation of Air Pollutant by Modified Nano Titanium Oxide (TiO2)in a Fluidized Bed Photoreactor: Optimizing and Kinetic Modeling. Chemosphere 2023, 319, 137995. [Google Scholar] [CrossRef] [PubMed]
- Haounati, R.; Ighnih, H.; Ouachtak, H.; Eshaghi Malekshah, R.; Hafid, N.; Jada, A.; Ait Addi, A. Z-Scheme G-C3n4/Fe3O4/Ag3PO4@Sep Magnetic Nanocomposites as Heterojunction Photocatalysts for Green Malachite Degradation and Dynamic Molecular Studies. SSRN Electron. J. 2023, 671, 131509. [Google Scholar] [CrossRef]
- Haounati, R.; Ighnih, H.; Malekshah, R.E.; Alahiane, S.; Alakhras, F.; Alabbad, E.; Alghamdi, H.; Ouachtak, H.; Addi, A.A.; Jada, A. Exploring ZnO/Montmorillonite Photocatalysts for the Removal of Hazardous RhB Dye: A Combined Study Using Molecular Dynamics Simulations and Experiments. Mater. Today Commun. 2023, 35, 105915. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Gao, X.; Ma, J.; Chen, Z.; Kang, B.; Liu, J.; Li, H.; Feng, Z.; Huang, J. Novel BP/BiOBr S-Scheme Nano-Heterojunction for Enhanced Visible-Light Photocatalytic Tetracycline Removal and Oxygen Evolution Activity. J. Hazard. Mater. 2020, 387, 121690. [Google Scholar] [CrossRef]
- Phillips, R.B.; James, R.R.; Magnuson, M.L. Functional Categories of Microbial Toxicity Resulting from Three Advanced Oxidation Process Treatments during Management and Disposal of Contaminated Water. Chemosphere 2020, 238, 124550. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, H.; Qin, Y.; Ma, J.; Kong, Y.; Komarneni, S. Copper Sulfide as an Excellent Co-Catalyst with K2S2O8 for Dye Decomposition in Advanced Oxidation Process. Sep. Purif. Technol. 2020, 233, 116057. [Google Scholar] [CrossRef]
- Warshagha, M.Z.A.; Muneer, M. Visible Light Active Boron Doped Phenyl-g-C3N4 Nanocomposites for Decomposition of Dyes. Surf. Interfaces 2021, 26, 101394. [Google Scholar] [CrossRef]
- Li, H.; Zhao, S.; Zhang, W.; Du, H.; Yang, X.; Peng, Y.; Han, D.; Wang, B.; Li, Z. Efficient Esterification over Hierarchical Zr-Beta Zeolite Synthesized via Liquid-State Ion-Exchange Strategy. Fuel 2023, 342, 127786. [Google Scholar] [CrossRef]
- Xia, G.; Zheng, Y.; Sun, Z.; Xia, S.; Ni, Z.; Yao, J. Fabrication of ZnAl-LDH Mixed Metal-Oxide Composites for Photocatalytic Degradation of 4-Chlorophenol. Environ. Sci. Pollut. Res. 2022, 29, 39441–39450. [Google Scholar] [CrossRef]
- Zhou, T.; Du, J.; Wang, Z.; Xiao, G.; Luo, L.; Faheem, M.; Ling, H.; Bao, J. Degradation of Sulfamethoxazole by MnO2/Heat-Activated Persulfate: Kinetics, Synergistic Effect and Reaction Mechanism. Chem. Eng. J. Adv. 2022, 9, 100200. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, R.; Chen, Z.; Gao, Z.; Zheng, S.; Song, H. Kinetics and Mechanisms of Diniconazole Degradation by α-MnO2 Activated Peroxymonosulfate. Sep. Purif. Technol. 2022, 281, 119850. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Lin, Q.; Cheng, B.; Liu, X.; Peng, F.; Ren, J. Preparation of Lignocellulose-Based Activated Carbon Paper as a Manganese Dioxide Carrier for Adsorption and in-Situ Catalytic Degradation of Formaldehyde. Front. Chem. 2019, 7, 808. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Gri, F.; Gasparotto, A.; Altantzis, T.; Gombac, V.; Fornasiero, P.; Maccato, C. Insights into the Plasma-Assisted Fabrication and Nanoscopic Investigation of Tailored MnO2 Nanomaterials. Inorg. Chem. 2018, 57, 14564–14573. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shen, M.; Xia, Y.; Lu, M. Au/MnO2 Nanostructured Catalysts and Their Catalytic Performance for the Oxidation of 5-(Hydroxymethyl)Furfural. Catal. Commun. 2015, 64, 37–43. [Google Scholar] [CrossRef]
- Wang, S.; Guan, A.; Wang, J.; Fu, X.; Guo, X.; Tian, Y.; Wang, K.; Cao, W.; Zhao, C. Highly Efficient Degradation of Rhodamine B by α-MnO2 Nanorods. Bull. Mater. Sci. 2022, 45, 35. [Google Scholar] [CrossRef]
- Kamran, U.; Heo, Y.J.; Min, B.G.; In, I.; Park, S.J. Effect of Nickel Ion Doping in MnO2/Reduced Graphene Oxide Nanocomposites for Lithium Adsorption and Recovery from Aqueous Media. RSC Adv. 2020, 10, 9245–9257. [Google Scholar] [CrossRef]
- Huang, Z.; Gu, X.; Cao, Q.; Hu, P.; Hao, J.; Li, J.; Tang, X. Catalytically Active Single-Atom Sites Fabricated from Silver Particles. Angew. Chem. Int. Ed. 2012, 51, 4198–4203. [Google Scholar] [CrossRef]
- Liu, P.; Duan, J.; Ye, Q.; Mei, F.; Shu, Z.; Chen, H. Promoting Effect of Unreducible Metal Doping on OMS-2 Catalysts for Gas-Phase Selective Oxidation of Ethanol. J. Catal. 2018, 367, 115–125. [Google Scholar] [CrossRef]
- Huang, L.; Hu, X.; Yuan, S.; Li, H.; Yan, T.; Shi, L.; Zhang, D. Photocatalytic Preparation of Nanostructured MnO2-(CO3O4)/TiO2 Hybrids: The Formation Mechanism and Catalytic Application in SCR DeNOx Reaction. Appl. Catal. B 2017, 203, 778–788. [Google Scholar] [CrossRef]
- Wang, X.; Tan, W.; Guo, K.; Ji, J.; Gao, F.; Tong, Q.; Dong, L. Evaluation of Manganese Oxide Octahedral Molecular Sieves for CO and C3H6 Oxidation at Diesel Exhaust Conditions. Front. Environ. Chem. 2021, 2, 672250. [Google Scholar] [CrossRef]
- Turner, S.; Buseck, P.R. Todorokites: A New Family of Naturally Occurring Manganese Oxides. Science 1981, 212, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Kanoh, H.; Ooi, K. Manganese Oxide Porous Crystals. J. Mater. Chem. 1999, 9, 319–333. [Google Scholar] [CrossRef]
- Kong, L.; Liu, Y.; Dong, L.; Zhang, L.; Qiao, L.; Wang, W.; You, H. Enhanced Red Luminescence in CaAl12O19:Mn4+: Via Doping Ga3+ for Plant Growth Lighting. Dalton Trans. 2020, 49, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Thenmozhi, E.; Harshavardhan, M.; Kamala-Kannan, S.; Janaki, V. Synthesis and Characterization of Mesoporous Silica-MnO2 Nanocomposite—An Efficient Nanocatalyst for Methylene Blue Degradation. Mater. Lett. 2022, 309, 131367. [Google Scholar] [CrossRef]
- Tong, W.; Wang, J.; Du, X.; Wang, X.; Wang, Y.; Zhang, Y. Tributyl Phosphate Degradation and Phosphorus Immobilization by MnO2: Reaction Condition Optimization and Mechanism Exploration. J. Hazard. Mater. 2022, 432, 128725. [Google Scholar] [CrossRef]
- Li, H.; Fu, B.; Huang, H.; Wu, S.; Ge, J.; Zhang, J.; Li, F.; Qu, P. Catalytic Degradation of Organic Pollutants by Manganese Oxides: A Comprehensive Review. Environ. Pollut. Bioavailab. 2022, 34, 395–406. [Google Scholar] [CrossRef]
- Rahmat, M.; Rehman, A.; Rahmat, S.; Bhatti, H.N.; Iqbal, M.; Khan, W.S.; Bajwa, S.Z.; Rahmat, R.; Nazir, A. Highly Efficient Removal of Crystal Violet Dye from Water by MnO2 Based Nanofibrous Mesh/Photocatalytic Process. J. Mater. Res. Technol. 2019, 8, 5149–5159. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Mumtaz, N.; Siddique, M.; Akram, N.; Hamayun, M. Ag-CO3O4: Synthesis, Characterization and Evaluation of Its Photo-Catalytic Activity towards Degradation of Rhodamine B Dye in Aqueous Medium. Chin. J. Chem. Eng. 2018, 26, 1264–1269. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Singh, M.; Javed, K. Comparative Study of Chemical Composition of Calotropis Gigantea Flower, Leaf and Fruit. Eur. Chem. Bull. 2015, 4, 477–480. [Google Scholar]
- Zafar, L.; Khan, A.; Kamran, U.; Park, S.J.; Bhatti, H.N. Eucalyptus (Camaldulensis) Bark-Based Composites for Efficient Basic Blue 41 Dye Biosorption from Aqueous Stream: Kinetics, Isothermal, and Thermodynamic Studies. Surf. Interfaces 2022, 31, 101897. [Google Scholar] [CrossRef]
- Warshagha, M.Z.A.; Muneer, M. Direct Z-Scheme AgBr/β-MnO2 Photocatalysts for Highly Efficient Photocatalytic and Anticancer Activity. ACS Omega 2022, 7, 30171–30183. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, H.; Zhang, C.; Sun, L.; Liu, S.; Wang, S. Antimicrobial Activity of Silver Loaded MnO2 Nanomaterials with Different Crystal Phases against Escherichia Coli. J. Environ. Sci. 2016, 41, 112–120. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y. Selected-Control Hydrothermal Synthesis of α- and β-MnO2 Single Crystal Nanowires. J. Am. Chem. Soc. 2002, 124, 2880–2881. [Google Scholar] [CrossRef]
- Mohamed Racik, K.K.; Prabakaran, P.; Madhavan, J.; Victor Antony Raj, M. Surfactant Free Hydrothermal Synthesis, Structural and Dielectric Studies of One-Dimensional ΒmnO2 Nanorods. Mater. Today Proc. 2019, 8, 162–168. [Google Scholar] [CrossRef]
- Luo, H.; Yang, Y.; Yang, B.; Xu, Z.; Wang, D. Silver/Manganese Dioxide Nanorod Catalyzed Hydrogen-Borrowing Reactions and Tert-Butyl Ester Synthesis. J. Chem. Res. 2021, 45, 708–715. [Google Scholar] [CrossRef]
- Gao, X.; Shang, Y.; Liu, L.; Fu, F. Multilayer Ultrathin Ag-δ-Bi2O3 with Ultrafast Charge Transformation for Enhanced Photocatalytic Nitrogen Fixation. J. Colloid Interface Sci. 2019, 533, 649–657. [Google Scholar] [CrossRef]
- Saeed, M.; Haq, A.U.; Muneer, M.; Ahmad, A.; Bokhari, T.H.; Sadiq, Q. Synthesis and Characterization of Bi2O3 and Ag-Bi2O3 and Evaluation of Their Photocatalytic Activities towards Photodegradation of Crystal Violet Dye. Phys. Scr. 2021, 96, 125707. [Google Scholar] [CrossRef]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic Degradation of Various Types of Dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in Water by UV-Irradiated Titania. Appl. Catal. B 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Xu, H.; Hou, X.; Xuan, L.; Jiang, Y.; Yuan, Y. Amorphous 3D Nanoflake Array-Assembled Porous 2D Cobalt-Oxalate Coordination Polymer Thin Sheets with Excellent Pseudocapacitive Performance. J. Mater. Chem. A Mater. 2015, 3, 1847–1852. [Google Scholar] [CrossRef]
- Asiri, S.M.M.; Cevik, E.; Sabit, H.; Bozkurt, A. Alginate-Guided Size and Morphology-Controlled Synthesis of MnO2 Nanoflakes. Soft Mater. 2020, 18, 46–54. [Google Scholar] [CrossRef]
- Worku, A.K.; Ayele, D.W.; Habtu, N.G.; Yemata, T.A. Engineering CO3O4/MnO2 Nanocomposite Materials for Oxygen Reduction Electrocatalysis. Heliyon 2021, 7, e08076. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.A.; Al-Thabaiti, S.A.; Al-Arjan, W.S.; Malik, M.A.; Khan, Z. Preparation of Ultra Long α-MnO2 and Ag@MnO2 Nanoparticles by Seedless Approach and Their Photocatalytic Performance. J. Mol. Struct. 2017, 1137, 495–505. [Google Scholar] [CrossRef]
- Kong, L.; Sun, H.; Nie, Y.; Yan, Y.; Wang, R.; Ding, Q.; Zhang, S.; Yu, H.; Luan, G. Luminescent Properties and Charge Compensator Effects of SrMo0.5W0.5O4:Eu3+ for White Light LEDs. Molecules 2023, 28, 2681. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Muthuraj, V.; Vadivel, S. Constructing Novel Ag Nanoparticles Anchored on MnO2 Nanowires as an Efficient Visible Light Driven Photocatalyst. RSC Adv. 2016, 6, 61357–61366. [Google Scholar] [CrossRef]
- Panimalar, S.; Logambal, S.; Thambidurai, R.; Inmozhi, C.; Uthrakumar, R.; Muthukumaran, A.; Rasheed, R.A.; Gatasheh, M.K.; Raja, A.; Kennedy, J.; et al. Effect of Ag Doped MnO2 Nanostructures Suitable for Wastewater Treatment and Other Environmental Pollutant Applications. Environ. Res. 2022, 205, 112560. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Kanimozhi, K.; Arularasu, M.V.; Ramalingam, G.; Kaviyarasu, K. Self-Cleaning Mechanism of Synthesized SnO2/TiO2 Nanostructure for Photocatalytic Activity Application for Waste Water Treatment. Surf. Interfaces 2019, 17, 100346. [Google Scholar] [CrossRef]
- Alhaji, N.M.I.; Nathiya, D.; Kaviyarasu, K.; Meshram, M.; Ayeshamariam, A. A Comparative Study of Structural and Photocatalytic Mechanism of AgGaO2 Nanocomposites for Equilibrium and Kinetics Evaluation of Adsorption Parameters. Surf. Interfaces 2019, 17, 100375. [Google Scholar] [CrossRef]
- Khan, I.; Luo, M.; Guo, L.; Khan, S.; Wang, C.; Khan, A.; Saeed, M.; Zaman, S.; Qi, K.; Liu, Q.L. Enhanced Visible-Light Photoactivities of Porous LaFeO3 by Synchronously Doping Ni2+ and Coupling TS-1 for CO2 reduction and 2,4,6-Trinitrophenol Degradation. Catal. Sci. Technol. 2021, 11, 6793–6803. [Google Scholar] [CrossRef]
- Khan, I.; Sun, N.; Wang, Y.; Li, Z.; Qu, Y.; Jing, L. Synthesis of SnO2/Yolk-Shell LaFeO3 Nanocomposites as Efficient Visible-Light Photocatalysts for 2,4-Dichlorophenol Degradation. Mater. Res. Bull. 2020, 127, 110857. [Google Scholar] [CrossRef]
- Khan, I.; Sun, N.; Zhang, Z.; Li, Z.; Humayun, M.; Ali, S.; Qu, Y.; Jing, L. Improved Visible-Light Photoactivities of Porous LaFeO3 by Coupling with Nanosized Alkaline Earth Metal Oxides and Mechanism Insight. Catal. Sci. Technol. 2019, 9, 3149–3157. [Google Scholar] [CrossRef]
- Kong, M.; Li, Y.; Chen, X.; Tian, T.; Fang, P.; Zheng, F.; Zhao, X. Tuning the Relative Concentration Ratio of Bulk Defects to Surface Defects in TiO2 Nanocrystals Leads to High Photocatalytic Efficiency. J. Am. Chem. Soc. 2011, 133, 16414–16417. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, X.M.; Tian, K.; Zhou, Z.W.; Wang, Y. Largely Improved Photocatalytic Properties of Ag/Tetrapod-like ZnO Nanocompounds Prepared with Different PEG Contents. Appl. Surf. Sci. 2011, 257, 7763–7770. [Google Scholar] [CrossRef]

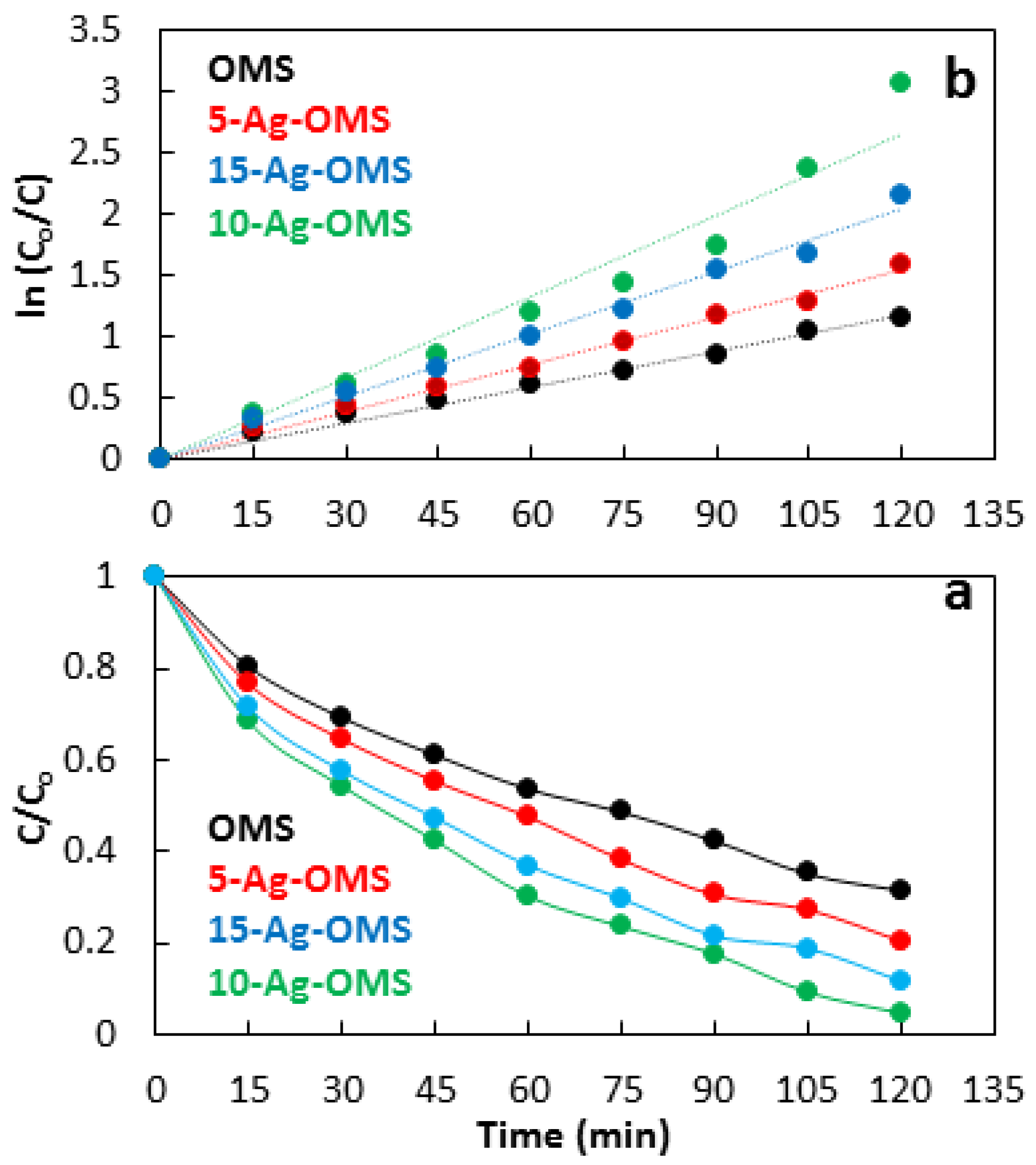
| Photocatalyst | Rate Constant (min−1) | R2 | Activity (%) |
|---|---|---|---|
| OMS | 0.009 | 0.996 | 68 |
| 5-Ag-OMS | 0.013 | 0.998 | 79 |
| 10-Ag-OMS | 0.022 | 0.986 | 95 |
| 15-Ag-OMS | 0.017 | 0.997 | 85 |
| Crystal Violet Dye (mg/L) | Rate Constant (min−1) | R2 | Activity (%) |
|---|---|---|---|
| 50 | 0.036 | 0.978 | 100 * |
| 100 | 0.022 | 0.986 | 95 ** |
| 150 | 0.011 | 0.997 | 71 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, M.; Pecho, R.D.C.; Panchal, S.; Alhag, S.K.; Al-Shuraym, L.A.; Al Syaad, K.M.; Bhutta, U.H. Synthesis of Ag-OMS Catalyst for Sunlight-Assisted Photodegradation of Crystal Violet Dye. Water 2023, 15, 2480. https://doi.org/10.3390/w15132480
Saeed M, Pecho RDC, Panchal S, Alhag SK, Al-Shuraym LA, Al Syaad KM, Bhutta UH. Synthesis of Ag-OMS Catalyst for Sunlight-Assisted Photodegradation of Crystal Violet Dye. Water. 2023; 15(13):2480. https://doi.org/10.3390/w15132480
Chicago/Turabian StyleSaeed, Muhammad, Renzon Daniel Cosme Pecho, Sandeep Panchal, Sadeq K. Alhag, Laila A. Al-Shuraym, Khalid M. Al Syaad, and Usman Hanif Bhutta. 2023. "Synthesis of Ag-OMS Catalyst for Sunlight-Assisted Photodegradation of Crystal Violet Dye" Water 15, no. 13: 2480. https://doi.org/10.3390/w15132480
APA StyleSaeed, M., Pecho, R. D. C., Panchal, S., Alhag, S. K., Al-Shuraym, L. A., Al Syaad, K. M., & Bhutta, U. H. (2023). Synthesis of Ag-OMS Catalyst for Sunlight-Assisted Photodegradation of Crystal Violet Dye. Water, 15(13), 2480. https://doi.org/10.3390/w15132480








