The Vital Roles of Parent Material in Driving Soil Substrates and Heavy Metals Availability in Arid Alkaline Regions: A Case Study from Egypt
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Specification
2.2. Soil Sampling and Laboratory Analysis
2.3. Mineralogical Analysis
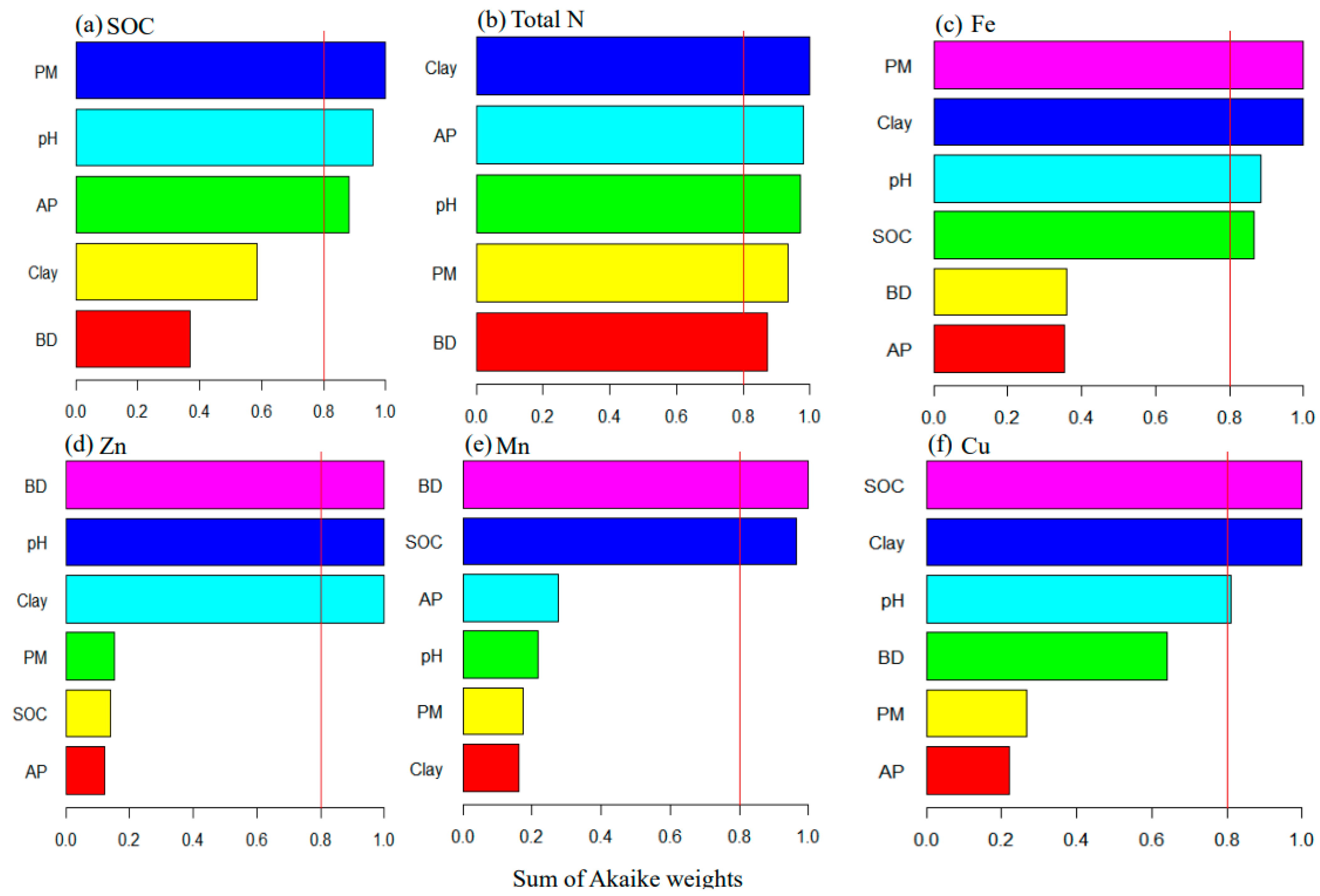
2.4. Data Analyses
3. Results
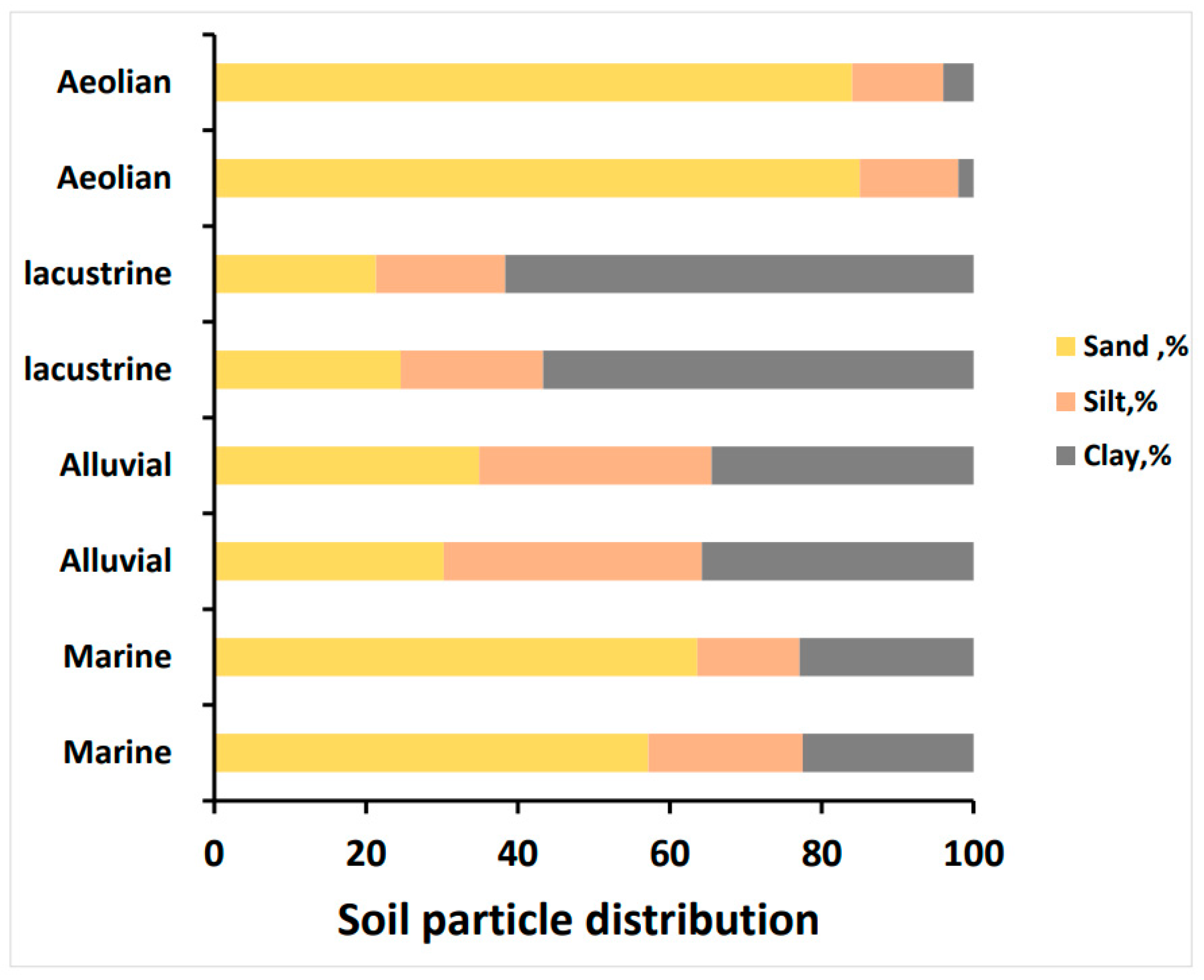
3.1. Morphological Properties of Studied Soils
3.2. Soil Taxonomy of the Studied Pedons
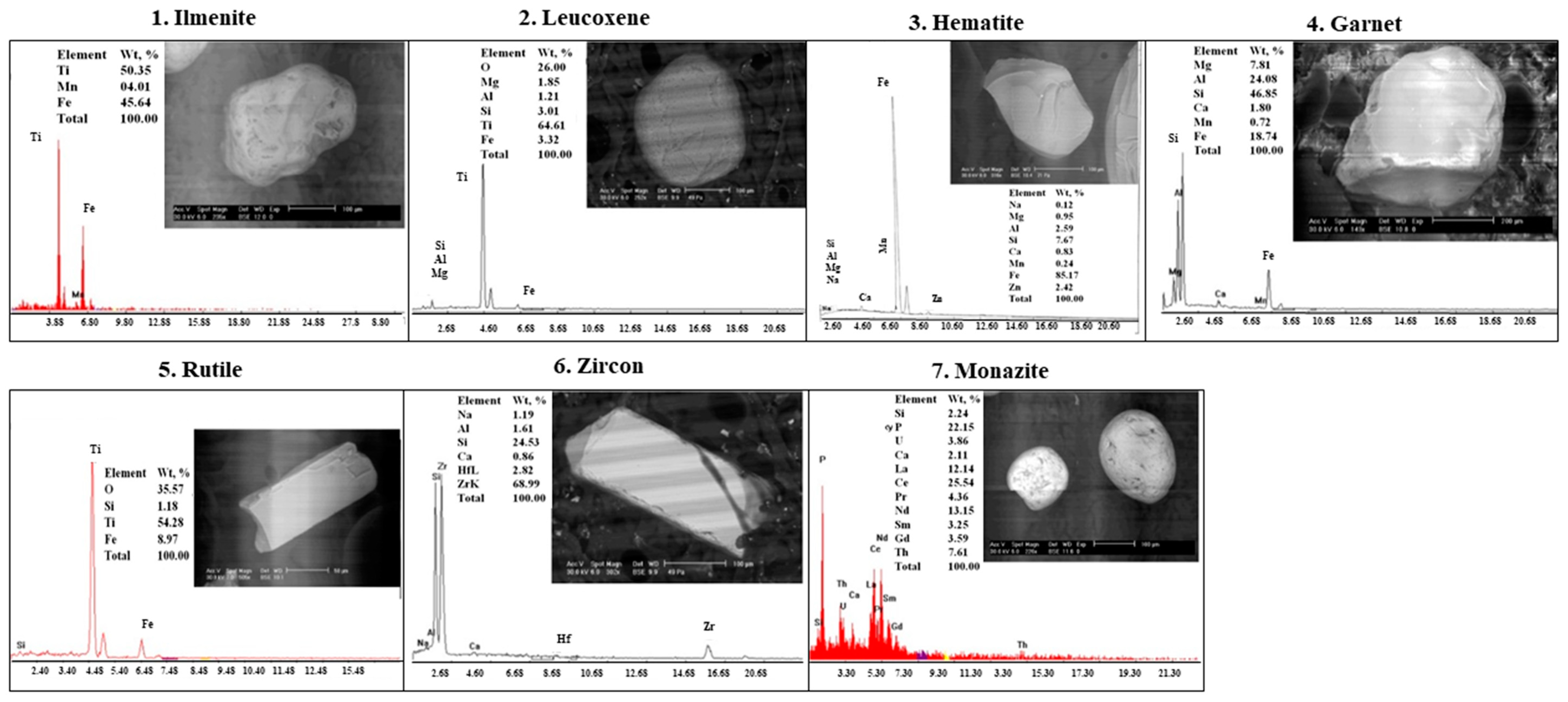
3.3. Mineralogy of Sand and Clay Fractions
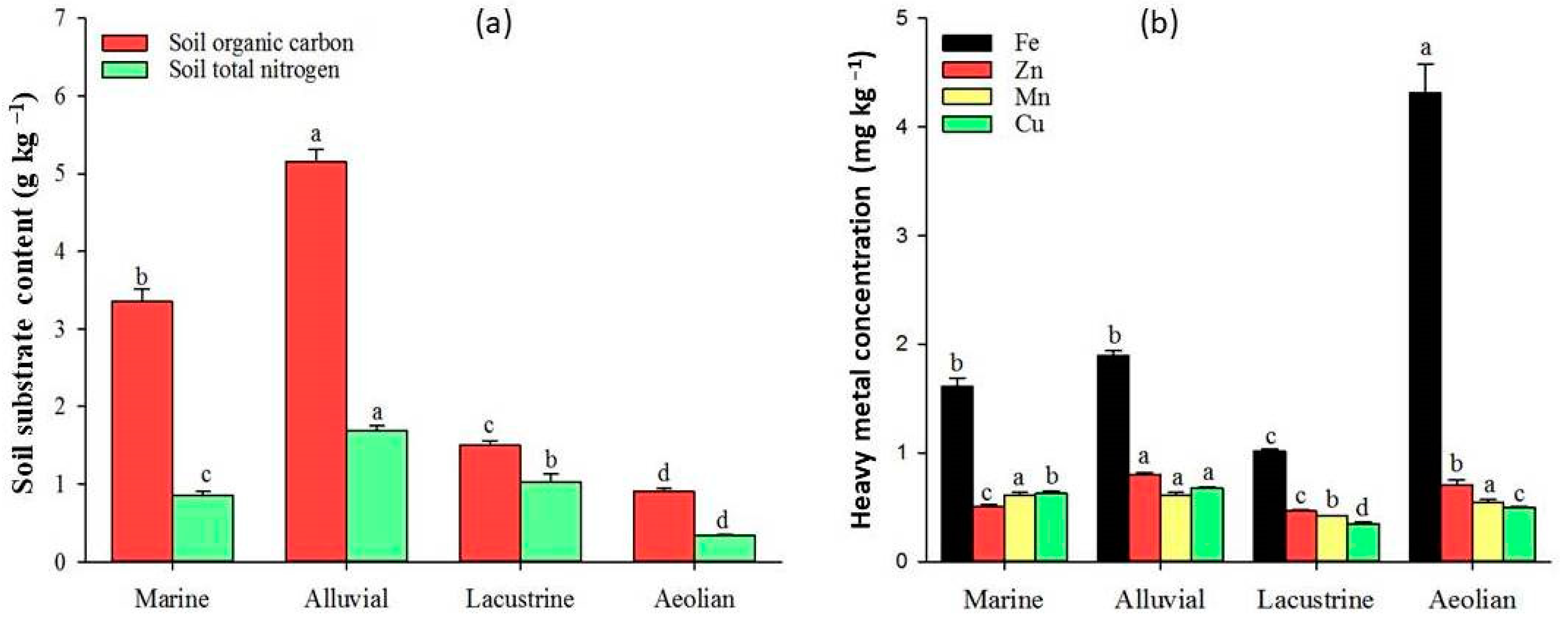
3.4. Patterns and Controlling Factors of Soil Substrate Availability in Arid Regions
3.5. Patterns and Controlling Factors of Heavy Metal Availability in Arid Regions
4. Discussion
4.1. The Role of Parent Material in Controlling Soil Substrate Availability in Arid Regions
4.2. Drivers of Heavy Metal Availability in Arid Regions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, M. The importance of parent material in soil classification: A review in a historical context. Catena 2019, 182, 104131. [Google Scholar] [CrossRef]
- Wysocki, D.A.; Lietzke, D.; Zelazny, L. Effects of parent material weathering on chemical and mineralogical properties of selected Hapludults in the Virginia Piedmont. Soil Sci. Soc. Am. J. 1988, 52, 196–203. [Google Scholar] [CrossRef]
- Suther, B.E.; Leigh, D.S.; West, L.T. Soil Chemistry and Clay Mineralogy of an Alluvial Chronosequence from the North Carolina Sandhills of the Upper Coastal Plain, USA. Soil Syst. 2021, 6, 1. [Google Scholar] [CrossRef]
- Irmak, S.; Surucu, A.; Aydogdu, I. Effects of different parent material on the mineral characteristics of soils in the arid region of Turkey. Pak. J. Biol. Sci. PJBS 2007, 10, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Murphy, B. Parent material and world soil distribution. In Proceedings of the 17th World Congress of Soil Science, Bangkok, Thailand, 14–21 August 2002; pp. 1–2215. [Google Scholar]
- Khorasani, M.; Ghorbani, R.R. Evaluation of carbon sequestration potential in corn fields with different management systems. Soil Tillage Res. 2013, 133, 2531. [Google Scholar]
- Alnaimy, M.A.; Shahin, S.A.; Afifi, A.A.; Ewees, A.A.; Junakova, N.; Balintova, M.; Abd Elaziz, M. Spatio Prediction of Soil Capability Modeled with Modified RVFL Using Aptenodytes Forsteri Optimization and Digital Soil Assessment Technique. Sustainability 2022, 14, 14996. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Yin, X.; Licht, M.A. Soil carbon and nitrogen changes as influenced by tillage and cropping systems in some Iowa soils. Agric. Ecosyst. Environ. 2005, 105, 635–647. [Google Scholar] [CrossRef]
- Angst, G.; Messinger, J.; Greiner, M.; Häusler, W.; Hertel, D.; Kirfel, K.; Kögel-Knabner, I.; Leuschner, C.; Rethemeyer, J.; Mueller, C.W. Soil organic carbon stocks in topsoil and subsoil controlled by parent material, carbon input in the rhizosphere, and microbial-derived compounds. Soil Biol. Biochem. 2018, 122, 19–30. [Google Scholar] [CrossRef]
- Gray, J.M.; Bishop, T.F. Change in soil organic carbon stocks under 12 climate change projections over New South Wales, Australia. Soil Sci. Soc. Am. J. 2016, 80, 1296–1307. [Google Scholar] [CrossRef]
- Xue, Z.; An, S. Changes in soil organic carbon and total nitrogen at a small watershed scale as the result of land use conversion on the loess plateau. Sustainability 2018, 10, 4757. [Google Scholar] [CrossRef]
- Elrys, A.S.; Desoky, E.-S.M.; Alnaimy, M.A.; Zhang, H.; Zhang, J.-B.; Cai, Z.-C.; Cheng, Y. The food nitrogen footprint for African countries under fertilized and unfertilized farms. J. Environ. Manag. 2021, 279, 111599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.-J.; Yang, C.; Zhan, L.-Q.; Wu, W.; Liu, H.-B. The stratification of soil organic carbon and total nitrogen affected by parent material and cropping system. Catena 2022, 210, 105898. [Google Scholar] [CrossRef]
- Van Eynde, E.; Groenenberg, J.E.; Hoffland, E.; Comans, R.N. Solid-solution partitioning of micronutrients Zn, Cu and B in tropical soils: Mechanistic and empirical models. Geoderma 2022, 414, 115773. [Google Scholar] [CrossRef]
- Elrys, A.S.; Merwad, A.-R.M.; Abdo, A.I.; Abdel-Fatah, M.K.; Desoky, E.-S.M. Does the application of silicon and Moringa seed extract reduce heavy metals toxicity in potato tubers treated with phosphate fertilizers? Environ. Sci. Pollut. Res. 2018, 25, 16776–16787. [Google Scholar] [CrossRef]
- Anderson, D. The effect of parent material and soil development on nutrient cycling in temperate ecosystems. Biogeochemistry 1988, 5, 71–97. [Google Scholar] [CrossRef]
- Zinn, Y.L.; de Faria, J.A.; de Araujo, M.A.; Skorupa, A.L.A. Soil parent material is the main control on heavy metal concentrations in tropical highlands of Brazil. Catena 2020, 185, 104319. [Google Scholar] [CrossRef]
- Zhenghu, D.; Honglang, X.; Zhibao, D.; Gang, W.; Drake, S. Morphological, physical and chemical properties of aeolian sandy soils in northern China. J. Arid. Environ. 2007, 68, 66–76. [Google Scholar] [CrossRef]
- Ugolini, F.; Hillier, S.; Certini, G.; Wilson, M. The contribution of aeolian material to an Aridisol from southern Jordan as revealed by mineralogical analysis. J. Arid. Environ. 2008, 72, 1431–1447. [Google Scholar] [CrossRef]
- Alnaimy, M.; Zelenakova, M.; Vranayova, Z.; Abu-Hashim, M. Effects of Temporal Variation in Long-Term Cultivation on Organic Carbon Sequestration in Calcareous Soils: Nile Delta, Egypt. Sustainability 2020, 12, 4514. [Google Scholar] [CrossRef]
- Ibrahim, A.; Gemail, K.S.; Bedair, S.; Saada, S.A.; Koch, M.; Nosair, A. An Integrated Approach to Unravel the Structural Controls on Groundwater Potentialities in Hyper-arid Regions Using Satellite and Land-Based Geophysics: A Case Study in Southwestern Desert of Egypt. Surv. Geophys. 2022, 44, 783–819. [Google Scholar] [CrossRef]
- Ferreira, E.P.; Anjos, L.H.C.d.; Pereira, M.G.; Valladares, G.S.; Cipriano-Silva, R.; Azevedo, A.C.d. Genesis and classification of soils containing carbonate on the Apodi Plateau, Brazil. Rev. Bras. Cienc. Solo 2016, 40, 1–20. [Google Scholar] [CrossRef]
- Wilford, J.; De Caritat, P.; Bui, E. Modelling the abundance of soil calcium carbonate across Australia using geochemical survey data and environmental predictors. Geoderma 2015, 259, 81–92. [Google Scholar] [CrossRef]
- Ismail, A. Soil properties and moisture characteristics and their relationship with crop mid-day stress in the Sudan Gezira. Geo J. 1991, 23, 233–237. [Google Scholar] [CrossRef]
- Caicedo, B.; Mendoza, C.; Lizcano, A.; Lopez-Caballero, F. Some contributions to mechanical behaviors of lacustrine deposit in Bogotá, Colombia. J. Rock Mech. Geotech. Eng. 2019, 11, 837–849. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Abo-Waly, M.E.; Ali, R.A. Classification, characterization, and management of some agricultural soils in the North of Egypt. In Developments in Soil Classification, Land Use Planning and Policy Implications: Innovative Thinking of Soil Inventory for Land Use Planning and Management of Land Resources; Springer: Berlin/Heidelberg, Germany, 2013; pp. 417–448. [Google Scholar]
- Elewa, H.H.; Nosair, A.M.; Zelenakova, M.; Mikita, V.; Abdel Moneam, N.A.; Ramadan, E.M. Environmental Sustainability of Water Resources in Coastal Aquifers, Case Study: El-Qaa Plain, South Sinai, Egypt. Water 2023, 15, 1118. [Google Scholar] [CrossRef]
- Omran, E.-S.E.; Negm, A.M. Adaptive management zones of Egyptian coastal lakes. In Egyptian Coastal Lakes and Wetlands: Part I: Characteristics and Hydrodynamics; Springer: Berlin/Heidelberg, Germany, 2019; pp. 37–60. [Google Scholar]
- US Department of Agriculture. Soil Survey Manual; US Department of Agriculture: Washington, DC, USA, 1993.
- Conoco, C. Geological Map of Egypt, Scale 1:500,000-NF 36 NE-Bernice, Egypt; The Egyptian General Petroleum Corporation: Cairo, Egypt, 1987. [Google Scholar]
- Klute, A. Water Retention: Laboratory Methods; SSSA Book Series; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 2018; pp. 635–662. [Google Scholar]
- Tedesco, M.; Gianello, G.; Bissani, C.; Bohnen, H.; Volkweiss, S. Análises de Solo, Plantas e Outros Materiais. Rev. e Ampliada; Departamento de Solos, Universidade Federal do Rio Grande do Sul-UFRGS: Porto Alegre, Brazil, 1995; Volume 174. [Google Scholar]
- van Reeuwijk, L.P. Procedures for Soil Analysis; No. 9; International Soil Reference and Information Center, Food and Agriculture Organization of the United Nations: Wageningen, The Netherlands, 1986; ISSN 0923-3792. [Google Scholar]
- Tan, Z.; Lal, R. Carbon sequestration potential with changes in land use and management in Ohio. Agric. Ecosyst. Environ. 2005, 110, 140–152. [Google Scholar] [CrossRef]
- Baruah, T.; Barthakur, H. Determination of available Potassium, Sulphur, Particle density and textural properties. In A Text Book of Soil Analysis; Vikas Publishing House Private Lim: New Delhi, India, 1997. [Google Scholar]
- Lindsay, W.; Norvell, W. Development of DTPA soils test for Zinc Cu, Fe and Mn. Soil Sci. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Brown, G. Crystal Structures of Clay Minerals and Their X-ray Identification; The Mineralogical Society of Great Britain and Ireland: London, UK, 1982; Volume 5. [Google Scholar]
- Dietrich, F.; Diaz, N.; Deschamps, P.; Ngatcha, B.N.; Sebag, D.; Verrecchia, E.P. Origin of calcium in pedogenic carbonate nodules from silicate watersheds in the Far North Region of Cameroon: Respective contribution of in situ weathering source and dust input. Chem. Geol. 2017, 460, 54–69. [Google Scholar] [CrossRef]
- Elrys, A.S.; Uwiragiye, Y.; Zhang, Y.; Abdel-Fattah, M.K.; Chen, Z.-X.; Zhang, H.-M.; Meng, L.; Wang, J.; Zhu, T.-B.; Cheng, Y. Expanding agroforestry can increase nitrate retention and mitigate the global impact of a leaky nitrogen cycle in croplands. Nat. Food 2023, 4, 109–121. [Google Scholar] [CrossRef]
- Huggett, R. Soil landscape systems: A model of soil genesis. Geoderma 1975, 13, 1–22. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, X.; Tao, J.; Wu, J.; Wang, J.; Shi, P.; Zhang, Y.; Yu, C. The impact of climate change and anthropogenic activities on alpine grassland over the Qinghai-Tibet Plateau. Agric. For. Meteorol. 2014, 189, 11–18. [Google Scholar] [CrossRef]
- Lal, R. Global potential of soil carbon sequestration to mitigate the greenhouse effect. Crit. Rev. Plant Sci. 2003, 22, 151–184. [Google Scholar] [CrossRef]
- Jenny, H. Factors of Soil Formation: A System of Quantitative Pedology; Courier Corporation: Chelmsford, NC, USA, 1994. [Google Scholar]
- Wagai, R.; Mayer, L.M.; Kitayama, K.; Knicker, H. Climate and parent material controls on organic matter storage in surface soils: A three-pool, density-separation approach. Geoderma 2008, 147, 23–33. [Google Scholar] [CrossRef]
- Chesworth, W. The parent rock effect in the genesis of soil. Geoderma 1973, 10, 215–225. [Google Scholar] [CrossRef]
- Babur, E. Effects of parent material on soil microbial biomass carbon and basal respiration within young afforested areas. Scand. J. For. Res. 2019, 34, 94–101. [Google Scholar] [CrossRef]
- Wilding, L. Factors of soil formation: Contributions to pedology. Factors Soil Form. A Fiftieth Anniv. Retrosp. 1994, 33, 15–30. [Google Scholar]
- Company, J.; Valiente, N.; Fortesa, J.; García-Comendador, J.; Lucas-Borja, M.E.; Ortega, R.; Miralles, I.; Estrany, J. Secondary succession and parent material drive soil bacterial community composition in terraced abandoned olive groves from a Mediterranean hyper-humid mountainous area. Agric. Ecosyst. Environ. 2022, 332, 107932. [Google Scholar] [CrossRef]
- Massas, I.; Kalivas, D.; Ehaliotis, C.; Gasparatos, D. Total and available heavy metal concentrations in soils of the Thriassio plain (Greece) and assessment of soil pollution indexes. Environ. Monit. Assess. 2013, 185, 6751–6766. [Google Scholar] [CrossRef]
- Horwath, W. Carbon cycling and formation of soil organic matter. In Soil Microbiology, Ecology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2007; pp. 303–339. [Google Scholar]
- John, K.; Abraham Isong, I.; Michael Kebonye, N.; Okon Ayito, E.; Chapman Agyeman, P.; Marcus Afu, S. Using machine learning algorithms to estimate soil organic carbon variability with environmental variables and soil nutrient indicators in an alluvial soil. Land 2020, 9, 487. [Google Scholar] [CrossRef]
- Song, K.; Zheng, X.; Lv, W.; Qin, Q.; Sun, L.; Zhang, H.; Xue, Y. Effects of tillage and straw return on water-stable aggregates, carbon stabilization and crop yield in an estuarine alluvial soil. Sci. Rep. 2019, 9, 4586. [Google Scholar] [CrossRef]
- Paul, E.; Collins, H.; Leavitt, S. Dynamics of resistant soil carbon of Midwestern agricultural soils measured by naturally occurring 14C abundance. Geoderma 2001, 104, 239–256. [Google Scholar] [CrossRef]
- Azam, S. Collapse and compressibility behaviour of arid calcareous soil formations. Bull. Eng. Geol. Environ. 2000, 59, 211–217. [Google Scholar] [CrossRef]
- Dickson, B.L.; Scott, K.M. Recognition of aeolian soils of the Blayney district, NSW: Implications for mineral exploration. J. Geochem. Explor. 1998, 63, 237–251. [Google Scholar] [CrossRef]
- McTainsh, G.; Strong, C. The role of aeolian dust in ecosystems. Geomorphology 2007, 89, 39–54. [Google Scholar] [CrossRef]
- Ravi, S.; D’Odorico, P.; Breshears, D.D.; Field, J.P.; Goudie, A.S.; Huxman, T.E.; Li, J.; Okin, G.S.; Swap, R.J.; Thomas, A.D. Aeolian processes and the biosphere. Rev. Geophys. 2011, 49, RG3001. [Google Scholar] [CrossRef]
- Bröder, L.; Keskitalo, K.; Zolkos, S.; Shakil, S.; Tank, S.E.; Kokelj, S.V.; Tesi, T.; Van Dongen, B.E.; Haghipour, N.; Eglinton, T.I. Preferential export of permafrost-derived organic matter as retrogressive thaw slumping intensifies. Environ. Res. Lett. 2021, 16, 054059. [Google Scholar] [CrossRef]
- Croffie, M.E.; Williams, P.N.; Fenton, O.; Fenelon, A.; Daly, K. Rubidium measured by XRF as a predictor of soil particle size in limestone and siliceous parent materials. J. Soils Sediments 2022, 22, 818–830. [Google Scholar] [CrossRef]
- Saiz, G.; Bird, M.I.; Domingues, T.; Schrodt, F.; Schwarz, M.; Feldpausch, T.R.; Veenendaal, E.; Djagbletey, G.; Hien, F.; Compaore, H. Variation in soil carbon stocks and their determinants across a precipitation gradient in W est A frica. Glob. Chang. Biol. 2012, 18, 1670–1683. [Google Scholar] [CrossRef]
- Pincus, L.; Ryan, P.; Huertas, F.; Alvarado, G. The influence of soil age and regional climate on clay mineralogy and cation exchange capacity of moist tropical soils: A case study from Late Quaternary chronosequences in Costa Rica. Geoderma 2017, 308, 130–148. [Google Scholar] [CrossRef]
- Torn, M.S.; Swanston, C.; Castanha, C.; Trumbore, S. Storage and turnover of organic matter in soil. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 219–272. [Google Scholar]
- Elnaggar, A. Spatial variability of soil physiochemical properties in Bahariya Oasis, Egypt. Egypt. J. Soil Sci. 2017, 57, 313–328. [Google Scholar]
- Vandenberghe, J.; Sun, Y.; Wang, X.; Abels, H.; Liu, X. Grain-size characterization of reworked fine-grained aeolian deposits. Earth Sci. Rev. 2018, 177, 43–52. [Google Scholar] [CrossRef]
- Ouda, S.; Sayed, M.; El-Afandi, G.; Khalil, F. Developing an adaptation strategy to reduce climate change risks on wheat grown in sandy soil in Egypt. In Meeting the Challenge of Sustainable Development in Drylands under Changing Climate-Moving from Global to Local, Proceedings of the Tenth International Conference on Development of Drylands, Cairo, Egypt, 12–15 December 2010; International Dryland Development Commission (IDDC): Cairo, Egypt, 2011; pp. 346–356. [Google Scholar]
- Kumar, A.; Yadav, D. Long-term effects of fertilizers on the soil fertility and productivity of a rice–wheat system. J. Agron. Crop Sci. 2001, 186, 47–54. [Google Scholar] [CrossRef]
- Elrys, A.S.; Wang, J.; Metwally, M.A.; Cheng, Y.; Zhang, J.B.; Cai, Z.C.; Chang, S.X.; Müller, C. Global gross nitrification rates are dominantly driven by soil carbon-to-nitrogen stoichiometry and total nitrogen. Glob. Chang. Biol. 2021, 27, 6512–6524. [Google Scholar] [CrossRef] [PubMed]
- Abdo, A.I.; Deng, Y.; Sun, D.; Chen, X.; Alnaimy, M.A.; El-Sobky, E.-S.E.; Wei, H.; Zhang, J. Maintaining higher grain production with less reactive nitrogen losses in China: A meta-analysis study. J. Environ. Manag. 2022, 322, 116018. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.; Arafat, S.; Ghar, M.A.; Khader, M. Physiographic soil map delineation for the Nile alluvium and desert outskirts in middle Egypt using remote sensing data of EgyptSat-1. Egypt. J. Remote Sens. Space Sci. 2010, 13, 129–135. [Google Scholar] [CrossRef]
- Yoothong, K.; Pukamphol, M.; Hutspardol, A. Distribution of Clay Minerals–Kaolinite, Illite and Montmorillonite in the Coarse and the Fine Clay Fractions in Some Alluvial Soils. Agric. Nat. Resour. 1986, 20, 285–299. [Google Scholar]
- Nosair, A.M.; Shams, M.Y.; AbouElmagd, L.M.; Hassanein, A.E.; Fryar, A.E.; Abu Salem, H.S. Predictive model for progressive salinization in a coastal aquifer using artificial intelligence and hydrogeochemical techniques: A case study of the Nile Delta aquifer, Egypt. Environ. Sci. Pollut. Res. 2022, 29, 9318–9340. [Google Scholar] [CrossRef]
- Abu Salem, H.S.; Gemail, K.S.; Junakova, N.; Ibrahim, A.; Nosair, A.M. An Integrated Approach for Deciphering Hydrogeochemical Processes during Seawater Intrusion in Coastal Aquifers. Water 2022, 14, 1165. [Google Scholar] [CrossRef]
- Youghly, N.A.; Elbous, M.M.; Elbanna, M.A.; Taher, H.S.; Hegazi, N.A.; Youssef, H.A.; Fouad, M.F. Assessment of heavy metal contamination and tolerant bacteria associated with halophyte Arthrocnemum Macrostachyum in Lake Manzala, Egypt. Alfarama J. Basic Appl. Sci. 2021, 2, 263–284. [Google Scholar] [CrossRef]
- Ali, S.; Ahmed, H.R. Comparative effects of different soil conditioners on wheat growth and yield grown in saline-sodic soils. Sains Malays. 2016, 45, 339–346. [Google Scholar]
- Abdel Monem, M.A.; Radojevic, B. Agricultural production in Egypt: Assessing vulnerability and enhancing adaptive capacity and resilience to climate change. In Climate Change Impacts on Agriculture and Food Security in Egypt: Land and Water Resources—Smart Farming—Livestock, Fishery, and Aquaculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 205–222. [Google Scholar]
- González-Ubierna, S.; Jorge-Mardomingo, I.; Carrero-González, B.; de la Cruz, M.T.; Casermeiro, M.Á. Soil organic matter evolution after the application of high doses of organic amendments in a Mediterranean calcareous soil. J. Soils Sediments 2012, 12, 1257–1268. [Google Scholar] [CrossRef]
- Ata, A.; Salem, T.N.; Hassan, R. Geotechnical characterization of the calcareous sand in northern coast of Egypt. Ain Shams Eng. J. 2018, 9, 3381–3390. [Google Scholar] [CrossRef]
- Lucena, J.J. Effects of bicarbonate, nitrate and other environmental factors on iron deficiency chlorosis. A review. J. Plant Nutr. 2000, 23, 1591–1606. [Google Scholar] [CrossRef]
- Ayyad, M. Vegetation and environment of the Western Mediterranean coastal land of Egypt: IV. The habitat of non-saline depressions. J. Ecol. 1976, 64, 713–722. [Google Scholar] [CrossRef]
- Mohamed, E.S.; Belal, A.; Saleh, A. Assessment of land degradation east of the Nile Delta, Egypt using remote sensing and GIS techniques. Arab. J. Geosci. 2013, 6, 2843–2853. [Google Scholar] [CrossRef]
- El Shinawi, A.; Zeleňáková, M.; Nosair, A.M.; Abd-Elaty, I. Geo-spatial mapping and simulation of the sea level rise influence on groundwater head and upward land subsidence at the rosetta coastal zone, nile delta, Egypt. J. King Saud Univ. Sci. 2022, 34, 102145. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, W.; Wang, H.; Liu, P.; Wang, X.; Huang, B. Spatial distribution, ecological risk and sources of heavy metals in soils from a typical economic development area, Southeastern China. Sci. Total Environ. 2021, 780, 146557. [Google Scholar] [CrossRef]
- Ramadan, E.M.; Badr, A.M.; Abdelradi, F.; Negm, A.; Nosair, A.M. Detection of Groundwater Quality Changes in Minia Governorate, West Nile River. Sustainability 2023, 15, 4076. [Google Scholar] [CrossRef]
- Zechmeister, H.G. Correlation between altitude and heavy metal deposition in the Alps. Environ. Pollut. 1995, 89, 73–80. [Google Scholar] [CrossRef]
- Fairbrother, A.; Wenstel, R.; Sappington, K.; Wood, W. Framework for metals risk assessment. Ecotoxicol. Environ. Saf. 2007, 68, 145–227. [Google Scholar] [CrossRef]
- Nahmani, J.; Hodson, M.E.; Black, S. A review of studies performed to assess metal uptake by earthworms. Environ. Pollut. 2007, 145, 402–424. [Google Scholar] [CrossRef]
- Soltanpour, P.; Schwab, A. A new soil test for simultaneous extraction of macro- and micro-nutrients in alkaline soils. Commun. Soil Sci. Plant Anal. 1977, 8, 195–207. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wierzbowska, J.; Kovačik, P.; Sienkiewicz, S.; Krzebietke, S.; Bowszys, T. Determination of heavy metals and their availability to plants in soil fertilized with different waste substances. Environ. Monit. Assess. 2018, 190, 567. [Google Scholar] [CrossRef] [PubMed]
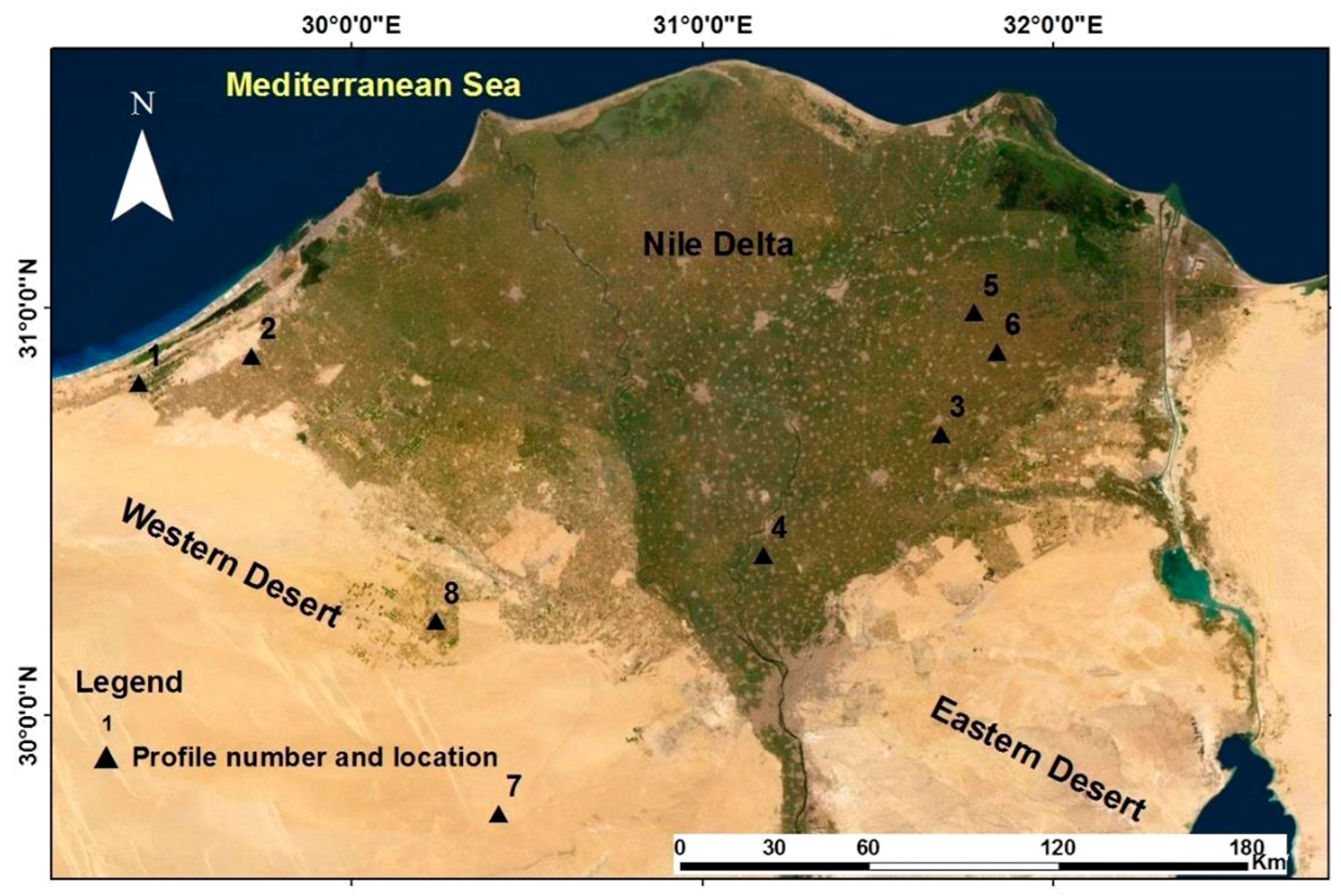

| Horizons | Depth, Topography, Munsell soil color (dry, moist), Soil texture, Soil structure, Stickiness (wet), Plasticity (wet), Soil consistency (moist), Soil consistency (dry); Calcium Carbonate, Clay and Sand Mineralogy * |
|---|---|
| P1 (parent material: Fluvio-Marine); land cover (alfalfa plant) | |
| A0 | 0–40 cm; flat; Dark grayish brown (10YR 4/1) dry; Dark gray (10YR 4/2) moist; SL; Ma; Sst; Spl; Lo; slightly hard dry; Moderately calcareous; quartz (+++), plagioclase (+), orthoclase (+). |
| B1 | 40–80 cm; Dark yellowish brown (10YR 3/6); Dark brown (10YR 3/2); SCL; Ma; Sst; Spl; Fi; slightly hard dry; Moderately calcareous; quartz (+++), plagioclase (+), orthoclase (+). |
| B2 | 80–120 cm; brown (10YR 5/3); Dark grayish brown (10YR 4/3); SCL; Sb; St; Pl; Vfi; hard dry; quartz (+++), plagioclase (+), orthoclase (+). |
| C | 120–150; brown (10YR 5/3); Dark grayish brown (10YR 4/3); SCL; Sb; St; Pl; Vfi; very hard dry; quartz (+++), plagioclase (+), orthoclase (+). |
| P2 (parent material: Fluvio-Marine); land cover (wheat) | |
| At | 0–30 cm; very pale brown (10YR 7/3) dry; yellowish brown (10YR 5/4) moist; L; Ma; Sst; Spl; Fi; slightly hard dry; extremely calcareous; quartz (+++), plagioclase (++), orthoclase (++). |
| B1 | 30–80 cm; light brown (7.5YR 6/4) dry; brown (10YR 5/6) moist; L; Sb; Vst; Vpl; Vfi; very hard dry; extremely calcareous; quartz (+++), plagioclase (++), orthoclase (++). |
| C | 80–150 cm; Yellowish brown (10YR 5/6) dry; brownish yellow (10 YR 6/6) moist; L; Sb; Vst; Vpl; Vfi; extremely hard dry; extremely calcareous; quartz (+++), plagioclase (++), orthoclase (++). |
| P3 (parent material: Nile alluvial deposit); land cover (alfalfa plant) | |
| A0 | 0–60 cm; Almost flat; Gray (10YR 5/1) dry; dark gray (10 YR 4/2) moist; CL; Gr; Sst; Spl; Fi; slightly hard dry; Slightly calcareous; Montmorillonite (++++), kaolinite (++), mica (++), plagioclase (+), orthoclase (+), Microcline (+), quartz (+), |
| B1 | 60–100 cm; Dark gray (10YR 4/1); very dark gray (10 YR 3/1); CL; Sb; St; Pl; Fi; very hard dry; Montmorillonite (++++), quartz (++), kaolinite (+), plagioclase (+), orthoclase (+), Microcline (+). |
| B2 | 100–150 cm; Dark gray (10YR 4/1); very dark gray (10 YR 3/1); lC; Sb; St; Pl; Vfi; very hard dry; Montmorillonite (++++), quartz (++), plagioclase (+), Albite (+), orthoclase (+), Microcline (+). |
| C | 150–200 cm; Dark gray (10 YR 4/1); very dark gray (10 YR 3/1); lC; Sb; Vst; Vpl; Vfi; extremely hard dry; Montmorillonite (++++), quartz (++), plagioclase (+), Albite (+), orthoclase (+), Microcline (+). |
| P4 (parent material: Nile alluvial deposit); land cover (vegetables) | |
| At | 0–30 cm; Light gray (10YR 7/2) dry; Light brownish gray (10 YR 6/2) moist; SL; Gr; Sst; Spl; Lo; soft dry; Non calcareous; Montmorillonite (+++), quartz (+), kaolinite (+), plagioclase (+), orthoclase (+), Microcline (+). |
| B1 | 30–60 cm; SL; Sb; Sst; Spl; Fi; soft dry; Montmorillonite (++++), quartz (++), kaolinite (+), plagioclase (+), orthoclase (+), Microcline (+). |
| C | 60–150 cm; SL; Sb; Sst; Spl; Fi; slightly hard dry; Montmorillonite (++++), quartz (++), kaolinite (+), plagioclase (+), orthoclase (+), Microcline (+). |
| P5 (parent material: Lacustrine); land cover (alfalfa plant) | |
| A1 | 0–20 cm; Almost flat; Grayish brown (10YR 5/2) dry; Dark gray (10 YR 4/1) moist; lC; Sb; St; Pl; Fi; hard dry; Moderately calcareous; Montmorillonite (++++), kaolinite (++), Vermiculite (+), illite (+), plagioclase (++), orthoclase (++), quartz (+). |
| A2 | 20–35 cm; grayish brown (10YR 5/2); Dark grayish brown (10 YR 4/2); lC; Sb;; Vst; Vpl; Vfi; very hard dry; Montmorillonite (++++), plagioclase (++), orthoclase (++), kaolinite (+), Muscovite (+), quartz (+). |
| BC | 35–85 cm; Dark clay (10YR 4/1); Very dark gray (10 YR 3/1); lC; Co; Vst; Vpl; Efi; very hard dry; Montmorillonite (++++), plagioclase (++), orthoclase (++), kaolinite (+), quartz (+). |
| C | 85–135 cm; Dark clay (10YR 4/1); Very dark gray (10 YR 3/1); lC; Co; Vst; Vpl; Efi; extremely hard dry; Montmorillonite (++++), plagioclase (++), orthoclase (++), kaolinite (+), quartz (+). |
| P6 (parent material: Lacustrine); land cover (onion) | |
| A1 | 0–10 cm; Almost flat; Grayish brown (10YR 5/2) dry; Dark gray (10 YR 4/1) moist; lC; Sb; St; Pl; Fi; slightly hard dry; Moderately calcareous; Montmorillonite (+++), kaolinite (+), Vermiculite (+), illite (+), plagioclase (+), orthoclase (+), quartz (+). |
| A2 | 10–35 cm; grayish brown (10YR 5/2); Dark grayish brown (10 YR 4/2); lC; Sb; Vst; Vpl; Vfi; hard dry; Montmorillonite (+++), plagioclase (+), orthoclase (+), kaolinite (+), Muscovite (+), quartz (+). |
| BC | 35–90 cm; Dark clay (10YR 4/1); Very dark gray (10 YR 3/1); lC; Ma; Vst; Vpl; Efi; very hard dry; Montmorillonite (++), plagioclase (+), orthoclase (+), kaolinite (+), quartz (+). |
| C | 85–135 cm; Dark clay (10YR 4/1); Very dark gray (10 YR 3/1); lC; Ma; Vst; Vpl; Efi; extremely hard dry; Montmorillonite (++), plagioclase (+), orthoclase (+), kaolinite (+), quartz (+). |
| P7 (parent material: Aeolian); land cover (Non-cultivated) | |
| A | 0–30 cm; Almost flat; Yellow (10YR 7/6) dry; Brownish yellow (10 YR 6/6) moist; S; We; Nst; Npl; Lo; loose dry; calcareous; quartz, opaques as; magnetite, ilmenite, leucoxene, hematite and the second is the non-opaques include zircon, garnet, monazite, rutile, silica (yellow and red silica) and green silicates (pyroxene and amphibole groups, epidote and mica group) (++++). |
| C | 30–50 cm; Rock. |
| CD | 50–100 cm; Hard rock |
| P8 (parent material: Aeolian); land cover (Non-cultivated) | |
| A | 0–30 cm; Almost flat; Pale brown (10YR 6/3) dry; Very pale brown (10YR 7/3) moist; S; We; Nst; Npl; Lo; soft dry; Calcareous; quartz, opaques as; magnetite, ilmenite, leucoxene, hematite and the second is the non-opaques include zircon, garnet, monazite, rutile, silica (yellow and red silica) and green silicates (pyroxene and amphibole groups, epidote and mica group) (++++). |
| CD | 30–60 cm; Hard rock. |
| Profile | Order, Suborder, Great Group, Subgroup, Family |
|---|---|
| P1 | Aridisols, Calcids, Petrocalcids, Typic Petrocalcids, Fine loamy, mixed, thermic |
| P2 | Aridisols, Calcids, Petrocalcids, Natric Petrocalcids, Fine loamy, mixed, thermic |
| P3 | Aridisols, Calcids, Haplocalcids, Typic Haplocalcid, mixed, thermic |
| P4 | Aridisols, Calcids, Haplocalcids, Typic Haplocalcid, mixed, thermic |
| P5 | Aridisols, Salids, Aquisalids, Typic Aquisalids |
| P6 | Vertisols, Torrerts, Haplotorrerts, Sodic Haplotorrerts |
| P7 | Entisols, Samments, Torripsamments, Lithic Torripsamments |
| P8 | Entisols, Samments, Torripsamments, Lithic Torripsamments |
| Profile | Ilmenite | Leucoxene | Rutile | Zircon | Garnet | Monazite | Red Silica | Green Silicates |
|---|---|---|---|---|---|---|---|---|
| P1 | 3.222 ± 0.23 | 0.145 ± 0.03 | 0.250 ± 0.04 | 0.302 ± 0.06 | 0.155 ± 0.02 | 0.010 ± 0.00 | 0.616 ± 0.03 | 0.219 ± 0.04 |
| P2 | 2.344 ± 0.31 | 0.205 ± 0.020 | 0.525 ± 0.07 | 0.306 ± 0.04 | 0.444 ± 0.02 | 0.030 ± 0.00 | 0.822 ± 0.06 | 0.323 ± 0.03 |
| P3 | 0.064 ± 0.01 | 0.027 ± 0.00 | 0.017 ± 0.00 | 0.013 ± 0.00 | 0.027 ± 0.00 | 0.000 ± 0.00 | 0.245 ± 0.02 | 0.135 ± 0.01 |
| P4 | 0.111 ± 0.00 | 0.062 ± 0.01 | 0.021 ± 0.00 | 0.003 ± 0.00 | 0.077 ± 0.01 | 0.011 ± 0.00 | 0.544 ± 0.06 | 0.212 ± 0.01 |
| P5 | 0.053 ± 0.00 | 0.022 ± 0.00 | 0.011 ± 0.00 | 0.009 ± 0.00 | 0.025 ± 0.00 | 0.000 ± 0.00 | 0.237 ± 0.01 | 0.126 ± 0.02 |
| P6 | 0.050 ± 0.00 | 0.024 ± 0.00 | 0.011 ± 0.00 | 0.009 ± 0.00 | 0.022 ± 0.00 | 0.000 ± 0.00 | 0.237 ± 0.01 | 0.124 ± 0.02 |
| P7 | 3.930 ± 0.42 | 0.494 ± 0.05 | 0.740 ± 0.13 | 0.607 ± 0.04 | 0.543 ± 0.03 | 0.060 ± 0.01 | 0.943 ± 0.08 | 0.678 ± 0.05 |
| P8 | 2.310 ± 0.22 | 0.159 ± 0.01 | 0.290 ± 0.06 | 0.212 ± 0.01 | 0.319 ± 0.04 | 0.000 ± 0.00 | 5.491 ± 0.35 | 0.794 ± 0.03 |
| Profile | Bulk Density (g cm−1) | CEC (cmolc kg−1) | CaCO3 (g kg−1) | EC (dS m−1) | pH | Available N (mg kg−1) | Available P (mg kg−1) | Available K (mg kg−1) | Total N (g kg−1) | Soil Organic C (g kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 1.67 ± 0.10 | 26.8 ± 1.27 | 336 ± 9.24 | 1.31 ± 0.06 | 8.40 ± 0.08 | 20.2 ± 0.44 | 12.2 ± 0.64 | 72.2 ± 1.85 | 0.87 ± 0.04 | 3.00 ± 0.02 |
| P2 | 1.44 ± 0.15 | 24.2 ± 0.69 | 428 ± 6.81 | 3.35 ± 0.08 | 8.21 ± 0.00 | 16.8 ± 0.14 | 6.39 ± 0.18 | 67.1 ± 1.56 | 0.83 ± 0.10 | 3.70 ± 0.06 |
| P3 | 1.30 ± 0.13 | 33.5 ± 1.10 | 4.90 ± 0.68 | 0.79 ± 0.12 | 7.83 ± 0.01 | 45.7 ± 1.79 | 19.8 ± 0.40 | 287 ± 7.16 | 1.67 ± 0.12 | 5.50 ± 0.08 |
| P4 | 1.30 ± 0.13 | 32.1 ± 1.10 | 4.40 ± 0.87 | 0.77 ± 0.11 | 7.80 ± 0.01 | 43.2 ± 1.79 | 20.7 ± 0.40 | 298 ± 8.78 | 1.69 ± 0.09 | 4.80 ± 0.06 |
| P5 | 1.38 ± 0.17 | 32.0 ± 1.04 | 36.7 ± 2.34 | 32.0 ± 2.34 | 8.50 ± 0.12 | 25.8 ± 0.64 | 6.70 ± 0.12 | 155 ± 4.16 | 1.02 ± 0.01 | 1.50 ± 0.06 |
| P6 | 1.40 ± 0.17 | 31.0 ± 1.04 | 36.9 ± 2.34 | 29.0 ± 2.34 | 8.40 ± 0.12 | 23.8 ± 0.64 | 6.10 ± 0.12 | 150 ± 4.16 | 1.02 ± 0.01 | 1.40 ± 0.06 |
| P7 | 1.80 ± 0.11 | 3.80 ± 0.27 | 820 ± 12.4 | 22.1 ± 1.73 | 8.07 ± 0.00 | 11.1 ± 0.28 | 4.60 ± 0.45 | 62.6 ± 1.56 | 0.34 ± 0.02 | 0.80 ± 0.03 |
| P8 | 1.70 ± 0.16 | 3.97 ± 0.36 | 149 ± 5.20 | 8.90 ± 0.96 | 8.16 ± 0.02 | 10.3 ± 0.86 | 4.80 ± 0.52 | 60.9 ± 1.21 | 0.33 ± 0.02 | 1.00 ± 0.03 |
| Clay | BD | CEC | EC | ESP | pH | CaCO3 | AP | AK | AN | TN | SOC | C/N | Fe | Zn | Mn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BD | −0.70 *** | |||||||||||||||
| CEC | 0.85 *** | −0.77 *** | ||||||||||||||
| EC | 0.27 ns | 0.22 ns | −0.24 ns | |||||||||||||
| ESP | 0.73 *** | −0.17 ns | 0.32 ns | 0.75 *** | ||||||||||||
| pH | 0.23 ns | 0.39 ns | −0.03 ns | 0.52 * | 0.71 *** | |||||||||||
| CaCO3 | −0.31 ns | 0.31 ns | −0.11 ns | −0.32 ns | −0.14 ns | 0.46 * | ||||||||||
| AP | 0.37 ns | −0.60 ** | 0.70 *** | −0.60 ** | −0.31 ns | −0.61 ** | −0.40 ns | |||||||||
| AK | 0.56 ** | −0.75 *** | 0.69 *** | −0.28 ns | −0.07 ns | −0.62 ** | −0.67 *** | 0.89 *** | ||||||||
| AN | 0.61 ** | −0.77 *** | 0.80 *** | −0.36 ns | −0.06 ns | −0.54 * | −0.54 * | 0.93 *** | 0.98 *** | |||||||
| TN | 0.65 ** | −0.77 *** | 0.87 *** | −0.40 ns | −0.02 ns | −0.46 * | −0.39 ns | 0.92 *** | 0.93 *** | 0.98 *** | ||||||
| SOC | 0.35 ns | −0.70 *** | 0.75 *** | −0.73 *** | −0.60 ** | −0.56 ** | −0.06 ns | 0.88 *** | 0.76 *** | 0.84 *** | 0.88 *** | |||||
| C/N | −0.33 ns | −0.004 ns | 0.08 ns | −0.79 *** | −0.36 ns | −0.17 ns | 0.71 *** | 0.16 ns | −0.12 ns | −0.01 ns | 0.10 ns | 0.50 * | ||||
| Fe | −0.80 *** | 0.67 *** | −0.89 *** | 0.05 ns | −0.45 * | −0.18 ns | −0.12 ns | −0.40 ns | −0.39 ns | −0.52 * | −0.62 ** | −0.54 * | −0.06 ns | |||
| Zn | −0.28 ns | −0.022 ns | −0.12 ns | −0.24 ns | −0.62 ** | −0.82 *** | −0.60 ** | 0.51 * | 0.55 ** | 0.44 * | 0.35 ns | 0.32 ns | −0.04 ns | 0.37 ns | ||
| Mn | −0.33 ns | 0.21 ns | 0.11 ns | −0.63 ** | −0.65 ** | −0.34 ns | 0.17 ns | 0.49 * | 0.17 ns | 0.27 ns | 0.29 ns | 0.49 * | 0.54 * | 0.05 ns | 0.44 * | |
| Cu | −0.18 ns | −0.25 ns | 0.33 ns | −0.93 *** | −0.75 *** | −0.57 ** | 0.24 ns | 0.67 *** | 0.40 ns | 0.49 * | 0.53 * | 0.83 *** | 0.80 *** | −0.13 ns | 0.41 ns | 0.73 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnaimy, M.A.; Elrys, A.S.; Zelenakova, M.; Pietrucha-Urbanik, K.; Merwad, A.-R.M. The Vital Roles of Parent Material in Driving Soil Substrates and Heavy Metals Availability in Arid Alkaline Regions: A Case Study from Egypt. Water 2023, 15, 2481. https://doi.org/10.3390/w15132481
Alnaimy MA, Elrys AS, Zelenakova M, Pietrucha-Urbanik K, Merwad A-RM. The Vital Roles of Parent Material in Driving Soil Substrates and Heavy Metals Availability in Arid Alkaline Regions: A Case Study from Egypt. Water. 2023; 15(13):2481. https://doi.org/10.3390/w15132481
Chicago/Turabian StyleAlnaimy, Manal A., Ahmed S. Elrys, Martina Zelenakova, Katarzyna Pietrucha-Urbanik, and Abdel-Rahman M. Merwad. 2023. "The Vital Roles of Parent Material in Driving Soil Substrates and Heavy Metals Availability in Arid Alkaline Regions: A Case Study from Egypt" Water 15, no. 13: 2481. https://doi.org/10.3390/w15132481
APA StyleAlnaimy, M. A., Elrys, A. S., Zelenakova, M., Pietrucha-Urbanik, K., & Merwad, A.-R. M. (2023). The Vital Roles of Parent Material in Driving Soil Substrates and Heavy Metals Availability in Arid Alkaline Regions: A Case Study from Egypt. Water, 15(13), 2481. https://doi.org/10.3390/w15132481










