Hydrochemical Characteristics, Water Quality, and Evolution of Groundwater in Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Analysis
2.3. Entropy-Weighted Water Quality Index (EWQI)
3. Results and Discussion
3.1. Hydrochemical Composition and Hydrochemical Types
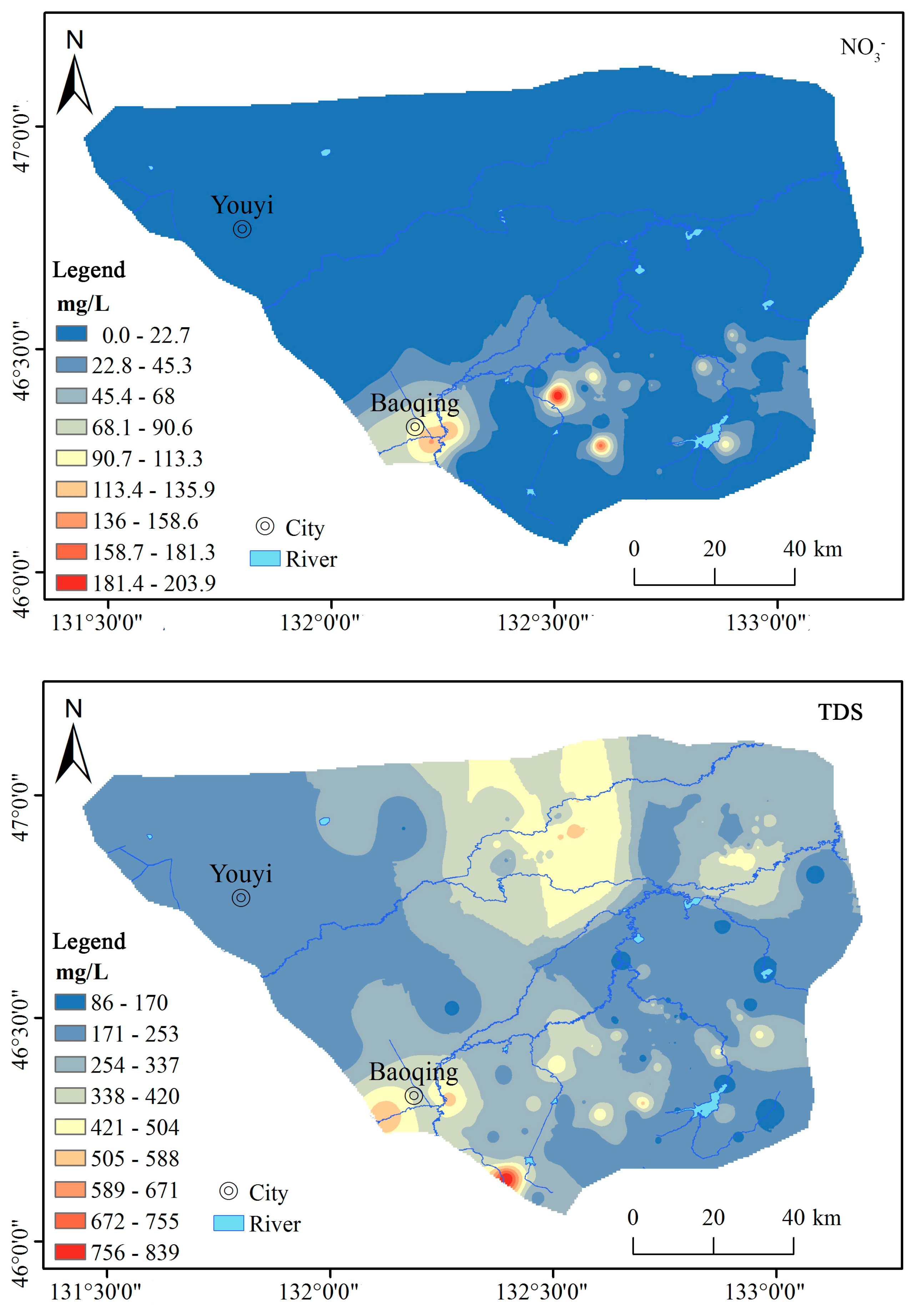
3.1.1. Chemical Composition Characteristics of Groundwater
3.1.2. Hydrochemical Type
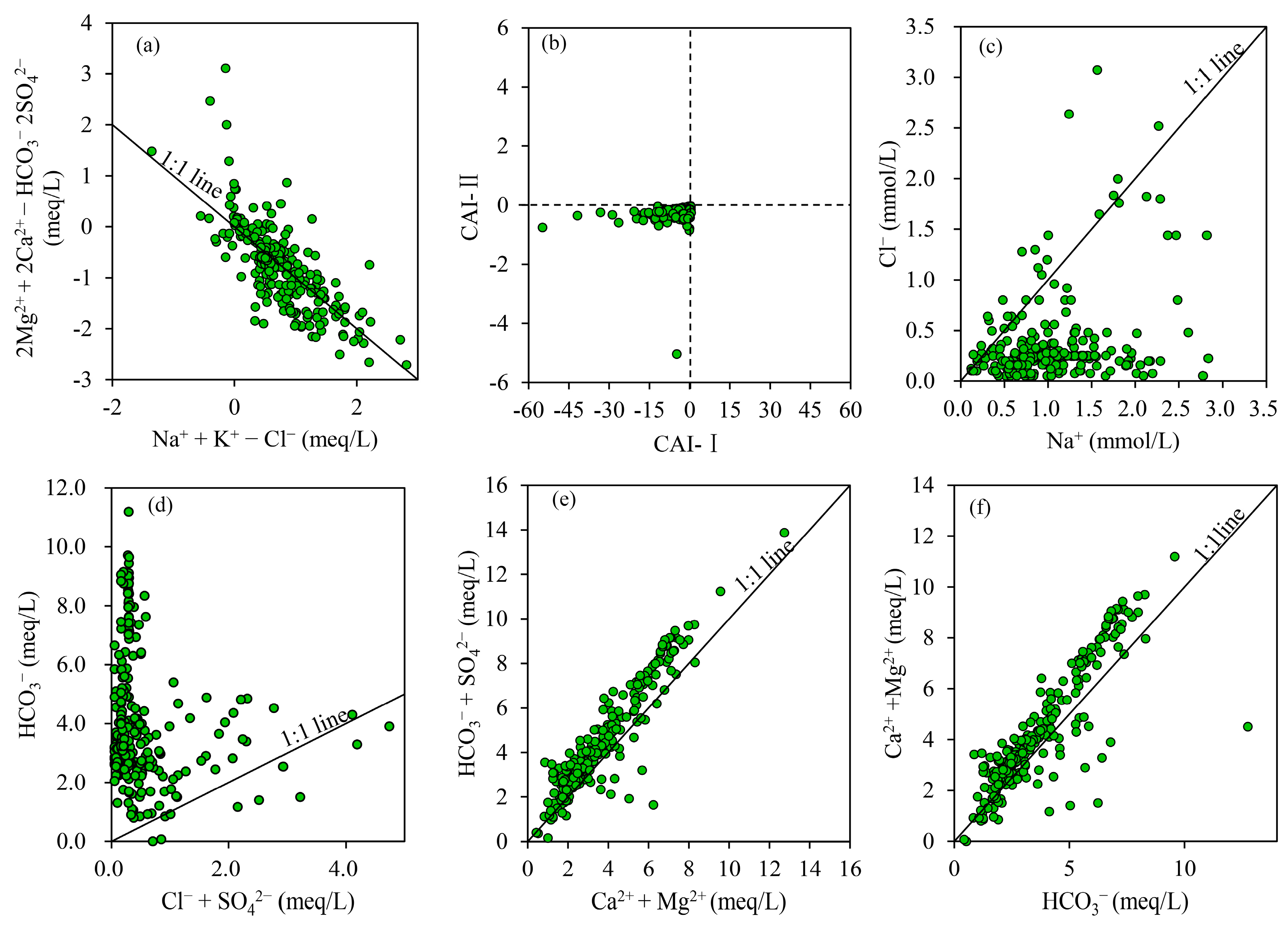
3.1.3. Relationship between Chemical Indexes
3.2. Control Factors of Groundwater Hydrochemistry
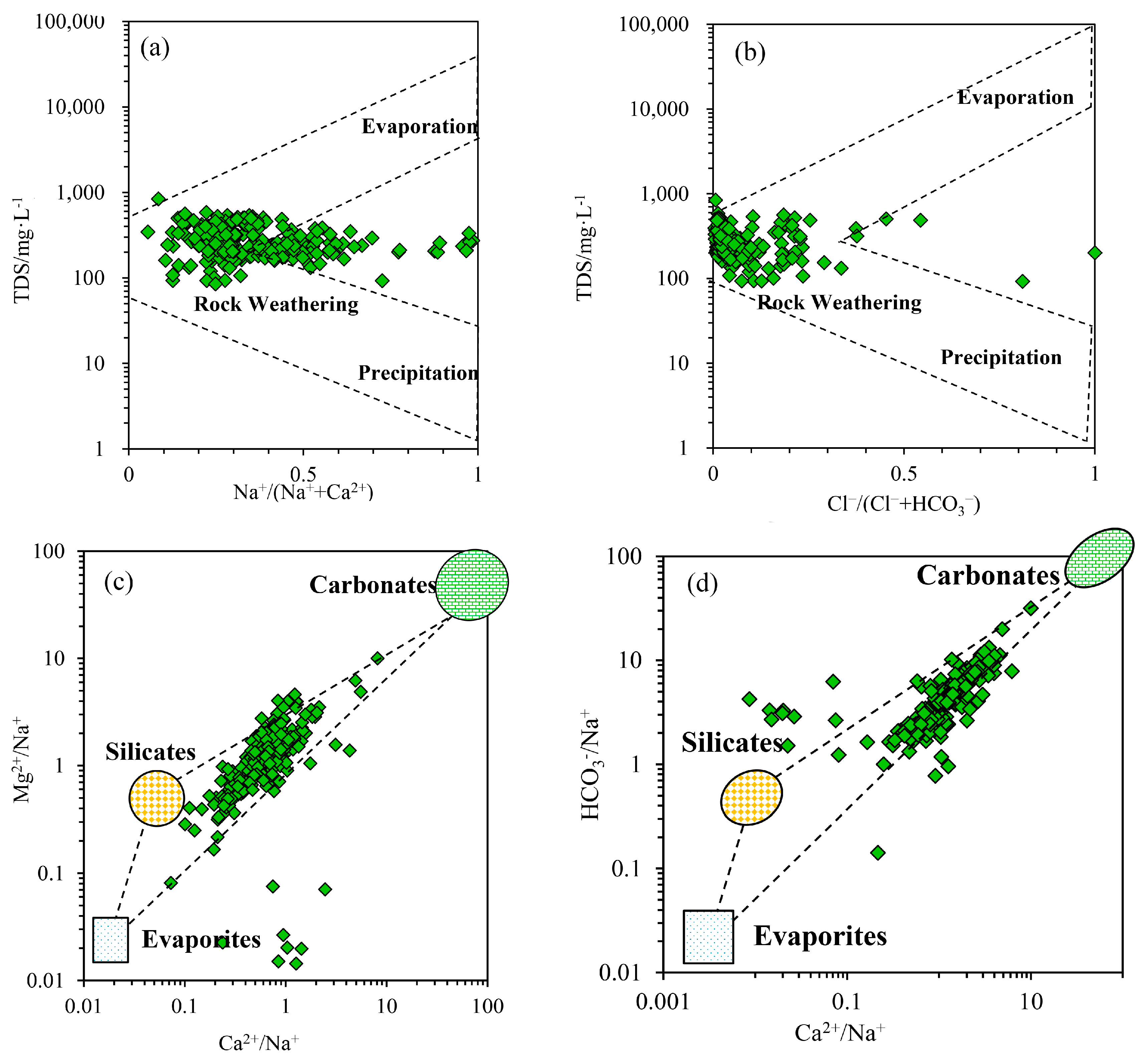
3.2.1. Natural Factors
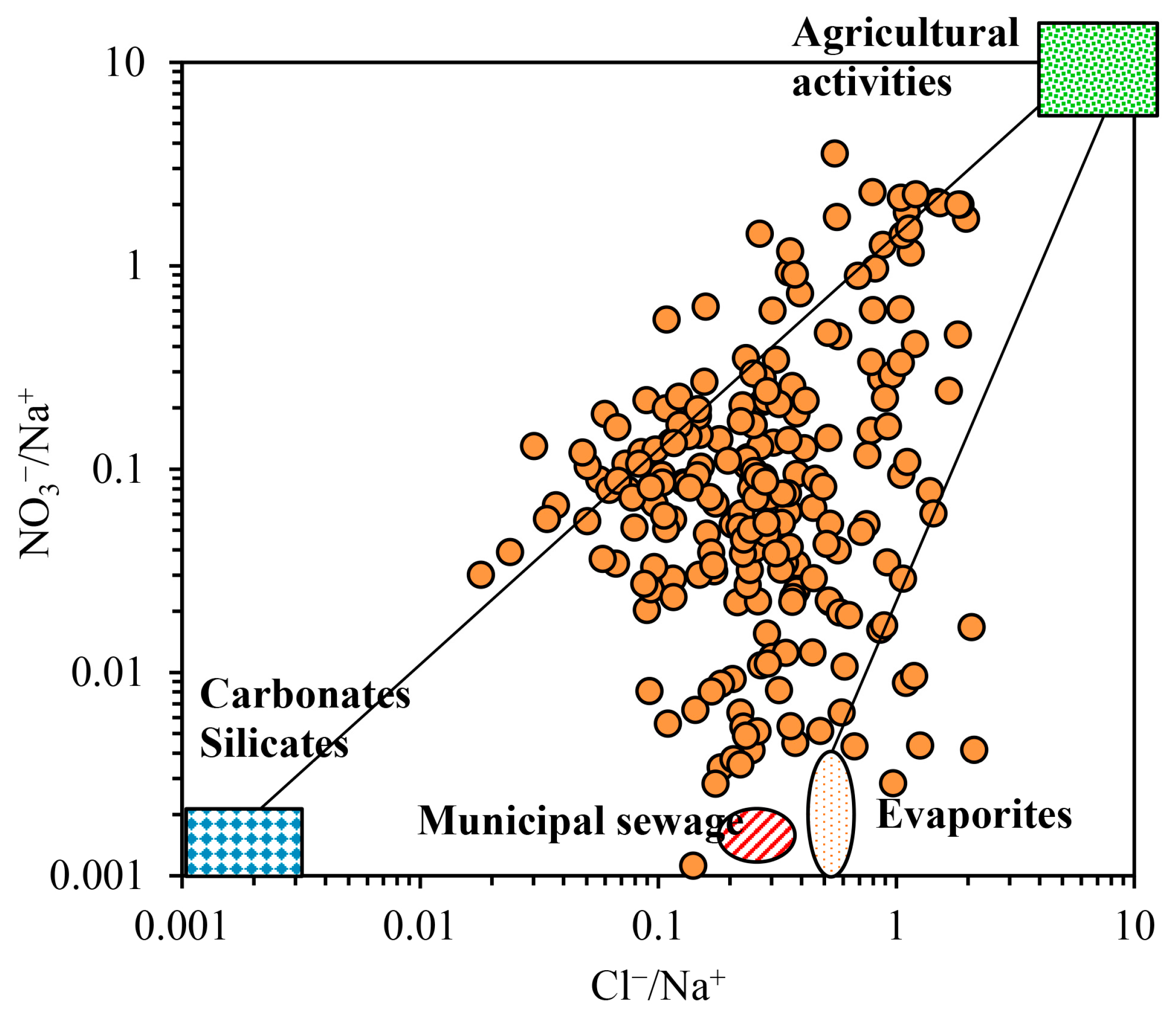
3.2.2. Human Activity Input
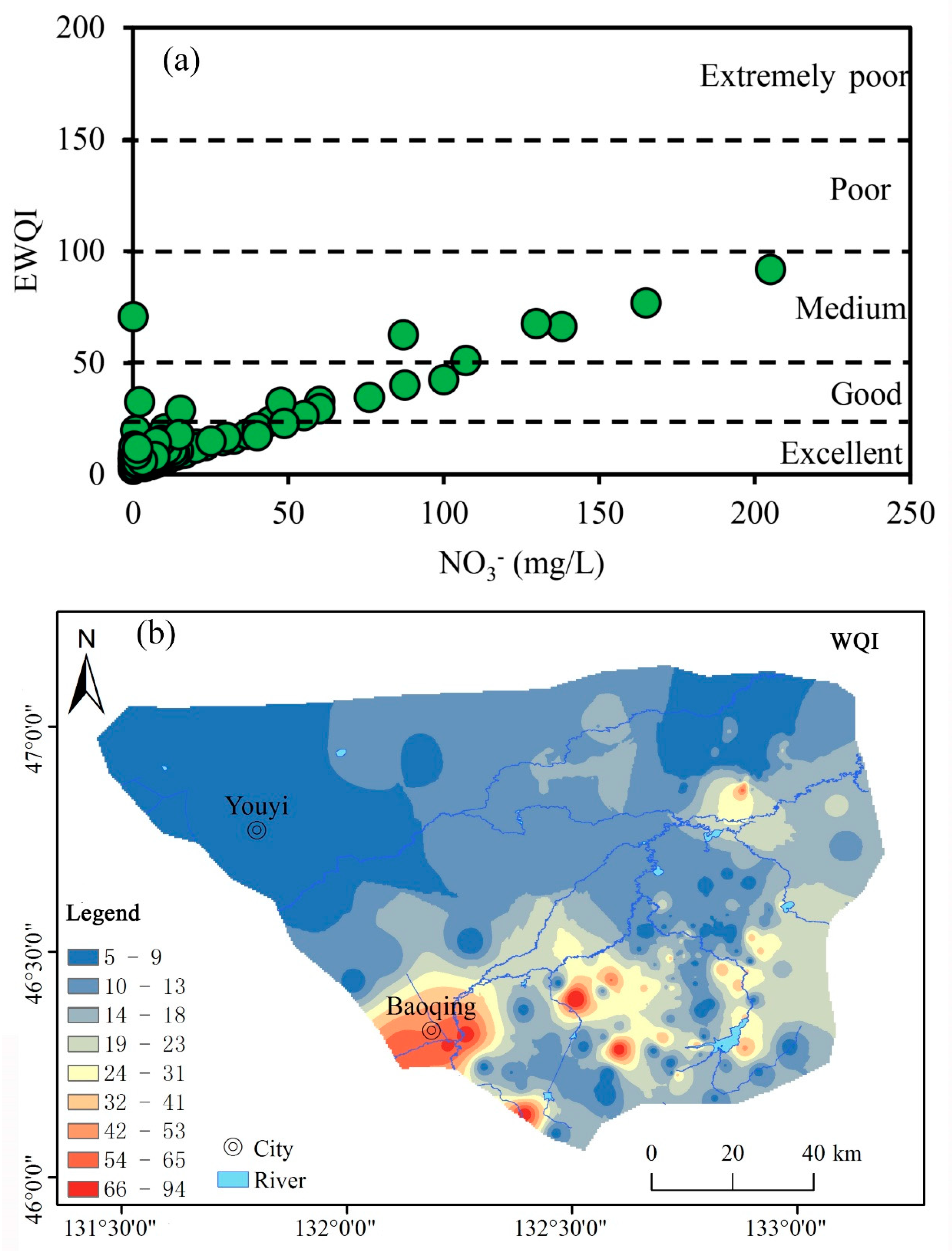
3.3. Groundwater Quality
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Döll, P.; Schmied, H.M.; Schuh, C.; Portmann, F.T.; Eicker, A. Global-scale assessment of groundwater depletion and related groundwater abstractions: Combining hydrological modeling with information from well observations and GRACE satellites. Water Resour. Res. 2014, 50, 5698–5720. [Google Scholar] [CrossRef]
- Islam, N.; Irshad, K. Artificial ecosystem optimization with Deep Learning Enabled Water Quality Prediction and Classification model. Chemosphere 2022, 309, 136615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cai, W.; Li, Y.; Geng, T.; Zhang, Z.; Lv, Y.; Zhao, M.; Liu, J. Ion chemistry of groundwater and the possible controls within Lhasa River Basin, SW Tibetan Plateau. Arab. J. Geosci. 2018, 11, 510. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, S.Q.; Li, H.F.; Fu, K.; Xu, Y. Groundwater pollution source identification and apportionment using PMF and PCA-APCA-MLR receptor models in a typical mixed land-use area in Southwestern China. Sci. Total Environ. 2020, 741, 140383. [Google Scholar] [CrossRef] [PubMed]
- Changmai, M.; Pasawan, M.; Purkait, M.K. A hybrid method for the removal of fluoride from drinking water: Parametric study and cost estimation. Sep. Purif. Technol. 2018, 206, 140–148. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, H.; Yang, Q. Hydrochemical interpretation of groundwater in Yinchuan basin using self-organizing maps and hierarchical clustering. Chemosphere 2022, 309, 136787. [Google Scholar] [CrossRef]
- Huang, G.; Song, J.; Han, D.; Liu, R.; Liu, C.; Hou, Q. Assessing natural background levels of geogenic contaminants in groundwater of an urbanized delta through removal of groundwaters impacted by anthropogenic inputs: New insights into driving factors. Sci. Total Environ. 2023, 857, 159527. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Huang, G.; Yang, M. Origins of groundwater nitrate in a typical alluvial-pluvial plain of North China plain: New insights from groundwater age-dating and isotopic fingerprinting. Environ. Pollut. 2023, 316, 120592. [Google Scholar] [CrossRef]
- Du, Y.; Deng, Y.; Ma, T.; Lu, Z.; Shen, S.; Gan, Y.; Wang, Y. Hydrogeochemical evidences for targeting sources of safe groundwater supply in arsenic-affected multi-level aquifer systems. Sci. Total Environ. 2018, 645, 1159–1171. [Google Scholar] [CrossRef]
- Smith, R.G.; Majumdar, S. Groundwater storage loss associated with land subsidence in western United States mapped using machine learning. Water Resour. Res. 2020, 56, e2019WR026621. [Google Scholar] [CrossRef]
- Harkness, J.S.; Jurgens, B.C. Effects of imported recharge on fluoride trends in groundwater used for public supply in California. Sci. Total Environ. 2022, 830, 154782. [Google Scholar] [CrossRef]
- Gill, B.; Webb, J.; Stott, K.; Cheng, X.; Wilkinson, R.; Cossens, B. Economic, social and resource management factors influencing groundwater trade: Evidence from Victoria, Australia. J. Hydrol. 2017, 550, 253–267. [Google Scholar] [CrossRef]
- Chen, R.H.; Tenga, Y.G.; Chen, H.Y.; Hu, B.; Yue, W.F. Groundwater pollution and risk assessment based on source apportionment in a typical cold agricultural region in Northeastern China. Sci. Total Environ. 2019, 696, 133972. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Ma, H.; Wang, L.; Martín, J.D. Identification of the hydrogeochemical processes and assessment of groundwater quality using classic integrated geochemical methods in the Southeastern part of Ordos basin, China. Environ. Pollut. 2016, 218, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Emenike, P.C.; Tenebe, I.T.; Neris, J.B.; Omole, D.O.; Afolayan, O.; Okeke, C.U.; Emenike, I.K. An integrated assessment of land-use change impact, seasonal variation of pollution indices and human health risk of selected toxic elements in sediments of River Atuwara, Nigeria. Environ. Pollut. 2020, 265, 114795. [Google Scholar] [CrossRef]
- Goswami, R.; Neog, N.; Bhagat, C.; Hdeib, R.; Mahlknecht, J.; Kumar, M. Arsenic in the groundwater of the Upper Brahmaputra floodplain: Variability, health risks and potential impacts. Chemosphere 2022, 306, 135621. [Google Scholar] [CrossRef]
- Devaraj, N.; Panda, B.; Chidambaram, S.; Prasanna, M.V.; Singh, D.K.; Ramanathan, A.L.R.; Sahoo, S.K. Spatio-temporal variations of uranium in groundwater: Implication to the environment and human health. Sci. Total Environ. 2021, 775, 145787. [Google Scholar]
- Li, X.; Huang, X.; Zhang, Y. Spatio-temporal analysis of groundwater chemistry, quality and potential human health risks in the Pinggu basin of North China Plain: Evidence from high-resolution monitoring dataset of 2015–2017. Sci. Total Environ. 2021, 800, 149568. [Google Scholar] [CrossRef]
- Elumalai, V.; Rajmohan, N.; Sithole, B.; Li, P.; Uthandi, S.; Tol, J. Geochemical evolution and the processes controlling groundwater chemistry using ionic ratios, geochemical modelling and chemometric analysis in uMhlathuze catchment, KwaZulu-Natal, South Africa. Chemosphere 2022, 312, 137179. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Y.; Qian, X.; Wang, Y. Hydrochemical Characteristics and Human Health Risk Assessment of Surface Water in the Danjiang River Source Basin of the Middle Route of China’s South-to-North Water Transfer Project. Water 2023, 15, 2203. [Google Scholar] [CrossRef]
- Wu, J.J.; Bian, J.M.; Wan, H.L.; Ma, Y.X.; Sun, X.Q. Health risk assessment of groundwater nitrogen pollution in Songnen Plain. Ecotoxicol. Environ. Saf. 2021, 207, 111245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, X.G.; Fang, X.; Zhang, N.; Xu, X.Q.; Li, L.H.; Liu, Y.; Su, X.; Xia, Y. Characterization of drinking groundwater quality in rural areas of Inner Mongolia and assessment of human health risks. Ecotoxicol. Environ. Saf. 2022, 234, 113360. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Bian, J.M.; Wang, Y. Impacts of urban land use on the spatial distribution of groundwater pollution, Harbin City, Northeast China. J. Contam. Hydrol. 2018, 215, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, W.; Li, F.; Zhang, F.; Liu, G.C. Hydrochemistry of waters in snowpacks, lakes and streams of Mt. Dagu, eastern of Tibet Plateau. Sci. Total Environ. 2018, 610–611, 641–650. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, D.; Yin, J.; Zhou, X.; Li, Y.; Chi, P.; Han, Y.; Ansari, U.; Cheng, Y. Sediment Instability Caused by Gas Production from Hydrate-bearing Sediment in Northern South China Sea by Horizontal Wellbore: Evolution and Mechanism. Nat. Resour. Res. 2023, 32, 1595–1620. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Jiang, B.; Zhuo, H.; Chen, W.; Chen, Y.; Li, X. Low-loading Pt nanoparticles combined with the atomically dispersed FeN4 sites supported by FeSA-N-C for improved activity and stability towards oxygen reduction reaction/hydrogen evolution reaction in acid and alkaline media. J. Colloid Interface Sci. 2023, 635, 514–523. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Yang, Y.; Ansari, U.; Han, Y.; Li, X.; Cheng, Y. Preliminary experimental investigation on long-term fracture conductivity for evaluating the feasibility and efficiency of fracturing operation in offshore hydrate-bearing sediments. Ocean Eng. 2023, 281, 114949. [Google Scholar] [CrossRef]
- Nixdorf, E.; Sun, Y.Y.; Lin, M.; Kolditz, O. Development and application of a novel method for regional assessment of groundwater contamination risk in the Songhua River Basin. Sci. Total Environ. 2017, 605–606, 598–609. [Google Scholar] [CrossRef]
- Zhang, B.; Song, X.F.; Zhang, Y.H.; Han, D.M.; Tang, C.Y.; Yang, L.H.; Wang, Z.L. The renewability and quality of shallow groundwater in Sanjiang and Songnen Plain, Northeast China. J. Integr. Agric. 2017, 16, 229–238. [Google Scholar] [CrossRef]
- Liu, Y.L.; Jin, M.G.; Wang, J.J. Insights into groundwater salinization from hydrogeochemical and isotopic evidence in an arid inland basin. Hydrol. Process. 2018, 32, 3108–3127. [Google Scholar] [CrossRef]
- GB/T 5750; Standards for Drinking Water Quality. Ministry of Health of the People’s Republic of China: Beijing, China, 2006.
- DZ/T 0064; MAGQ, Methods for Analysis of Groundwater Quality. Ministry of Natural Resources of the People’s Republic of China: Beijing, China, 2021.
- Paca, J.M.; Santos, F.M.; Pires, J.C.M.; Leitão, A.A.; Boaventura, R.A.R. Quality assessment of water intended for human consumption from Kwanza, Dande and Bengo rivers (Angola). Environ. Pollut. 2019, 254, 113037. [Google Scholar] [CrossRef] [PubMed]
- Adimalla, N. Application of the entropy weighted water quality index (EWQI) and the pollution index of groundwater (PIG) to assess groundwater quality for drinking purposes: A case study in a rural area of Telangana State, India. Arch. Environ. Contam. Toxicol. 2021, 80, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Gao, Z.J.; Zhang, Y.Q.; Sun, Z.B.; Sun, T.Z.; Fan, H.B.; Wu, B.; Li, M.B.; Qian, L.L. Hydrochemical evaluation of groundwater quality and human health risk assessment of nitrate in the largest peninsula of China based on high-density sampling: A case study of Weifang. J. Clean. Prod. 2021, 322, 129164. [Google Scholar] [CrossRef]
- GB/T 14848-2017; Standards for Groundwater Quality. National Standard of People’s Republic of China: Beijing, China, 2017.
- Chai, N.; Yi, X.; Xiao, J.; Liu, T.; Liu, Y.; Deng, L.; Jin, Z. Spatiotemporal variations, sources, water quality and health risk assessment of trace elements in the Fen River. Sci. Total Environ. 2021, 757, 143882. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, Y.; Wang, Y.; Huang, X.; Xiao, Y.; Pei, Q. Hydrochemistry, quality and potential health risk appraisal of nitrate enriched groundwater in the Nanchong area, southwestern China. Sci. Total Environ. 2021, 784, 147186. [Google Scholar] [CrossRef]
- Ke, X.; Li, Y.; Wang, W.; Niu, F.; Gao, Z. Hydrogeochemical characteristics and processes of thermokarst lake and groundwater during the melting of the active layer in a permafrost region of the Qinghai-Tibet Plateau, China. Sci. Total Environ. 2022, 851, 158183. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P. Appraisal of shallow groundwater quality with human health risk assessment in different seasons in rural areas of the Guanzhong Plain (China). Environ. Res. 2022, 207, 112210. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, G.R.; Chai, N.P.; Hu, J.; Jin, Z.D. Hydrochemistry and source apportionment of boron, sulfate, and nitrate in the Fen River, a typical loess covered area in the eastern Chinese Loess Plateau. Environ. Res. 2022, 206, 112570. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Qian, H.; Zhou, Y.H.; Chen, J.; Wang, H.K.; Ren, W.H.; Qu, W.G. Cumulative health risk assessment of multiple chemicals in groundwater based on deterministic and Monte Carlo models in a large semiarid basin. J. Clean. Prod. 2022, 352, 131567. [Google Scholar] [CrossRef]
- Sunkari, E.D.; Seidu, J.; Ewusi, A. Hydrogeochemical evolution and assessment of groundwater quality in the Togo and Dahomeyan aquifers, Greater Accra Region, Ghana. Environ. Res. 2022, 208, 112679. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, M.; Yang, L.; Yang, T.; Li, J.; Chen, Y. Distribution Characteristics and Genesis of Iron and Mangnese Ions in Groundwater of Eastern Sanjiang Plain, China. Water 2023, 15, 2068. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Shen, B.B.; Wu, J.L.; Zhan, S.E.; Jin, M.; Saparov, A.S.; Abuduwaili, J. Spatial variations and controls on the hydrochemistry of surface waters across the Ili-Balkhash Basin, arid Central Asia. J. Hydrol. 2021, 600, 126565. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, J.; Hu, B.X.; Liu, H.; Zhang, H.; Ma, Z. Hydrogeochemical characterization and groundwater quality assessment in intruded coastal brine aquifers (Laizhou Bay, China). Environ. Sci. Pollut. Res. 2017, 24, 21073–21090. [Google Scholar] [CrossRef] [PubMed]
- Argamasilla, M.; Barbera, J.A.; Andreo, B. Factors controlling groundwater salinization and hydrogeochemical processes in coastal aquifers from southern Spain. Sci. Total Environ. 2017, 580, 50–68. [Google Scholar] [CrossRef]
- Ha, Q.K.; Ngoc, T.D.T.; Vo, P.L.; Nguyen, H.Q.; Dang, D.H. Groundwater in Southern Vietnam: Understanding geochemical processes to better preserve the critical water resource. Sci. Total Environ. 2022, 807, 151345. [Google Scholar] [CrossRef]
- Borrok, D.M.; Lenz, R.M.; Jennings, J.E.; Gentry, M.L.; Steensma, J.; Vinson, D.S. Applied Geochemistry the origins of high concentrations of iron, sodium, bicarbonate, and arsenic in the Lower Mississippi River Alluvial Aquifer. Appl. Geochem. 2018, 98, 383–392. [Google Scholar] [CrossRef]
- Wang, H.L.; Hussaini, K.M.; Zhang, H.; Rinklebe, J. Hydrogeochemical and health risk evaluation of arsenic in shallow and deep aquifers along the different floodplains of Punjab, Pakistan. J. Hazard. Mater. 2021, 402, 124074. [Google Scholar]
- Brindha, K.; Paul, R.; Walter, J.; Tan, M.L.; Singh, M.K. Trace metals contamination in groundwater and implications on human health: Comprehensive assessment using hydrogeochemical and geostatistical methods. Environ. Geochem. Health 2020, 42, 3819–3839. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Su, Q.; Wang, S.; Gao, Z.J.; Liu, J.T. Spatial distribution and health risk assessment of dissolved heavy metals in groundwater of eastern China coastal zone. Environ. Pollut. 2021, 290, 118016. [Google Scholar] [CrossRef] [PubMed]
- Adimalla, N.; Qian, H. Groundwater chemistry, distribution and potential health risk appraisal of nitrate enriched groundwater: A case study from the semi-urban region of South India. Ecotoxicol. Environ. Saf. 2021, 207, 111277. [Google Scholar] [CrossRef] [PubMed]


| Chemical Parameter | Mean | Standard Deviation | Min | Max | NSPRC (NSPRC, 2017) | Coefficient Variation |
|---|---|---|---|---|---|---|
| K+/mg·L−1 | 3.01 | 4.83 | 0.08 | 61.17 | — | 1.61 |
| Na+/mg·L−1 | 23.23 | 12.53 | 2.75 | 65.22 | 200.00 | 0.54 |
| Ca2+/mg·L−1 | 45.76 | 25.59 | 0.34 | 142.28 | — | 0.56 |
| Mg2+/mg·L−1 | 16.13 | 10.74 | 2.52 | 67.47 | — | 0.67 |
| Cl−/mg·L−1 | 12.45 | 15.31 | 1.77 | 109.02 | 250.00 | 1.23 |
| SO42−/mg·L−1 | 9.31 | 32.04 | 0.00 | 450.00 | 250.00 | 0.54 |
| HCO3−/mg·L−1 | 255.28 | 138.65 | 0.00 | 682.63 | — | 3.16 |
| NO3−/mg·L−1 | 10.21 | 24.57 | 0.00 | 205.00 | 88.60 | 2.41 |
| TDS/mg·L−1 | 285.94 | 118.59 | 85.24 | 839.64 | 1000.00 | 0.41 |
| TH/mg·L−1 | 178.33 | 93.62 | 20.80 | 474.95 | 450.00 | 0.52 |
| pH | 7.15 | 0.42 | 5.46 | 9.04 | 6.5–8.5 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wang, P.; He, J.; Liu, D.; Wang, M.; Wang, M.; Xia, S. Hydrochemical Characteristics, Water Quality, and Evolution of Groundwater in Northeast China. Water 2023, 15, 2669. https://doi.org/10.3390/w15142669
Zhang T, Wang P, He J, Liu D, Wang M, Wang M, Xia S. Hydrochemical Characteristics, Water Quality, and Evolution of Groundwater in Northeast China. Water. 2023; 15(14):2669. https://doi.org/10.3390/w15142669
Chicago/Turabian StyleZhang, Tao, Pei Wang, Jin He, Dandan Liu, Min Wang, Mingguo Wang, and Shibin Xia. 2023. "Hydrochemical Characteristics, Water Quality, and Evolution of Groundwater in Northeast China" Water 15, no. 14: 2669. https://doi.org/10.3390/w15142669
APA StyleZhang, T., Wang, P., He, J., Liu, D., Wang, M., Wang, M., & Xia, S. (2023). Hydrochemical Characteristics, Water Quality, and Evolution of Groundwater in Northeast China. Water, 15(14), 2669. https://doi.org/10.3390/w15142669







