Sequestration of Toxic Metal Ions from Industrial Effluent Using the Novel Chelating Resin Tamarind Triazine Amino Propanoic Acid (TTAPA)
Abstract
:1. Introduction
2. Methodology
2.1. Reagents and Chemicals
2.2. Experiment
Synthesis of Tamarind Triazine Amino Propanoic Acid (TTAPA) Resin
- (i)
- Preparation of tamarind triazine ether (TTE)
- (ii)
- Preparation of TTAPA
2.3. Characterization
2.4. Physical Analysis of the Synthesized H-TTAPA Resin
2.4.1. Moisture Content Analysis
2.4.2. Bulk Density Analysis
2.4.3. Swelling Analysis
2.4.4. Ion-Exchange Capacity of the H-TTAPA Resin
2.4.5. Estimation of the Nitrogen Content of H-TTAPA
2.5. Batch Adsorption Experiments
2.6. Recovery of Metal Ions from a Column Using a Suitable Eluent
3. Results and Discussion
3.1. Physical Analysis of the Synthesized H-TTAPA Resin
3.2. Characterization
3.2.1. FTIR Analysis
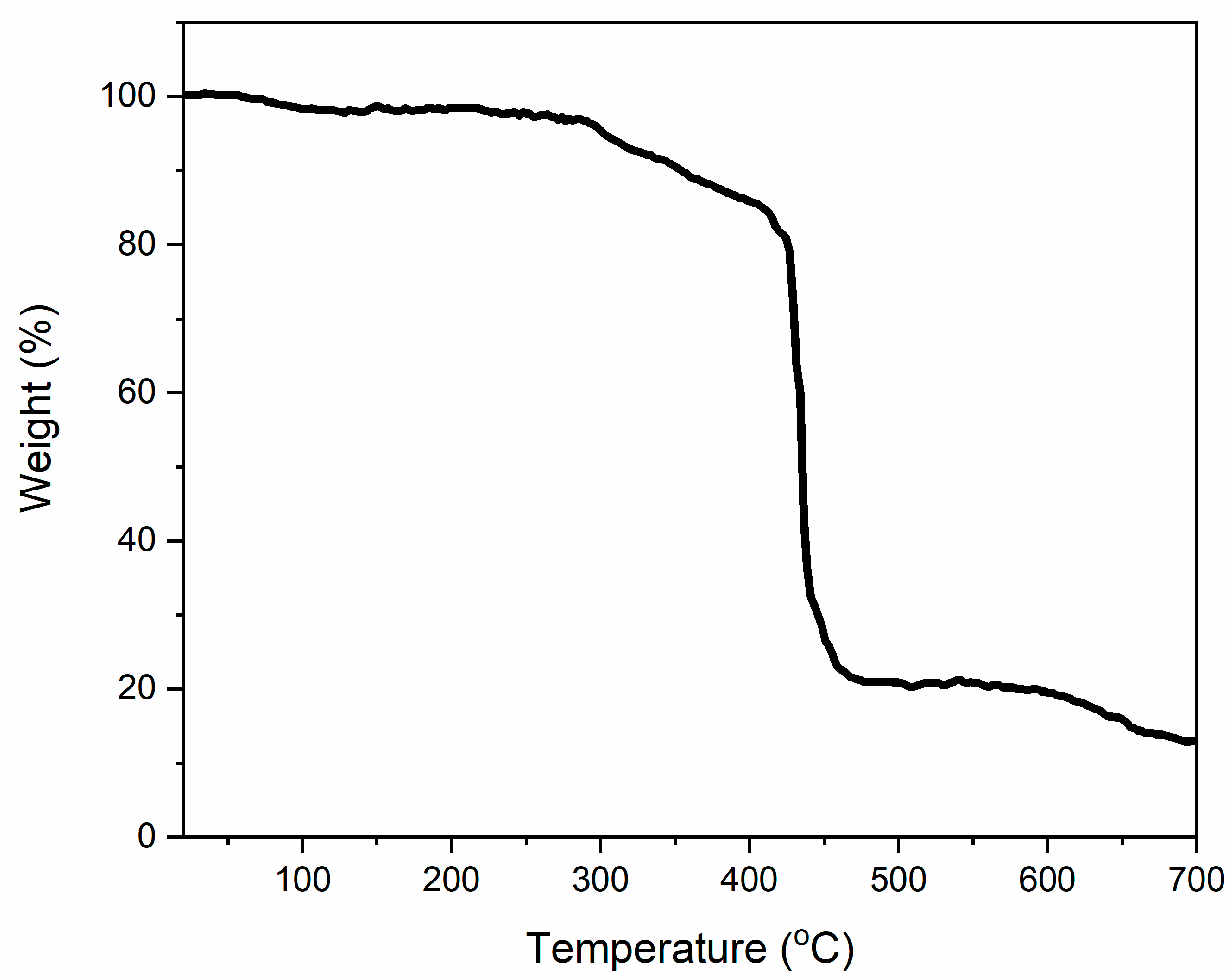
3.2.2. Thermogravimetric Analysis
3.2.3. X-ray Diffraction Analysis
3.2.4. Surface Morphological Analysis
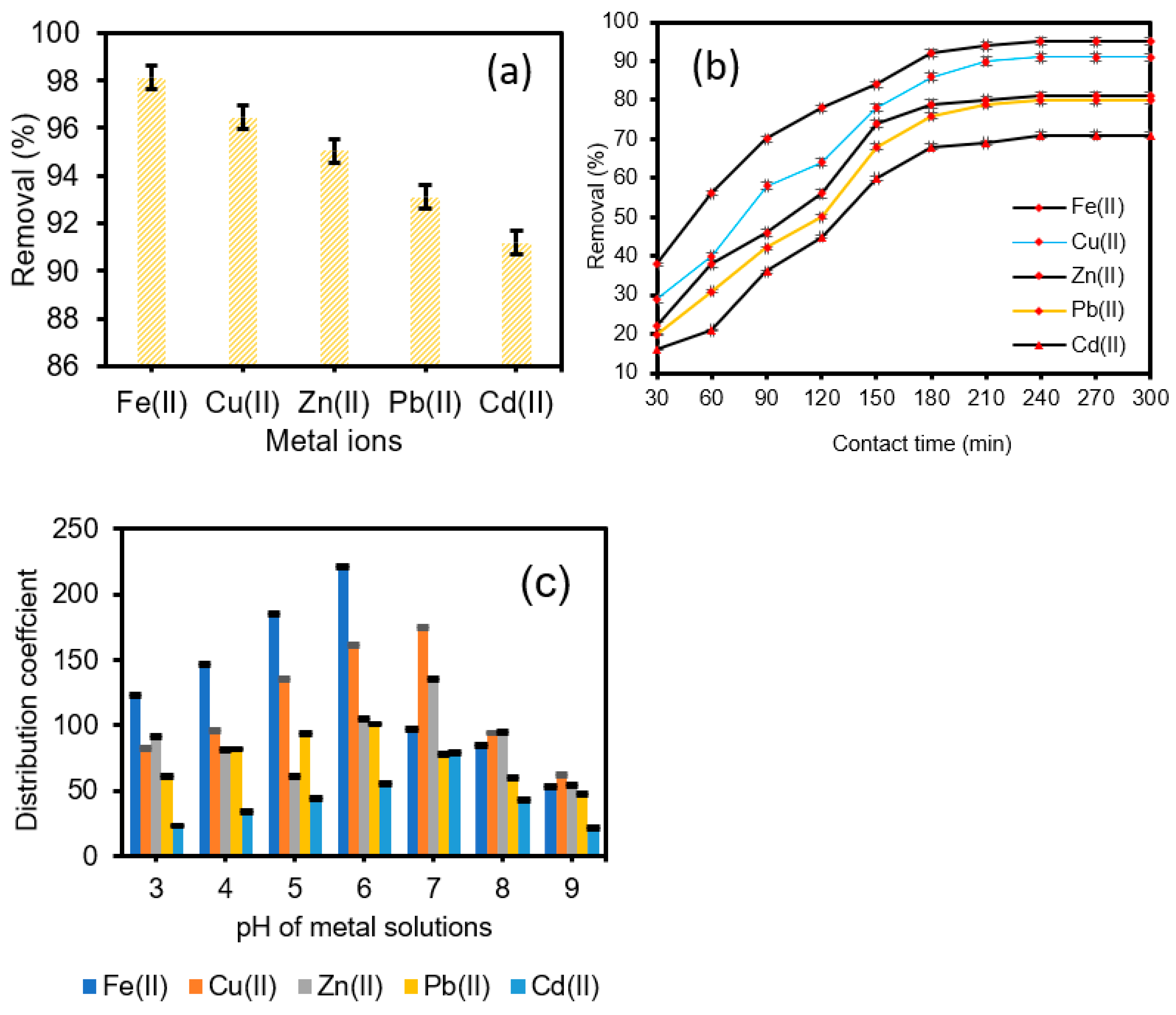
3.3. Metal Ion Removal from Natural Water
3.4. Effect of Contact Time on the Removal of Metal Ions
3.5. Effect of pH on Adsorption and the Distribution Coefficient
3.6. Recovery of Metal Ions from a Column Using a Suitable Eluent
3.7. Resin Durability and Reproducibility
3.8. Comparative Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, R.; Siddiqui, S.I.; Al Alwan, B.; Almesfer, M.; Khanna, M.K.; Fatima, B.; Mishra, R.; Ansari, M.A.; Oh, S. Biodegradable acid-based nanocomposite-CuO-ZnO-Ni(OH)2/PA: A novel material for water cleansing. J. Clean. Prod. 2022, 341, 130860. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. Arsenic: Toxic Effects and Remediation. In Advanced Materials for Wastewater Treatment; Islam, S.U., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Ravi, R.; Rathi, G.; Tara, N.; ul-Islam, S.; Chaudhry, S.A. Decolorization of textile wastewater using composite materials. In Nano Materials in the Wet Processing of Textiles; Islam, S.U., Butola, B.S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 187–218. [Google Scholar]
- Siddiqui, S.I.; Chaudhry, S.A. Iron oxide and its modified forms as an adsorbent for arsenic removal: A comprehensive recent advancement. Process Saf. Environ. Protect. 2017, 111, 592–626. [Google Scholar]
- Chaudhry, S.A.; Zaidi, Z.; Siddiqui, S.I. Isotherm, kinetic and thermodynamics of arsenic adsorption onto Iron-Zirconium Binary Oxide-Coated Sand (IZBOCS): Modelling and process optimization. J. Mol. Liq. 2017, 229, 230–240. [Google Scholar]
- Choudhry, A.; Sharma, A.; Siddiqui, S.I.; Ahamad, I.; Sajid, M.; Khan, T.A.; Chaudhry, S.A. Origanum vulgare manganese ferrite nanocomposite: An advanced multifunctional hybrid material for dye remediation. Environ. Res. 2023, 220, 115193. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.I.; Ravi, R.; Chaudhry, S.A. Removal of arsenic from water using graphene oxide nano-hybrids. In A New Generation Material Graphene: Applications in Water Technology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 221–237. [Google Scholar]
- Siddiqui, S.I.; Naushad, M.; Chaudhry, S.A. Promising prospects of nanomaterials for arsenic water remediation: A comprehensive review. Process Saf. Environ. Protect. 2019, 126, 60–97. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. A review on graphene oxide and its composites preparation and their use for the removal of As3+ and As5+ from water under the effect of various parameters: Application of isotherm, kinetic and thermodynamics. Process Saf. Environ. Protect. 2018, 119, 138–163. [Google Scholar] [CrossRef]
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Alharbi, T.; Al-Kahtany, K.; Nour, H.E.; Giacobbe, S.; El-Sorogy, A.S. Contamination and health risk assessment of arsenic and chromium in coastal sediments of Al-Khobar area, Arabian Gulf, Saudi Arabia. Mar. Pollut. Bull. 2022, 185, 114255. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K. Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environ. Monit. Assess. 2015, 187, 63. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar]
- Ullah, S.; Ohsawa, O.; Ishaq, T.; Hashmi, M.; Sarwar, M.N.; Zhu, C.; Ge, Y.; Jang, Y.; Kim, I.S. Fabrication of Novel Hemp Charcoal Nanofiber Membrane for Effectual Adsorption of Heavy Metal Ions from Wastewater. Sustainability 2023, 15, 9365. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, Á.; Ortega-Toro, R. Removal of Metals and Dyes in Water Using Low-Cost Agro-Industrial Waste Materials. Appl. Sci. 2023, 13, 8481. [Google Scholar] [CrossRef]
- Kumari, S.; Agrawal, N.K.; Agarwal, A.; Kumar, A.; Malik, N.; Goyal, D.; Rajput, V.D.; Minkina, T.; Sharma, P.; Garg, M.C. A Prominent Streptomyces sp. Biomass-Based Biosorption of Zinc (II) and Lead (II) from Aqueous Solutions: Isotherm and Kinetic. Separations 2023, 10, 393. [Google Scholar] [CrossRef]
- Sherugar, P.; Padaki, M.; Naik, N.S.; George, S.D.; Murthy, D.H.K. Biomass-derived versatile activated carbon removes both heavy metals and dye molecules from wastewater with near-unity efficiency: Mechanism and kinetics. Chemosphere 2022, 287, 132085. [Google Scholar] [CrossRef] [PubMed]
- Khoo, P.S.; Ilyas, R.A.; Uda, M.N.A.; Hassan, S.A.; Nordin, A.H.; Norfarhana, A.S.; Ab Hamid, N.H.; Rani, M.S.A.; Abral, H.; Norrrahim, M.N.F.; et al. Starch-Based Polymer Materials as Advanced Adsorbents for Sustainable Water Treatment: Current Status, Challenges, and Future Perspectives. Polymers 2023, 15, 3114. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Mousa, R.H.; Alshehri, S.M. Fabrication of Starch-Salicylaldehyde Based Polymer Nanocomposite (PNC) for the Removal of Pollutants from Contaminated Water. Int. J. Biol. Macromol. 2020, 165, 2731–2738. [Google Scholar] [CrossRef]
- Bai, W.; Fan, L.; Zhou, Y.; Zhang, Y.; Shi, J.; Lv, G.; Wu, Y.; Liu, Q.; Song, J. Removal of Cd2+ Ions from Aqueous Solution Using Cassava Starch–based Superabsorbent Polymers. J. Appl. Polym. Sci. 2017, 134, 44758. [Google Scholar] [CrossRef]
- Yadav, V.K.; Amari, A.; Gacem, A.; Elboughdiri, N.; Eltayeb, L.B.; Fulekar, M.H. Treatment of Fly-Ash-Contaminated Wastewater Loaded with Heavy Metals by Using Fly-Ash-Synthesized Iron Oxide Nanoparticles. Water 2023, 15, 908. [Google Scholar] [CrossRef]
- Ankrah, A.F.; Tokay, B.; Snape, C.E. Heavy Metal Removal from Aqueous Solutions Using Fly-Ash Derived Zeolite NaP1. Int. J. Environ. Res. 2022, 16, 17. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, L.; Li, Y.; Li, L.; Li, S.; Zhu, G. Highly Efficient Removal of Mercury Ions from Aqueous Solutions by Thiol-Functionalized Graphene Oxide. Water 2023, 15, 2529. [Google Scholar] [CrossRef]
- Tamizharasan, S.; Muralidharan, R.; Abirami, N.; Leelavathi, H.; Siva, A.; Kumarasamy, A.; Arulmozhi, R. Biomass derived carbon blended ion-exchange resins for the removal of toxic metal ions from waste water. Optik 2023, 283, 170930. [Google Scholar] [CrossRef]
- Paudyal, H.; Adhikari, S.; Ghimire, K.N. Synthesis, characterization and cation exchange performance of chemically modified pineapple waste biomass for the removal of Fe(II) from water. Res. Chem. 2022, 4, 100608. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Wołowicz, A.; Hubicki, Z. Strongly Basic Anion Exchange Resin Based on a Cross-Linked Polyacrylate for Simultaneous C.I. Acid Green 16, Zn(II), Cu(II), Ni(II) and Phenol Removal. Molecules 2022, 27, 2096. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Comparison of ion-exchange resins for efficient cobalt(II) removal from acidic streams. Chem. Eng. Commun. 2018, 205, 1207–1225. [Google Scholar] [CrossRef]
- Dąbrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, F.; Liu, X.; Su, J. Preparation of corn starch/acrylic acid/itaconic acid ion exchange hydrogel and its adsorption properties for copper and lead ions in wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131668. [Google Scholar] [CrossRef]
- Wong, C.W.; Barford, J.P.; Chen, G.; McKay, G. Kinetics and equilibrium studies for the removal of cadmium ions by ion exchange resin. J. Environ. Chem. Eng. 2014, 2, 698–707. [Google Scholar] [CrossRef]
- Elkady, M.F.; Abu-Saied, M.A.; Rahman, A.A.; Soliman, E.A.; Elzatahry, A.A.; Yossef, M.E.; Eldin, M.M. Nano-sulphonated poly (glycidyl methacrylate) cations exchanger for cadmium ions removal: Effects of operating parameters. Desalination 2011, 279, 152–162. [Google Scholar] [CrossRef]
- Vinco, J.H.; Junior, A.B.B.; Duarte, H.A.; Espinosa, D.C.R.; Tenório, J.A.S. Kinetic modeling of adsorption of vanadium and iron from acid solution through ion exchange resins. Trans. Nonferrous Met. Soc. China 2022, 32, 2438–2450. [Google Scholar] [CrossRef]
- Hart, G.; Fuller, D.R.; Brown, J.A.; Dale, S. Plant, Sulfonated poly(styrene-co-divinylbenzene) ion-exchange resins: Acidities and catalytic activities in aqueous reactions. J. Mol. Catal. A Chem. 2002, 182–183, 439–445. [Google Scholar] [CrossRef]
- Hamed, M.G.; El-khalafawy, A.; Youssef, M.A.; Borai, E.H. Competitive sorption behavior of mono, di, and trivalent ions in highly acidic waste by polymeric resin based on crotonic acid prepared by gamma radiation. Radiat. Phys. Chem. 2023, 212, 111159. [Google Scholar] [CrossRef]
- Liu, Z.; Solliec, M.; Papineau, I.; Lompe, K.M.; Mohseni, M.; Bérubé, P.R.; Sauvé, S.; Barbeau, B. Elucidating the removal of organic micropollutants on biological ion exchange resins. Sci. Total Environ. 2022, 808, 152137. [Google Scholar] [CrossRef] [PubMed]
- How to Transform a Popular Fruit into a Plastic, Nature Portfolio, Nature Research Custom Media. Available online: https://www.nature.com/articles/d42473-023-00019-6 (accessed on 25 July 2023).
- Hoque, M.; Sarkar, P.; Ahmed, J. Preparation and characterization of tamarind kernel powder/ZnO nanoparticle-based food packaging films. Indus. Crops Prod. 2022, 178, 114670. [Google Scholar] [CrossRef]
- Khanna, M.N.; Sarin, R.C. J.P.S. standardization of tamarind seed polysaccharide for pharmaceutical use. Indian Drugs 1987, 24, 268–269. [Google Scholar]
- Lee, H.; Neville, K. Handbook of Epoxy Resins; McGraw-Hill: New York, NY, USA, 1990. [Google Scholar]
- Blake, G.R. Bulk density. In Methods of Soil Analysis; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; Volume 1, pp. 374–390. [Google Scholar]
- Mohan, M.Y.; Sudhakar, K.; Keshava Murthy, P.S.; Mohan Raju, K. Swelling and diffusion properties of poly (acrylamide-co-maleic acid) hydrogels: A study with different crosslinking agents. Int. J. Polym. Mater. 2006, 55, 513. [Google Scholar] [CrossRef]
- Vogel, A. Textbook of Qualitative Chemical Analysis, 5th ed.; Longman Group: London, UK, 1989. [Google Scholar]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An overview of the Kjeldahl method of nitrogen determination. Part II. Sample preparation, working scale, instrumental finish, and quality control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Shehnaz; Prasher, I.B.; Ahmad, N.; Ahmed, M.; Raghuwanshi, S.; Kumar, V.; Siddiqui, S.I.; Oh, S. Live Biomass of Rigidoporus vinctus: A Sustainable Method for Decoloration and Detoxification of Dyes in Water. Microorganisms 2023, 11, 1435. [Google Scholar] [CrossRef]
- Fatima, B.; Alwan, B.A.; Siddiqui, S.I.; Ahmad, R.; Almesfer, M.; Khanna, M.K.; Mishra, R.; Ravi, R.; Oh, S. Facile Synthesis of Cu-Zn Binary Oxide Coupled Cadmium Tungstate (Cu-ZnBO-Cp-CT) with Enhanced Performance of Dye Adsorption. Water 2021, 13, 3287. [Google Scholar] [CrossRef]
- Narasimharao, K.; Al-Thabaiti, S.; Rajor, H.K.; Mokhtar, M.; Alsheshri, A.; Alfaifi, S.Y.; Siddiqui, S.I.; Abdulla, N.K. Fe3O4@ date seeds powder: A sustainable nanocomposite material for wastewater treatment. J. Mater. Res. Technol. 2022, 18, 3581–3597. [Google Scholar] [CrossRef]
- Fatima, B.; Siddiqui, S.; Ahmed, R.; Chaudhry, S.A. Preparation of functionalized CuO nanoparticles using Brassica rapa leave extract for water purification. Desalin. Water Treat. 2019, 164, 192–205. [Google Scholar] [CrossRef]
- Abdulla, N.K.; Siddiqui, S.I.; Fatima, B.; Sultana, R.; Tara, N.; Hashmi, A.A.; Ahmad, R.; Mohsin, M.; Nirala, R.K.; Linh, N.T.; et al. Silver based hybrid nanocomposite: A novel antibacterial material for water cleansing. J. Clean. Prod. 2021, 284, 124746. [Google Scholar] [CrossRef]
- Dev, K.; Rao, G.N. Synthesis and analytical properties of a chelating resin functionalized with bis-(N, N′-salicylidene) 1,3-propanediamine ligands. Talanta 1996, 43, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Kumawat, I.K. Preparation and characterisation of tamarind 4-hydroxybenzoic acid (THBA) resin and its use in extraction of heavy metal ions from industrial wastewater. Water 2012, 38, 529–536. [Google Scholar] [CrossRef]
- Tara, N.; Siddiqui, S.I.; Nirala, R.K.; Abdulla, N.K.; Chaudhry, S.A. Synthesis of antibacterial, antioxidant and magnetic Nigella sativa-graphene oxide based nanocomposite BC-GO@Fe3O4 for water treatment. Colloid Interface Sci. Commun. 2020, 37, 100281. [Google Scholar] [CrossRef]
- Tara, N.; Siddiqui, S.I.; Bach, Q.V.; Chaudhry, S.A. Reduce graphene oxide-manganese oxide-black cumin based hybrid composite (rGO-MnO2/BC): A novel material for water remediation. Mater. Today Commun. 2020, 25, 101560. [Google Scholar] [CrossRef]
- Rathi, G.; Siddiqui, S.I.; Pham, Q. Nigella sativa seeds based antibacterial composites: A sustainable technology for water cleansing—A review. Sustain. Chem. Pharm. 2020, 18, 100332. [Google Scholar] [CrossRef]
- Sahmetlioglu, E.; Mart, H.; Yuruk, H.; Sürme, Y. Synthesis and characterization of oligosalicylaldehyde-based epoxy resins. Chem. Zvesti. 2006, 60, 65–68. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I. Mechanical Properties of Epoxy–Rubber Blends. In Handbook of Epoxy Blends; Parameswaranpillai, J., Hameed, N., Pionteck, J., Woo, E., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Bargujar, S.; Gambhir, G.; Raigar, M.B.; Hooda, S.; Arya, D.K.; Bhatia, M. A new polysaccharide-based ion-exchange resin for industrial wastewater treatment. Polimery 2022, 67, 212–219. [Google Scholar] [CrossRef]
- Badawy, N.A.; El-Bayaa, A.A.; Abdel-Aal, A.Y.; Garamon, S.E. Chromatographic separations and recovery of lead ions from a synthetic binary mixture of some heavy metal using cation exchange resin. J. Hazard. Mater. 2009, 166, 1266–1271. [Google Scholar] [CrossRef]
- Zewail, T.M.; Yousef, N.S. Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed. Alex. Eng. J. 2015, 54, 83–90. [Google Scholar] [CrossRef]







| Order | Analysis | Result |
|---|---|---|
| 1. | Moisture content | 4.59% |
| 2. | Bulk density | 0.713 g/cm3 |
| 3. | Swelling | 9.9% |
| 4. | Ion-exchange capacity | 1.5556 meq/g of H+ ions |
| 5. | Nitrogen content | 2.65% |
| Order | Element | Theoretical Value (%) | Experimental Value (%) | Error Function (χ) |
|---|---|---|---|---|
| 1. | C | 36.36 | 36.04 | 0.008801 |
| 2. | H | 3.03 | 3.00 | 0.009901 |
| 3. | N | 28.28 | 27.98 | 0.010608 |
| 4. | O | 32.32 | 32.02 | 0.009282 |
| Order | Ion Exchanger | Experimental Conditions | Ions | % Removal | References |
|---|---|---|---|---|---|
| 1. | Tamarind-tripropylamine resin (TTA) | pH = 7.0; Dose = 0.4 g/L; Contact time = 3 h; 25 °C | Pb(II) | 53.38 | [56] |
| Cd(II) | 71.35 | ||||
| Zn(II) | 74.88 | ||||
| Cu(II) | 77.84 | ||||
| Fe(II) | 83.42 | ||||
| 2. | Raw pineapple waste biomass ion exchange (RPWB) | pH = 3.5; 30 mg of RPWB with 20 mL of 11 mg/L Fe(II) solution | Fe(II) | 47% | [25] |
| 3. | Saponified pineapple waste biomass ion exchange (SPWB) | pH = 3.0; 30 mg of SPWB with 20 mL of 11 mg/L Fe(II) solution | 94% | ||
| 4. | Purolite C 100 | - | Pb(II) | 99.17 | [57] |
| 5. | Amberjet 1200 Na | 800, 1000, 1250 mg/L | Pb(II) | 99.0 | [58] |
| 6. | TTAPA | pH = 7.0; Dose = 1.0 g; Contact time = 4 h; 25 °C | Fe(II) | 98.11 | This study |
| Cu(II) | 96.44 | ||||
| Zn(II) | 95.06 | ||||
| Pb(II) | 93.10 | ||||
| Cd(II) | 91.17 | ||||
| 7. | TKP | pH = 7.0; Dose = 1.0 g; Contact time = 4 h; 25 °C | Fe(II) | 67.21 | |
| Cu(II) | 57.41 | ||||
| Zn(II) | 61.24 | ||||
| Pb(II) | 66.60 | ||||
| Cd(II) | 64.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, K.; Abomuti, M.A.; Al-Harbi, S.A.; Tejasvi, S.; Park, S.; Raigar, M.B.; Oh, S. Sequestration of Toxic Metal Ions from Industrial Effluent Using the Novel Chelating Resin Tamarind Triazine Amino Propanoic Acid (TTAPA). Water 2023, 15, 2924. https://doi.org/10.3390/w15162924
Mandal K, Abomuti MA, Al-Harbi SA, Tejasvi S, Park S, Raigar MB, Oh S. Sequestration of Toxic Metal Ions from Industrial Effluent Using the Novel Chelating Resin Tamarind Triazine Amino Propanoic Acid (TTAPA). Water. 2023; 15(16):2924. https://doi.org/10.3390/w15162924
Chicago/Turabian StyleMandal, Kalpa, May Abdullah Abomuti, Sami A. Al-Harbi, Sarika Tejasvi, Sangeun Park, Madhu Bala Raigar, and Seungdae Oh. 2023. "Sequestration of Toxic Metal Ions from Industrial Effluent Using the Novel Chelating Resin Tamarind Triazine Amino Propanoic Acid (TTAPA)" Water 15, no. 16: 2924. https://doi.org/10.3390/w15162924
APA StyleMandal, K., Abomuti, M. A., Al-Harbi, S. A., Tejasvi, S., Park, S., Raigar, M. B., & Oh, S. (2023). Sequestration of Toxic Metal Ions from Industrial Effluent Using the Novel Chelating Resin Tamarind Triazine Amino Propanoic Acid (TTAPA). Water, 15(16), 2924. https://doi.org/10.3390/w15162924






