Comparative Analysis of Composition and Porosity of the Biogenic Powder Obtained from Wasted Crustacean Exoskeletonsafter Carotenoids Extraction for the Blue Bioeconomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Sourcing and Preparation
2.2. Analysis of the Extract
2.3. Analysis of Native and Post-Extraction Exoskeleton Powders
3. Results
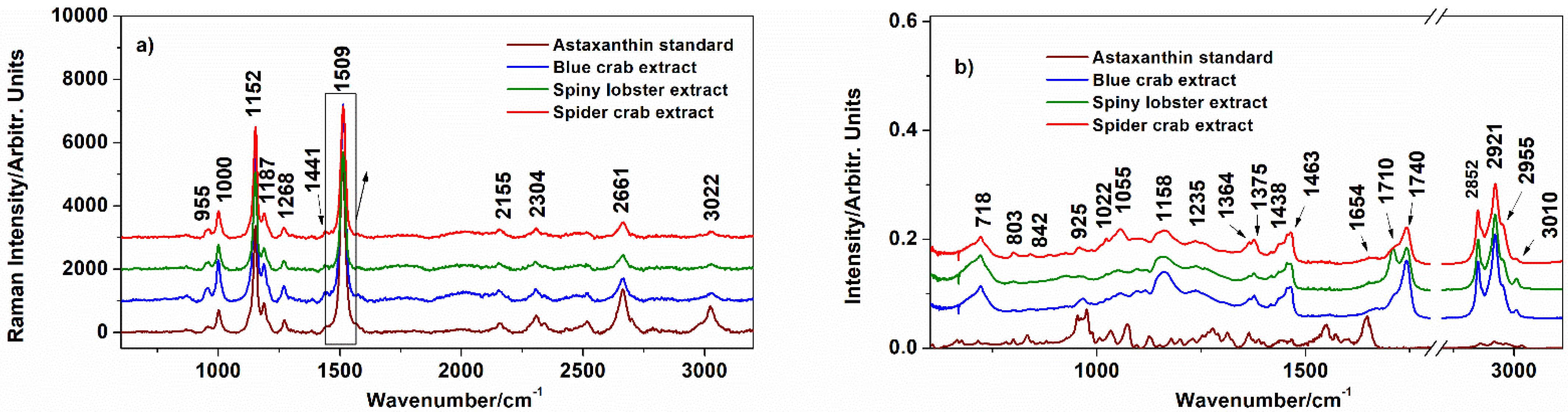
3.1. Characterization of the Extracts
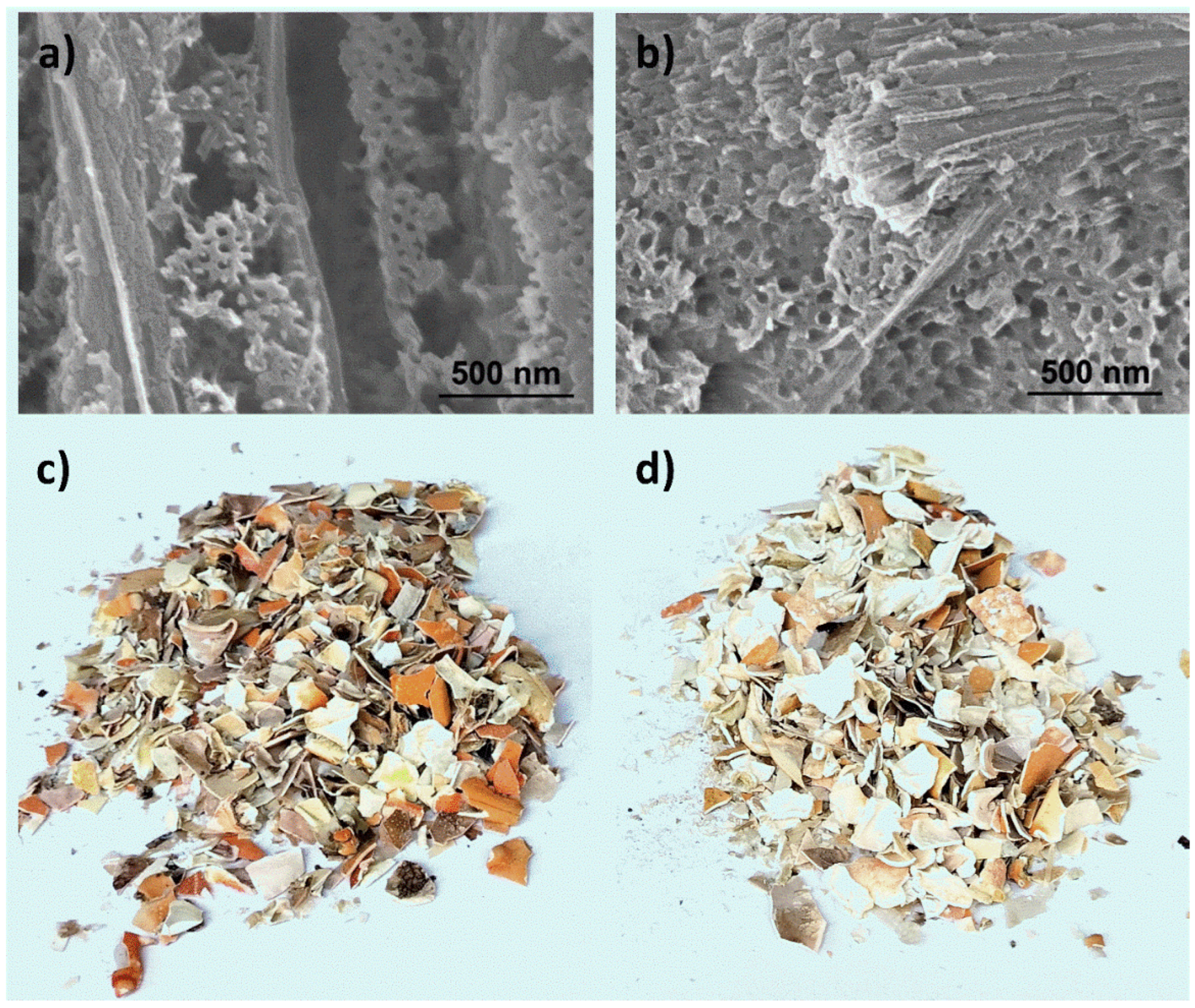
3.2. Exoskeleton Ultrastructure
3.3. Exoskeleton Powder Porosity
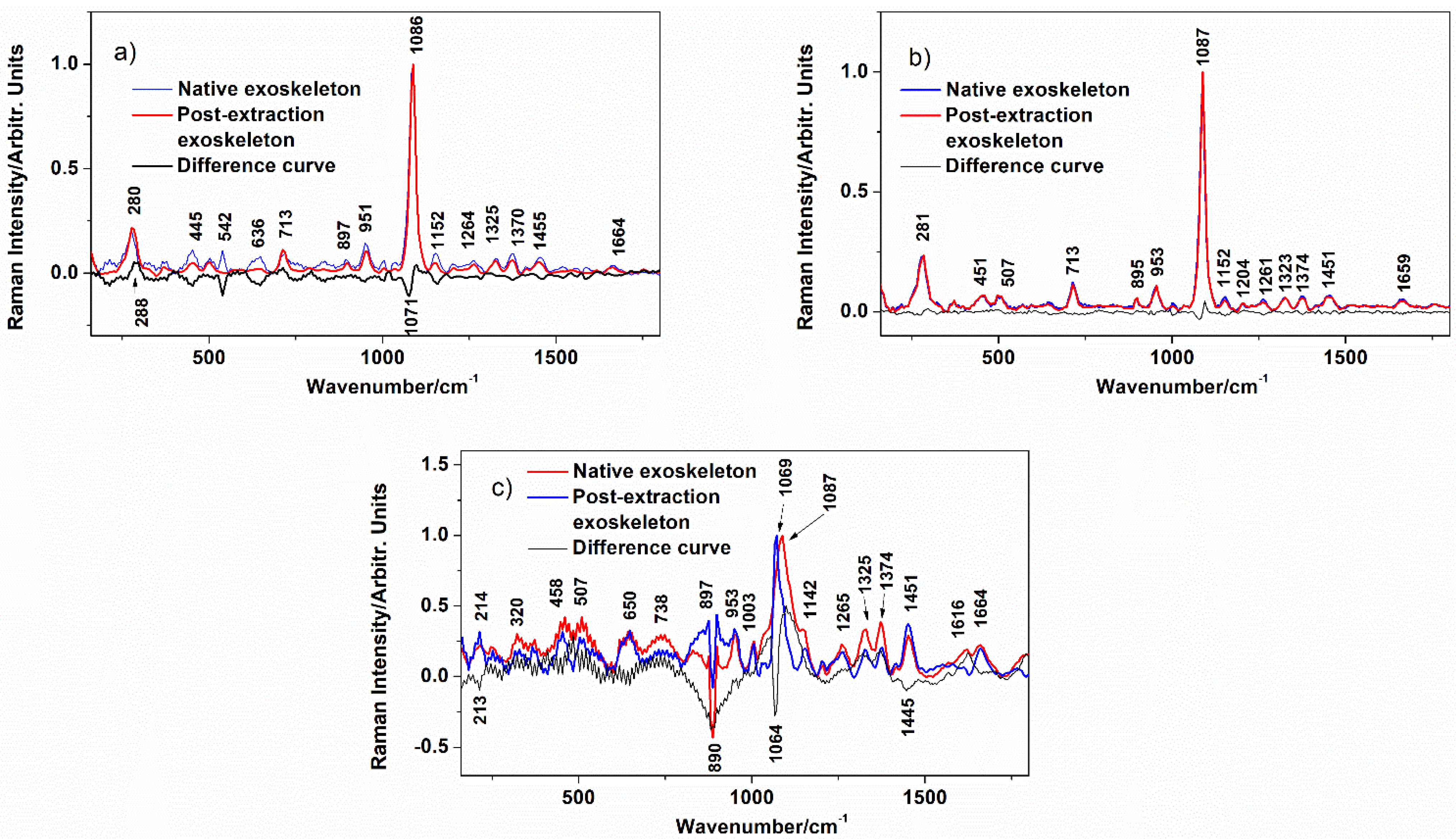
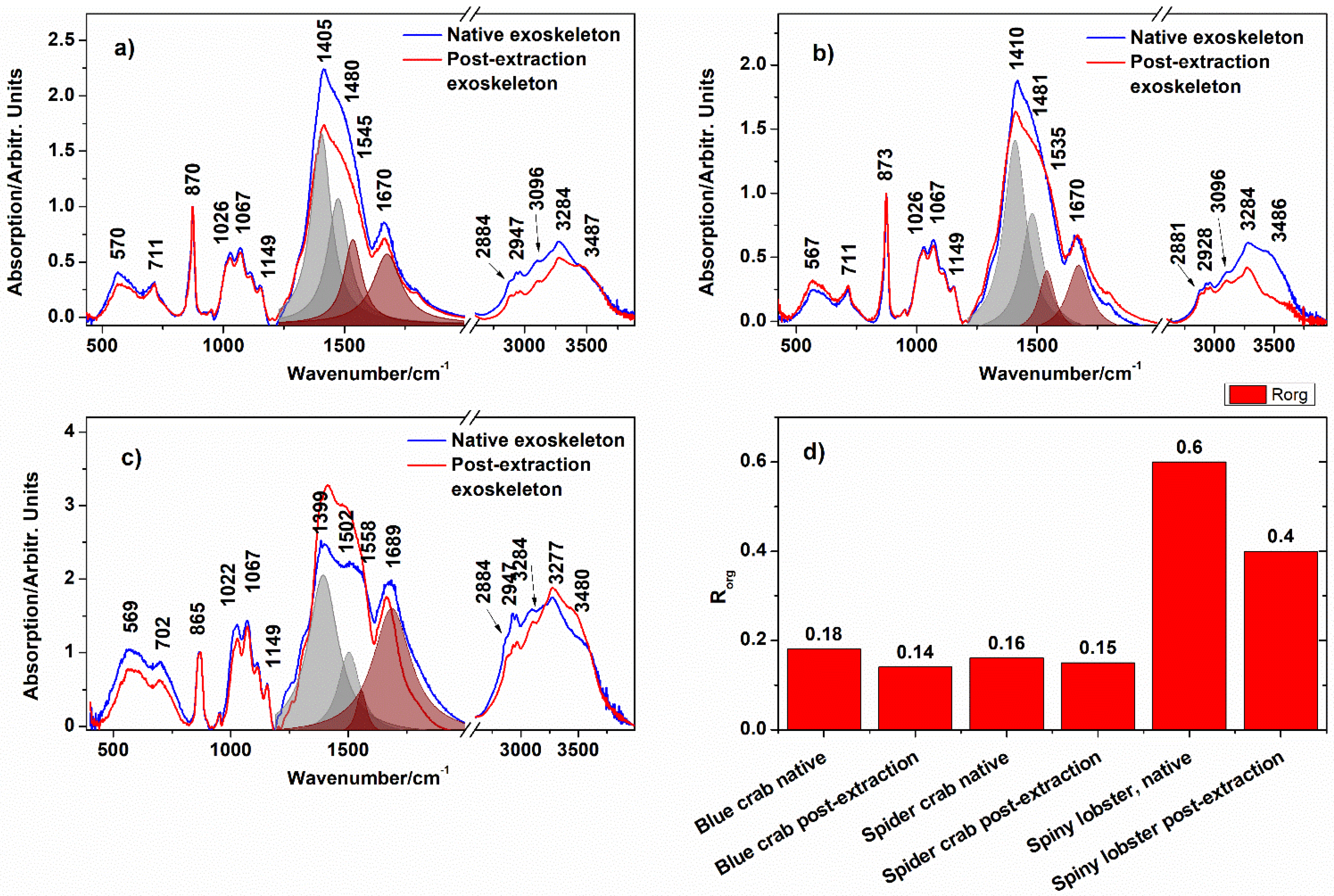
3.4. Exoskeleton Powder Chemical Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elleh-Ali-Komi, D.; Hamblin, M. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Sci. (Indore) 2017, 4, 411–427. [Google Scholar]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and Biomedical Applications of Chitin and Chitosan Nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Aneesh, P.A.; Anandan, R.; Kumar, L.R.G.; Ajeeshkumar, K.K.; Kumar, K.A.; Mathew, S. A step to shell biorefinery—Extraction of astaxanthin-rich oil, protein, chitin, and chitosan from shrimp processing waste. Biomass- Convers. Biorefinery 2020, 13, 205–214. [Google Scholar] [CrossRef]
- The BlueBioSustain Project. Available online: https://bluebiosustain.granturi.ubbcluj.ro/index.html (accessed on 3 January 2023).
- Sajid, M.A.; Shahzad, S.A.; Hussain, F.; Skene, W.G.; Khan, Z.A.; Yar, M. Synthetic modifications of chitin and chitosan as multipurpose biopolymers: A review. Synth. Commun. 2018, 48, 1893–1908. [Google Scholar] [CrossRef]
- Trobajo, R.; Rovira, L.; Ector, L.; Wetzel, C.E.; Kelly, M.; Mann, D.G. Morphology and identity of some ecologically important small Nitzschia species. Diatom Res. 2012, 28, 37–59. [Google Scholar] [CrossRef]
- Weinkauf, M.F.G.; Zwick, M.M.; Kučera, M. Constraining the Role of Shell Porosity in the Regulation of Shell Calcification Intensity in the Modern Planktonic Foraminifer Orbulina Universa d’Orbigny. J. Foraminifer. Res. 2020, 50, 195–203. [Google Scholar] [CrossRef]
- Lauer, C.; Sillmann, K.; Haussmann, S.; Nickel, K.G. Strength, elasticity and the limits of energy dissipation in two related sea urchin spines with biomimetic potential. Bioinspiration Biomim. 2018, 14, 016018. [Google Scholar] [CrossRef]
- Gautron, J.; Stapane, L.; Le Roy, N.; Nys, Y.; Rodriguez-Navarro, A.B.; Hincke, M.T. Avian eggshell biomineralization: An update on its structure, mineralogy and protein tool kit. BMC Cell Biol. 2021, 22, 11. [Google Scholar] [CrossRef]
- Nekvapil, F.; Pinzaru, S.C.; Barbu–Tudoran, L.; Suciu, M.; Glamuzina, B.; Tamaș, T.; Chiș, V. Color-specific porosity in double pigmented natural 3d-nanoarchitectures of blue crab shell. Sci. Rep. 2020, 10, 3019. [Google Scholar] [CrossRef] [Green Version]
- Bentov, S.; Abehsera, S.; Sagi, A. Chapter 5: The Mineralized Exoskeletons of Crustaceans. In Extracellular Composite Matrices in Arthropods; Cohen, E., Moussian, B., Eds.; Springer: New York, NY, USA, 2016; pp. 137–163. [Google Scholar]
- Nekvapil, F.; Aluas, M.; Barbu-Tudoran, L.; Suciu, M.; Bortnic, R.-A.; Glamuzina, B.; Cintă Pinzaru, S. From Blue Bioeconomy toward Circular Economy through High-Sensitivity Analytical Research on Waste Blue Crab Shells. ACS Sustain. Chem. Eng. 2019, 7, 16820–16827. [Google Scholar] [CrossRef]
- Lazar, G.; Nekvapil, F.; Hirian, R.; Glamuzina, B.; Tamas, T.; Barbu-Tudoran, L.; Pinzaru, S.C. Novel Drug Carrier: 5-Fluorouracil Formulation in Nanoporous Biogenic Mg-calcite from Blue Crab Shells—Proof of Concept. ACS Omega 2021, 6, 27781–27790. [Google Scholar] [CrossRef] [PubMed]
- Raabe, D.; Sachs, C.; Romano, P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005, 53, 4281–4292. [Google Scholar] [CrossRef]
- Romano, P.; Fabritius, H.-O.; Raabe, D. The exoskeleton of the lobster Homarus americanus as an example of a smart anisotropic biological material. Acta Biomater. 2007, 3, 301–309. [Google Scholar] [CrossRef]
- Nekvapil, F.; Ganea, I.-V.; Ciorîță, A.; Hirian, R.; Tomšić, S.; Martonos, I.; Pinzaru, S.C. A New Biofertilizer Formulation with Enriched Nutrients Content from Wasted Algal Biomass Extracts Incorporated in Biogenic Powders. Sustainability 2021, 13, 8777. [Google Scholar] [CrossRef]
- Morris, A.; Sneddon, J. Use of Crustacean Shells for Uptake and Removal of Metal Ions in Solution. Appl. Spectrosc. Rev. 2011, 46, 242–250. [Google Scholar] [CrossRef]
- Fabbricino, M.; Pontoni, L. Use of non-treated shrimp-shells for textile dye removal from wastewater. J. Environ. Chem. Eng. 2016, 4, 4100–4106. [Google Scholar] [CrossRef]
- Rissouli, L.; Beenicha, M.; Chafik, T.; Chabbi, M. Decontamination of water polluted with pesticide using biopolymers: Adsorption of glyphosate by chitin and chitosan. J. Mater. Environ. Sci. 2017, 8, 4544–4549. [Google Scholar] [CrossRef]
- Inthapanya, X.; Wu, S.; Han, Z.; Zeng, G.; Wu, M.; Yang, C. Adsorptive removal of anionic dye using calcined oyster shells: Isotherms, kinetics, and thermodynamics. Environ. Sci. Pollut. Res. 2019, 26, 5944–5954. [Google Scholar] [CrossRef]
- Faizal, A.N.M.; Putra, N.R.; Zaini, M.A.A. Scylla Sp. Shell: A potential green adsorbent for wastewater treatment. Toxin Rev. 2022, 41, 1280–1289. [Google Scholar] [CrossRef]
- Londono-Zuluaga, C.; Jameel, H.; Gonzalez, R.W.; Lucia, L. Crustacean shell-based biosorption water remediation platforms: Status and perspectives. J. Environ. Manag. 2018, 231, 757–762. [Google Scholar] [CrossRef]
- FAO—United Nations Food and Agriculture Organization. The State of World Fisheries and Aquaculture—Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Nekvapil, F.; Ganea, I.-V.; Ciorîță, A.; Hirian, R.; Ogresta, L.; Glamuzina, B.; Roba, C.; Pinzaru, S.C. Wasted Biomaterials from Crustaceans as a Compliant Natural Product Regarding Microbiological, Antibacterial Properties and Heavy Metal Content for Reuse in Blue Bioeconomy: A Preliminary Study. Materials 2021, 14, 4558. [Google Scholar] [CrossRef]
- EC—European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions on The European Green Deal. COM/2019/640 final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 1 December 2021).
- EC—European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions on a new approach for a sustainable blue economy in the EU: Transforming the EU’s Blue Economy for a Sustainable Future. COM/240/2021 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM:2021:240:FIN (accessed on 1 December 2021).
- EPA—United States Environmental Protection Agency. National Recycling Strategy—Part One of a Series on Building a Circular Economy for All. Available online: https://www.epa.gov/system/files/documents/2021-11/final-national-recycling-strategy.pdf (accessed on 19 December 2022).
- EC—Commission of the European Communities. Green Paper on the Management of Bio-Waste in the European Union. COM(2008) 811 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52008DC0811&from=EN (accessed on 19 December 2022).
- Ogresta, L.; Nekvapil, F.; Tǎmaş, T.; Barbu-Tudoran, L.; Suciu, M.; Hirian, R.; Aluaş, M.; Lazar, G.; Levei, E.; Glamuzina, B.; et al. Rapid and Application-Tailored Assessment Tool for Biogenic Powders from Crustacean Shell Waste: Fourier Transform-Infrared Spectroscopy Complemented with X-ray Diffraction, Scanning Electron Microscopy, and Nuclear Magnetic Resonance Spectroscopy. ACS Omega 2021, 6, 27773–27780. [Google Scholar] [CrossRef]
- Antunes-Valcareggi, S.A.; Ferreira, S.R.S.; Hense, H. Enzymatic Hydrolysis of Blue Crab (Callinectes Sapidus) Waste Processing to Obtain Chitin, Protein, and Astaxanthin-Enriched Extract. Int. J. Environ. Agricult. Res. 2017, 3, 81–92. [Google Scholar]
- Montoya, J.M.; Mata, S.V.; Acosta, J.L.; Herrera Cabrera, B.E.; Lopez Valdez, L.G.; Reyes, C.; Barrales Cureno, H.J. Obtaining of Astaxanthin from Crab Exoskeletons and Shrimp Head Shells. Biointerface Res. Appl. Chem. 2021, 11, 13516–13523. [Google Scholar] [CrossRef]
- Lindberg, D.; Solstad, R.G.; Arnesen, J.A.; Helmers, A.K.; Whitaker, R.D. Lab scale sustainable extraction of components from snow crab (Chionoecetes opilio) co-products, and estimation of processing costs based on a small-scale demonstration plant (Biotep). Ǿkonomisk Fisk. 2021, 31, 42–57. [Google Scholar]
- Rodrigues, L.; Pereira, C.; Leonardo, I.; Fernández, N.; Gaspar, F.; Silva, J.; Reis, R.; Duarte, A.R.C.; Paiva, A.; Matias, A. Terpene-Based Natural Deep Eutectic Systems as Efficient Solvents To Recover Astaxanthin from Brown Crab Shell Residues. ACS Sustain. Chem Eng. 2020, 8, 2246–2259. [Google Scholar] [CrossRef]
- Nunes, A.N.; Roda, A.; Gouveia, L.F.; Fernandez, N.; Bronze, M.R.; Matias, A.A. Astaxanthin Extraction from Marine Crustacean Waste Streams: An Integrated Approach between Microwaves and Supercritical Fluids. ACS Sustain. Chem. Eng. 2021, 9, 3050–3059. [Google Scholar] [CrossRef]
- Salares, V.R.; Young, N.M.; Bernstein, H.J.; Carey, P.R. Resonance Raman spectra of lobster shell carotenoproteins and a model astaxanthin aggregate. A possible photobiological function for the yellow protein. Biochemistry 1977, 16, 4751–4756. [Google Scholar] [CrossRef]
- Pinzaru, S.C.; Müller, C.; Tomšić, S.; Venter, M.M.; Cozar, B.I.; Glamuzina, B. New SERS feature of β-carotene: Consequences for quantitative SERS analysis. J. Raman Spectrosc. 2015, 46, 597–604. [Google Scholar] [CrossRef]
- Nekvapil, F.; Brezestean, I.; Lazar, G.; Firta, C.; Pinzaru, S.C. Resonance Raman and SERRS of fucoxanthin: Prospects for carotenoid quantification in live diatom cells. J. Mol. Struct. 2021, 1250, 131608. [Google Scholar] [CrossRef]
- Guillen, A.D.; Cabo, N. Infrared Spectroscopy in the Study of Edible Oils and Fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Perrin, J.; Vielzeuf, D.; Laporte, D.; Ricolleau, A.; Rossman, G.R.; Floquet, N. Raman characterization of synthetic magnesian calcites. Am. Mineral. 2016, 101, 2525–2538. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Motylenko, M.; Langer, E.; Bazhenov, V.V.; Galli, R.; Stelling, A.L.; Kljajić, Z.; Szatkowski, T.; Kutsova, V.Z.; et al. Renewable chitin from marine sponge as a thermostable biological template for hydrothermal synthesis of hematite nanospheres using principles of extreme biomimetics. Bioinspired Mater. 2015, 1, 12–22. [Google Scholar] [CrossRef]
- Andersen, F.A.; Brecevic, L.; Beuter, G.; Dell’Amico, D.B.; Calderazzo, F.; Bjerrum, N.J.; Underhill, A.E. Infrared Spectra of Amorphous and Crystalline Calcium Carbonate. Acta Chem. Scand. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Lavall, R.L.; Assis, O.B.G.; Campana-Filho, S.P. β-Chitin from the pens of Loligo sp.: Extraction and characterization. Bioresour. Technol. 2007, 98, 2465–2472. [Google Scholar] [CrossRef]
- Coleyshaw, E.E.; Crump, G.; Griffith, W.P. Vibrational spectra of the hydrated carbonate minerals ikaite, monohydrocalcite, lansfordite and nesquehonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2231–2239. [Google Scholar] [CrossRef]
- Dotto, G.L.; McKay, G. Current scenario and challenges in adsorption for water treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
- Yao, H.; Zheng, G.; Li, W.; McDowell, M.T.; Seh, Z.W.; Liu, N.; Lu, Z.; Cui, Y. Crab Shells as Sustainable Templates from Nature for Nanostructured Battery Electrodes. Nano Lett. 2013, 13, 3385–3390. [Google Scholar] [CrossRef]
- Fortune Business Insights. Carotenoids Market Size, Share & COVID-19 Impact Analysis, By Type (Astaxan-thin, Beta-Carotene, Lutein, Zeaxanthin, Lycopene, Canthaxanthin, and others), Source (Synthetic and Natural), Application (Animal Feed, Food & Beverages, Dietary Supplements, Personal Care & Cosmetics, and Pharmaceuti-cals), and Regional Forecast 2020–2027. Available online: https://www.fortunebusinessinsights.com/industry-reports/carotenoids-market-100180 (accessed on 3 January 2023).

| Sample, Treatment | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) |
|---|---|---|
| Blue crab exoskeleton powder, native | 8.2 | 0.049 |
| Blue crab exoskeleton powder, post-extraction | 32.9 | 0.135 |
| Spider crab exoskeleton powder, native | 3.2 | 0.051 |
| Spider crab exoskeleton powder, post-extraction | 32.6 | 0.138 |
| Spiny lobster exoskeleton powder, native | 0 | 0.012 |
| Spiny lobster exoskeleton powder, post-extraction | 1.4 | 0.030 |
| Band Position/cm−1 | |||
|---|---|---|---|
| Blue Crab | Spider Crab | Spiny Lobster | Assignment |
| 280 | 281 | L (libration) CaCO3 | |
| 300–600 | 300–600 | 300–600 | chitin skeletal chains |
| 713 | 713 | V4(CO32−) in-plane bending | |
| 892 | 895 | 897 | chitin skeletal chain |
| 951 | 953 | 953 | Chitin |
| 1003 | protein trace | ||
| 1069 | v1 symm(CO32−) MHC | ||
| 1086 | 1087 | 1087 | v1 symm(CO32−) calcite |
| 1152 | 1152 | 1142 | chitin |
| 1204 | chitin | ||
| 1264 | 1261 | 1265 | ρ(C-H) chitin |
| 1325 | 1323 | 1325 | ρ(C-H) chitin |
| 1370 | 1374 | 1374 | ρ(C-H) chitin |
| 1455 | 1451 | 1451 | ρ(C-H) chitin |
| 1616 | Chitin | ||
| 1664 | 1659 | 1664 | Amide I of chitin |
| Band Position/cm−1 | ||||
|---|---|---|---|---|
| Blue Crab | Spider Crab | Spiny Lobster | Ogresta et al. [29] | Assignment |
| 582 | 569 | 561 | 576 | MHC (lattice water) |
| 707 | 714 | 696 | 700, 714 | v4b(CO32−) out-of-plane bending |
| 873 | 873 | 866 | 864 | v2 asymm(CO32−) calcite + HMC; δ(C−H) chitin |
| 1029 | 1029 | 1026 | 1026 | C−O asym. stretch in the phase ring |
| 1067 | 1067 | 1067 | 1068 | v1(CO32−) MHC; CH2CO stretch chitin |
| 1149 | 1149 | 1149 | 1154 | asym. bridge oxygen stretching |
| 1405 | 1402 | 1399 | 1414 | v3b asym(CO32−) calcite + MHC + ACC |
| 1480 | 1482 | 1492 | 1472 | vasym(CO32−) MHC |
| 1670 | 1682 | 1682 | 1662 | Amide I of chitin |
| 2884 | 2881 | 2884 | v(CH2,3) | |
| 2957 | 2964 | 2960 | vsymm(CH2,3) | |
| 3096 | 3096 | 3096 | v(CH2,3) | |
| 3279 | 3282 | 3276 | 3272 | v(NH) chitn v(O-H) MHC (structural water) |
| 3487 | 3486 | 3480 | 3436 | v(O-H) MHC (structural water) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekvapil, F.; Mihet, M.; Lazar, G.; Pinzaru, S.C.; Gavrilović, A.; Ciorîță, A.; Levei, E.; Tamaș, T.; Soran, M.-L. Comparative Analysis of Composition and Porosity of the Biogenic Powder Obtained from Wasted Crustacean Exoskeletonsafter Carotenoids Extraction for the Blue Bioeconomy. Water 2023, 15, 2591. https://doi.org/10.3390/w15142591
Nekvapil F, Mihet M, Lazar G, Pinzaru SC, Gavrilović A, Ciorîță A, Levei E, Tamaș T, Soran M-L. Comparative Analysis of Composition and Porosity of the Biogenic Powder Obtained from Wasted Crustacean Exoskeletonsafter Carotenoids Extraction for the Blue Bioeconomy. Water. 2023; 15(14):2591. https://doi.org/10.3390/w15142591
Chicago/Turabian StyleNekvapil, Fran, Maria Mihet, Geza Lazar, Simona Cîntă Pinzaru, Ana Gavrilović, Alexandra Ciorîță, Erika Levei, Tudor Tamaș, and Maria-Loredana Soran. 2023. "Comparative Analysis of Composition and Porosity of the Biogenic Powder Obtained from Wasted Crustacean Exoskeletonsafter Carotenoids Extraction for the Blue Bioeconomy" Water 15, no. 14: 2591. https://doi.org/10.3390/w15142591
APA StyleNekvapil, F., Mihet, M., Lazar, G., Pinzaru, S. C., Gavrilović, A., Ciorîță, A., Levei, E., Tamaș, T., & Soran, M.-L. (2023). Comparative Analysis of Composition and Porosity of the Biogenic Powder Obtained from Wasted Crustacean Exoskeletonsafter Carotenoids Extraction for the Blue Bioeconomy. Water, 15(14), 2591. https://doi.org/10.3390/w15142591










