Age and Growth of Hedinichthys yarkandensis (Day, 1877) in the Hotan River
Abstract
:1. Introduction
2. Materials and Methods
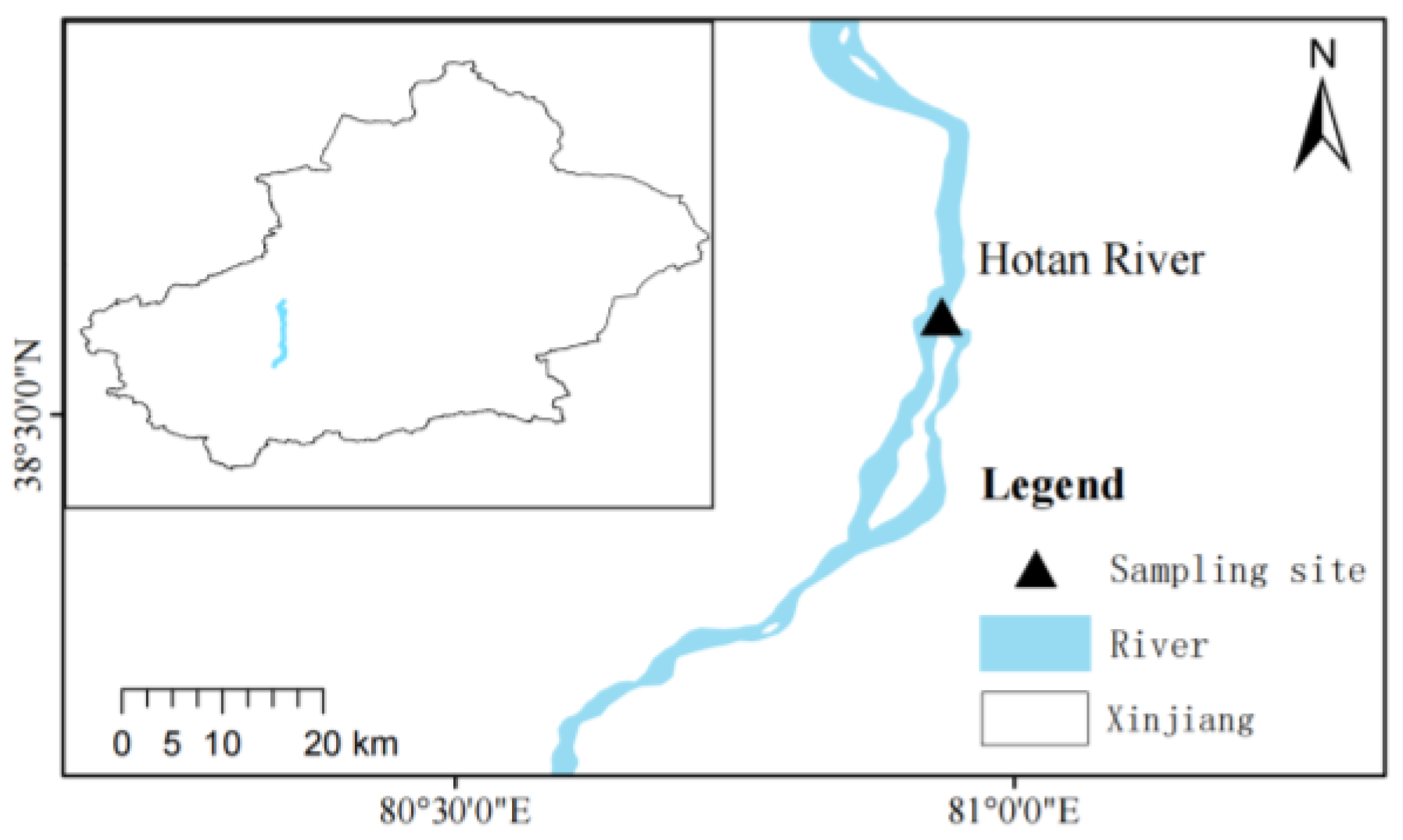
2.1. Sample Collection
2.2. Otolith Characteristics
2.3. Age Identification
2.4. First-Round Confirmation and Annual Cycle of Formation
- Otolith First-Round Confirmation
- 2.
- Annual whorl formation cycle
2.5. Growth Pattern
2.6. Growth Equations, Growth Rate, and Growth Acceleration Equations
2.7. Relationship between Otolith Morphology and Standard Length and Body Mass
3. Results
3.1. Otolith Morphology
3.2. Age Structure
3.3. First-Round Confirmation and Annual Round Formation Cycle
- Otolith first-round confirmation
- 2.
- Annual whorl formation cycle
3.4. Distribution of Standard Length and Weight
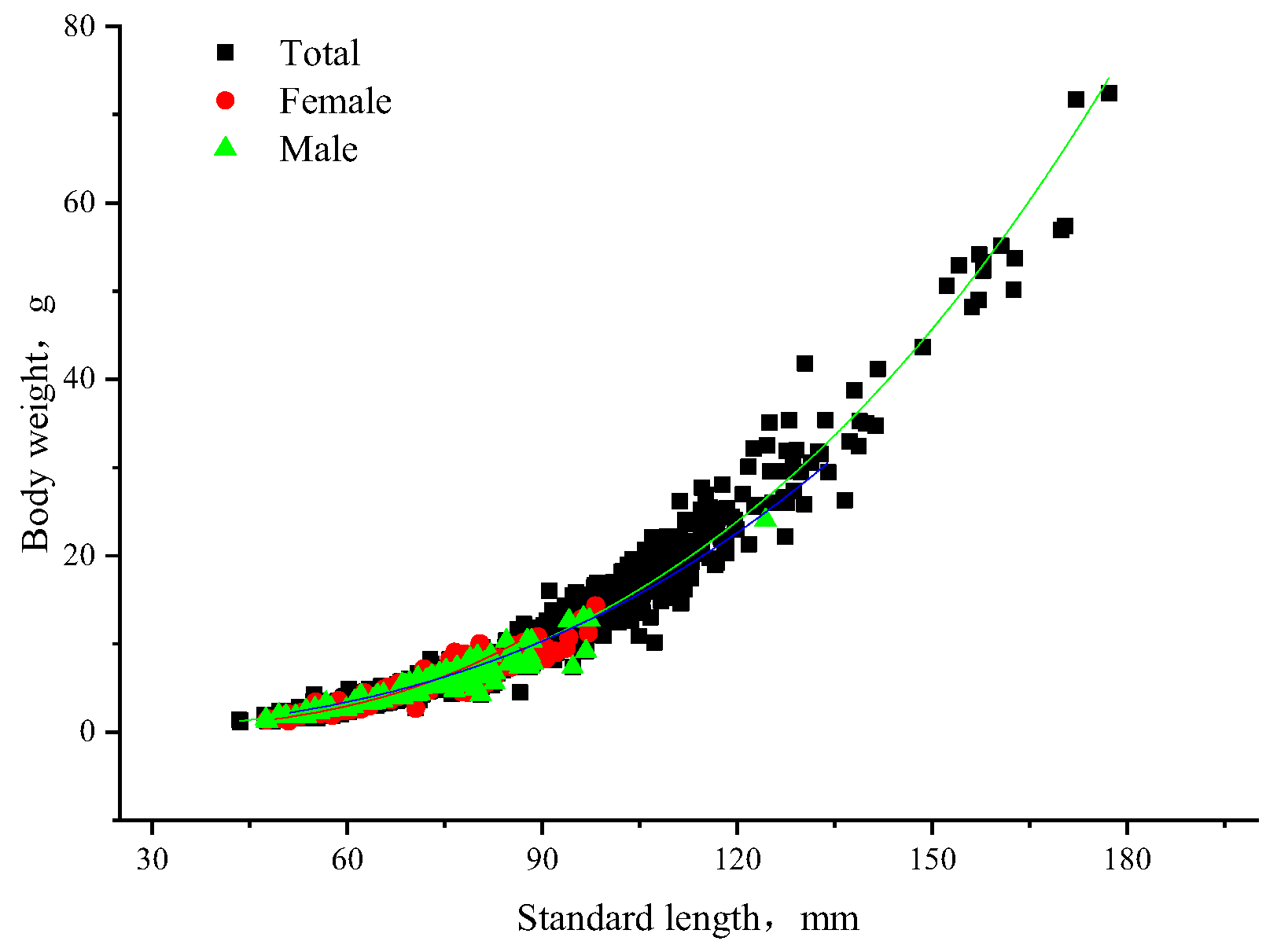
3.5. Correlation Analysis of Standard Length and Body Weight
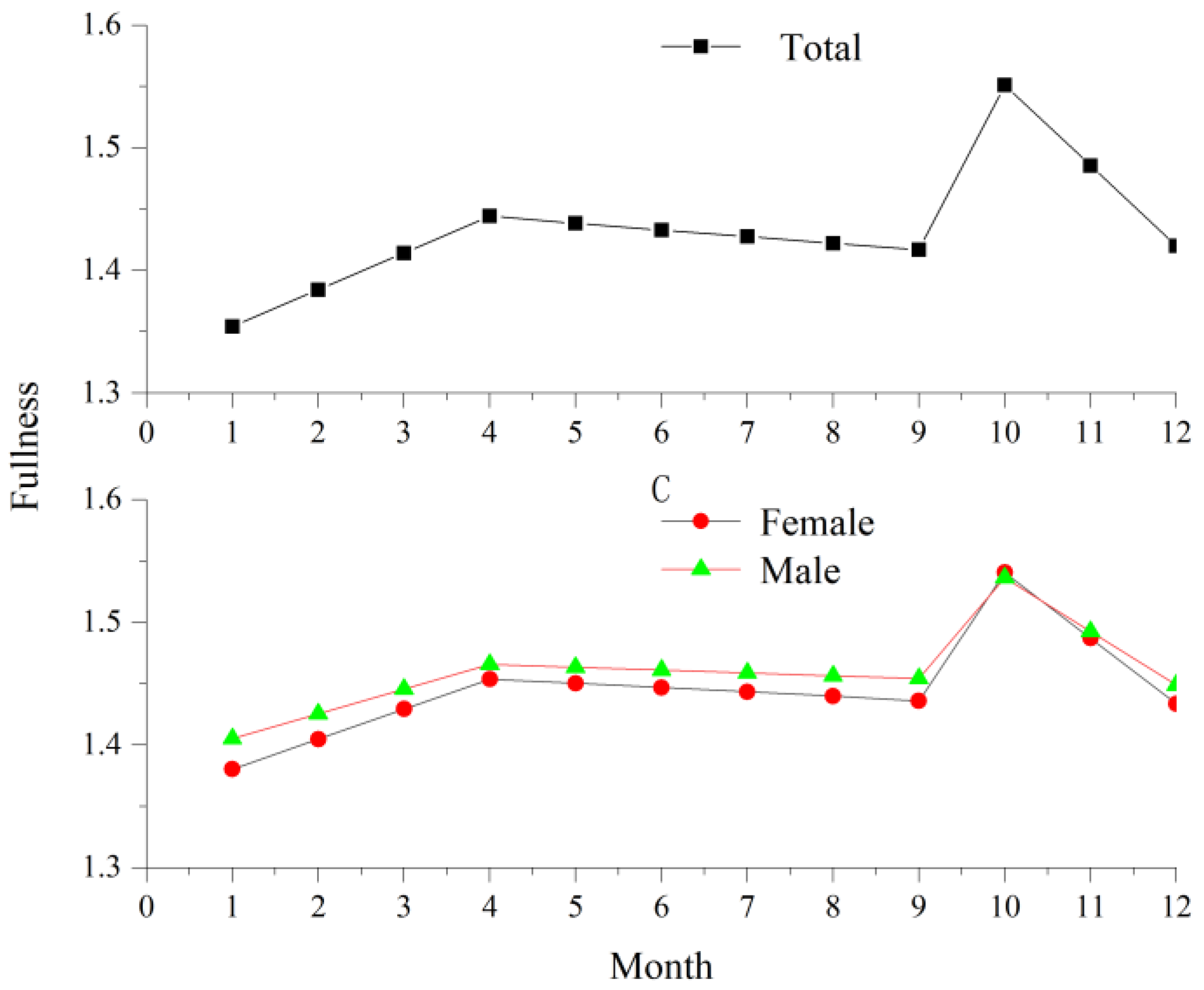
3.6. Fulton Condition Index
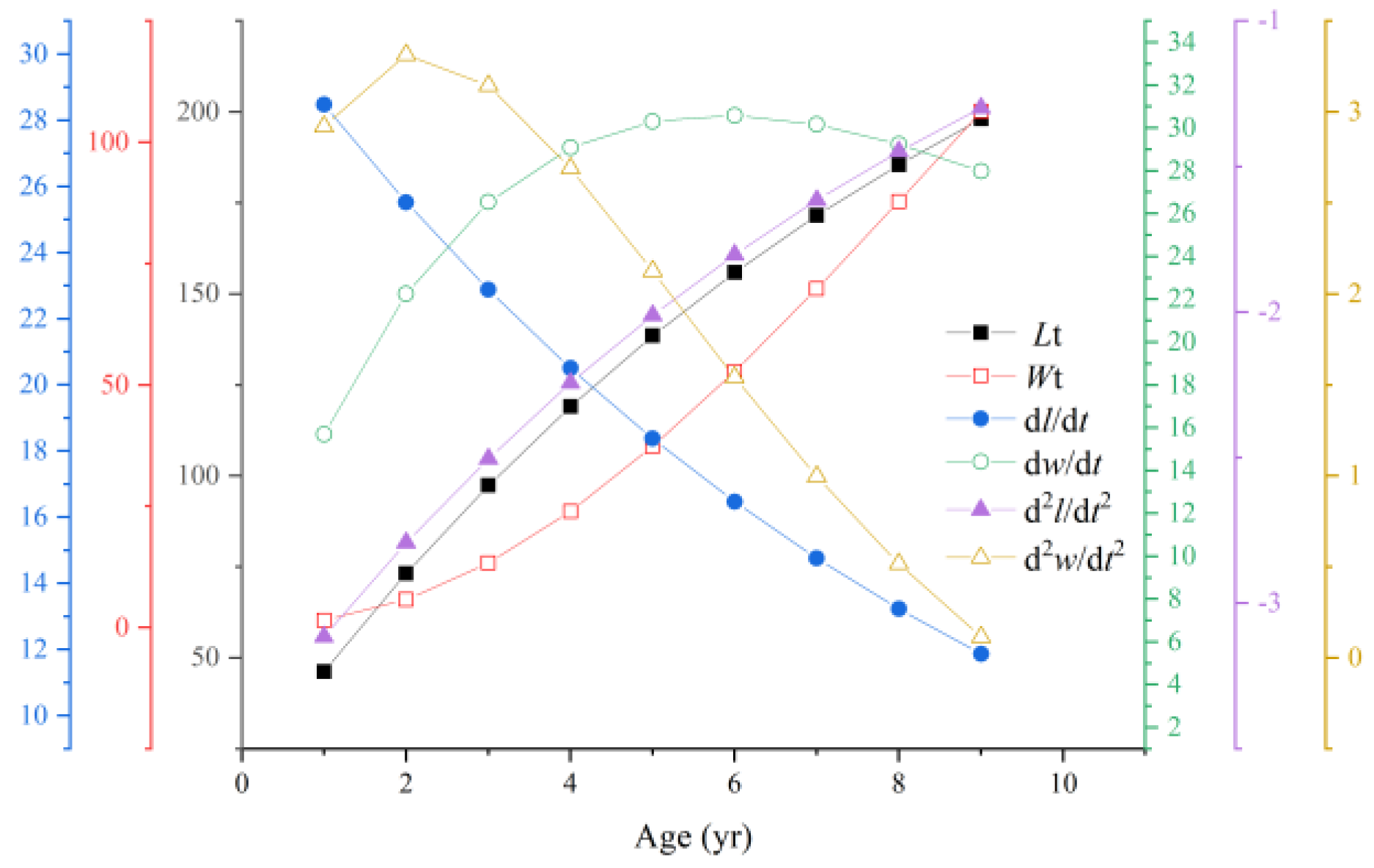
3.7. Growth Equations, Growth Rate, and Growth Acceleration Equations
3.8. Correlation between Otolith Morphology and Growth in Fish
4. Discussion
4.1. Correlation between Otolith Morphology and Growth in Fish
4.2. Age Identification Analysis
4.3. Growth Characteristics Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, B.G.; Mao, W.Y.; Feng, Y.R.; Chang, T.; Zhang, L.P.; Zao, L. Study on the Change of Air Temperature, Precipitation and Runoff Volume in the Yarkant River Basin. Arid Zone Res. 2006, 23, 203–209. [Google Scholar]
- Nazakaiti, T.; Hudan, T.; Jiao, P.; Milishati, M.; Chen, J. Study on the evolution characteristics of main hydrometeorological factors and runoff in Hetian river basin in recent 60 years. J. China Hydrol. 2022, 42, 85–89. [Google Scholar]
- Zhen, Y.Q. Study on Water Resources Protection Planning of Hotan River Basin. Master’s Thesis, Xi’an University of Technology, Xi’an, China, 2005. [Google Scholar]
- Song, B.Y. A study on the changes of Khotan River since the Wei and Jin Dynasties. Master’s Thesis, Northwest Minzu University, Lanzhou, China, 2018. [Google Scholar]
- Záhorská, E.; Kováč, V.; Falka, I.; Beyer, K.; Katina, S.; Copp, G.H.; Gozlan, R.E. Morphological variability of the Asiatic cyprinid, topmouth gudgeon Pseudorasbora parva, in its introduced European range. J. Fish Biol. 2009, 74, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Gorgonio, R.C.; Faustino, C.R.; Alejandro, V.R.; Sergio, S.G.; Jorge De La, R.V. Morphometric variation of wild trout populations from northwestern Mexico (Pisces: Salmonidae). Rev. Fish Biol. Fish. 2003, 13, 91–110. [Google Scholar]
- Xie, C.G.; Ma, Y.W.; Guo, Y. Analysis of Biogeography of Fishes in Tarim Basin. Chin. J. Fish. 2015, 28, 40–46. [Google Scholar]
- Zhao, W.H.; Yi, S.K.; Su, J.X.; Zhou, Q.; Shen, J.Z.; Li, D.P.; Zhao, X.Y. Genetic diversity of Triplophysa Yarkandensis populations in Tarim river basin in Xinjiang. Acta Hydrobiol. Sin. 2022, 46, 364–374. [Google Scholar]
- Zhao, W.H.; Yi, S.K.; Zhou, Q.; Shen, J.Z.; Li, D.P.; Zhao, X.Y. Population Genetic Study of Triplophysa yarkandensis in Tarim River Basin in Xinjiang. Fish. Sci. 2023, 42, 664–673. [Google Scholar]
- Zhao, J.F.; Qiu, L.H.; Zhou, Q.; Zhou, X.Y.; Shen, J.Z. Age structure and growth characteristics of Triplophysa yarkandensis in the Qarqan River, Xinjiang. J. Huazhong Agric. Univ. 2022, 46, 1–9. [Google Scholar]
- Wang, X.Y.; Chen, S.A.; Wang, C.X.; Zi, F.Z.; Chang, D.S.; Xu, H.; Li, D.P. Otolith Morphology and Population Discrimination of Triplophysa yarkandensis. Prog. Fish. Sci. 2023, 44, 201–211. [Google Scholar]
- Yin, M.C. Fish Ecology; China Agriculture Press: Beijing, China, 1995. [Google Scholar]
- Chen, S.A. Study on Population Ecology of Triplophysa yarkandensis (Day) in Tarim River. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2012. [Google Scholar]
- Steven, E.C.; Simon, R.T. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar]
- Gaemers, P.A.M. Taxonomic position of Cichlidae (Pisces, Perciformes) as demonstrated by the morphology of their otoliths. Neth. J. Zool. 1983, 34, 566–595. [Google Scholar]
- Haas, R.E.; Recksiek, C.W. Age verification of winter flounder in Narragansett Bay. Trans. Am. Fish. Soc. 1995, 124, 103–111. [Google Scholar]
- Huo, B. Study on the Biology and Population Dynamics of Oxygymnocypris stewartii. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2014. [Google Scholar]
- Pauly, D. The relationship between gill surface area and growth performance in fish: A generalization of von Bertalanffy’s theory of growth. Meeresforsch 1981, 28, 251–282. [Google Scholar]
- Ren, B.; Ma, Y.W.; Tu, E.X.; Guo, Y.; Zhang, R.M.; A, B.D.; Ai, Z.Z.; Liu, Y. The study on the biology of Triplophysa (Hedinichthgs) yarkandensis (Day) in Akesu River. Chin. J. Fish. 2004, 17, 46–52. [Google Scholar]
- Ma, B.S. Study on the biology and population dynamics of Schizothorax O’connori. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2011. [Google Scholar]
- Sergio, B. Fish age classification based on length, weight, sex and otolith morphological features. Fish. Res. 2007, 84, 270–274. [Google Scholar]
- Ding, L.; Tao, J.; Ding, C. Hydrogeomorphic factors drive differences in otolith morphology in fish from the Nu-Salween River. Ecol. Freshw. Fish 2019, 28, 132–140. [Google Scholar]
- Morrison, C.M.; Kunegel, L.M.; Gallagher, C.P. Decoupling of otolith and somatic growth during anadromous migration in a northern salmonid. Can. J. Fish. Aquat. Sci. 2019, 76, 1940–1953. [Google Scholar] [CrossRef]
- Miloevi, D.; Bigovi, M.; Mrdak, D. Otolith morphology and microchemistry fingerprints of European eel, Anguilla anguilla (Linnaeus, 1758) stocks from the Adriatic Basin in Croatia and Montenegro. Sci. Total Environ. 2021, 786, 147478. [Google Scholar]
- Souza, A.T.; Soukalova, K.; Ded, V. Ontogenetic and interpopulation differences in otolith shape of the European perch (Perca fluviatilis). Fish. Res. 2020, 230, 105673. [Google Scholar] [CrossRef]
- Lombarte, A.; Cruz, A. Otolith size trends in marine fish communities from different depth strata. J. Fish Biol. 2007, 71, 53–76. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, H.H.; Bi, X.J. Sagittal otolith growth and development at different development stages in larval and juvenile Coilia mystus in the Yangtze estuary. J. Fish. Sci. China 2017, 24, 1315–1322. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Xue, Y.; Xu, B.D. Relationships between otolith size and fish size for twelve prey fish species from Jiaozhou bay. Acta Hydrobiol. Sin. 2013, 37, 481–487. [Google Scholar]
- Peng, L.; Jiang, Y.E.; Xu, S.N. Otolith morphology of Nemipterus virgatus and its relation to body length and mass in continental shelf of northern South China Sea. South China Fish. Sci. 2018, 14, 27–33. [Google Scholar]
- Battaglia, P.; Malara, D.; Romeo, T. Relationships between otolith size and fish size in some mesopelagic and bathypelagic species from the Mediterranean Sea (Strait of Messina, Italy). Sci. Mar. 2010, 74, 605–612. [Google Scholar] [CrossRef]
- Duan, M.; Wei, L.; Zhu, G.P. Morphometric features of sagittal otolith for Alaska pollock Gadus chalcogrammus in the western Bering Sea. J. Dalian Ocean Univ. 2018, 33, 492–498. [Google Scholar]
- Fey, D.P. The effect of temperature and somatic growth on otolith growth the discrepancy between two clupeid species from a similar environment. J. Fish Biol. 2006, 69, 794–806. [Google Scholar] [CrossRef]
- Mangel, M.; Munch, S.A. Life-History Perspective on Short-and Long-Term Consequences of Compensatory Growth. Am. Nat. 2005, 166, E155–E176. [Google Scholar] [CrossRef]
- Mitsui, S.; Strussmann, C.A.; Yokota, M.; Yamamoto, Y. Comparative otolith morphology and species identification of clupeids from Japan. Ichthyol. Res. 2020, 67, 502–513. [Google Scholar] [CrossRef]
- Campana, S.E. How Reliable are Growth Back-Calculations Based on Otoliths? J. Can. Des Sci. Halieut. Et Aquat. 1990, 47, 2219–2227. [Google Scholar]
- Zhang, X.J.; Chen, J.H. Survey on study of the fish age determination. Mar. Fish. 2009, 31, 92–99. [Google Scholar]
- Polat, N.; Bostanci, D.; Yilmaz, S. Comparable Age Determination in Different Bony Structures of Pleuronectes flesus luscus Pallas, 1811 Inhabiting the Black Sea. Turk. J. Zool. 2001, 25, 441–446. [Google Scholar]
- Liu, S.P. A study on the biology of Pseudobagrus fulvidraco in Poyang Lake. Chin. J. Zool. 1997, 32, 10–16. [Google Scholar]
- Duan, Z.H.; Sun, J.Y. Study on the age and growth of Pelteobagrus vachelli (Richardson). Acta Hydrobiol. Sin. 1999, 23, 617–623. [Google Scholar]
- Zhu, X.F.; Chen, Y.F. Preliminary study on the age and growth characteristics of Schizothorax macropogon. Chin. J. Zool. 2009, 44, 76–82. [Google Scholar]
- Liu, Y.C.; Liu, S.Y.; Liu, H.P. Values of eight structures as age determination of Ptychobarbus dipogon, Tibet autonomous region. Acta Hydrobiol. Sin. 2019, 43, 579–588. [Google Scholar]
- Cai, Z.L.; Duan, Y.J.; Xu, J.C.; He, Y.; Geng, J.Z.; Liu, X.; Han, M.X.; Liu, Y.H. Comparison of five structure meterials in age determination of Amur ide Leuciscus waleckii in Dali Lake. J. Dalian Ocean Univ. 2020, 35, 121–125. [Google Scholar]
- Zeng, L.; Tang, W.Q. Discussion on age determination methods for two esquamate Triplophysa fishes. Chin. J. Zool. 2010, 45, 94–103. [Google Scholar]
- Jia, Y.; Chen, Y. Otolith microstructure of Oxygymnocypris stewartii (Cypriniformes, Cyprinidae, Schizothoracinae) in the Lhasa River in Tibet, China. Environ. Biol. Fishes 2009, 86, 45–52. [Google Scholar] [CrossRef]
- Zeng, L.; Tang, W.Q. Age, body, growth and reproductive characteristics of Triplophysa yarkandensis. Chin. J. Zool. 2010, 45, 29–38. [Google Scholar]
- Wang, X.Y. Age, Growth, Reproduction and Population Discrimination of Triplophysa yarkandensis. Master’s Thesis, Tarim University, Alar, China, 2022. [Google Scholar]
- Chen, S.A.; Yao, N.; Ren, D.Q.; Xie, C.X.; Fan, Z.M. Study on the population structure of Triplophysa (Hedinichthys) yarkandensis (Day) in the upper reaches of Tarim river. J. Northeast Agric. Univ. 2014, 45, 90–97. [Google Scholar]
- Chen, S.A. Early Development and Physiological Mechanism of Salinity-Alkalinity Adaption of Triplophysa yarkandensis (Day). Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2019. [Google Scholar]







| Sampling Time | Sampling Site | Number | Standard Length/mm | Body Weight/g | ||
|---|---|---|---|---|---|---|
| Range | Mean ± S.D. | Range | Mean ± S.D. | |||
| 2020.04 (spring) | E 80°80′ N 38°32′ | 326 | 67.72~177.24 | 104.60 ± 17.52 | 4.62~72.44 | 17.82 ± 10.26 |
| 2021.08 (summer) | 311 | 51.82~131.42 | 70.68 ± 12.95 | 2.09~30.58 | 5.43 ± 3.68 | |
| 2020.10 (autumn) | 321 | 43.34~137.34 | 90.41 ± 13.49 | 1.09~35.11 | 11.06 ± 4.84 | |
| 2021.01 (winter) | 317 | 47.46~119.93 | 73.14 ± 12.51 | 0.91~22.95 | 5.79 ± 2.95 | |
| Age | n | Weight (g) | Length (mm) | ||
|---|---|---|---|---|---|
| Mean ± S.D. | Range | Mean ± S.D. | Range | ||
| 1 | 236 | 2.98 ± 0.76 | 1.09–4.90 | 57.64 ± 4.21 | 43.34–63.49 |
| 2 | 627 | 5.94 ± 1.85 | 2.68–12.23 | 73.91 ± 7.29 | 63.51–90.21 |
| 3 | 291 | 14.58 ± 3.10 | 7.34–26.17 | 100.00 ± 5.80 | 90.24–111.26 |
| 4 | 76 | 25.92 ± 5.98 | 14.53–41.78 | 122.05 ± 8.61 | 111.33–141.70 |
| 5 | 13 | 52.09 ± 3.62 | 43.64–57.32 | 159.08 ± 6.05 | 148.61–170.51 |
| 6 | 1 | 71.73 | 171.21 | ||
| 7 | 1 | 72.44 | 177.24 | ||
| Morphological Parameter | Linear | Logarithmic | Power | Exponential |
|---|---|---|---|---|
| OA | −3050.17470 | −3052.38664 | −3041.71708 | −321.12924 |
| Rmax | −2080.81676 | −3749.73248 | −3747.55544 | −442.77639 |
| Rmin | −3100.08822 | −3120.88221 | −3111.15243 | 114.34746 |
| OP | −1512.86269 | −1533.82343 | −1524.22967 | 1598.85819 |
| OL | −2937.54239 | −2952.68046 | −2945.12256 | 105.19167 |
| OW | −3524.07473 | −3531.11478 | −3529.89282 | −422.78094 |
| Morphological Parameter | Linear | Logarithmic | Power | Exponential |
|---|---|---|---|---|
| OA | −291.59416 | −3024.81257 | −3013.91078 | −684.76113 |
| Rmax | −3626.86108 | −3736.8819 | −3735.04495 | −442.77639 |
| Rmin | −2911.45940 | −3072.18751 | −3066.26107 | 120.35009 |
| OP | −1376.49575 | −1507.31532 | −1503.05033 | 1598.85819 |
| OL | −2776.46259 | −2909.16640 | −2904.39940 | 111.19148 |
| OW | −3424.90780 | −3513.42209 | −3512.37929 | −422.78094 |
| Relationship between Morphological Parameters of Otolith and Body Weight | R2 | Relationship between Morphological Parameters of Otolith and Standard Length | R2 |
|---|---|---|---|
| OA = 0.2388lnW + 0.1690 | 0.5605 | OA = 0.72lnL − 2.5024 | 0.5859 |
| Rmax = 0.1233lnW + 0.5094 | 0.4822 | Rmax = 0.3688lnL − 0.8572 | 0.4921 |
| Rmin = 0.2423lnW + 0.6352 | 0.5878 | Rmin = 0.7381lnL − 2.1076 | 0.6168 |
| OP = 0.6058lnW + 1.8893 | 0.5077 | OP = 1.8297lnL − 4.9011 | 0.5239 |
| OL = 0.2444lnW + 0.6206 | 0.5909 | OL = 0.7449lnL − 2.1479 | 0.6197 |
| OW = 0.1289lnW + 0.5066 | 0.4868 | OW = 0.3858lnL − 0.9232 | 0.5012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-Y.; Chen, S.-A.; Song, Y.; Wang, C.-X.; Liu, F. Age and Growth of Hedinichthys yarkandensis (Day, 1877) in the Hotan River. Water 2023, 15, 2948. https://doi.org/10.3390/w15162948
Wang X-Y, Chen S-A, Song Y, Wang C-X, Liu F. Age and Growth of Hedinichthys yarkandensis (Day, 1877) in the Hotan River. Water. 2023; 15(16):2948. https://doi.org/10.3390/w15162948
Chicago/Turabian StyleWang, Xin-Yue, Sheng-Ao Chen, Yong Song, Cheng-Xin Wang, and Fei Liu. 2023. "Age and Growth of Hedinichthys yarkandensis (Day, 1877) in the Hotan River" Water 15, no. 16: 2948. https://doi.org/10.3390/w15162948
APA StyleWang, X.-Y., Chen, S.-A., Song, Y., Wang, C.-X., & Liu, F. (2023). Age and Growth of Hedinichthys yarkandensis (Day, 1877) in the Hotan River. Water, 15(16), 2948. https://doi.org/10.3390/w15162948






