Response of Upstream Behavior and Hydrodynamic Factors of Anguilla Japonica in a Combined Bulkhead Fishway under Tidal Conditions
Abstract
1. Introduction
2. Materials and Methods
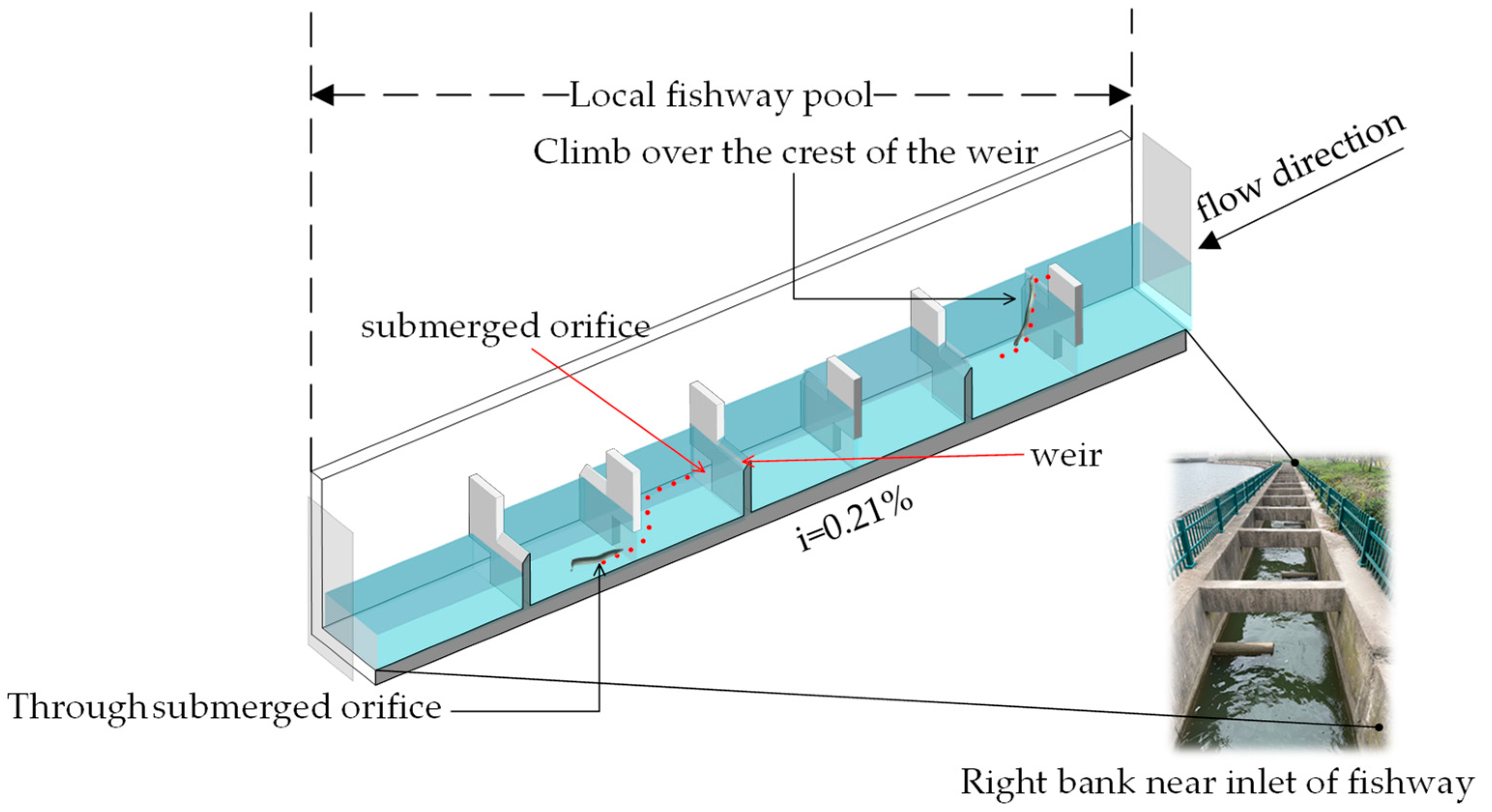
2.1. Research Fishway Overview
2.2. Scaling Scale Experiment
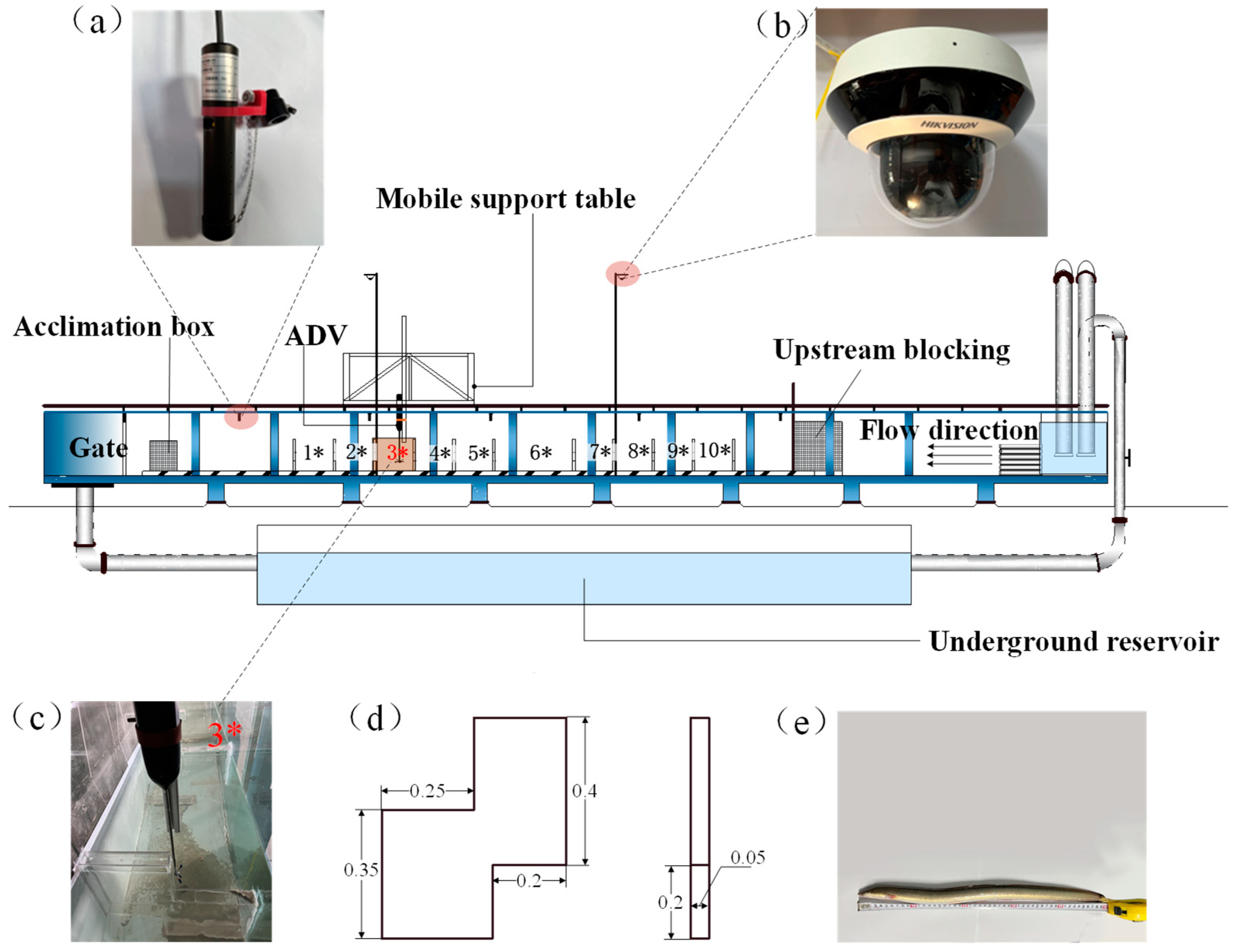
2.2.1. Experimental Facilities
2.2.2. Hydraulics
2.2.3. Fish
2.2.4. Test Method
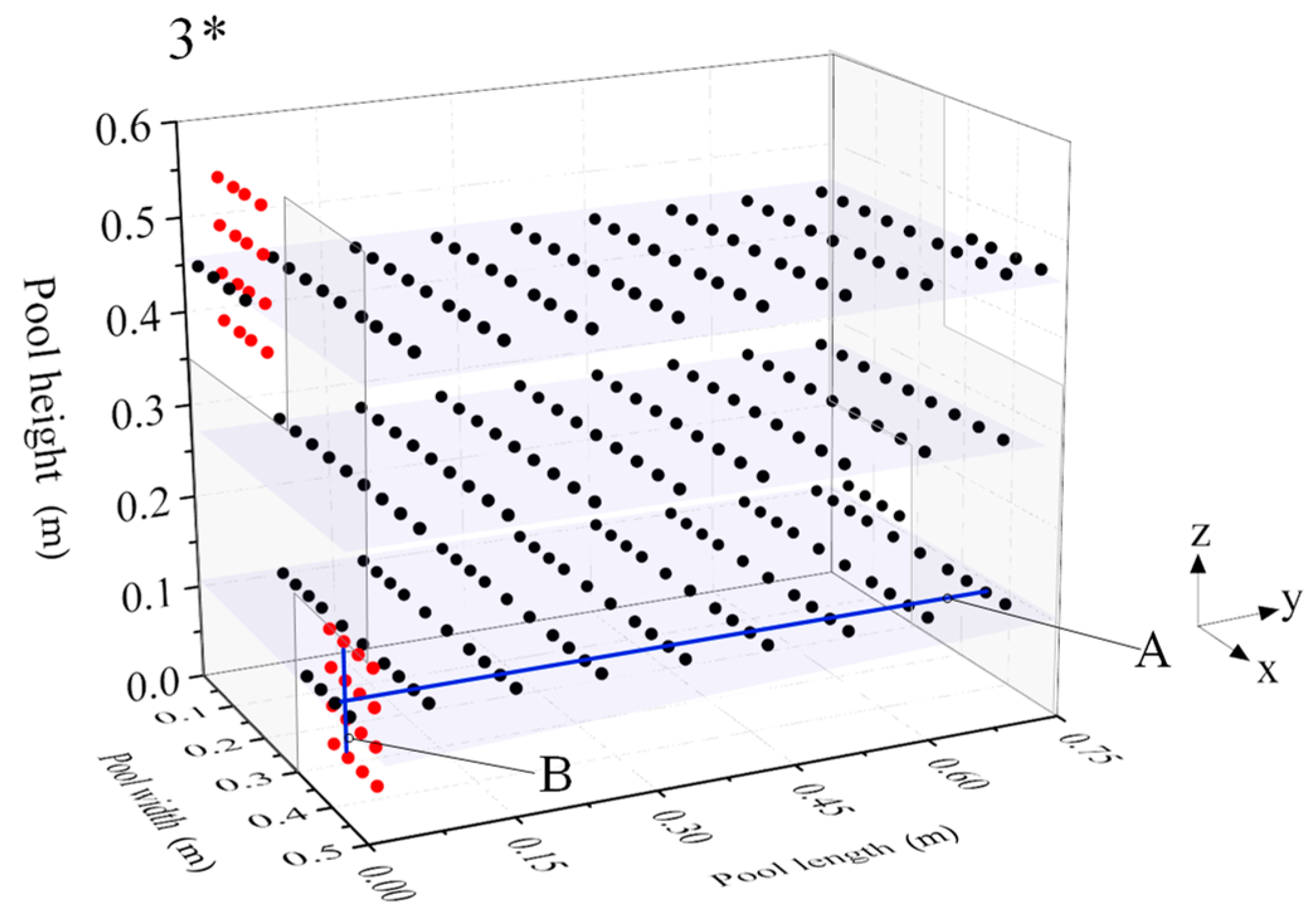
2.3. Data Analysis
3. Results
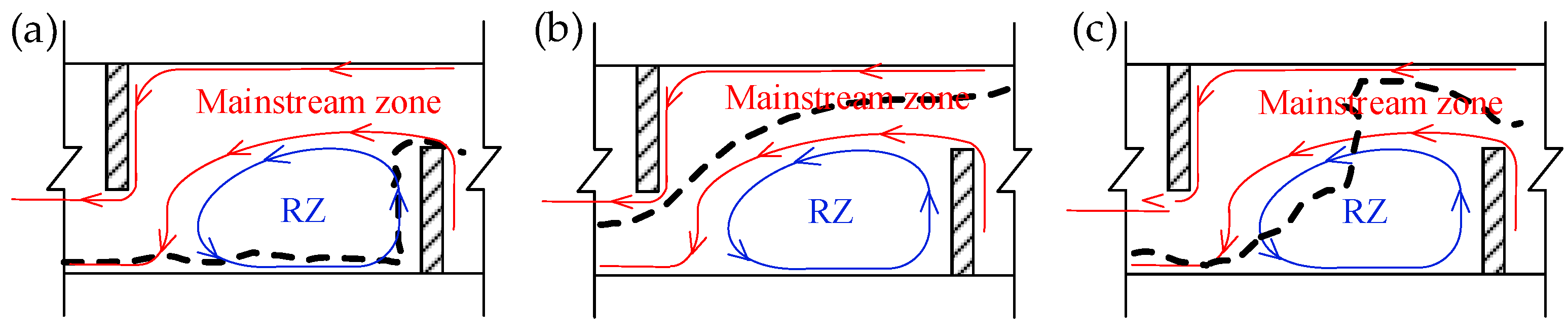
3.1. Flow Field Results Verification
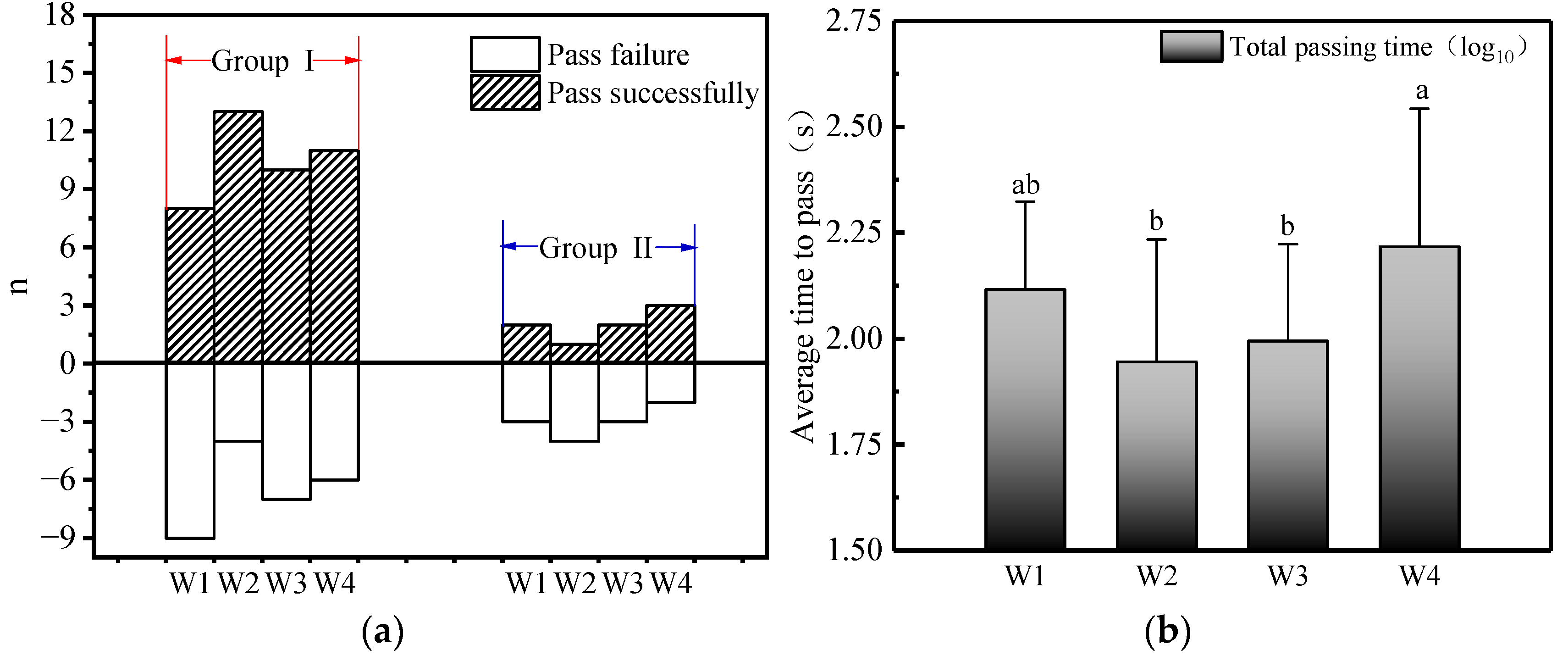
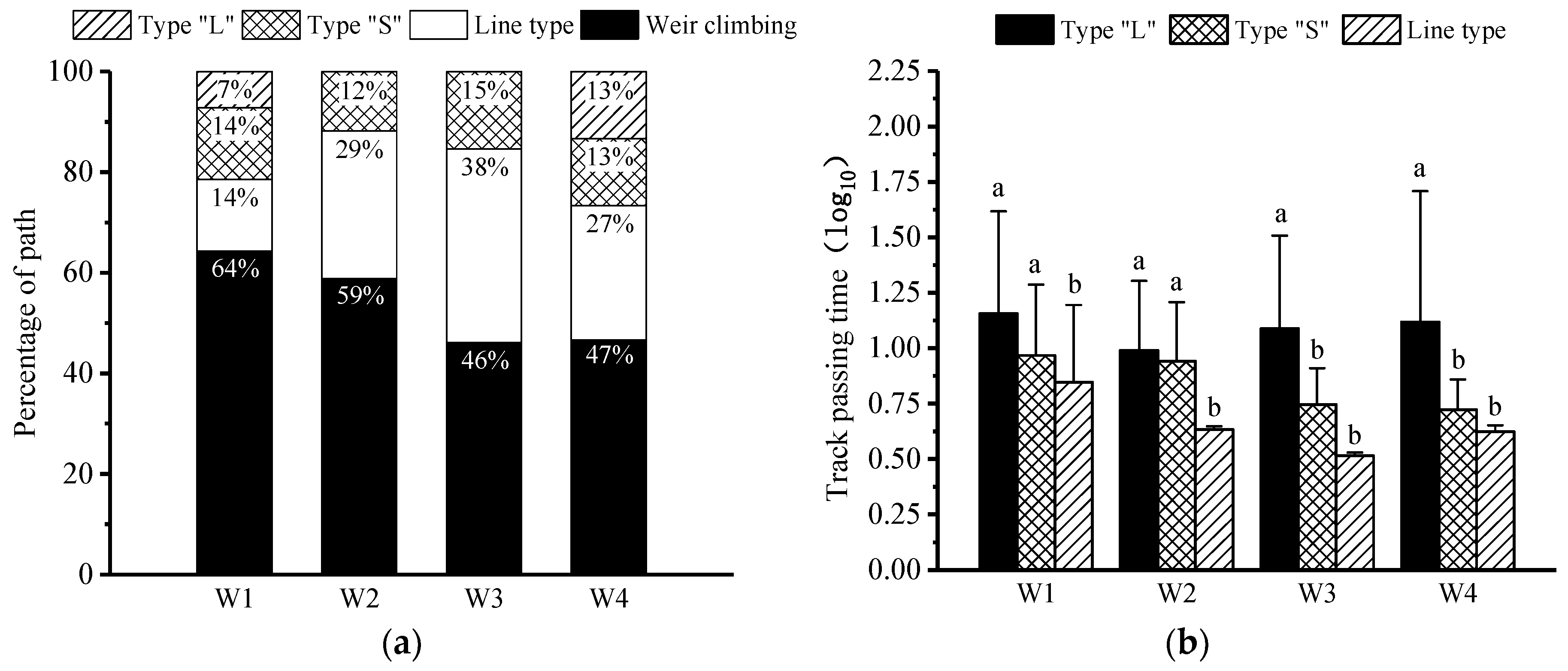
3.2. Pass Rate and Pass Time
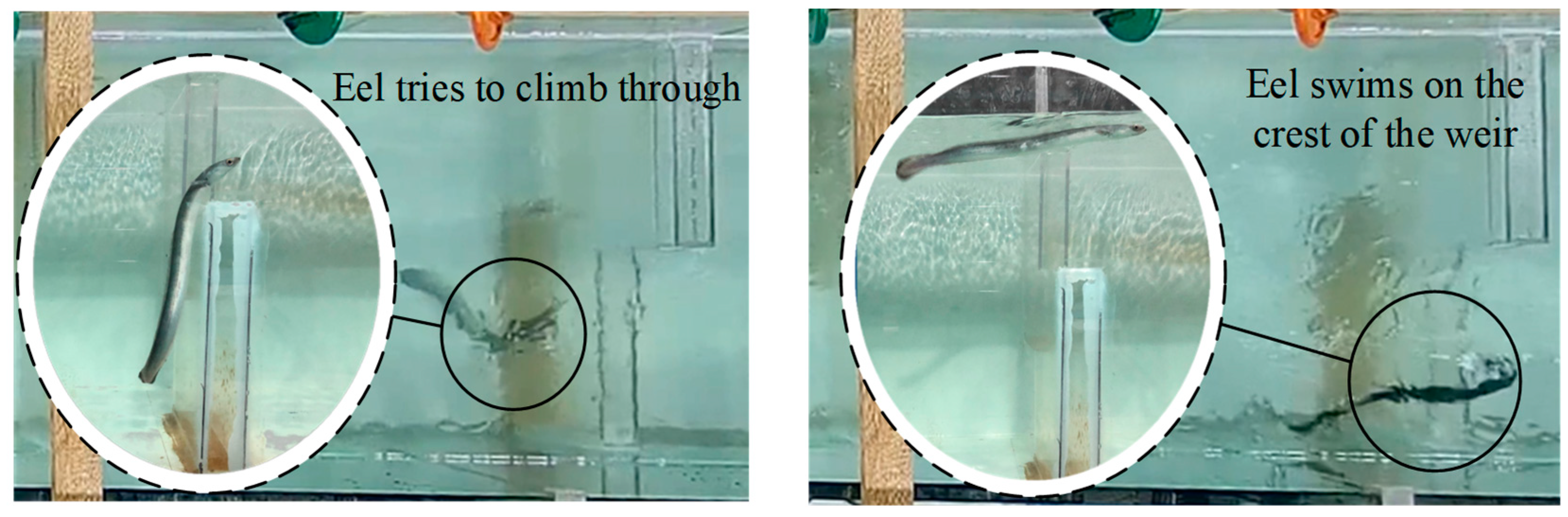
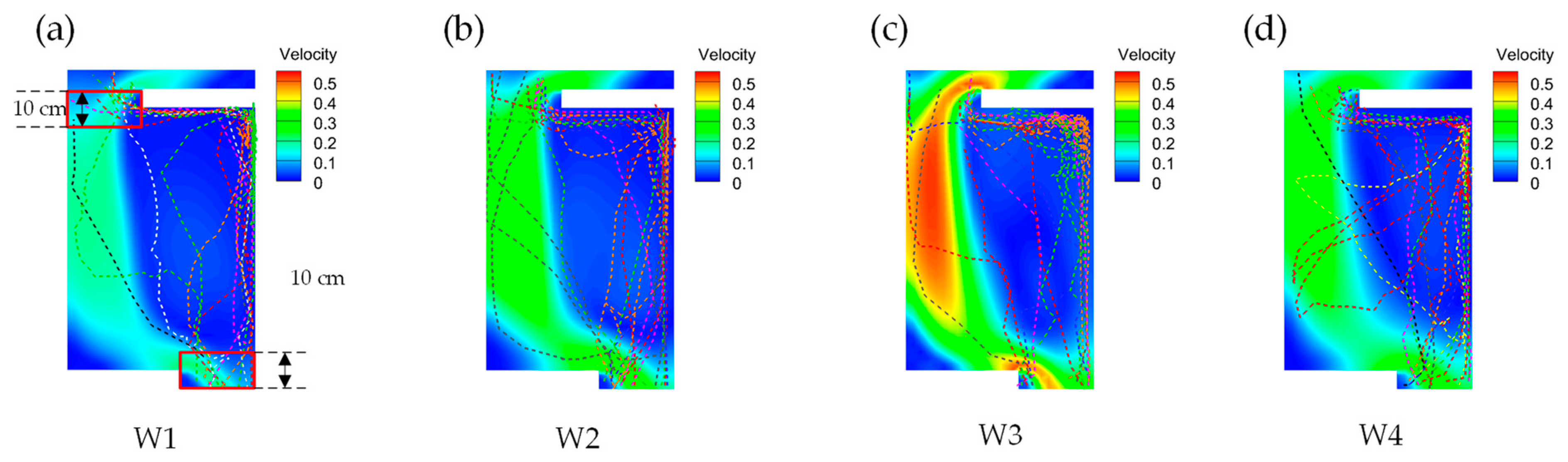
3.3. Upstream Paths and Access Strategies
3.4. Upward Preference
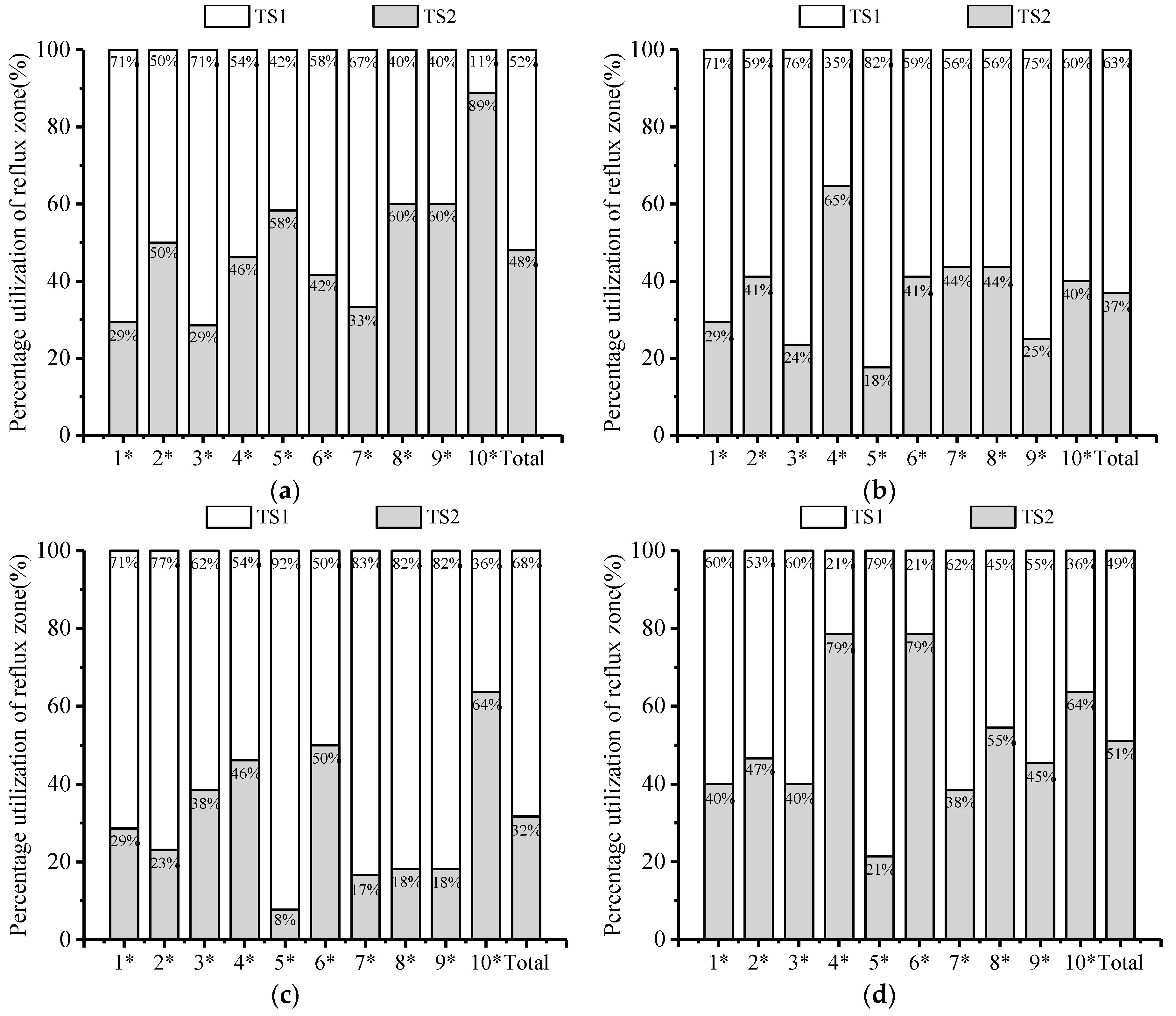
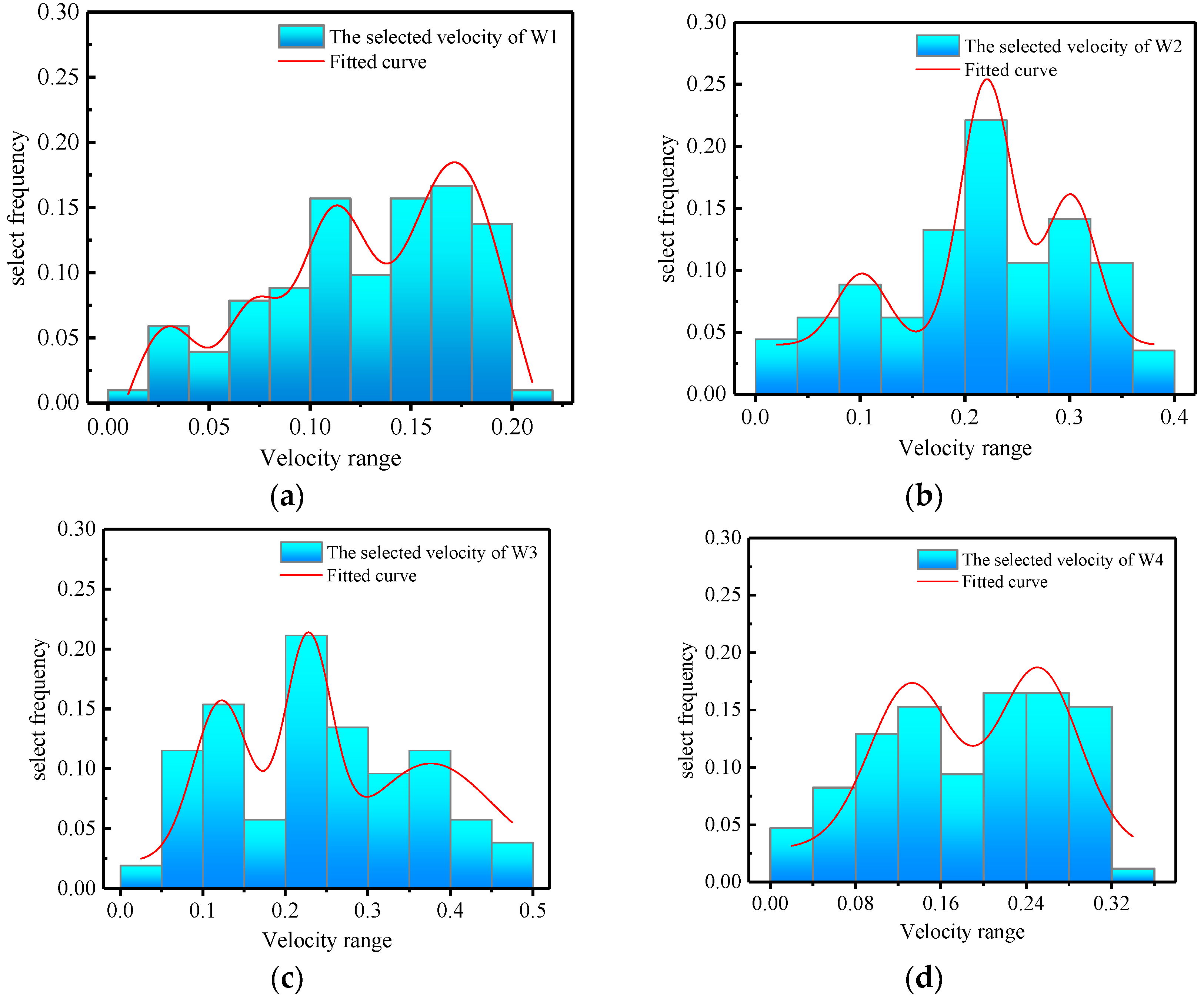
3.4.1. Flow Rate Selection Preference
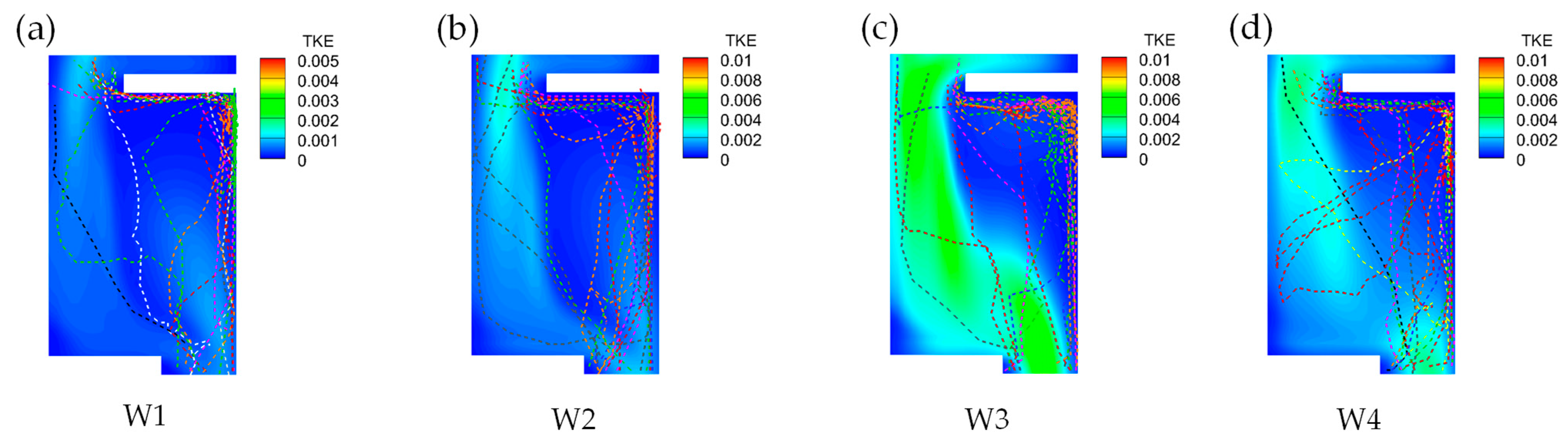
3.4.2. Disordered Turbulent Kinetic Energy Selection Preference
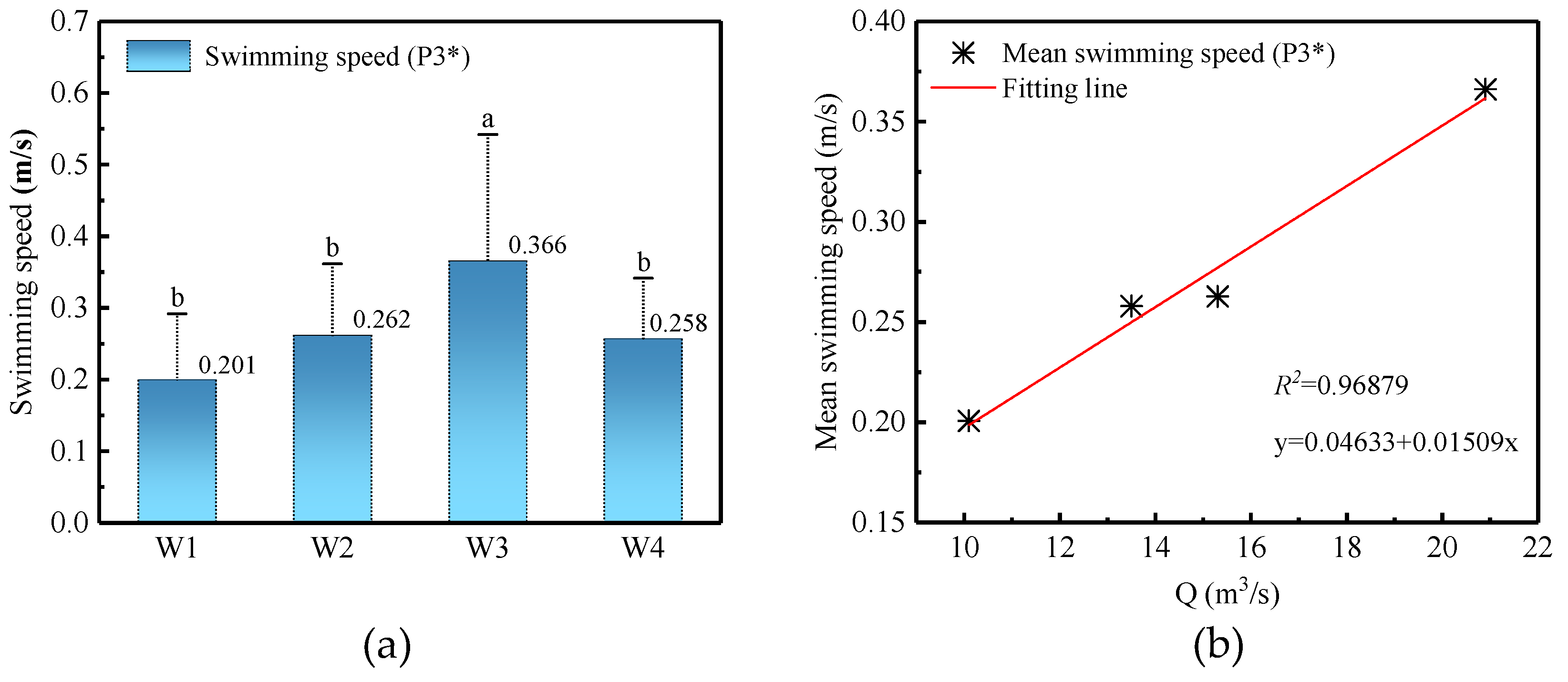
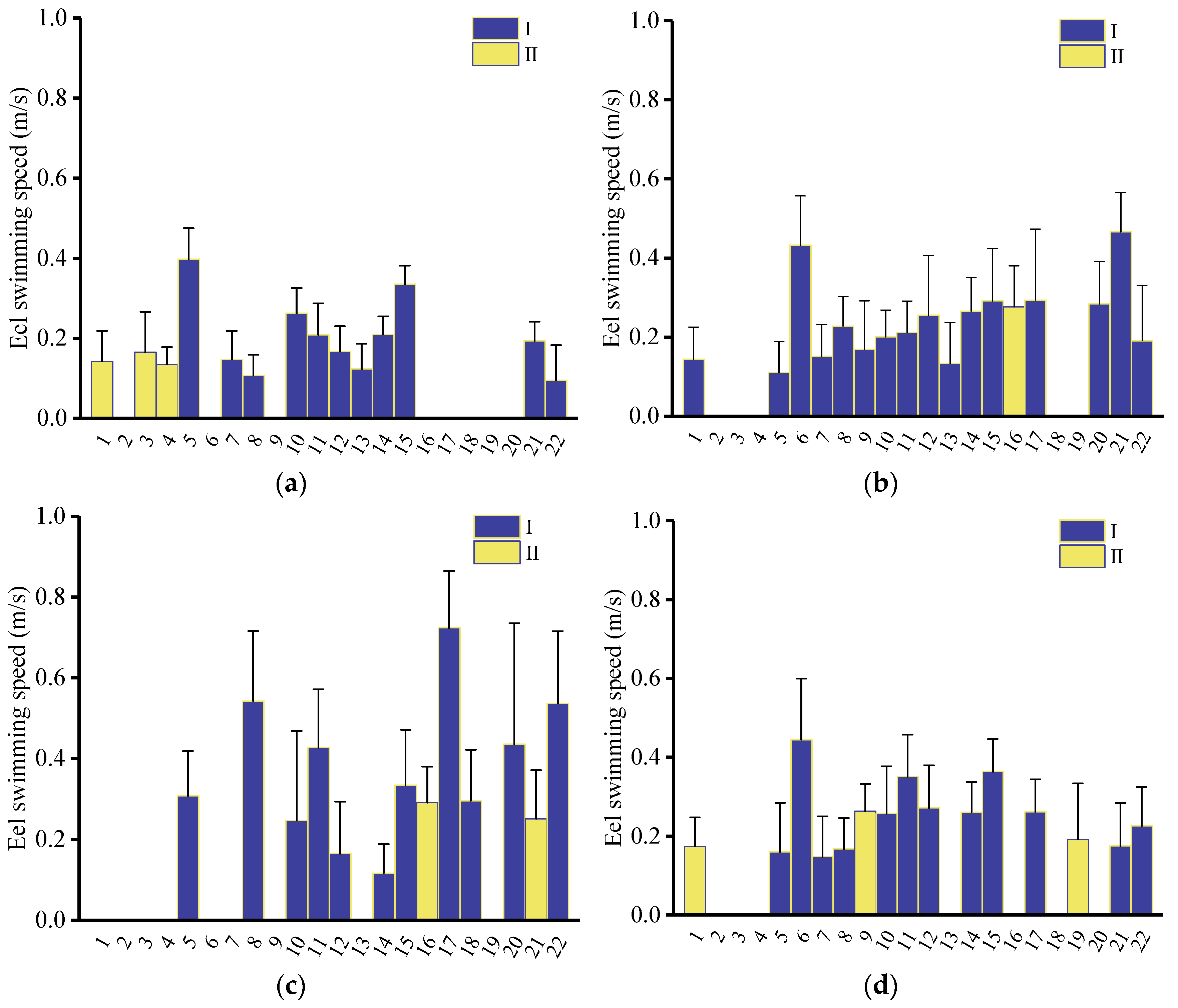
3.4.3. Swimming Speed
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, X. Review of fishway research in China. Ecol. Eng. 2018, 115, 91–95. [Google Scholar] [CrossRef]
- Schilt, C.R. Developing fish passage and protection at hydropower dams. Appl. Anim. Behav. Sci. 2007, 104, 295–325. [Google Scholar] [CrossRef]
- Zielinski, D.P.; Freiburger, C. Advances in fish passage in the Great Lakes basin. J. Great Lakes Res. 2021, 47, S439–S447. [Google Scholar] [CrossRef]
- Brooks, D.A. The hydrokinetic power resource in a tidal estuary: The Kennebec River of the central Maine coast. Renew. Energy 2011, 36, 1492–1501. [Google Scholar] [CrossRef]
- Rolls, R.J.; Faggotter, S.J.; Roberts, D.T.; Burford, M.A. Simultaneous assessment of two passage facilities for maintaining hydrological connectivity for subtropical coastal riverine fish. Ecol. Eng. 2018, 124, 77–87. [Google Scholar] [CrossRef]
- Zielinski, D.P.; Gaden, M.; Muir, A.M. Editorial: Global fish passage issues. Aquac. Fish. 2021, 6, 111–112. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Tong, J.L.; Huang, Z. Turbulence characteristics in a weir-orifice-slot combined fishway with an identical layout. J. Turbul. 2022, 23, 327–351. [Google Scholar] [CrossRef]
- Halvorsen, S.; Korslund, L.; Gustavsen, P.Ø.; Slettan, A. Environmental DNA analysis indicates that migration barriers are decreasing the occurrence of European eel (Anguilla anguilla) in distance from the sea. Glob. Ecol. Conserv. 2020, 24, e01245. [Google Scholar] [CrossRef]
- Kaifu, K. Challenges in assessments of Japanese eel stock. Mar. Policy 2019, 102, 1–4. [Google Scholar] [CrossRef]
- Kaifu, K.; Yokouchi, K. Increasing or decreasing?—Current status of the Japanese eel stock. Fish. Res. 2019, 220, 105348. [Google Scholar] [CrossRef]
- Kaifu, K.; Yokouchi, K.; Miller, M.J.; Washitani, I. Management of glass eel fisheries is not a sufficient measure to recover a local Japanese eel population. Mar. Policy 2021, 134, 104806. [Google Scholar] [CrossRef]
- Bernas, R.; Debowski, P.; Skora, M.; Radtke, G.; Morzuch, J.; Kapusta, A. Low mortality rate in silver eels (Anguilla anguilla L.) passing through a small hydropower station. Mar. Freshw. Res. 2017, 68, 2081–2086. [Google Scholar] [CrossRef]
- Heisey, P.G.; Mathur, D.; Phipps, J.L.; Avalos, J.C.; Hoffman, C.E.; Adanns, S.W.; De-Oliveira, E. Passage survival of European and American eels at Francis and propeller turbines. J. Fish Biol. 2019, 95, 1172–1183. [Google Scholar] [CrossRef]
- Trancart, T.; Carpentier, A.; Acou, A.; Charrier, F.; Mazel, V.; Danet, V.; Feunteun, E. When “safe” dams kill: Analyzing combination of impacts of overflow dams on the migration of silver eels. Ecol. Eng. 2020, 145, 105741. [Google Scholar] [CrossRef]
- McCleave, J.D. Swimming performance of European eel (Anguilla anguilla (L.)) elvers. J. Fish Biol. 1980, 16, 445–452. [Google Scholar] [CrossRef]
- Kerr, J.R.; Karageorgopoulos, P.; Kemp, P.S. Efficacy of a side-mounted vertically oriented bristle pass for improving upstream passage of European eel (Anguilla anguilla) and river lamprey (Lampetra fluviatilis) at an experimental Crump weir. Ecol. Eng. 2015, 85, 121–131. [Google Scholar] [CrossRef]
- Foulds, W.L.; Lucas, M.C. Extreme inefficiency of two conventional, technical fishways used by European river lamprey (Lampetra fluviatilis). Ecol. Eng. 2013, 58, 423–433. [Google Scholar] [CrossRef]
- Feunteun, E.; Acou, A.; Guillouet, J.; Laffaille, P.; Legault, A. Spatial distribution of an eel population (Anguilla anguilla L.) in a small coastal catchment of northern Brittany (France). Consequences of hydraulic works. Knowl. Manag. Aquat. Ecosyst. 1998, 349, 129–139. [Google Scholar] [CrossRef]
- Cowx, I.G.; O’Grady, K.T.; Knights, B.; White, E.M. Enhancing immigration and recruitment of eels: The use of passes and associated trapping systems. Fish. Manag. Ecol. 1998, 5, 459–471. [Google Scholar]
- Ballu, A.; Pineau, G.; Calluaud, D.; David, L. Experimental-Based Methodology to Improve the Design of Vertical Slot Fishways. J. Hydraul. Eng. 2019, 145, 04019031. [Google Scholar] [CrossRef]
- Cao, P.; Mu, X.; Li, X.; Baiyin, B.; Wang, X.; Zhen, W. Relationship between Upstream Swimming Behaviors of Juvenile Grass Carp and Characteristic Hydraulic Conditions of a Vertical Slot Fishway. Water 2021, 13, 1299. [Google Scholar] [CrossRef]
- Li, G.; Sun, S.; Liu, H.; Zheng, T. Schizothorax prenanti swimming behavior in response to different flow patterns in vertical slot fishways with different slot positions. Sci. Total Environ. 2021, 754, 142142. [Google Scholar] [CrossRef]
- Daneshfaraz, R.; Aminvash, E.; Bagherzadeh, M.; Ghaderi, A.; Kuriqi, A.; Najibi, A.; Ricardo, A.M. Laboratory Investigation of Hydraulic Parameters on Inclined Drop Equipped with Fishway Elements. Symmetry 2021, 13, 1643. [Google Scholar] [CrossRef]
- Daneshfaraz, R.; Aminvash, E.; Abraham, J. Hydraulic Characteristics of Fish-passes on Inclined Drops with Multifarious Configurations: An Experimental Study. Res. Dev. Sci. Technol. 2022, 4, 108–123. [Google Scholar] [CrossRef]
- Dockery, D.R.; McMahon, T.E.; Kappenman, K.M.; Blank, M. Evaluation of swimming performance for fish passage of longnose dace Rhinichthys cataractae using an experimental flume. J. Fish Biol. 2017, 90, 980–1000. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.A.; Caudill, C.C.; Syms, J.C.; Tonina, D. Context-dependent responses to turbulence for an anguilliform swimming fish, Pacific lamprey, during passage of an experimental vertical-slot weir. Ecol. Eng. 2017, 106, 296–307. [Google Scholar] [CrossRef]
- Newbold, L.R.; Shi, X.; Hou, Y.; Han, D.; Kemp, P.S. Swimming performance and behaviour of bighead carp (Hypophthalmichthys nobilis): Application to fish passage and exclusion criteria. Ecol. Eng. 2016, 95, 690–698. [Google Scholar] [CrossRef]
- Goring, D.G.; Nikora, V.I. Despiking Acoustic Doppler Velocimeter Data. J. Hydraul. Eng. 2002, 128, 117–126. [Google Scholar] [CrossRef]
- Fuentes-Pérez, J.F.; Quaresma, A.L.; Pinheiro, A.; Sanz-Ronda, F.J. OpenFOAM vs FLOW-3D: A comparative study of vertical slot fishway modelling. Ecol. Eng. 2022, 174, 106446. [Google Scholar] [CrossRef]
- Zhong, Z.; Ruan, T.; Hu, Y.; Liu, J.; Liu, B.; Xu, W. Experimental and numerical assessment of hydraulic characteristic of a new semi-frustum weir in the pool-weir fishway. Ecol. Eng. 2021, 170, 106362. [Google Scholar] [CrossRef]
- Kjærås, H.; Baktoft, H.; Silva, A.T.; Gjelland, K.Ø.; Økland, F.; Forseth, T.; Szabó-Mészáros, M.; Calles, O. Three-dimensional migratory behaviour of European silver eels (Anguilla anguilla) approaching a hydropower plant. J. Fish Biol. 2023, 102, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Welsh, S.A.; Liller, H.L. Environmental Correlates of Upstream Migration of Yellow-Phase American Eels in the Potomac River Drainage. Trans. Am. Fish. Soc. 2013, 142, 483–491. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Jiang, L.B.; Mao, B. Experimental study on turbulent flow structure of vertical slit type fish passage with heterolateral arrangement. J. Hydroecol. 2021, 42, 129–136. [Google Scholar] [CrossRef]
- Fuentes-Pérez, J.F.; Eckert, M.; Tuhtan, J.A.; Ferreira, M.T.; Kruusmaa, M.; Branco, P. Spatial preferences of Iberian barbel in a vertical slot fishway under variable hydrodynamic scenarios. Ecol. Eng. 2018, 125, 131–142. [Google Scholar] [CrossRef]
- Cai, L.; Chen, J.; Johnson, D.; Tu, Z.; Huang, Y. Effect of body length on swimming capability and vertical slot fishway design. Glob. Ecol. Conserv. 2020, 22, e00990. [Google Scholar] [CrossRef]
- Shiau, J.; Watson, J.R.; Cramp, R.L.; Gordos, M.A.; Franklin, C.E. Interactions between water depth, velocity and body size on fish swimming performance: Implications for culvert hydrodynamics. Ecol. Eng. 2020, 156, 105987. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, R.; Wang, Y.; Yuan, Q.; Zhang, Z.; Li, K. Study on the swimming ability of endemic fish in the lower reaches of the Yangtze River: A case study. Glob. Ecol. Conserv. 2020, 22, e01014. [Google Scholar] [CrossRef]
- Silva, A.T.; Santos, J.M.; Ferreira, M.T.; Pinheiro, A.N.; Katopodis, C. Effects of water velocity and turbulence on the behaviour of Iberian barbel (Luciobarbus bocagei, Steindachner 1864) in an experimental pool-type fishway. River Res. Appl. 2011, 27, 360–373. [Google Scholar] [CrossRef]
- Barbin, G.P.; Krueger, W.H. Behaviour and swimming performance of elvers of the American eel, Anguitta rostrata, in an experimental flume. J. Fish Biol. 1994, 45, 111–121. [Google Scholar] [CrossRef]


| Group | H1 a (m) | H2 b (m) | ∆H c (cm) | ∆Z d (cm) |
|---|---|---|---|---|
| W1 | 0.575 | 0.561 | 1.5 | 0.6 |
| W2 | 0.525 | 5 | 1.8 | |
| W3 | 0.475 | 10 | 3.6. | |
| W4 | 0.500 | 0.450 | 5 | 1.8 |
| Group ID | L cm | L ± SE cm | W g | W ± SE cm |
|---|---|---|---|---|
| I | 26.6~39.5 | 4.4 | 80.0~119.2 | 9.8 |
| II | 42.5~68.4 | 7.8 | 131~334 | 62.2 |
| Group | Number of Samples | Passage Rate | Total Number of Turns Back | Turnback Rate | Number of Weir Crossings | Over-Weir Occurrence Rate |
|---|---|---|---|---|---|---|
| W1 | 22 | 45.45% | 32 | 84.21% | 2 | 10.53% |
| W2 | 22 | 68.18% | 15 | 31.82% | 3 | 12.50% |
| W3 | 22 | 50.00% | 25 | 75.00% | 12 | 18.75% |
| W4 | 22 | 54.55% | 23 | 62.50% | 9 | 37.50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.; Lian, X.; Bai, F.; Hao, D.; Li, D.; Fang, Z. Response of Upstream Behavior and Hydrodynamic Factors of Anguilla Japonica in a Combined Bulkhead Fishway under Tidal Conditions. Water 2023, 15, 2585. https://doi.org/10.3390/w15142585
Ye Z, Lian X, Bai F, Hao D, Li D, Fang Z. Response of Upstream Behavior and Hydrodynamic Factors of Anguilla Japonica in a Combined Bulkhead Fishway under Tidal Conditions. Water. 2023; 15(14):2585. https://doi.org/10.3390/w15142585
Chicago/Turabian StyleYe, Zhou, Xin Lian, Fuqing Bai, Di Hao, Dongfeng Li, and Zhihao Fang. 2023. "Response of Upstream Behavior and Hydrodynamic Factors of Anguilla Japonica in a Combined Bulkhead Fishway under Tidal Conditions" Water 15, no. 14: 2585. https://doi.org/10.3390/w15142585
APA StyleYe, Z., Lian, X., Bai, F., Hao, D., Li, D., & Fang, Z. (2023). Response of Upstream Behavior and Hydrodynamic Factors of Anguilla Japonica in a Combined Bulkhead Fishway under Tidal Conditions. Water, 15(14), 2585. https://doi.org/10.3390/w15142585







