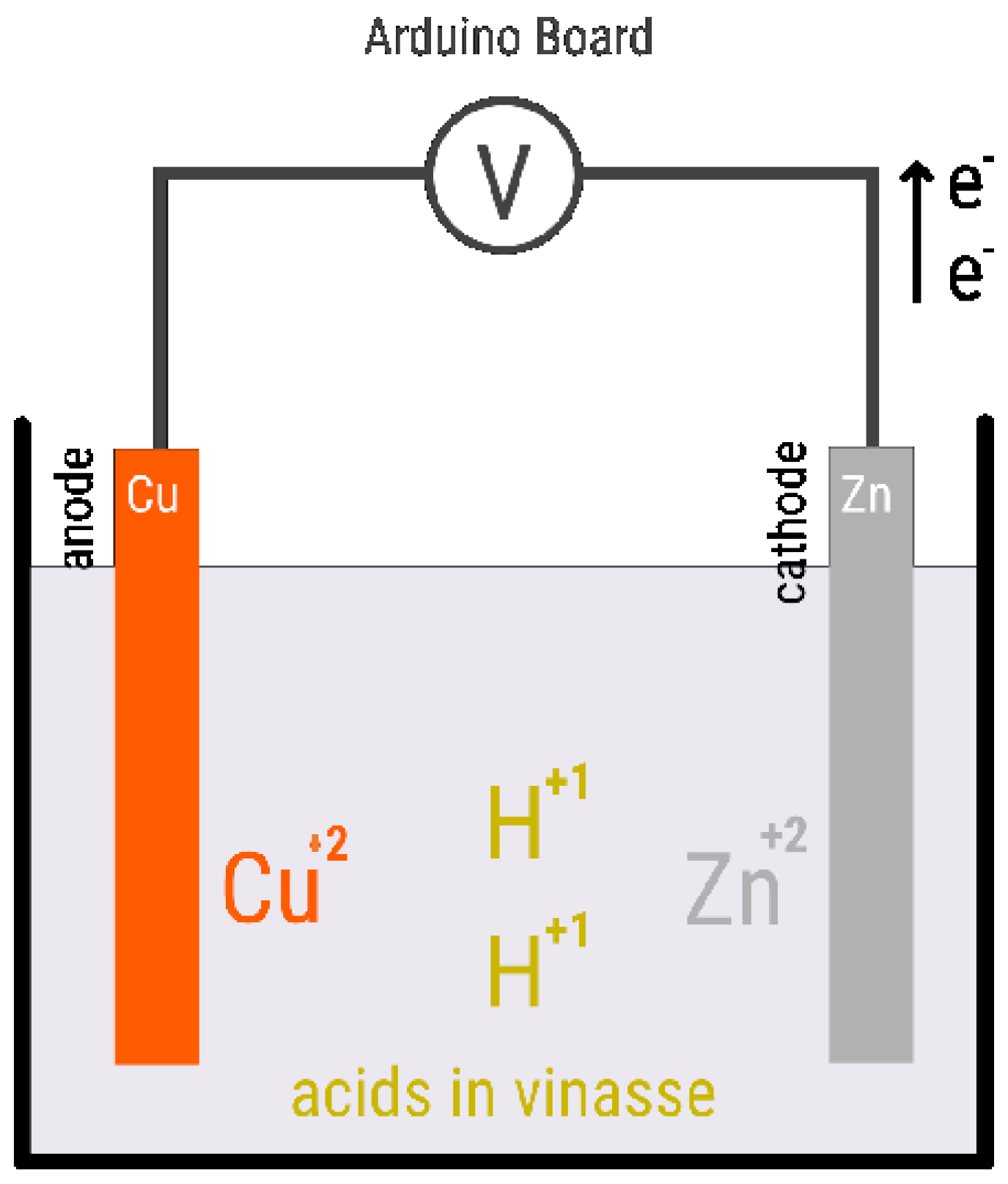
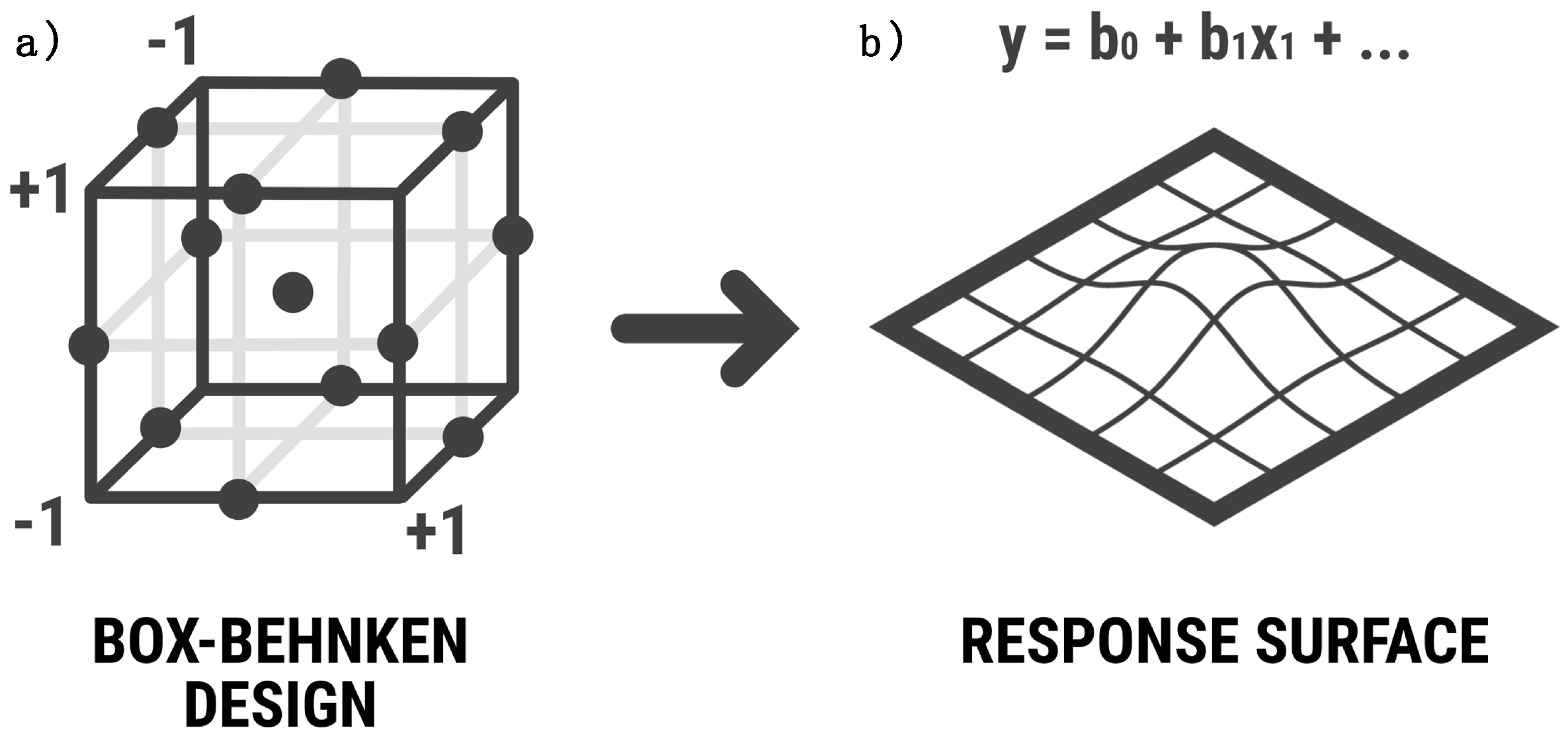
1. Introduction
Experimental design is an approximation commonly employed to make experimentation more efficient. It is based on the combination of mathematical models and graphical representations that help to identify the optimal combination of variables to achieve the best results [
1]. In chemical or biological engineering, it is common to employ chemometric tools for process optimization. They present advantages such as a reduction in the number of experiments carried out, which leads to considerably less work in the laboratory and a consequent cost reduction. These tools allow for the development of models that facilitate the evaluation of the results, obtaining statistical parameters, and identifying the interaction between different factors to check the significant variations that may occur in the parameters of interest. Optimization techniques arise as a set of methods to improve the operation of systems and to identify the optimal values of variables influencing their functioning. Data processing techniques are used to compare data with others to identify trends or recognize patterns aiming at classifying the data into clusters or to predict future observations.
Among the design of experiments (DoE) techniques, one of the best-known today is the use of factorial and fractional factorial designs [
2]. These methods allow for deducing a behavior from various data observed. The process needs a careful examination of the variables that influence the process and the selection of which values they can obtain. When conducting these experiments, several values may be modified simultaneously instead of just one. In this method, a mesh is made in which there are 2
k points, where
k is the number of factors to vary. In the fractional case, only 2
k-p values of the mesh will be taken into consideration. In this equation, the superscript
k-
p defines the resolution of the system, where
k is the number of factors and
p is the number of their interactions. However, these systems can become very complex if the number of variables increases. To carry out an optimizing technology with a considerable number of variables, multivariate optimization techniques will have to be applied on a larger scale. This work highlights the importance of the Plackett–Burman and Box–Behnken designs, which have similar characteristics but different purposes. The first one facilitates the selection of the most influential parameters and the second one allows for their combination to provide their optimal experimentation to maximize or minimize the response.
In this study, Plackett–Burman and Box–Behnken designs were applied to obtain bioelectricity from winery residues. The European Union is the world’s largest wine producer, with some of its countries leading the wine business, such as France, Spain and Italy [
3,
4]. Although wine production has been interpreted as a respectful process for the environment, winemaking generates large amounts of solid and liquid wastes [
5]. Tons of bagasse, lees and vinasses, among others, are generated each year and valorization strategies are encouraged in order to mitigate the huge environmental impact they produce. In this sense, fermentation of grape marc, trimming vine shoot or vinification lees may be employed to produce lactic acid, biosurfactants, xylitol, ethanol and other compounds. Also, grape marc and seeds may exhibit antioxidant properties, and vinasse contains tartaric acid that can be extracted and commercialized [
6]. The complexity of these procedures has led some producers to manufacture compost, which is another by-product of agricultural interest [
7]. Vinasses present favorable chemical characteristics for their use in the production of sustainable energy since they present conditions that help with electricity production: they have a remarkable acidity (pH = 2–6); high salinity; high values of dissolved oxygen; and high content of organic matter (900–35,000 mg/L) due to its high carbon, nitrogen and potassium concentrations, in addition to phenolic substances and heavy metals [
8,
9].
Bioelectricity using vinasse residues was analyzed by other authors, although there are scarce references about winery materials. In the viticulture and winemaking processes, the winery residues are rich in organic compounds such as polyphenols, tannins and volatile compounds. While these compounds contribute to the desirable sensory attributes of wine, they also possess the potential to exert harmful effects on soil, water and biota when not properly managed. Using microbial fuel cells (MFCs) to treat disposal materials presents the advantage that this technique is not affected by the toxicity of the materials, e.g., fungicide [
10] or pyretroid drugs [
11], among other agrochemical products.
In relation to the MFC configuration, previous results showed that these complex residues operate more efficiently with an inoculum concentration of 20 to 30%. This dilution rate is necessary to avoid the high organic load, low pH and high polyphenol contents [
12], which may even inhibit the energy production. Also, a short distance between electrodes, between 1 cm [
13] and up to 6 cm [
14], was documented to favor redox reactions. Seasonality in the composition of this wastewater must also be considered as nutrient or microorganisms’ content may vary [
15], although Liu et al. [
16] treated the winery sources with organic amendments to stabilize the electrical energy recovered by cycle.
Therefore, the main objective of this study was to employ winery residues to produce bioelectricity to generate renewable energy. Red wine residues were selected, as they are the most common wine species produced in our study area. The Plackett–Burman design was employed as a previous screening to a series of factors that affect the MFC configuration (concentration of the electrolyte, pH, temperature, stirring, addition of NaCl, yeast dose and electrode:solution ratio). Next, the Box–Behnken design was used to evaluate the capacity of the three variables that most influenced the process to maximize bioelectricity production.
Also, the secondary benefits are the reduction in waste production and disposal, as management of these residues can be a challenge to wineries, and they can benefit from an environmentally friendly approach, which will also contribute to reducing their carbon footprint. Finally, reducing waste can bring benefits from an economical perspective, as wineries can reduce their energy costs by using their residues to generate bioelectricity, becoming more self-sufficient.
3. Results
3.1. Phase 1: Analysis of Plackett–Burman Results to Identify Patterns
The vinasse concentration, pH, temperature, stirring, NaCl concentration, yeast dose and electrode:solution ratio were tested as influential variables in the bioelectricity production. The measurements were taken at multiple time points, starting from 1 min and continuing at 5, 10 and 15 min. Measuring the bioelectricity at short intervals, ranging from 1 to 15 min, is important in microbial fuel cell experiments. The dynamic nature of bioelectrochemical processes occurring within these systems necessitates frequent monitoring to capture the transient behavior and fluctuations in electrical output and provides valuable data for comprehensive analysis, improves experimental control and enhances the understanding of bioelectrochemical processes in microbial fuel cells.
Table 3 shows the average values obtained for the six experiments of the Plackett–Burman design with measurements registered every 10 s.
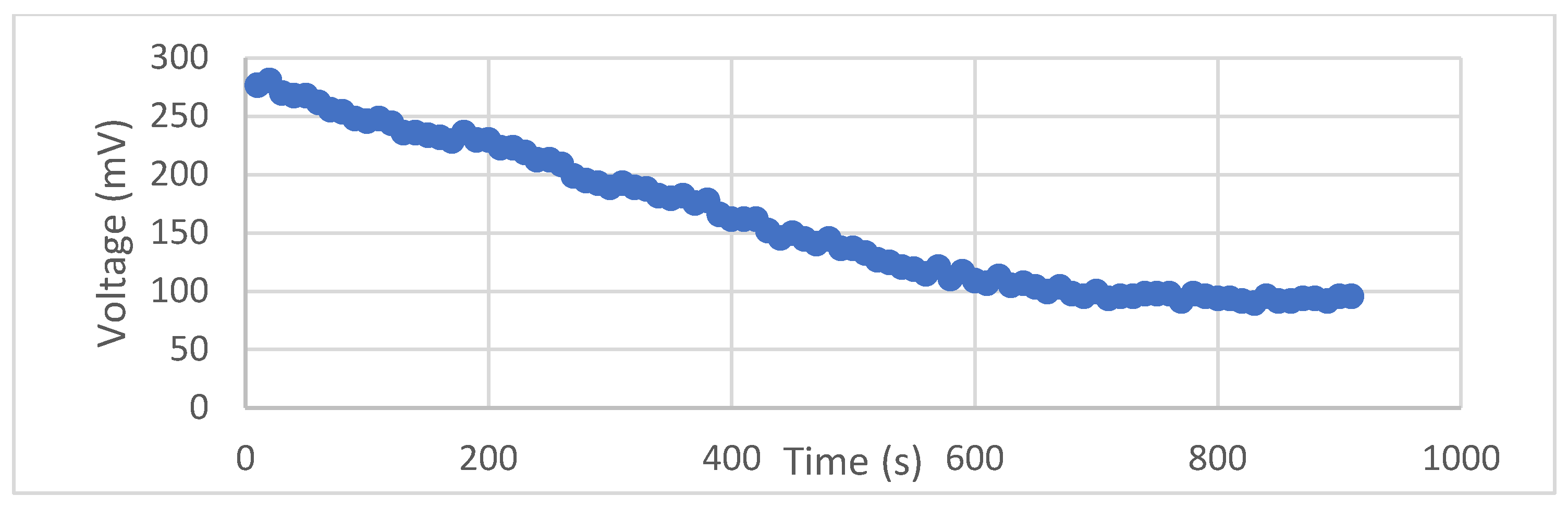
The observed millivolt values exhibited a gradual decline over time, except for experiment 1, where the minimum values decreased by up to 60% of the maximum peaks at the 15 min mark (
Figure 4). The maximum value was observed in experiment 1, reaching over 306 mV. However, in the other six experiments, the millivolt values were sustained at around 90% of their maximums after 15 min. Notably, the minimum values consistently remained above 75% of the maximum values, except for the aforementioned exception.
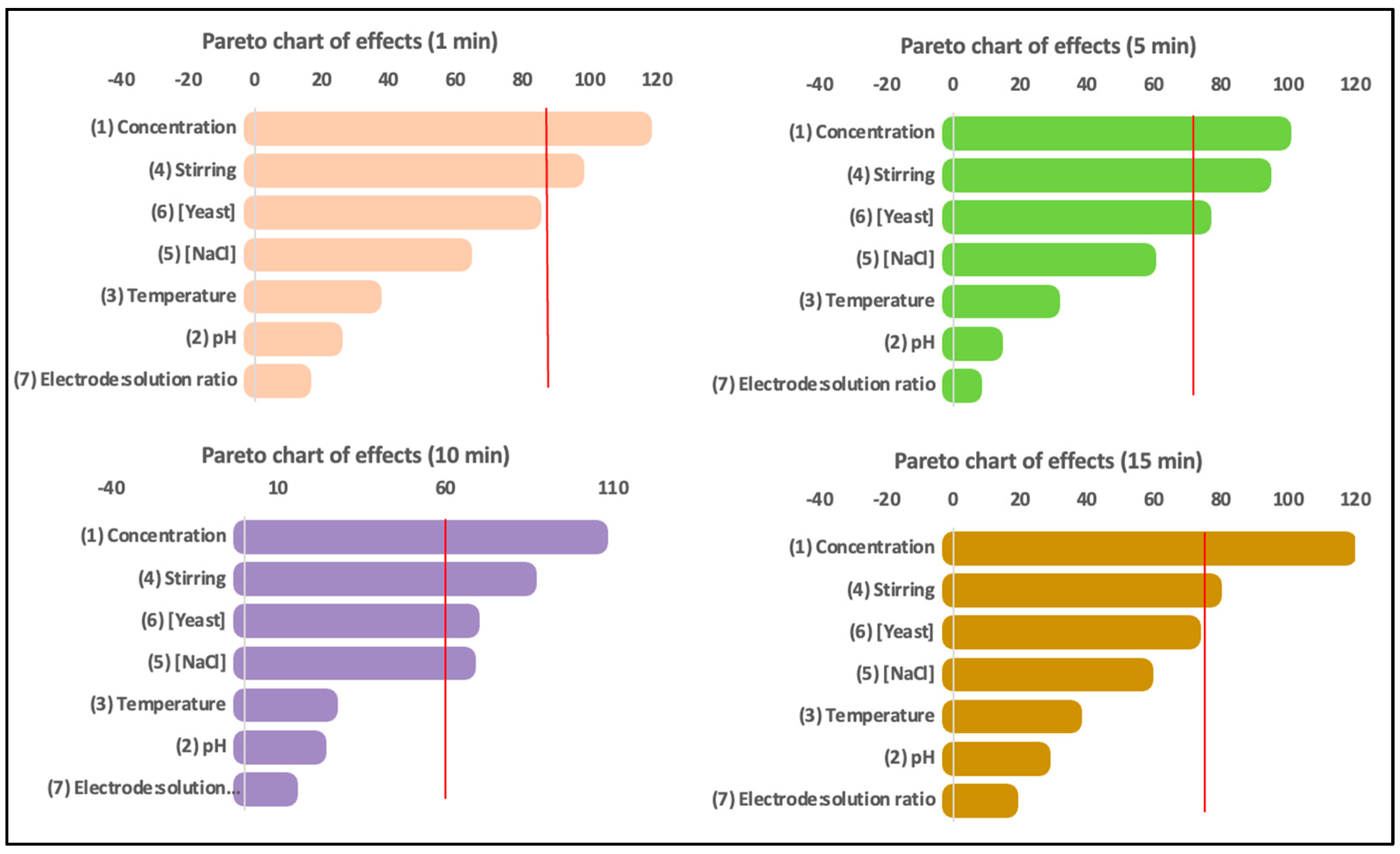
The Plackett–Burman design experiments were further analyzed using Pareto charts, as depicted in
Figure 5, which illustrate the influence of the analyzed variables on the millivolt production. The data reveal that the concentration, stirring, and yeast were the most influential variables, while temperature showed a negative correlation. Within 1 min of the experiment starting, these three variables accounted for up to 70% of the millivolt variability. However, after 15 min, their influence increased to approximately 83%. As the experiment progressed, stirring became more important, while the influence of concentration and yeast decreased. The pH showed a slight positive influence on the conductivity of the solution, and the concentration of NaCl increased over time. Additionally, the concentration was found to have an inverse relationship with stirring.
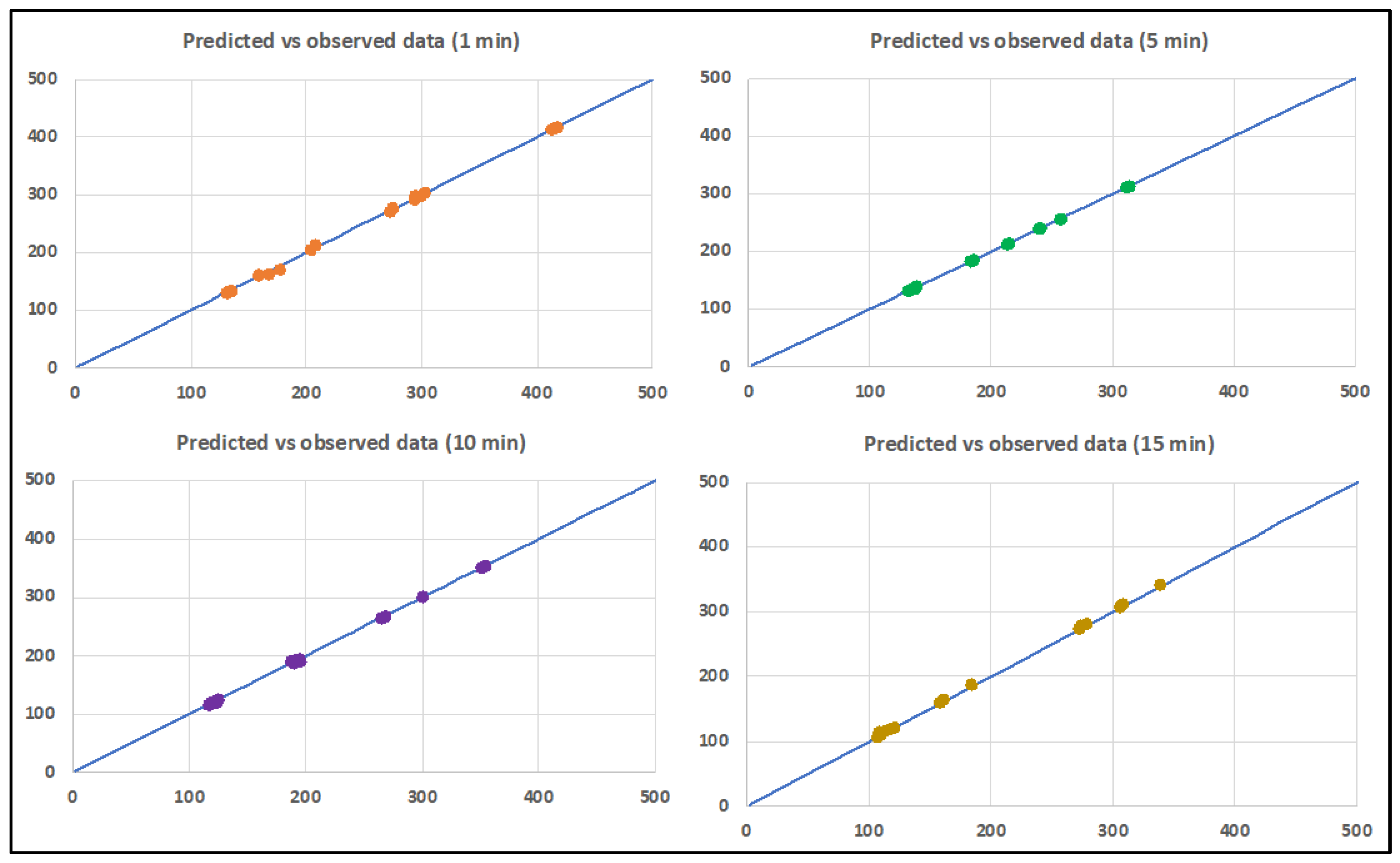
Validation of the statistical analysis is shown in
Figure 6, which shows the relation between the observed and predicted data for the millivolt production, showing good reproduction of the experimental data in all cases.
Also, the significance of each coefficient was determined using
p-values for the variables A to G, corresponding to bioelectricity production after 1, 5, 10 and 15 min, respectively (
Table 4).
Based on the obtained results, the Plackett–Burman model successfully identified the most important parameters for optimizing the process of electricity production from a solution. Concentration and stirring consistently appeared as important factors in the process. However, yeast concentration appeared as an important factor only during the first five minutes of the experiments and its importance decayed in favor of NaCl, whose influence seemed more important for larger experiments. Therefore, for the second stage of the experiment, stirring, concentration, and sodium chloride were chosen as the relevant parameters. Stirring was considered the most important parameter, although its use required energy. Concentration positively influenced the electricity production, but finding the optimal concentration was challenging due to the balance between density and the ability to agitate or dilute the solution. Sodium chloride was chosen due to its lower cost, ability to work for an extended time, and ease of instant preparation compared with yeast. Furthermore, the use of seawater instead of distilled water and sodium chloride was suggested to increase sustainability and reduce costs.
In summary, the study identified the key variables that influenced bioelectricity production and highlighted the importance of stirring, concentration, and yeast. The results of the Plackett–Burman design experiments provided insights into the optimal parameters for the electricity production process. The microbial fuel cell experiment conducted in this study was performed at a small scale, which allowed for controlled conditions and ease of monitoring. However, scaling up the microbial fuel cell system holds the potential to enhance electricity generation and address the cost associated with stirring. By increasing the scale, a higher volume of microbial biomass and substrate can be accommodated, leading to increased power output. The larger surface area and increased number of electrochemically active microorganisms can result in greater electron transfer rates and improved the overall performance. Moreover, as the scale increases, the need for mechanical stirring can be reduced or eliminated. At larger scales, natural convection and diffusion processes can play a more significant role in providing adequate mixing and substrate availability throughout the system. This equilibrium between electricity production and reduced stirring costs makes scaling up microbial fuel cells an attractive avenue for achieving higher energy yields and improving the economic feasibility of the technology.
3.2. Phase 2: Optimization of the Process to Maximize Milivolt Production
The Plackett–Burman design allowed for identifying the concentration, stirring and yeast as the most important factors that affect the process. This analysis allowed for screening many factors at once with a minimum number of experimental runs. After the Plackett–Burman analysis, the most significant factors were selected for further optimization using a Box–Behnken design. Overall, the combined findings from the Plackett–Burman design and subsequent Box–Behnken design highlighted the influential factors in bioelectricity production and provided insights into their optimal settings. By systematically varying and analyzing these factors, this study aimed to optimize the process and achieve the desired bioelectricity output.
The Box–Behnken design, which is a type of response surface methodology, enables the optimization of multiple factors concurrently while minimizing the number of experimental runs required. This approach involves systematically varying each factor across low, medium and high levels to determine their optimal values. The goal is to identify the combination of factor settings that yield the desired response or outcome. The Box–Behnken Design typically involves a series of experimental runs, where the response of interest is measured and used to construct a mathematical model to predict the optimal settings for each factor.
In the experiments conducted, the observed millivolt values exhibited notable differences depending on the specific experiment (
Table 5). For example, experiment 1 displayed low millivolt values, as it was carried out with an intermediate vinasse concentration, along with the lowest stirring and NaCl values. Conversely, experiments 13 to 15, which utilized intermediate values of concentration, NaCl and stirring, significantly increased the millivolt values. The maximum bioelectricity production was observed in experiment 2, with an average value of 431.1 mV. This experiment employed the highest stirring value, intermediate concentration and minimum NaCl dose. Importantly, the minimum millivolt values consistently remained above 75% of the maximum values throughout the experiments.
In this study, a Pareto chart was employed as a tool to detect the influence of the variables. The use of a Pareto chart of standardized effects in the analysis of influential variables in bioelectricity production provides valuable insights into the significance of each factor. The Pareto chart allows for a visual representation of the magnitude of the effects, helping to prioritize and focus on the most influential factors for process optimization. The use of a standardized effects chart allows for a fair comparison of the influence of different variables, as the effects are normalized to a common scale. This normalization enables researchers to identify the most critical factors that significantly impact the response variable, regardless of the differences in their units or scales. In this study, the standardized effects chart provided a clear representation of the relative importance of the variables, highlighting the concentration, stirring and yeast as the primary drivers of millivolt production.
In the presented study, the Pareto chart of standardized effects (
Figure 7) revealed that the vinasse concentration and stirring were the most influential variables in the bioelectricity production process at the beginning of the process. These variables exhibited positive effects on millivolt production, indicating that higher levels of concentration and stirring were associated with increased bioelectricity generation. These variables showed positive significant influence, both in linear and quadratic forms.
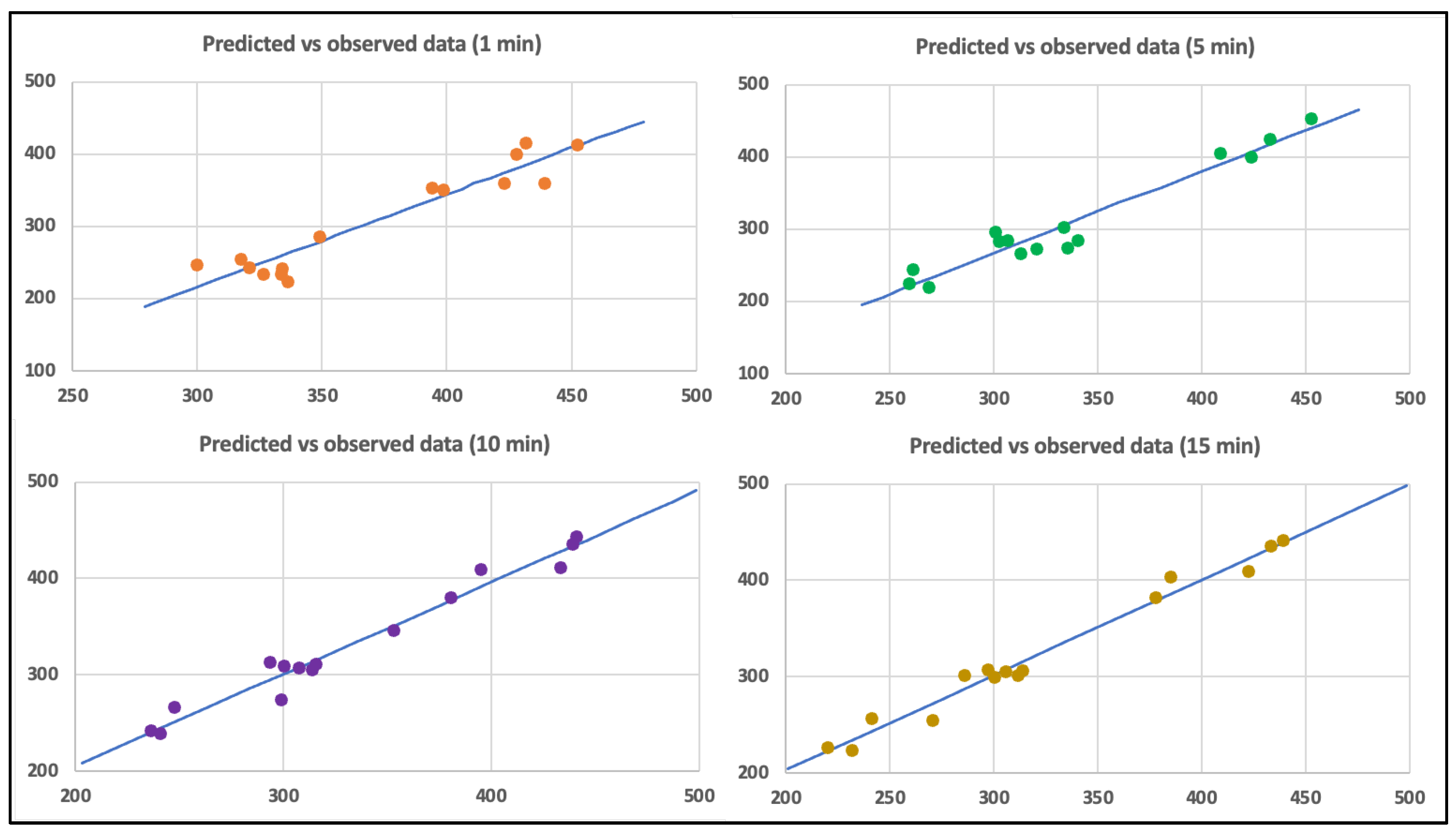
Validation of the statistical analysis is shown in
Figure 8, which shows the relationship between the observed and predicted data for the millivolt production, demonstrating a good reproduction of the experimental data in all cases. Also, the significance of each coefficient was determined using
p-values for the variables A to G, corresponding to bioelectricity production after 1, 5, 10 and 15 min, respectively (
Table 6).
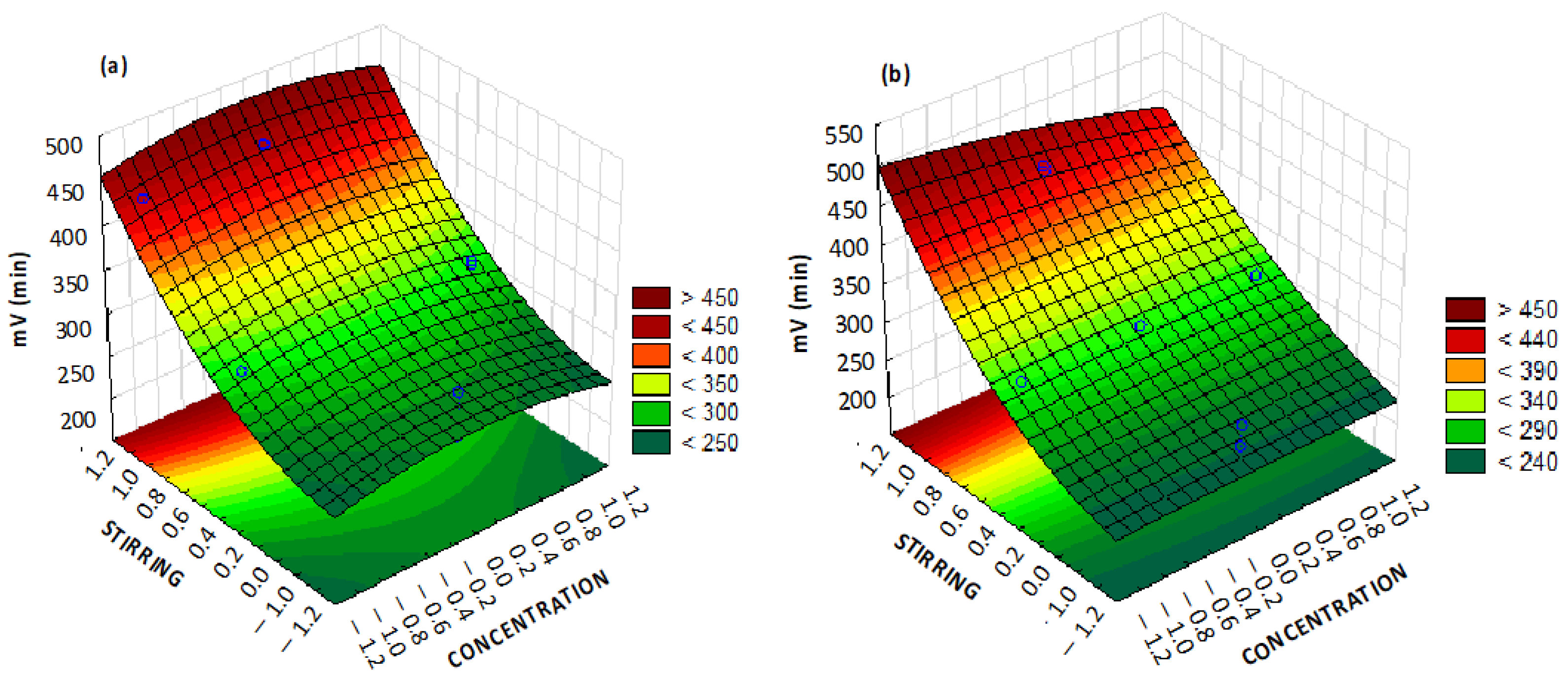
Once the main variables were determined, surface response graphs were generated to determine their magnitude. Surface response graphs play a crucial role in a Box–Behnken design, providing valuable insights into the relationship between multiple variables and the response of a system. These graphs illustrate the behavior of the response surface by depicting contours or three-dimensional plots, enabling scientists to visualize and analyze the interactions between factors within the design space. The utility of surface response graphs lies in their ability to identify optimal settings for the variables, aiding in process optimization and improving product quality. For short times, the model shows its maximum point with 8% NaCl concentration and near-maximum stirring, resulting in approximately 500 mV.
For the analysis of the ten-minute interval, stirring and concentration were used as independent axes (
Figure 9a,b), as these two variables were the most important variables at larger times. From 10 to 15 min, stirring appeared as the most important factor, together with the vinasse concentration. When interpreted together with the Pareto diagram, as more time elapsed, stirring became a more influential factor than NaCl addition. With stirring predominating, the response surfaces became less curved. Although the concentration had a greater influence, it was observed that the highest voltage was obtained at its average value (concentration 1:2), yielding approximately 460 mV, which was slightly lower than at shorter time intervals. Finally, after 15 min of the experiment, the model predicted that the target voltage of 500 mV may be obtained (
Figure 7d) with maximum stirring and the minimum concentration (1:4). The selection of the minimum concentration was rationalized by the observation that heightened stirring promotes earlier attainment of homogeneity within the solution. Consequently, it is imperative to avoid a dense solution that may induce irregularities in the fluid dynamics.
4. Discussion
The Plackett–Burman and Box–Behnken designs play pivotal roles in experimental design and optimization studies, offering valuable insights into identifying the most influential variables in a system. The Plackett–Burman design efficiently screens numerous factors, aiding in narrowing down key factors that significantly impact the response of interest. By exploring a wide parameter space with minimal experimental runs, it provides valuable insights in the initial stages of research, enhancing our understanding of the system’s behavior.
In contrast, the Box–Behnken design allows for a thorough examination of the response surface, considering both main effects and second-order interactions. This design facilitates the construction of response surface models and process parameter optimization, enabling us to understand complex interactions between multiple variables [
22]. It provides valuable information on the curvature, optimal factor levels, and regions of maximum or minimum response, contributing to process optimization, quality improvement and efficient resource allocation [
23].
In our study, we employed both the Plackett–Burman and Box–Behnken designs to identify the most influential variables in a microbial fuel cell (MFC) for maximizing its performance and efficiency. Through systematic optimization, we enhanced the power output, increased the conversion efficiency and improved the overall MFC performance. Our findings align with previous research that highlighted the crucial role of these designs in narrowing down critical variables and developing robust models for process optimization.
Among the variables analyzed, the composition of vinasse emerged as a crucial factor in the MFC performance. Excessive microorganism growth during treatment can lead to a decline in MFC activity. To mitigate this issue, previous studies suggested various strategies, such as the addition of a polymer to increase the viscosity [
24] or utilizing porous solids to prevent electrolyte sedimentation [
25,
26,
27]. Also, having an electrolyte that may sediment necessitates continuous stirring, which affects MFC yield. Furthermore, the effect of flow velocity on bioelectricity generation should also be considered [
28]. These approaches help maintain stable MFC operation and enhance the yield. However, electricity production using MFCs remains a substantial problem for practical implementations owing to the difficulty in balancing the yield with overall system upscaling [
29].
Temperature also emerged as a significant factor that affected the MFC energy production. High temperatures were found to negatively impact the MFC performance due to excessive microorganism growth. To optimize the energy production, maintaining MFCs at values close to room temperature was recommended in previous studies [
30,
31]. Room temperature offers advantages, such as power savings, low solid production and higher treatment efficiency, contributing to sustainable MFC operation [
32]. Similarly, Cano et al. [
33] found additional implications regarding temperature, as wastewater treated at high values may require a cooling step prior to discharge to avoid negative effects on the natural system.
In contrast, the salt concentration showed the least influence on the MFC process. Surprisingly, experiment 2 conducted with 2% of salt exhibited the highest millivolt values when combined with maximum stirring and medium vinasse dose. Additionally, experiment 4 conducted with the maximum salt concentration, medium vinasse dose and the lowest stirring yielded values comparable to experiment 2. These findings suggest the possibility of expanding MFC applications to saline wastewater without adversely affecting MFC yield. Vijay et al. [
33] analyzed saline environments as critical issues to be addressed due to the presence of halophilic bacteria, which alter the composition of the medium and could impact the electrochemical activity. This is an important finding, as yeast presents a higher ability to grow in harsh environments compared with most bacteria [
34].
The results highlighted the significance of variables such as the vinasse composition, temperature and salt concentration in influencing MFC operation. Furthermore, by incorporating theoretical frameworks and empirical findings, we advanced our understanding of complex interactions within the MFC system. The use of MFC for wastewater treatment is an area of great potential, which has demonstrated its ability to treat a wide range of contaminants, such as drugs [
35], organic removal [
36], dyes [
37], or contribute to desalination [
38] and bioelectricity generation [
39,
40,
41]. These insights not only contribute to the development of sustainable energy technologies but also pave the way for further research on optimizing MFC operation, improving waste treatment processes, and exploring the potential synergies between waste valorization and renewable energy generation.