Abstract
Groundwater resources are an essential component of global water resources. Long-term consumption of groundwater exceeding the standard levels for iron and manganese can lead to chronic diseases, posing a threat to human health. The Sanjiang Plain is the largest swamp wetland area in China and has high levels of iron and manganese in the groundwater, but the cause still needs to be clarified. Based on the results of water quality tests of 41 groundwater samples in the Eastern Sanjiang Plain, this paper analyzes the distribution characteristics and causes of iron and manganese from the perspectives of the original strata environment, redox conditions, pH conditions, and hydrochemical indicator factors in the research area, using statistical methods and GIS technology. The results show that high iron and manganese content in groundwater is prevalent in the Eastern Sanjiang Plain, and the exceedance rate of manganese is higher than that of iron (87.80% and 82.90%, respectively). The primary sources of iron and manganese in groundwater are iron and manganese minerals in the original strata environment. Influenced by factors such as acidic conditions, reducing environment, and rich organic matter, insoluble high-valent iron and manganese oxides are reduced to low-valent and soluble divalent iron and manganese. At the same time, groundwater’s high mineralization and evaporation concentration are conducive to increased iron and manganese content, while the influence of human activities is small.
1. Introduction
Iron and manganese are elements known for their variable valence and are richly present in the Earth’s crust. In groundwater, the soluble divalent ions of these elements are widespread [1,2,3,4,5]. While iron and manganese are essential trace elements for human health, long-term exposure to high concentrations of these elements in groundwater has been linked to chronic poisoning, which poses a severe threat to human health. This can lead to several diseases affecting the digestive, nervous, and skeletal systems, as well as memory loss and inattentiveness [6,7,8,9,10]. At the same time, high iron and manganese content in water can affect crop yield, animal growth, and development [11,12]. To mitigate these risks, the Chinese Drinking Water Hygiene Standard (GB5749–2022) has set maximum permissible limits of 0.3 mg/L for iron and 0.1 mg/L for manganese [13]. The European Drinking Water Directive (2020/2184/UE) sets the upper limits of iron and manganese ions in drinking water at 0.2 mg/L and 0.05 mg/L, respectively [14]. The World Health Organization (WHO) recommends a critical value of manganese in water of 0.4 mg/L [15]. However, high-manganese groundwater remains a persistent problem worldwide, including in New Zealand, Sweden, Bangladesh, Nigeria, India, and the United States [11,16,17,18]. Moreover, in several regions of China, such as northeastern Heilongjiang Province, southern Hebei, northern Ningxia, Shaanxi, Shanxi, Qinghai, Inner Mongolia, and the Pearl River Delta, the concentration of manganese in groundwater significantly exceeds the permissible level [19,20,21,22,23,24]. This is a cause for concern, as nearly 20% of China’s groundwater resources are impacted by this issue, mainly in densely populated alluvial plains and inland basins. Notably, the primary reason for the high iron and manganese content in groundwater is primarily a natural outcome of the geographical and geological environment [25]. Given the potential adverse consequences of high iron and manganese levels in groundwater, the “water-quality type water shortage” phenomenon has become increasingly prevalent in some regions [26].
The Sanjiang Plain, located in northeastern Heilongjiang Province, China, has abundant water resources, with groundwater resources accounting for a significant portion, i.e., 27.4% of a total volume of 18.764 billion cubic meters. High iron and manganese levels in groundwater in the Sanjiang Plain have been reported for a long time. Jiamusi, the core city of the Sanjiang Plain, relies entirely on groundwater for its drinking water needs. Investigations by Wang Lan [27] in 2006 revealed that 100% of the iron and manganese content of the four groundwater drinking water sources in Jiamusi City exceeded the permissible limits. Similarly, Ma Hongwei [28] found that 77.78% of shallow groundwater samples collected from the eastern and northern regions of the Sanjiang Plain exceeded the permissible limit for iron, while 66.67% exceeded the permissible limit for manganese. However, there are few detailed studies on the influencing factors and genetic mechanism of Fe and Mn content in the groundwater of the Sanjiang Plain.
Trace elements in groundwater are mainly affected and restricted by factors such as aquifer media, groundwater flow field, REDOX conditions, pH, microorganisms, and anthropogenic activities [16,29,30,31,32]. High-Fe/Mn groundwater occurrence can primarily be attributed to natural factors, such as the hydrological environment, aquifer media, and groundwater recharge and discharge conditions. Owing to leaching, manganese minerals in soil or aquifer media enter groundwater [18,33,34]. Under low pH and Eh conditions, soluble forms of Fe2+ and Mn2+ prevail, leading to their presence in groundwater [35].
Statistical analysis, ion ratio, spatial analysis technology, correlation analysis, and principal component analysis are mainly used to analyze the distribution characteristics and causes of iron and manganese ions in groundwater. Wei et al. [21] employed statistical and spatial analysis techniques to investigate the formation of groundwater containing high levels of Fe and Mn in the Songnen Plain, northeast China. The study revealed that the properties of soils and aquifers were the contributing factors to the occurrence of Fe and Mn in the groundwater, with the use of fertilizer in Changchun resulting in an increased nitrate concentration and decreased Fe and Mn content. Rizwan et al. [36] evaluated groundwater quality in the Gadilam River basin using Geographical Information Technology (GIS), risk assessment, and statistical analysis, finding that higher Mn concentrations in tertiary formation were possibly caused by mining and related activities. Bacquart et al. [37] conducted spatial and correlation analyses and health effect evaluations of heavy metals, including Fe and Mn, in groundwater samples from Central Burma’s Myingyan Township, which showed that there were multiple toxic inorganic contaminants present in well water, exceeding levels of concern for public health, which might have originated from natural geological deposits in the region. Solano S et al. [38] utilized hierarchical clustering analysis to study chemical concentrations and profile the groundwater quality of the Central Pacific, Costa Rica, which linked significant levels of Fe and Mn to the dissolution of hydrothermally altered volcanic rocks. In Luo et al. [39]’s research, pollution factors, comprehensive water quality index, PCA, and other methods were used to assess heavy metal elements in groundwater and pollutant sources.
The Sanjiang Plain is an essential ecological protection barrier and grain production area in northeast China. Still, the reason for high-iron and high-manganese groundwater formation in this area remains unclear. Therefore, this study took Shengli Farm and its surrounding areas in the east of the Sanjiang Plain as the research area, and 41 groups of groundwater samples were collected. Based on multivariate statistics and GIS spatial analysis techniques, the ion ratio, correlation analysis, and principal component analysis were adopted to achieve the following research objectives: (1) to determine the iron and manganese content level and the pollution status of groundwater in the study area; (2) to elucidate the primary factors and mechanisms affecting the migration and transformation of iron and manganese in groundwater in the study area; and (3) to reveal the source of Fe and Mn substances in groundwater with high Fe and Mn levels. The research results can provide theoretical support for protecting groundwater resources and preventing pollution.
2. Study Area
2.1. Overview
The Sanjiang Plain, situated in northeastern China, resulted from the accumulation of alluvial deposits from the Heilongjiang, Wusuli, and Songhua Rivers. As China’s largest marsh wetland region, it covers an extensive area of 10.89 × 104 square kilometers. This area is well known for its rich soil, which produces 15 million tons of grain annually, making it a critical commodity grain base in China. The study area is primarily located in the eastern section of the Sanjiang Plain, with Shengli Farm positioned as the center. The research area was appropriately extended beyond the jurisdictional boundaries of Shengli Farm. Encompassing roughly 1000 square km and with a population of 20,000, the area is characterized as a classic agricultural site.
The study area is in a temperate humid and semi-humid continental monsoon climate. The area is characterized by short sunlight duration, severe cold temperatures, and dryness during the winter. Spring sees a rapid increase in temperature along with strong winds, rendering the area vulnerable to droughts and floods. The summer season is short and warm and tends to experience abundant rainfall, while high precipitation levels typify the autumn season. The mean annual precipitation in the study area falls between 500 and 550 mm, with 50–70% of total rainfall observed between May and September. Notably, precipitation between March and May constitutes merely 12.5% of the total annual rainfall. Water surface evaporation ranges from 580 to 650 mm, with the highest rates recorded between May and September and the lowest in December and January. The mean annual temperature in the area is 1.3 °C, with the coldest month seeing an average temperature of −16.2 °C, while the warmest month has an average temperature of 20.2 °C. The region experiences around 190 freezing days annually, with the frozen layer thickness ranging from 1.4 to 2.0 m [40].
The study area features an east-to-west sloping topography, with the western section of the region characterized by alluvial plains and terraces formed by rivers (Figure 1). The presence of extensive swamps and marshy meadows is also prevalent in the region. Conversely, the eastern part of the region is composed of residual hills. The Naoli River flows through the southeastern sector of the area, while the Bielahong River courses through its northwestern part. The central-eastern part of the region is typified by residual hills such as Shuangshan Mt., Xinan Mt., Fuyang Mt., Laochi Mt., Xiaogu Mt., Kaerka Mt., and other hills. The study area predominantly consists of outcrops of the Upper Triassic Dajiahe Formation siliceous rocks; Upper Triassic-Lower Jurassic Dalingqiao Formation sandstones, shales, and siliceous rocks; Upper Neogene basalt; and locally, Lower Cretaceous Dongshan Formation andesite and tuff. In the surrounding area, the sand layer of the unconsolidated Quaternary aquifer is distributed below the 0–18 m thick clay layer, which mainly comprises silty clay and viscous soil [41].
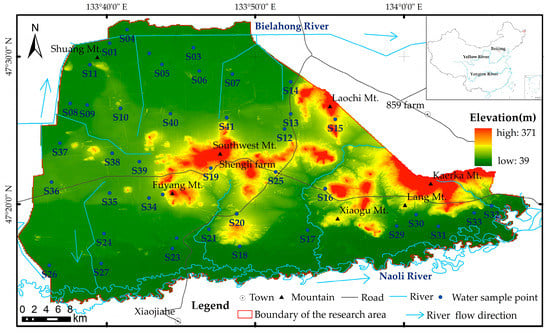
Figure 1.
Topographic map of the research area and sample points’ distribution.
2.2. Groundwater
The regional groundwater flows roughly from south to north, and the flow field in the research area is consistent with the trend of regional flow direction (Figure 2). The primary aquifer is the quaternary unconsolidated porous aquifer, with a typical groundwater depth ranging from 2.1 to 27.8 m. In the central and eastern residual mountainous areas, the aquifer is a fractured aquifer, which is typically low in water yield. From east to west in the study area, the water yield of the aquifer increases, and the groundwater depth becomes shallower. Quaternary groundwater is the primary source of drinking and irrigation water. Based on the single well water usage Q, the distribution of unconsolidated aquifer in the area can be divided into four types: extremely abundant (Q > 5000 m3/d), abundant (5000 m3/d > Q > 1000 m3/d), moderate (1000 m3/d > Q > 100 m3/d), and scarce (Q < 100 m3/d). The fractured rock porous aquifer in the study area has poor water yield (Figure 3).
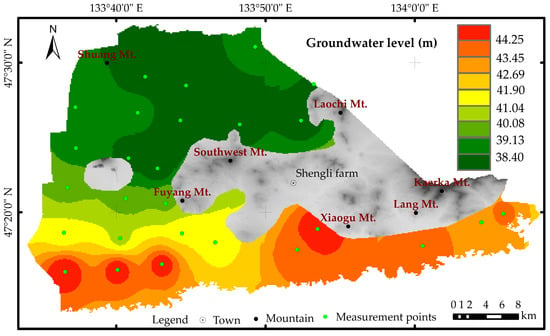
Figure 2.
Diagram of the water table level in September in the study area.
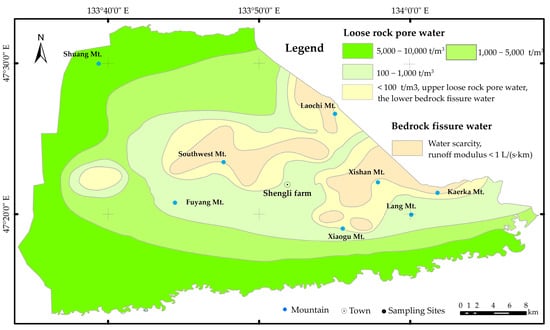
Figure 3.
Hydrogeology setting of the study area.
Atmospheric precipitation infiltration is the most important source of recharge for unconsolidated rock porous water in the study area, and irrigation infiltration is also an essential component of groundwater recharge. Artificial extraction is the main means of groundwater discharge, while a small amount of evaporation discharge also exists. In addition, the northwest and south of the study area can receive surface water recharge from rivers during the flood season, while groundwater discharges into the rivers during the dry season.
3. Materials and Methods
3.1. Sampling and Analyzing
In this study, 41 groundwater samples were collected in September 2022, as illustrated in Figure 1. The sampling depths ranged from 2.1 to 27.8 m, and the samples were primarily obtained from the loose porous water in the Quaternary system. Before collection, the wells were purged for at least 10 min, and the pH and Eh values were measured on-site using a portable multi-parameter water quality analyzer (SmarTROLL MP, In-Situ, Fort Collins, CO, USA). The cation analysis samples were acidified with 1:1 nitric acid to a pH value of less than 2 and stored accordingly. The collection, storage, and transportation of groundwater samples were conducted in strict accordance with the Technical Specifications for Groundwater Environmental Monitoring (HJ164–2020) [42] and tested following the Hygienic Standard for Drinking Water (GB5750–2006) [43].
The samples were filtered using 0.45 μm acetate fiber membranes on site and sealed in 500 mL high-density Teflon plastic sample bottles. Samples for cation analysis were acidified with hydrochloric acid to pH less than 2, and all samples were stored under 4 °C sealed and protected from light. The concentrations of K+, Na+, Mg2+, Ca2+, and Fe2+ were measured using inductively coupled plasma optical emission spectrometry (ICP-OES) (Vista MPX, Varian Instruments, Walnut Creek, CA, USA). When conducting ICP-OES analysis, 1000 PPM Cs must be added to correct for ionization of Na and K in the plasma. The ion chromatography (IC) method was used to measure Cl−, NO3−, and SO42− (DionexTM ICS-1100, Thermo Fisher Scientific Inc., Waltham, MA, USA). The concentrations of HCO3− and CO32− were determined by hydrochloric acid titration, and the total dissolved solids (TDS) were determined using the gravimetric method. All measurements were conducted at the Laboratory of the Center for Hydrogeology and Environmental Geology Survey.
Table 1 shows the precision and accuracy metrics for each chemical parameter. The precision was assessed using the relative standard deviation (RSD) and the relative deviation (RD), and all water samples had NICB values below 5%.

Table 1.
Precision and accuracy metrics of each chemical parameter.
3.2. Data Analysis
All statistical analyses and plots were made using SPSS v 17.0 and ArcGIS V 9.2. A statistical program, SPSS v 17.0, evaluated the relationships between various hydrochemical parameters and principal component analysis. ArcGIS V 9.2 was used to determine the spatial distributions of the pH, Eh, Fe, and Mn values. Ion ratio plots were finished using Microsoft® Excel.
4. Results and Discussion
4.1. Characteristics of Iron and Manganese Contents
The main hydrochemical parameters of the groundwater samples are presented in Table 2. The groundwater was primarily composed of Ca·Mg-HCO3, Ca·Mg-SO4·HCO3, Ca·Mg-HCO3·SO4, and Ca-HCO3, with some samples containing Mg·Ca-HCO3, Ca·Mg-NO3·Cl, Ca·Mg-SO4, Ca·Mg-HCO3·Cl, and Ca-HCO3·SO4. According to the Groundwater Quality Standard (GB/T14848-2017), the detection rate of Fe in the groundwater samples was 95.12%, with a concentration range of ND (not detected) to 31.55 mg/L, an average concentration of 3.78 mg/L, and a non-compliance rate of 87.80%. The detection rate of Mn was 92.68%, with a concentration range of ND–1.41 mg/L, an average concentration of 0.40 mg/L, and a non-compliance rate of 82.90%. The Drinking Water Standard for Residents (GB5749-2006) sets the limits for Fe and Mn concentrations in drinking water at 0.3 mg/L and 0.1 mg/L, respectively. Similar to the Groundwater Quality Standard (GB/T14848-2017), groundwater exceeding these limits is classified as high-Fe and high-Mn groundwater. In the study area, there were 36 and 34 groups of high-Fe and high-Mn groundwater, respectively, with non-compliance rates exceeding 80%, indicating a significant impact on residents’ drinking water safety.

Table 2.
Statistical table for hydrochemical parameters (in mg/L).
To investigate the spatial distribution characteristics of Fe and Mn concentrations in the groundwater, isopleth maps were generated using ArcGIS V9.2 software (Figure 4). The results showed that the overall background concentrations of Fe and Mn were high, with high-background areas distributed in Daohuzhuang, Shuang Mt., Laochi Mt., and west of Fuyang Mt. High-background areas of Mn were also observed in the southeast of Kaerka Mt. in the east. Low-value points were sporadically distributed in the central and western parts of the study area. Combined with Figure 2, along the direction of groundwater flow, both Fe and Mn concentrations showed a significant increasing trend, indicating a similar distribution pattern. Fe and Mn concentrations, with a broader distribution range, exceeded the standard limits.
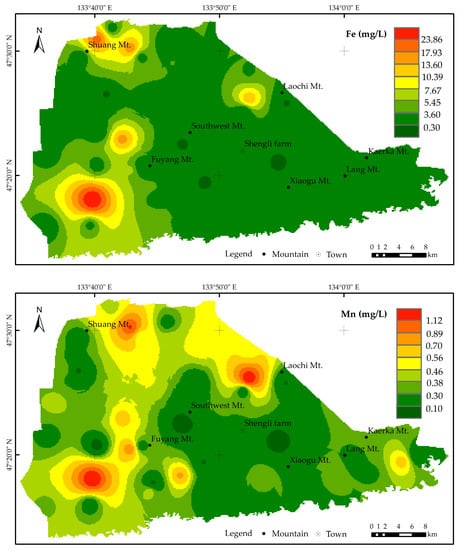
Figure 4.
Spatial variation in iron and manganese content.
A particular relationship exists between the changes in the concentration of each ion in the same groundwater system. Correlation analysis can be used to investigate the source of ions in hydrochemistry research. The correlation between components from the same source is strong. Otherwise, the correlation could be poor [33,34].
The results of the correlation analysis are presented in Figure 5. There is a positive correlation between Fe2+, Mn2+, and SO42−, with the correlations between Fe2+ vs. Mn2+, Fe2+ vs. SO42−, and Mn2+ vs. SO42− being 0.771, 0.600, and 0.642, respectively, indicating a common source. There is a significant negative correlation between Fe2+, Mn2+, and pH, meaning that a decrease in pH favors an increase in the content of Fe2+ and Mn2+ ions in groundwater. Usually, Cl− and NO3− can reflect the impact of human activities [33], but Fe2+ and Mn2+ have no significant correlation with them, indirectly indicating that the content of Fe2+ and Mn2+ is less affected by human activities.
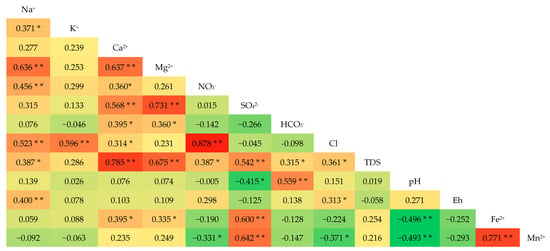
Figure 5.
Person correlation coefficients of groundwater hydrochemical parameters in the Eastern Sanjiang Plain. (** Significant at the 0.01 level. * Significant at the 0.05 level).
4.2. Cause Analysis of High-Iron and High-Manganese Groundwater in Northeastern Sanjiang Plain
4.2.1. Primary Stratigraphic Environment
High-Fe and high-Mn groundwater, as types of geogenic poor-quality water, result from long-term interactions between the groundwater and surrounding geological formations, which are influenced by various factors, such as aquifer media and soil chemical composition [19,44]. The Gibbs diagram can qualitatively determine the hydrochemical evolution mechanism controlled by atmospheric precipitation, rock filtration, and evaporation concentration [45]. The Gibbs diagram of groundwater in the study area (Figure 6) showed that the groundwater samples were mainly within the TDS concentration range of 10–1000 mg/L, with Na+/(Na++Ca2+) ratios mainly distributed between 0.1 and 0.7, and Cl−/(Cl− + HCO3−) ratios mainly distributed between 0.0 and 0.5, falling between “rock filtration control” and “evaporation concentration control”, indicating that the hydrochemical evolution of groundwater was largely controlled by rock filtration and evaporation concentration, with some groundwater samples falling outside the Gibbs model, possibly due to human activities or cation exchange [46].
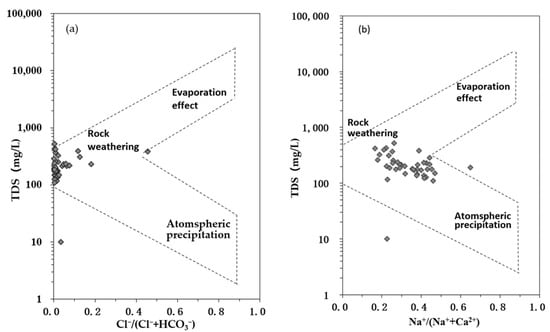
Figure 6.
Gibbs plots of groundwater samples in the Eastern Sanjiang plain.
The northern and western peripheries of the study area are rich in iron and manganese mineral resources, with the ore deposit type being shallow sea sedimentary metamorphic. The proven geological reserves of iron ore are 54 million tons, with an average iron content of about 30%, mainly composed of pyrite and magnetite. At the same time, manganese minerals mainly include manganese oxide and manganese carbonate [38]. These minerals are weathered and undergo water–rock interactions and are ultimately transported by rivers to the plains, where some of their components dissolve into the groundwater, becoming the main source of Fe and Mn in the groundwater.
At the same time, the Sanjiang Plain has a strong Late Pleistocene pedogenesis, with the development of iron-rich aluminous soils with high magnetic susceptibility and leached soils rich in iron and manganese nodules since the Quaternary, which could also provide abundant sources of Fe and Mn for the groundwater [47].
4.2.2. REDOX Condition
In a relatively closed groundwater environment, a reducing environment (low Eh value) is conducive to dissolving and releasing Fe and Mn oxides in sediments. The insoluble high-valence Fe and Mn oxides in the formation will be reduced to lower-valence and more soluble Fe2+ and Mn2+. In an oxidizing environment (high Eh value), Fe2+ and Mn2+ in groundwater will be oxidized and precipitated, decreasing Fe and Mn content in groundwater [48]. To analyze the relationship between Eh and the Fe and Mn content in groundwater, an Eh contour map of groundwater in the Kaerka Mt. area was drawn based on the sample’s Eh value (Figure 7). The Eh values in the study area are mostly negative, ranging from −78.30 to 64.31 mV, with an average value of −15.76 mV. The runoff of the Bielahong River and the Naoli River flows from west to east in the study area, which dilutes the upstream groundwater, lowers the Eh value, favors the formation of Fe2+ and Mn2+, and tilts towards a reducing environment. The points where iron and manganese exceed the standard in the groundwater of the plain area are mainly located in areas with developed water systems, such as alluvial plains and valleys, where the groundwater flow rate is slow (the groundwater environment is still in the transitional stage or reducing environment). In low-lying alluvial and alluvial areas, iron and manganese content in surface sediments is relatively high.
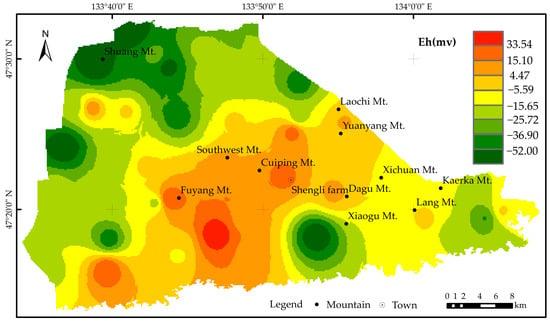
Figure 7.
Isogram of Eh in groundwater in the Eastern Sanjiang Plain.
4.2.3. Acid–Base Conditions of Groundwater
Under normal circumstances, Fe2+ and Mn2+ can relatively easily enter groundwater from formations, while it is relatively difficult for Fe3+ and Mn4+ (iron and manganese oxides) to enter groundwater from formations. However, under acidic conditions, Fe2+, Mn2+, Fe3+, and Mn4+ are more likely to enter groundwater [49]. The pH contour map of groundwater in the Eastern Sanjiang Plain (Figure 8) shows that the pH values of groundwater in the study area are between 5.94 and 7.24, and weakly acidic groundwater is mainly concentrated in the northeast and southwest of Shengli Farm. This is because the area is primarily marsh soil, and the soil (0–1.5 m) is also acidic. Under acidic conditions, Fe and Mn will undergo dissolution reactions, which are conducive to the dissolution and enrichment of Fe2+ and Mn2+. The groundwater in the southern part of the study area is close to neutral, and the lithology is mainly limestone. The Eh–pH diagram of groundwater at Shengli Farm (Figure 9) shows that Mn and Fe mainly exist as Fe2+ and Mn2+ in groundwater, which causes severe and widespread exceedance of Fe and Mn content. As groundwater is closely related to human activities, it is generally believed that the excessive content of Fe and Mn in the aquifer is closely associated with human activities.
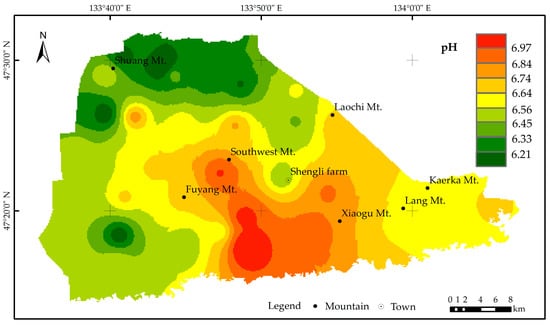
Figure 8.
Isogram of pH in groundwater in the Eastern Sanjiang Plain.
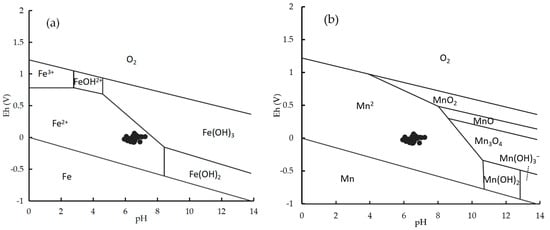
Figure 9.
Eh–pH diagram of Fe (a) and Mn (b) of groundwater in the Northeastern Sanjiang Plain.
The overlying layers in the plain area of the study area are mainly white loess, meadow soil, and marsh soil, which contain abundant organic matter [50]. This is conducive to enriching Fe and Mn in groundwater through chemical reactions. Li Xiao et al.’s research shows that the average NH4+ concentration in shallow groundwater in the northeast of the Sanjiang Plain is 0.73 mg/L, which is higher than the groundwater quality standard of 0.5 mg/L [13]. The high NH4+ concentration indicates that groundwater tends to be in a reducing environment, which is conducive to forming Fe2+ and Mn2+, resulting in iron and manganese exceedance.
4.3. Main Ion Source Analysis
The suitability of the hydrochemical parameters of groundwater in the Northeastern Sanjiang Plain for principal component analysis was tested using Bartlett’s test of sphericity and the KMO test [37,51]. The results of the Bartlett sphericity test (significance 0.00 < 0.05) and KMO measurement test (0.516 > 0.5) indicated that principal component analysis was suitable for each element. After the Kkaise normalized factors were subjected to orthogonal rotation by Varimax, four principal components with eigenvalues greater than 1 were obtained, and their contribution rates reached 74.03%, which could reflect the main information of all heavy metals.
Based on the principal component (PC) loadings, 13 parameters were grouped into three PCs (F1–F3, Table 3). The main loadings of F1 were Mg2+, Ca2+, and TDS, which were strongly correlated with SO42−, K+, and Fe2+ (with loadings of 0.784, 0.601, and 0.543, respectively). This indicated that high mineralization and evaporation concentrations were conducive to increased Fe2+ content in groundwater. The main loading of F2 was Cl−, which was strongly correlated with NO3−, Fe2+, Mn2+, pH, and Eh (with loadings of 0.692, −0.666, −0.775, 0.572, and 0.560, respectively). The positive correlation between F2 and Fe2+ and Mn2+, combined with Cl− (Figure 8), suggested that human activities had a relatively small impact on the excessive iron and manganese content in groundwater. The main loading of F3 was HCO3−, which was well correlated with pH (with a loading of 0.618).

Table 3.
Principal component analysis matrix.
5. Conclusions
This study analyzed 41 groundwater samples from the Eastern Sanjiang Plain to investigate their distribution characteristics and main controlling factors. The main conclusions are as follows:
- (1)
- The range of iron content in groundwater in the study area is ND–31.55 mg/L, with a mean value of 3.78 mg/L and an excessive rate of 87.80%; the range of manganese content is ND–1.41 mg/L, with a mean value of 0.38 mg/L and an excessive rate of 82.90%; the excessive rate of iron in groundwater is higher than that of manganese, and the distribution range is wider. To ensure the safety of drinking water for residents, it is recommended that the groundwater within the area be appropriately treated before consumption.
- (2)
- The Fe and Mn in groundwater in the study area exist in the form of Fe2+ and Mn2+, respectively. The primary sources of Fe and Mn in groundwater are the Fe and Mn minerals in the original geological environment of the study area. Groundwater tends to be reduced due to surface runoff and soil organic matter, leading to excessive Fe and Mn content.
- (3)
- High mineralization and evaporation concentrations are conducive to increased Fe content, as indicated by the factor analysis results of hydrochemical indicators. There is no significant correlation between Fe2+, Mn2+ and Cl−, NO3−. The excessive content of Fe2+ and Mn2+ in groundwater is relatively less affected by human activities.
This study systematically investigated and discussed the distribution, influencing factors, and sources of groundwater iron and manganese at Shengli Farm of the Eastern Sanjiang Plain, providing hydrochemical evidence for water safety, food security, and ecological security and offering a reference for the analysis of iron and manganese sources in similar regions. At the same time, further research and discussions are needed on the treatment methods and strategies for high-iron and high-manganese groundwater, and dynamic monitoring of iron and manganese content in groundwater should be strengthened. Research on the relationship between agricultural activities and iron and manganese content should be conducted to ensure sustainable agricultural development.
Author Contributions
Conceptualization, L.Y. and M.W. (Mingguo Wang); Data curation, M.W. (Min Wang); Formal analysis, M.W. (Mingguo Wang) and M.W. (Min Wang); Investigation, T.Y. and J.L.; Methodology, M.W. (Mingguo Wang) and L.Y.; Project administration, M.W. (Mingguo Wang); Resources, T.Y. and Y.C.; Software, T.Y. and J.L.; Supervision, L.Y.; Validation, M.W. (Mingguo Wang); Writing—original draft, L.Y.; Writing—review and editing, L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Geological Survey project of China Geological Survey, No. DD20230456 and DD20221754. The APC was funded by China Geological Survey.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rushdi, M.I.; Basak, R.; Das, P.; Ahamed, T.; Bhattacharjee, S. Assessing the health risks associated with elevated manganese and iron in groundwater in Sreemangal and Moulvibazar Sadar, Bangladesh. J. Hazard. Mater. Adv. 2023, 10, 100287. [Google Scholar] [CrossRef]
- Zhai, Y.; Han, Y.; Xia, X.; Li, X.; Lu, H.; Teng, Y.; Wang, J. Anthropogenic Organic Pollutants in Groundwater Increase Releases of Fe and Mn from Aquifer Sediments: Impacts of Pollution Degree, Mineral Content, and pH. Water 2021, 13, 1920. [Google Scholar] [CrossRef]
- Podgorski, J.; Araya, D.; Berg, M. Geogenic manganese and iron in groundwater of Southeast Asia and Bangladesh—Machine learning spatial prediction modeling and comparison with arsenic. Sci. Total Environ. 2022, 833, 155131. [Google Scholar] [CrossRef]
- Guo, Y.M.; Huang, T.L.; Wen, G.; Cao, X. The simultaneous removal of ammonium and manganese from groundwater by iron-manganese co-oxide filter film: The role of chemical catalytic oxidation for ammonium removal. Chem. Eng. J. 2017, 308, 322–329. [Google Scholar] [CrossRef]
- Shvartsev, S.L. Geochemistry of fresh groundwater in the main landscape zones of the Earth. Geochem. Int. 2008, 46, 1285–1398. [Google Scholar] [CrossRef]
- Wei, M.; Huang, Q.; Dai, Y.; Zhou, H.; Cui, Y.; Song, W.; Di, D.; Zhang, R.; Li, C.; Wang, Q.; et al. Manganese, iron, copper, and selenium co-exposure and osteoporosis risk in Chinese adults. J. Trace Elem. Med. Biol. 2022, 72, 126989. [Google Scholar] [CrossRef]
- Gupta, A. Iron overload in human disease. N. Engl. J. Med. 2012, 366, 1549. [Google Scholar]
- Bhutiani, R.; Kulkarni, D.B.; Khanna, D.R.; Gautam, A. Water quality, pollution source apportionment and health risk assessment of heavy metals in groundwater of an industrial area in north India. Expo. Health 2016, 8, 3–18. [Google Scholar] [CrossRef]
- Yadav, K.K.; Kumar, S.; Pham, Q.B.; Gupta, N.; Rezania, S.; Kamyab, H.; Yadav, S.; Vymazal, J.; Kumar, V.; Tri, D.Q.; et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review. Ecotoxicol. Environ. Saf. 2019, 182, 109362. [Google Scholar] [CrossRef] [PubMed]
- Yunker, L.M.; Parboosingh, J.S.; Conradson, H.E.; Faris, P.; Bridge, P.J.; Buithieud, J.; Title, L.M.; Charbonneaub, F.; Verma, S.; Lonn, E.M.; et al. The effect of iron status on vascular health. Vasc. Med. 2006, 8, 3–18. [Google Scholar] [CrossRef]
- Jaafar, M.; Shrivastava, A.; Bose, S.R.; Felipe-Sotelo, M.; Ward, N.I. Transfer of arsenic, manganese and iron from water to soil and rice plants: An evaluation of changes in dietary intake caused by washing and cooking rice with groundwater from the Bengal Delta, India. J. Food Compos. Anal. 2021, 96, 103748. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, I.; Nagpal, A.K. Estimation of arsenic, manganese and iron in mustard seeds, maize grains, groundwater and associated human health risks in Ropar wetland, Punjab, India, and its adjoining areas. Environ. Monit. Assess. 2018, 190, 385. [Google Scholar] [CrossRef] [PubMed]
- GB5749-202022; Ministry of Health of the People’s Republic of China; China National Standardization Administration. Standards for Drinking Water Quality. Standard Press of China: Beijing, China, 2003. (In Chinese)
- Official J EU23.12.2020.435; Directive 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast). Official Journal of the European Union: Luxembourg, 2020.
- World Health Organization. Guidelines for Drinking-Water Quality (Edition, F); World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Vega, M.A.; Kulkarni, H.V.; Johannesson, K.H.; Taylor, R.J.; Datta, S. Mobilization of co-occurring trace elements (CTEs) in arsenic contaminated aquifers in the Bengal Basin. Appl. Geochem. 2020, 122, 104709. [Google Scholar] [CrossRef]
- Johnson, C.; Nandi, A.; Joyner, T.; Luffman, I. Iron and Manganese in Groundwater: Using Kriging and GIS to Locate High Concentrations in Buncombe County, North Carolina. Groundwater 2017, 56, 87–95. [Google Scholar] [CrossRef]
- Sharma, G.K.L.; Jena, R.K.; Ray, P.; Yadav, K.K.; Moharana, P.C.; Cabral-Pinto, M.M.; Bordoloi, B. Evaluating the geochemistry of groundwater contamination with iron and manganese and probabilistic human health risk assessment in endemic areas of the world’s largest River Island, India. Environ. Toxicol. Pharmacol. 2021, 87, 103690. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, P.; Chen, C. Spatial and temporal distribution and affecting factors of iron and manganese in the groundwater in the middle area of the Yangtze River Basin, China. Environ. Sci. Pollut. Res. Int. 2022, 29, 61204–61221. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, F.; Meng, L. Occurrence, distribution, and prediction of iron and manganese in groundwater of opencast mines: An example from Inner Mongolia, China. Environ. Monit. Assess. 2021, 193, 544. [Google Scholar]
- Zhang, Z.; Xiao, C.; Yang, W.; Adeyeye, O.A.; Liang, X. Effects of the natural environment and human activities on iron and manganese content in groundwater: A case study of Changchun city, Northeast China. Environ. Sci. Pollut. Res. Int. 2021, 28, 41109–41119. [Google Scholar] [CrossRef]
- Huang, B.; Li, Z.; Chen, Z.; Chen, G.; Zhang, C.; Huang, J.; Nie, X.; Xiong, W.; Zeng, G. Study and health risk assessment of the occurrence of iron and manganese in groundwater at the terminal of the Xiangjiang River. Environ. Sci. Pollut. Res. Int. 2015, 22, 19912–19921. [Google Scholar] [CrossRef]
- Yin, X.; Jiang, B.; Feng, Z.; Yao, B.; Shi, X.; Sun, Y.; Wu, J. Comprehensive evaluation of shallow groundwater quality in central and southern Jiangsu Province, China. Environ. Earth Sci. 2017, 76, 400. [Google Scholar] [CrossRef]
- Huang, S.; Chen, L.; Li, J.; Xu, J.; Xie, W.; Zhang, C. The effects of colloidal Fe and Mn on P distribution in groundwater system of Jianghan Plain, China. Sci. Total Environ. 2023, 854, 158739. [Google Scholar] [CrossRef]
- Zhai, Y.; Cao, X.; Xia, X.; Wang, B.; Teng, Y.; Li, X. Elevated Fe and Mn Concentrations in Groundwater in the Songnen Plain, Northeast China, and the Factors and Mechanisms Involved. Agronomy 2021, 11, 2392. [Google Scholar] [CrossRef]
- Wu, A.; Hao, A.; Guo, H.; Liu, J.; Zhang, E.; Wang, H.; Wang, X.; Wen, X.; Zhang, C. Main progress and prospect for China’s hydrogeological survey. J. Groundw. Sci. Eng. 2020, 8, 195–209. [Google Scholar]
- Wang, L.; Meng, X.; Xu, H. Analysis of Causes of Superstandard Fe and Mn Content in source water of Catchment Area in Jiamusi. Environ. Sci. Manag. 2006, 31, 152–153. (In Chinese) [Google Scholar]
- Ma, H.; Li, X.; Cui, J.; Yang, Z. A Preliminary Study on The Organic and Inorganic Pollution of Groundwater of Jiansanjiang Farm of Sanjiang Plain. Geol. Resour. 2014, 23, 38–41. (In Chinese) [Google Scholar]
- Ilyushin, Y.V.; Asadulagi, M.-A.M. Development of a Distributed Control System for the Hydrodynamic Processes of Aquifers, Taking into Account Stochastic Disturbing Factors. Water 2023, 15, 770. [Google Scholar] [CrossRef]
- Martirosyan, A.V.; Martirosyan, K.V.; Mir-Amal, A.M.; Chernyshev, A.B. Assessment of a Hydrogeological Object’s Distributed Control System Stability. In Proceedings of the 2022 Conference of Russian Young Researchers in Electrical and Electronic Engineering (ElConRus), Saint Petersburg, Russia, 25–28 January 2022; pp. 768–771. [Google Scholar]
- Liu, J.; Peng, Y.; Li, C.; Gao, Z.; Chen, S. Characterization of the hydrochemistry of water resources of the Weibei Plain, Northern China, as well as an assessment of the risk of high groundwater nitrate levels to human health. Environ. Pollut. 2021, 268, 115947. [Google Scholar] [CrossRef]
- Xing, W.; Wei, L.; Ma, W.; Li, J.; Liu, X.; Hu, J.; Wang, X. Geochemistry and Sources Apportionment of Major Ions and Dissolved Heavy Metals in a Small Watershed on the Tibetan Plateau. Water 2022, 14, 3856. [Google Scholar] [CrossRef]
- Abesser, C.; Robinson, R.; Soulsby, C. Iron and manganese cycling in the storm runoff of a Scottish upland catchment. J. Hydrol. 2006, 326, 59–78. [Google Scholar] [CrossRef]
- Palmucci, W.; Rusi, S.; Di Curzio, D. Mobilisation processes responsible for iron and manganese contamination of groundwater in Central Adriatic Italy. Environ. Sci. Pollut. Res. Int. 2016, 23, 11790–11805. [Google Scholar] [CrossRef]
- Ewusi, A.; Sunkari, E.D.; Seidu, J.; Coffie-Anum, J. Hydrogeochemical characteristics, sources and human health risk assessment of heavy metal dispersion in the mine pit water–surface water-groundwater system in the largest manganese mine in Ghana. Environ. Technol. Innov. 2022, 26, 102312. [Google Scholar] [CrossRef]
- Rizwan, K.M.; Thirukumaran, V.; Suresh, M. Assessment and source identification of heavy metal contamination of groundwater using geospatial technology in Gadilam River basin, Tamil Nadu, India. Appl. Water Sci. 2021, 11, 102. [Google Scholar] [CrossRef]
- Bacquart, T.; Frisbie, S.; Mitchell, E.; Grigg, L.; Cole, C.; Small, C.; Sarkar, B. Multiple inorganic toxic substances contaminating the groundwater of Myingyan Township, Myanmar: Arsenic, manganese, fluoride, iron, and uranium. Sci. Total Environ. 2015, 517, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Solano, S.C.; Ingrid, V.-A.; Rolando, C.-M.; Huapaya, R.P.S. Multivariate data analysis applied to groundwater geochemical characterization, Central Pacific, Costa Rica. Appl. Geochem. 2023, 151, 105599. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, Y.; Li, H.; Hu, W.; Xiao, K.; Yu, S.; Zheng, C.; Wang, X. Pollution assessment and sources of dissolved heavy metals in coastal water of a highly urbanized coastal area: The role of groundwater discharge. Sci. Total Environ. 2022, 807, 151070. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Y.; Pan, X.; Qi, P.; Dai, C.; Zhang, G. Evolution characteristics of meteorological and hydrological drought in Sanjiang Plain. Res. Soil Water Conserv. 2019, 26, 177–184+189. (In Chinese) [Google Scholar]
- Shu, L.; Yin, X.; Yuan, Y.; Lu, Y.; Lu, C.; Liu, B. Temporal and spatial variation of water quantity exchange between surface water and ground water in typical district of Sanjiang Plain. Shuilixuebao 2021, 52, 1151–1162. (In Chinese) [Google Scholar]
- HJ164-2020; Ministry of Ecology and Environment of the People’s Republic of China. Technical Specifications for Environmental Monitoring of Groundwater. China Environment Publishing Group: Beijing, China, 2020. (In Chinese)
- GB5750-2006; Ministry of Health of the People’s Republic of China; China National Standardization Administration. Sanitary Standard Test Method for Drinking Water. Standard Press of China: Beijing, China, 2007. (In Chinese)
- Chakraborty, M.; Mishra, A.K.; Mukherjee, A. Influence of hydrogeochemical reactions along flow paths on contrasting groundwater arsenic and manganese distribution and dynamics across the Ganges River. Chemosphere 2022, 287, 132144. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Wang, Y. Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci. Total Environ. 2015, 508, 155–165. [Google Scholar] [CrossRef]
- Peng, C.; Liu, Y.; Chen, H.; Yuan, Q.; Chen, Q.; Mei, S.; Wu, Z. Analysis of Hydrogeochemical Characteristics of Tunnel Groundwater Based on Multivariate Statistical Technology. Geofluids 2021, 2021, 4867942. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, E.; Chen, R.; Shen, J. Lacustrine carbon cycling since the last interglaciation in northeast China: Evidence from n-alkanes in the sediments of Lake Xingkai. Quat. Int. 2019, 523, 101–108. [Google Scholar] [CrossRef]
- Khozyem, H.; Hamdan, A.; Tantawy, A.A.; Emam, A.; Elbadry, E. Distribution and origin of iron and manganese in groundwater: Case study, Balat-Teneida area, El-Dakhla Basin, Egypt. Arab. J. Geosci. 2019, 12, 523. [Google Scholar] [CrossRef]
- Carretero, S.; Kruse, E. Iron and manganese content in groundwater on the northeastern coast of the Buenos Aires Province, Argentina. Environ. Earth Sci. 2015, 73, 1983–1995. [Google Scholar] [CrossRef]
- Mao, D.H.; Wang, Z.M.; Li, L.; Miao, Z.H.; Ma, W.H.; Song, C.C.; Ren, C.Y.; Jia, M.M. Soil organic carbon in the Sanjiang Plain of China: Storage, distribution and controlling factors. Biogeosciences 2015, 12, 1635–1645. [Google Scholar] [CrossRef]
- Wang, M.; Yang, L.; Li, J.; Liang, Q. The Evaluation and Sources of Heavy Metal Anomalies in the Surface Soil of Eastern Tibet. Minerals 2023, 13, 86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).