Abstract
Marimo is a type of microalgal-bacterial granular sludge (MBGS) that exists in natural water bodies. For the first time, this paper explored the feasibility of marimo in real wastewater effluent polishing, focusing on nutrient removal as compared with MBGS. The results showed that the color of marimo gradually darkened during a 21-day experiment, and the chlorophyll content increased significantly. Although marimo and MBGS showed fairly similar removal performance in terms of NO3−-N and TN, marimo exhibited better phosphate removal as compared to MBGS. Marimo and MBGS contained different algae but the same bacterial phylum of Proteobacteria, including denitrifiers. In addition, marimo had a higher relative abundance of nitrite reductase than MBGS, suggesting that the denitrification process might also happen in addition to assimilation. This study is expected to initiate the application of marimo for wastewater effluent polishing and reclamation, shedding light on nature-based wastewater self-purification technology in the era of carbon neutrality.
1. Introduction
Recently, the world has been facing various water crises, of which the shortage of water resources and water pollution are the most prominent [1,2]. On the one hand, the global demand for water resources is constantly expanding, and a growing number of cities are facing water scarcity issues. According to statistics, the population of cities facing water scarcity worldwide will reach 2.065 billion by 2050 [3,4,5]. On the other hand, wastewater from cities, agriculture, medicine, and industries is rich in nitrogen and phosphorus, which are the main factors leading to the eutrophication of water bodies [6,7]. The huge discharge volume of effluent from wastewater treatment plants and unreasonable discharge standards are also intensifying the water crisis due to water pollution. Therefore, reclaimed water has been developed as a new water resource. In 2019, China’s unconventional water resources reached 9 billion cubic meters, of which over 80% came from reclaimed water [8]. Reclaimed water can simply be derived from the advanced treatment of effluent from wastewater treatment plants, which can not only save water resources but also alleviate pollution of the water environment.
However, there are some difficulties in developing environment-sustainable wastewater reuse technology. Advanced wastewater treatment technology often has complex processes, which makes its management more cumbersome, and maintenance becomes more expensive [9,10]. In addition, the issues of energy consumption and the accompanying greenhouse gas emissions during the treatment process cannot be ignored [11,12,13]. Some common biological methods for treating wastewater will generate greenhouse gases, which is a major challenge faced by current wastewater treatment technology [14]. Therefore, it is crucial to seek for a simple and economically sustainable method for effluent polishing.
The main purpose of advanced wastewater treatment is to remove nitrogen and phosphorus [15]. As an aggregate formed by microalgae and bacteria, the symbiotic relationship between them provides a guarantee for wastewater treatment [16,17]. Microalgal-bacterial granular sludge (MBGS) has been developed for municipal wastewater treatment with greater environmental and economic sustainability as compared to conventional activated sludge [18,19]. However, it has not been reported for effluent polishing. Aegagropila linnaei, along with its bacteria in the phycosphere, is a kind of natural MBGS that exists in natural water bodies, and it is also commonly called “marimo” [20]. Marimo can achieve self-sustaining growth by absorbing external nutrients, thereby achieving the goal of purifying water quality [20]. Additionally, it possesses a special type of zoospore, whose presence helps it maintain an aggregated form while absorbing nutrients [21]. Meanwhile, microalgae usually produce lipids, which can potentially serve as biofuels [22,23]. Considering marimo can grow in lake water with low nutrient concentrations, it can be deduced that it may also adapt well to wastewater effluent. It seems that marimo has the advantages of low energy consumption and environmental sustainability when applied to advanced wastewater treatment. However, until now, this kind of nature-based technology has not been reported. Therefore, the development of this low-cost and natural-based technology is particularly crucial.
Consequently, this paper investigated the granular characteristics, removal performance, and changes in the microbial structure and function of marimo and MBGS for effluent polishing of wastewater treatment plants. This study is expected to provide a theoretical basis for the application of natural and artificial microalgal-bacterial granular sludge in effluent polishing and wastewater reclamation. In addition, this study intends to shed light on nature-based wastewater self-purification technology under the era of carbon neutrality.
2. Materials and Methods
2.1. Wastewater Effluent
The wastewater effluent used in this experiment came from the effluent of the Chongxian Wastewater Treatment Plant, Hangzhou, Zhejiang. The effluent mainly contained 7.3 mg/L total nitrogen (TN) mainly as nitrate nitrogen (NO3−-N), 0.04 mg/L total phosphorus (TP), and 12.0 mg/L chemical oxygen demand (COD). The pH of the effluent was approximately 7.2.
2.2. Experimental Setup
The marimo and MBGS used in this experiment were derived from the internet and our previous experiment [24], respectively. The granule sizes of marimo and MBGS were about 7.1 and 7.0 mm, respectively. The 5-min sludge volume index (SVI5) values of marimo and MBGS were about 20.9 and 41.8 mL/g, respectively, and the initial volatile suspended solids (VSS) of marimo and MBGS were about 3.9 and 2.5 g/L, respectively. The hydraulic retention time (HRT) was set as 4 h. The experimental device was a continuous flow system shown in Figure 1, with an effective volume of 340 mL and a water depth of 5 cm for the runway pool. The influent flowed into the lower inlet of the device and discharged from the 5 cm high outlet. The experiment was conducted under a fixed artificial light source for 12 h/12 h of light/dark cycles, with an indoor temperature of 20 °C and a light intensity of 180 μmol/m2/s. The effluent samples were collected from one light cycle and one dark cycle per day and passed through a 0.45 μm filter for further analysis. At the beginning and end of the experiment, granule samples were collected for physicochemical analysis and metagenomics sequencing.
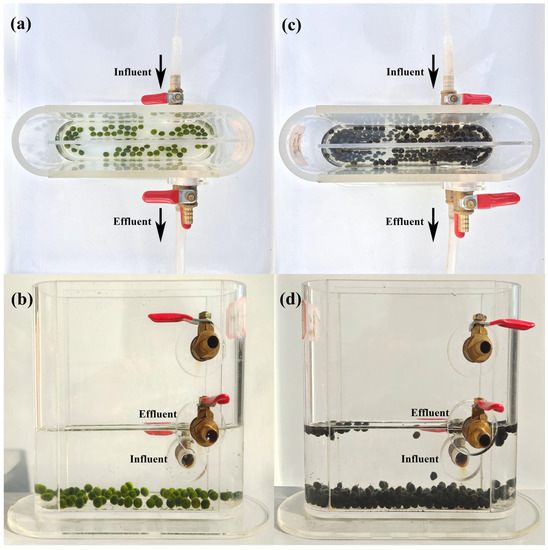
Figure 1.
Experiment setup for marimo (a,b) and MBGS (c,d) for wastewater effluent polishing.
2.3. Analytical Methods
The NO3−-N, TN, TP, COD, and SVI5 were determined by standard methods [25]. The content of chlorophyll (Chl) was determined by acetone extraction [26]. The pH values were measured using the STARTER3100 pH meter (Ohaus, Parsippany, NJ, USA), and the dissolved oxygen (DO) concentration was measured using the YSI5100 DO meter (Yellow Springs, Ohio, USA). The light intensity and temperature were measured using a light quantum meter (TES-1339P, Taibei, Taiwan) and a thermometer (MITIR TP-677, Wenzhou, China), respectively. Microalgae were examined using optical microscopy (RX50, SOPTOP, Zhejiang, China). The total genome DNA was extracted by using a FastDNA Spin Kit for Soil (MP Biomedicals, Santa Ana, CA, USA) for metagenomic sequencing, and the metabolic pathways were analyzed using the KEGG. The original sequence data can be obtained in NCBI with login no.PRJNA967691.
2.4. Statistical Analysis
Data were statistically analyzed using SPSS software (IBM SPSS Statistics 27), and the results were tested for statistical differences using one-way analysis of variance (ANOVA). The difference analysis results are statistically significant at p < 0.01.
3. Results and Discussion
3.1. Granular Characteristics and Changes in Chl Content
The granules of marimo and MBGS were recorded at the beginning and end of the experiment, respectively. As shown in Figure 2, compared to MBGS, the granule color of marimo significantly darkened as the experiment progressed, transitioning from light green to dark green, which was ascribed to the significant increase in its Chl content (Figure 3b). As can be observed in Figure 2c, marimo had a basic structural unit consisting of filamentous cells wrapped in a sheath, resembling a straw bag or teardrop [21]. The main microorganisms were eukaryotic algae of Chlorophyta. In MBGS, there were multitudes of Cyanobacteria and a small amount of green algae (Figure 2d). As shown in Figure 3a, the granule sizes of marimo and MBGS increased by 0.5 mm and 0.8 mm, respectively. This suggested that marimo had a more compact structure than MBGS during the cultivation process [20]. In addition, the SVI5 of marimo decreased by 10.3% (Figure 3b), indicating an improvement in its settling performance. In the experiment, it was intuitively observed that MBGS continuously floated up during the experiment, and until the end of the experiment, most granules of MBGS were in a floating state, while the granules of marimo always remained precipitated at the bottom of the reactor. The main factor causing MBGS to float was the generation of dissolved oxygen [27], with the growth of SVI5, while marimo’s excellent settling performance could be ascribed to its compact structure. Its excellent settling performance will be an advantage of the application of marimo in wastewater treatment, especially in continuous flow system [28].
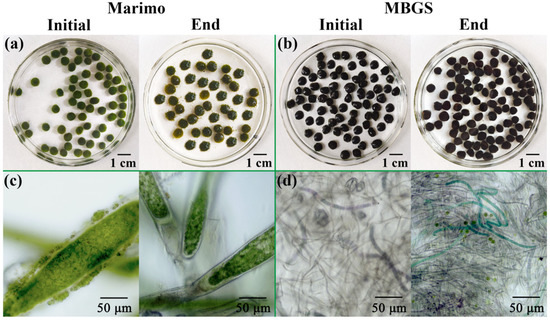
Figure 2.
The morphology and micro images of marimo (a,c) and MBGS (b,d) at the beginning and end of the experiment.
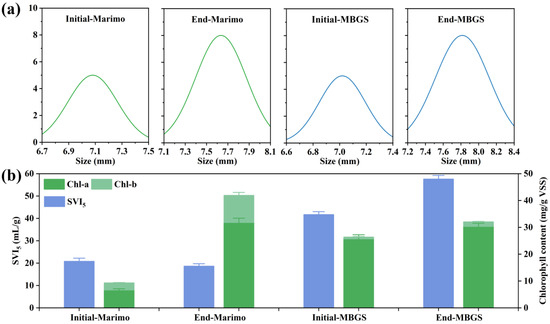
Figure 3.
The granule size (a), SVI5 value and Chl content (b) of marimo and MBGS at the beginning and end of the experiment.
Usually, the removal performance of microalgae is closely related to the intensity of its photosynthesis. As shown in Figure 3b, due to the presence of a large amount of Cyanobacteria in MBGS, which only contained chlorophyll-a (Chl-a) [29], the content of Chl-a in MBGS was much higher than that in marimo during the initial stage of the experiment. On the contrary, the main algae in marimo were eukaryotic algae of Chlorophyta, which resulted in a higher chlorophyll-b (Chl-b) content than MBGS in the early stage of the experiment. At the end of the experiment, both of Chl-a and Chl-b contents in marimo and MBGS increased (Figure 3b), suggesting the increase in photosynthesis efficiency.
3.2. Reactor Performance
An intuitive comparison of the removal rates between marimo and MBGS was conducted. As shown in Figure 4a,b, there were significant differences in the removal rates of NO3−-N and TN between marimo and MBGS during the day (ANOVA, p < 0.01). In addition, it could be seen that the concentration changes in NO3−-N and TN were quite similar. The concentrations of NO3−-N and TN in the treated wastewater had decreased by an average of less than 2 mg/L (Figure 5a,b). The nitrogen component of the effluent of the wastewater plant was mainly NO3−-N, and the removal of NO3−-N usually depends on microbial denitrification as well as assimilation [30,31]. Usually, the optimal C/N ratio for aerobic denitrification is around 7.5; it is not conducive to NO3−-N removal in an environment with a low C/N ratio [32,33]. In this study, the C/N ratio in the wastewater used in the experiment was around 0.62. In addition, the high DO environment might also hinder the reduction of nitrate nitrogen. Usually, DO values below 0.7 ± 0.1 mg/L is necessary for denitrification [34,35], but aerobic denitrification can also happen at DO concentrations of around 4 mg/L [36]. As shown in Table 1, the average DO concentration in this study was around 5.4 mg/L. Therefore, based on the above analyses and taken the biofilm property of bio-granules into account, it is speculated that both marimo and MBGS could remove nitrite from wastewater mainly through microbial assimilation but denitrification might also happen, which was also verified by the subsequent microbial and metagenomics analyses.
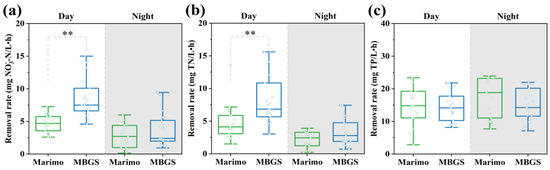
Figure 4.
Differences in removal rates of NO3−-N (a), TN (b), and TP (c) between marimo and MBGS during the day and night. ** symbols for p < 0.01.
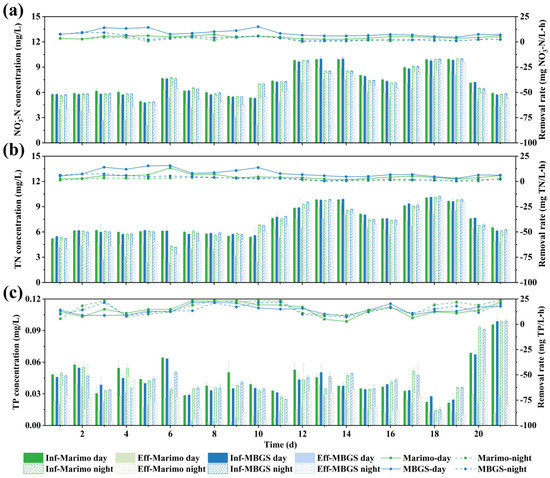
Figure 5.
NO3−-N (a), TN (b), and TP (c) influent and effluent concentrations (left axis) and removal rates (right axis) by marimo and MBGS.

Table 1.
Dissolved oxygen concentrations in influent and effluent of marimo and MBGS systems during the day and night.
Contrary to the results of NO3−-N and TN, Figure 4c indicated that there was no significant difference in the removal rates of TP between marimo and MBGS (ANOVA, p > 0.05); the average removal rates of TP during the day were 16.1% and 16.5%, which were 18.1% and 16.2% at night, respectively. The influent concentration of TP was as low as around 0.04 mg/L because the raw wastewater in this experiment came from the wastewater treatment plant treated by chemicals. However, after advanced treatment by marimo, the concentration of TP became even lower than before. In addition, it could also be seen that the performance of marimo in advanced wastewater treatment of phosphorus was comparable to MBGS during the day and even slightly better than MBGS at night. Under the condition of light/dark cycles, MBGS was found to remove phosphorus mainly through cell assimilation and polyphosphate accumulation [37]. Considering that the HRT in this study was only 4 h, it can be assumed that by extending the HRT, the phosphorus removal efficiency could be further improved when treating effluent with a higher phosphorus concentration.
Moreover, the average concentrations of NO3−-N in the effluent of marimo and MBGS were 6.19 and 5.54 mg/L, respectively (Figure 5a), and the average concentrations of TN in effluent were 6.29 and 5.66 mg/L, respectively (Figure 5b). Excessive nitrogen emissions often bring some negative environmental impacts, such as nitrate and ammonia emissions causing pollution to water bodies, while nitrogen oxides can cause air pollution [38]. The NO3−-N and TN concentrations in this experiment were reduced to some extent. As shown in Figure 5c, the average concentrations of TP in the effluent of marimo and MBGS were both around 0.02 mg/L. Phosphorus is considered to be a limiting element for preventing eutrophication [6]. In this study, the extremely low concentration of phosphorus allows the treated wastewater to be returned to natural water to maintain its healthy state. Due to the influent COD concentration being less than 15 mg/L in this experiment, only the COD data from the first ten days were measured, indicating that COD were also reduced by 25% to 40% by marimo and MBGS during the light/dark cycles, respectively.
3.3. Microbial Community and Gene Functional Analysis
As shown in Figure 6a, at the phylum level, marimo mainly consisted of Proteobacteria at the initial stage of the experiment, followed by Chlorophyta. At the end of the experiment, the relative abundance of Proteobacteria became higher, with Proteobacteria and Chlorophyta accounting for more than 75% of the total. The increase in Proteobacteria might be due to that the low nutrient conditions were more suitable for their growth [39]. A small amount of Bacteroidota was also detected. Proteobacteria and Bacteroidota can play the role of denitrification in wastewater treatment [40]. In addition, the presence of Proteobacteria can play an important role in removing anti-inflammatory and antimicrobial substances [41]. Therefore, it was speculated that Proteobacteria acted as one of the main contributors in the wastewater treatment process [42]. The dominant phylum transformed from Proteobacteria into Cyanobacteria for MBGS, which was consistent with the previous study [43]. At a finer scale, α-proteobacteria and γ-proteobacteria occupied the main position in MBGS, and by the end of the experiment, the dominant class had passed from γ-proteobacteria to α-proteobacteria. Interestingly, it could be observed that, at the end of the experiment, marimo and MBGS were quite similar in microbial composition, both containing higher relative abundances of Burkholdericeae, Acetobacteraceae, Rhodobacteraceae, and Xanthomonadaceae. Compared with the initial data, there was a significant increase in the relative abundance of Rhodobacteriaceae in marimo, which was a kind of denitrification bacteria with the nirS gene [44]. In contrast, MBGS had little change in Rhodobacteriaceae, which might suggest that the denitrification potential of marimo slightly increased in the later stage of the experiment.
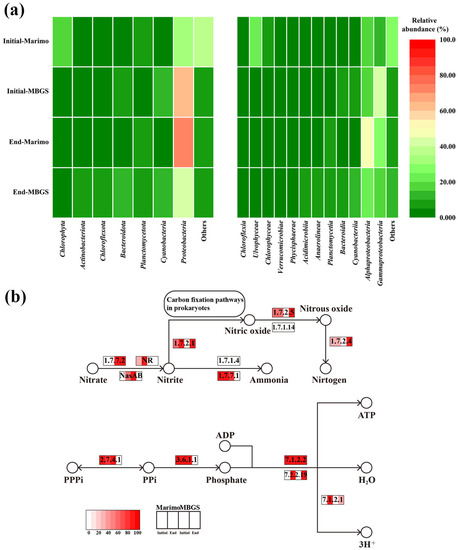
Figure 6.
Changes in microbial communities (a) and key metabolic pathways of nitrate and phosphate (b) of marimo and MBGS.
According to Figure 6b, it was speculated that marimo might also have a mechanism of nitrogen removal through denitrification in addition to assimilation. It could be observed that the abundance of Pithopora in marimo was significantly reduced, while Roseomonas and Hydrogenophaga showed a downward trend. In addition, marimo and MBGS both detected the presence of Thauera, which was also a famous denitrification bacterium, and it might be the main contributor to nitrogen removal via N2 in MBGS [45]. Recently, it was also found that some Thauera belong to denitrifying phosphorus-accumulating organisms [46]. Polyphosphate accumulation is usually considered to be the main avenue of biological phosphorus removal [37]. In this experiment, it is speculated that phosphorus-accumulating organisms in marimo might adopt available oxygen as the electron acceptor to decompose polyhydroxyalkanoates and used the energy generated by this reaction to absorb phosphate.
Changes in key metabolic pathways of nitrate and phosphate were further analyzed for marimo and MBGS based on the metagenomics. As shown in Figure 6b, the genes of nitrite reductase (EC 1.7.7.1) contained in marimo had higher relative abundance, while MBGS had higher relative abundance of nitrate reductase (EC 1.7.7.2). In the denitrification pathway, the relative abundances of nitric oxide reductase (EC 1.7.2.5) and nitrous oxide reductase (EC 1.7.2.4) in marimo remained stable, while at the end of the experiment, the relative abundances of both enzymes in MBGS increased. Based on the above results, it can be reasonably speculated that, at the end of the experiment, both of marimo and MBGS could remove nitrogen mainly through assimilation, but the denitrification might also happen due to the present of complete enzymes responsible for denitrification (Figure 6b). In the key metabolic pathway of phosphate, according to Figure 6b, the relative abundances of polyphosphotransferase (2.7.4.1) and diphosphate hydrolase (3.6.1.1) in marimo were higher than those in MBGS, which might explained for why the phosphorus removal performance of marimo was slightly better than MBGS in the later stage of the experiment.
4. Conclusions
The advanced wastewater treatment performances of marimo on NO3−-N, TN, and TP were first evaluated and compared with MBGS in this study. The results showed that the pollutant removal performance of marimo was close to that of MBGS. Especially in the removal of phosphate, marimo had an even more excellent performance than MBGS. Overall, marimo experienced a slight increase in granule size and a significant increase in Chl content. During the day, marimo had the capacity to remove NO3−-N, TN, and TP during real wastewater effluent polishing at an HRT of 4 h. Due to the HRT being 4 h in this study, it can be assumed that extending HRT might further promote the pollutant removal. This paper speculated that marimo could remove nitrogen through microbial assimilation, and denitrification might also happen during wastewater effluent polishing. The high abundances of polyphosphotransferase and diphosphate hydrolase in marimo might be the possible reason for its excellent phosphorus removal efficiency. In addition, marimo can simply be acquired from nature and had a certain oxygen production capacity when it was applied for effluent polishing, making it economically and environmentally sustainable for advanced wastewater treatment and reclamation.
Author Contributions
Y.W.: Investigation, Writing—original draft, Data curation, Software. P.S.: Investigation, Writing—original draft, Data curation, Formal analysis. M.L.: Resources. Q.H.: Writing—review and editing. B.J.: Conceptualization, Writing—review and editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This experiment was funded by the National Natural Science Foundation of China (51808416).
Data Availability Statement
Experimental data can be provided as required.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Z.; Wu, G.; Wu, Y.; Wu, Q.; Shi, Q.; Ngo, H.H.; Vargas Saucedo, O.A.; Hu, H. Water Eco-Nexus Cycle System (WaterEcoNet) as a key solution for water shortage and water environment problems in urban areas. Water Cycle 2020, 1, 71–77. [Google Scholar] [CrossRef]
- Wang, D.; Hubacek, K.; Shan, Y.; Gerbens-Leenes, W.; Liu, J. A Review of Water Stress and Water Footprint Accounting. Water 2021, 13, 201. [Google Scholar] [CrossRef]
- UN-Water. The United Nations World Water Development Report 2018; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2018. [Google Scholar]
- Capodaglio, A.G. Fit-for-purpose urban wastewater reuse: Analysis of issues and available technologies for sustainable multiple barrier approaches. Crit. Rev. Env. Sci. Tec. 2020, 51, 1619–1666. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef]
- Diatta, J.; Waraczewska, Z.; Grzebisz, W.; Niewiadomska, A.; Tatuśko-Krygier, N. Eutrophication Induction Via N/P and P/N Ratios Under Controlled Conditions—Effects of Temperature and Water Sources. Water Air Soil Pollut. 2020, 231, 149. [Google Scholar] [CrossRef]
- Preisner, M.; Neverova-Dziopak, E.; Kowalewski, Z. Analysis of eutrophication potential of municipal wastewater. Water Sci. Technol. 2020, 81, 1994–2003. [Google Scholar] [CrossRef]
- Khilchevskyi, V.; Karamushka, V. Global Water Resources: Distribution and Demand. Clean Water Sanit. 2021, 1, 11. [Google Scholar] [CrossRef]
- Gul, A.; Khaligh, N.G.; Julkapli, N.M. Surface modification of Carbon-Based Nanoadsorbents for the Advanced Wastewater Treatment. J. Mol. Struct. 2021, 1235, 130148. [Google Scholar] [CrossRef]
- Lugo, A.; Xu, X.; Abeysiriwardana-Arachchige, I.S.A.; Bandara, G.L.C.L.; Nirmalakhandan, N.; Xu, P. Techno-economic assessment of a novel algal-membrane system versus conventional wastewater treatment and advanced potable reuse processes: Part II. J. Environ. Manag. 2023, 331, 117189. [Google Scholar] [CrossRef]
- Ross, B.N.; Lancellotti, B.V.; Brannon, E.Q.; Loomis, G.W.; Amador, J.A. Greenhouse gas emissions from advanced nitrogen-removal onsite wastewater treatment systems. Sci. Total Environ. 2020, 737, 140399. [Google Scholar] [CrossRef]
- Du, W.; Lu, J.; Hu, Y.; Xiao, J.; Yang, C.; Wu, J.; Huang, B.; Cui, S.; Wang, Y.; Li, W. Spatiotemporal pattern of greenhouse gas emissions in China’s wastewater sector and pathways towards carbon neutrality. Nat. Water 2023, 1, 166–175. [Google Scholar] [CrossRef]
- Ghimire, U.; Sarpong, G.; Gude, V.G. Transitioning Wastewater Treatment Plants toward Circular Economy and Energy Sustainability. ACS Omega 2021, 6, 11794–11803. [Google Scholar] [CrossRef]
- Zaborowska, E.; Czerwionka, K.; Mąkinia, J. Integrated plant-wide modelling for evaluation of the energy balance and greenhouse gas footprint in large wastewater treatment plants. Appl. Energ. 2021, 282, 116126. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, H.; Jia, L.; Wu, W.; Wang, J. Simultaneous biological removal of nitrogen and phosphorus from secondary effluent of wastewater treatment plants by advanced treatment: A review. Chemosphere 2022, 296, 134054. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Pei, H. Algal–bacterial consortia for bioproduct generation and wastewater treatment. Renew. Sustain. Energ. Rev. 2021, 149, 111395. [Google Scholar] [CrossRef]
- Ji, B.; Liu, Y. Assessment of Microalgal-Bacterial Granular Sludge Process for Environmentally Sustainable Municipal Wastewater Treatment. ACS EST Water 2021, 1, 2459–2469. [Google Scholar] [CrossRef]
- Ji, B.; Shi, Y.; Yılmaz, M. Microalgal-bacterial granular sludge process for sustainable municipal wastewater treatment: Simple organics versus complex organics. J. Water Process Eng. 2022, 46, 102613. [Google Scholar] [CrossRef]
- Nakayama, K.; Komai, K.; Ogata, K.; Yamada, T.; Sato, Y.; Sano, F.; Horii, S.; Somiya, Y.; Kumamoto, E.; Oyama, Y. The structure and formation of giant Marimo (Aegagropila linnaei) in Lake Akan, Japan. Sci. Rep. 2021, 11, 22017. [Google Scholar] [CrossRef]
- Umekawa, T.; Wakana, I.; Ohara, M. Reproductive behavior and role in maintaining an aggregative form of the freshwater green alga Marimo, Aegagropila linnaei, in Lake Akan, Hokkaido, Japan. Aquat. Bot. 2021, 168, 103309. [Google Scholar] [CrossRef]
- Song, Q.; Kong, F.; Liu, B.; Song, X.; Ren, N.; Ren, H. Insights into the Effect of Rhamnolipids on the Anaerobic Fermentation and Microalgae Lipid Production of Waste Activated Sludge: Performance and Mechanisms. ACS EST Eng. 2023, 3, 438–448. [Google Scholar] [CrossRef]
- Song, X.; Kong, F.; Liu, B.; Song, Q.; Ren, N.; Ren, H. Thallium-mediated NO signaling induced lipid accumulation in microalgae and its role in heavy metal bioremediation. Water Res. 2023, 239, 120027. [Google Scholar] [CrossRef]
- Sun, P.; Liu, C.; Li, A.; Ji, B. Using carbon dioxide-added microalgal-bacterial granular sludge for carbon-neutral municipal wastewater treatment under outdoor conditions: Performance, granule characteristics and environmental sustainability. Sci. Total Environ. 2022, 848, 157657. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Lee, C.S.; Lee, S.; Ko, S.; Oh, H.; Ahn, C. Effects of photoperiod on nutrient removal, biomass production, and algal-bacterial population dynamics in lab-scale photobioreactors treating municipal wastewater. Water Res. 2015, 68, 680–691. [Google Scholar] [CrossRef]
- Shi, Y.; Ji, B.; Zhang, X.; Liu, Y. Auto-floating oxygenic microalgal-bacterial granular sludge. Sci. Total Environ. 2023, 856, 159175. [Google Scholar] [CrossRef]
- Jia, Y.; Wen, Z.; Shang-Guan, Y.; Li, Z. Aerobic granulation in an oxidation ditch using the residual sludge after extracting slime-extracellular polymer substances. J. Water Process Eng. 2023, 54, 103978. [Google Scholar] [CrossRef]
- Chini Zittelli, G.; Mugnai, G.; Milia, M.; Cicchi, B.; Silva Benavides, A.M.; Angioni, A.; Addis, P.; Torzillo, G. Effects of blue, orange and white lights on growth, chlorophyll fluorescence, and phycocyanin production of Arthrospira platensis cultures. Algal Res. 2022, 61, 102583. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, C.; Huang, X.; Liu, J.; Lu, L.; Peng, K.; Li, S. Research progress in solid carbon source–based denitrification technologies for different target water bodies. Sci. Total Environ. 2021, 782, 146669. [Google Scholar] [CrossRef]
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.N.; Bemment, C.D. Nitrate attenuation in groundwater: A review of biogeochemical controlling processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, H.; Cai, L.; Sun, P.; Ma, B.; Li, J.; Chen, G.; Ruan, Y. C/N ratios inform sustainable aerobic denitrification for nitrogen pollution conctrol: Insights into the key parameter from a view of metabolic division. J. Clean Prod. 2023, 414, 137565. [Google Scholar] [CrossRef]
- Gu, X.; Leng, J.; Zhu, J.; Zhang, K.; Zhao, J.; Wu, P.; Xing, Q.; Tang, K.; Li, X.; Hu, B. Influence mechanism of C/N ratio on heterotrophic nitrification- aerobic denitrification process. Bioresour. Technol. 2022, 343, 126116. [Google Scholar] [CrossRef]
- Hu, B.; Quan, J.; Huang, K.; Zhao, J.; Xing, G.; Wu, P.; Chen, Y.; Ding, X.; Hu, Y. Effects of C/N ratio and dissolved oxygen on aerobic denitrification process: A mathematical modeling study. Chemosphere 2021, 272, 129521. [Google Scholar] [CrossRef]
- Yan, L.; Liu, S.; Liu, Q.; Zhang, M.; Liu, Y.; Wen, Y.; Chen, Z.; Zhang, Y.; Yang, Q. Improved performance of simultaneous nitrification and denitrification via nitrite in an oxygen-limited SBR by alternating the DO. Bioresour. Technol. 2019, 275, 153–162. [Google Scholar] [CrossRef]
- Ji, B.; Yang, K.; Zhu, L.; Jiang, Y.; Wang, H.; Zhou, J.; Zhang, H. Aerobic denitrification: A review of important advances of the last 30 years. Biotechnol. Bioproc. E. 2015, 20, 643–651. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, M.; Wang, L.; Wang, S.; Liu, Y. Removal mechanisms of phosphorus in non-aerated microalgal-bacterial granular sludge process. Bioresour. Technol. 2020, 312, 123531. [Google Scholar] [CrossRef]
- Yu, C.; Huang, X.; Chen, H.; Godfray, H.C.J.; Wright, J.S.; Hall, J.W.; Gong, P.; Ni, S.; Qiao, S.; Huang, G.; et al. Managing nitrogen to restore water quality in China. Nature 2019, 567, 516–520. [Google Scholar] [CrossRef]
- Gomila, M.; GascÃ, J.; Busquets, A.; Gil, J.; Bernabeu, R.; Buades, J.M.; Lalucat, J. Identification of culturable bacteria present in haemodialysis water and fluid. Fems. Microbiol. Ecol. 2005, 52, 101–114. [Google Scholar] [CrossRef]
- Guo, J.; Li, Q.; Gao, Q.; Shen, F.; Yang, Y.; Zhang, X.; Luo, H. Comparative study on the treatment of swine wastewater by VFCW-MFC and VFCW: Pollutants removal, electricity generation, microorganism community. J. Environ. Manag. 2023, 342, 118299. [Google Scholar] [CrossRef]
- Xu, H.; Song, H.; Singh, R.P.; Yang, Y.; Xu, J.; Yang, X. Simultaneous reduction of antibiotics leakage and methane emission from constructed wetland by integrating microbial fuel cell. Bioresour. Technol. 2021, 320, 124285. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Macedo, G.; Silva, A.M.T.; Manaia, C.M.; Nunes, O.C. Proteobacteria become predominant during regrowth after water disinfection. Sci. Total Environ. 2016, 573, 313–323. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, M.; Gu, J.; Ma, Y.; Liu, Y. A self-sustaining synergetic microalgal-bacterial granular sludge process towards energy-efficient and environmentally sustainable municipal wastewater treatment. Water Res. 2020, 179, 115884. [Google Scholar] [CrossRef]
- Chen, Z.; Chang, Z.; Qiao, L.; Wang, J.; Yang, L.; Liu, Y.; Song, X.; Li, J. Nitrogen removal performance and microbial diversity of bioreactor packed with cellulosic carriers in recirculating aquaculture system. Int. Biodeter. Biodegr. 2021, 157, 105157. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, X.; Zhang, X.; Zhu, C.; Pan, Y.; Sun, T.; Wang, L. Denitrification for acidic wastewater treatment: Long-term performance, microbial communities, and nitrous oxide emissions. J. Biosci. Bioeng. 2022, 134, 513–520. [Google Scholar] [CrossRef]
- Wang, Q.; He, J. Complete nitrogen removal via simultaneous nitrification and denitrification by a novel phosphate accumulating Thauera sp. strain SND5. Water Res. 2020, 185, 116300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).