Impacts of Habitat Quality on the Physiology, Ecology, and Economical Value of Mud Crab Scylla sp.: A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Synthesis and Analyses
3. Economic and Biotechnological Values of Scylla sp. under Varied Water Physicochemical Factors
3.1. Nutritional Values of Mud Crab Meat
3.2. Value and Environmental Water Management of Crab Shell Bio-Waste
3.3. Antimicrobial Proteins in Mud Crabs under Varied Water Temperature
| Innate Immune Molecules | Expression Upregulation at Temperature | M.W. | Highly Expressing Tissue | Response to Bacteria or Antigens | Response to WSSV | Antibacterial Activity | References |
|---|---|---|---|---|---|---|---|
| SSAPs | 35 °C | 11 kDa | Ejaculatory duct | UP: Bacteria | ND | G+, G- | [44] |
| Sc-ALF | 25 and 35 °C | 11.17 kDa | Hemolymph | UP: Bacteria, LPS | ND | ND | [47] |
| Ss Toll | ND | NA | NA | Up: Peptidoglycan, LPS | ND | ND | [48] |
| LRR | ND | NA | Various tissues | Up: Bacteria | Up-regulation | G- | [49] |
| Vg-2 | 25 °C | NA | Testicular spermatozoa | ND | Up-regulation | ND | [50,51] |
| Ss ALF | 25 and 35 °C | NA | Hemocyte, heart and, muscle | Up: Bacteria and LPS | ND | G+, G- | [52] |
| B-GBP | ND | 100 kDa | Hemolymph | Up: Bacteria | ND | G+, G- | [41] |
3.4. Allergens in Mud Crab Meat and Their Modulation under Abiotic Factors
3.5. Anti-Cancer Molecules in Mud Crab and Their Variation under Habitat Water
4. Status of Mud Crab Industries in Asian Countries
5. Mud Crab and Its Importance in the Brackish Water Aquatic Environment
5.1. Direct Contribution of Mud Crabs into Habitat
5.2. Role of Habitat Water on Ecology and Life Cycle of Mud Crabs
| Water Physicochemical Factors | Location | Ranges | Duration (days) | Effects on Crab | Reference |
|---|---|---|---|---|---|
| pH | Coimbatore, Tamil Nadu, India | 8.2 | 60 days | Normal growth, feed intake, and survival rate | [87] |
| 7.8 | |||||
| 7.6 | Decrease in growth rate, survival rate, and feed intake | ||||
| 7.2 | |||||
| 7.0 | |||||
| Chantaburi, Thailand | Hemolymph osmolality (%) | [88] | |||
| 4–6 | 10 days | 11% decrease | |||
| 6–12 | 10 days | 15% increase | |||
| Temperature | Growth rate (%) | [89] | |||
| Terengganu, Malaysia | 24 °C | 45 days | 7.28 ± 1.31 | ||
| 28 °C | 45 days | 9.69 ± 0.75 | |||
| 32 °C | 45 days | 7.83 ± 0.56 | |||
| 27–30 °C | 45 days | 9.48 ± 1.02 | |||
| Northern Territory of Australia | 20 °C/20 ppt | 1 day | 7.75 ± 1.28 | [90] | |
| 25 °C/20 ppt | 1 day | 12.68 ± 0.77 | |||
| 30 °C/20 ppt | 1 day | 15.98 ± 0.36 | |||
| 35 °C/20 ppt | 1 day | 12.59 ± 0.60 | |||
| Salinity | Queensland, Australia | Hemolymph osmolality (mOsm kg−1) | [91] | ||
| 4 ppt | NA | 415 ± 12 (hyperregulated) | |||
| 12 ppt | 312 ± 8 (hyperregulated) | ||||
| 20 ppt | 194 ± 15 (hyperregulated) | ||||
| 28 ppt | 122 ± 12 (hyperregulated) | ||||
| Iilan, Taiwan | 14 ppt | 1 day | 772.38 (stabilized) | [21] | |
| 24 ppt | 3 days | 803.50 (stabilized) | |||
| 34 ppt | 0 day | 1034.50 (stabilized) | |||
| 44 ppt | 1 day | 1274 (stabilized) | |||
| Queensland, Australia | 30 ppt | 4 days | 968.73 ± 8.85 (stabilized) | [92] | |
| Odisha, India | Mitochondrial respiration rate complex I and II (nmol) | [93] | |||
| 10 ppt | 21 days | 4.42 ± 0.88 and 6.41 ± 1.69 | |||
| 17 ppt | 21 day | 1.69 ± 0.41 and 4.04 ± 0.58 | |||
| 35 ppt | 21 day | 2.19 ± 0.55 and 4.42 ± 0.88 |
5.3. Predatory Contribution to Food Chain under Varied Water Habitats
5.4. Behavioural Contribution to Ecosystem
5.5. Contribution as Biomarkers and Bio-Indicators
6. Adaptive Responses of Scylla sp. to Water Physicochemical Factors
6.1. Migration in Saline Water Bodies
6.2. Reproduction Maturity in Natural Saline Water
6.3. Breeding and Induced Breeding in Mud Crab and the Role of Habitat Water
6.4. Regulatory Proteins and Their Regulation in Reproduction
6.5. Harvesting of Crabs as a Function of Seasonal Variation of Habitat Water
7. Mud Crab Physiology under Fluctuating Water and Pollution Stress
7.1. Effects of Water Salinity
7.2. Effects of Water Temperature
7.3. Effects of Inorganic and Organic Metals/Pollutants in Habitat Water on Mud Crabs
7.3.1. Effects of Heavy Metal in Habitat Water on Crab Physiology
| Inorganic Toxicants | Season | Site of Accumulation | Range of Accumulation | Effect | References |
|---|---|---|---|---|---|
| As, Cu, Zn | Wet | Muscle | High | Tissue damage | [138] |
| Cd, Cr, Pb | Wet | Na | Low | NA | [138] |
| Zn, Hg | Wet | Whole body | Balanced | Muscle damage | [144] |
| Pb and As | Summer | Whole body | High in female | [139] | |
| Pb | Summer | Highest in hp, lowest in muscle | Too high | Body damage | [139] |
| Ag+2, Cd+2 | Wet | Muscle | High | Structural changes in sperm | [142] |
7.3.2. Effects of Toxic Chemicals in Habitat Water on Mud Crab’s Physiology
7.4. Endocrine Systems under Habitat Water Fluctuation
7.5. Neurotransmitter Changes under Habitat Water Fluctuation
7.6. Immunity and Disease Aspects under Habitat Water Fluctuation
8. Molecular Response under Physicochemical Variation of Water Quality
9. Habitat Water Quality Management and Techniques to Enhance Mud Crab Production
10. Future Prospective of Scylla sp. with Respect to Mangrove/Estuarine Habitat Water
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- FAO. FAO Yearbook. Fishery and Aquaculture Statistics 2019/FAO Annuaire. Statistiques des Pêches et de L’aquaculture 2019/FAO Anuario. Estadísticas de Pesca y Acuicultura 2019; Food and Agriculture Organization: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Retnaningdyah, C.; Ridlo, I.A.; Febriansyah, S.C.; Nusantara, O.B. Water quality evaluation of some mangrove ecosystems with variations of time restoration in South Malang, East Java, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 743, 012011. [Google Scholar] [CrossRef]
- Hillman, J.R.; Stephenson, F.; Thrush, S.F.; Lundquist, C.J. Investigating changes in estuarine ecosystem functioning under future scenarios. Ecol. Appl. 2020, 30, e02090. [Google Scholar] [CrossRef]
- Silva, A.M.M.; Glover, H.E.; Josten, M.E.; Gomes, V.J.C.; Ogston, A.S.; Asp, N.E. Implications of a Large River Discharge on the Dynamics of a Tide-Dominated Amazonian Estuary. Water 2023, 15, 849. [Google Scholar] [CrossRef]
- Nidzieko, N.J. Allometric scaling of estuarine ecosystem metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 6733–6738. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.D.C.; Arrivabene, H.P.; Craig, C.-A.; Midwood, A.J.; Thornton, B.; Matsumoto, S.T.; Elliott, M.; Wunderlin, D.A.; Monferrán, M.V.; Fernandes, M.N. Interrogating pollution sources in a mangrove food web using multiple stable isotopes. Sci. Total. Environ. 2018, 640–641, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Govender, J.; Naidoo, T.; Rajkaran, A.; Cebekhulu, S.; Bhugeloo, A.; Sershen, S. Towards Characterising Microplastic Abundance, Typology and Retention in Mangrove-Dominated Estuaries. Water 2020, 12, 2802. [Google Scholar] [CrossRef]
- Bal, A.; Panda, F.; Pati, S.G.; Anwar, T.N.; Das, K.; Paital, B. Influence of Anthropogenic Activities on Redox Regulation and Oxidative Stress Responses in Different Phyla of Animals in Coastal Water via Changing in Salinity. Water 2022, 14, 4026. [Google Scholar] [CrossRef]
- Le, C.T.; Van Do, D.; Nguyen, D.B.; Wang, P. A Laboratory Scale of the Physical Model for Inclined and Porous Breakwaters on the Coastline of Soc Trang Province (Mekong Delta). Water 2023, 15, 1366. [Google Scholar] [CrossRef]
- Muro-Torres, V.M.; Amezcua, F.; Soto-Jiménez, M.; Balart, E.F.; Serviere-Zaragoza, E.; Green, L.; Rajnohova, J. Primary Sources and Food Web Structure of a Tropical Wetland with High Density of Mangrove Forest. Water 2020, 12, 3105. [Google Scholar] [CrossRef]
- Barbier, E.B. Valuing Coastal Habitat–Fishery Linkages under Regulated Open Access. Water 2019, 11, 847. [Google Scholar] [CrossRef]
- Sampantamit, T.; Ho, L.; Van Echelpoel, W.; Lachat, C.; Goethals, P. Links and Trade-Offs between Fisheries and Environmental Protection in Relation to the Sustainable Development Goals in Thailand. Water 2020, 12, 399. [Google Scholar] [CrossRef]
- Abdelzaher, M.A.; Farahat, E.M.; Abdel-Ghafar, H.M.; Balboul, B.A.A.; Awad, M.M. Environmental Policy to Develop a Conceptual Design for the Water–Energy–Food Nexus: A Case Study in Wadi-Dara on the Red Sea Coast, Egypt. Water 2023, 15, 780. [Google Scholar] [CrossRef]
- Teegalapalli, K.; Hiremath, A.J.; Jathanna, D. Burrowing Activity and Distribution of Scylla serrata Forskal from Hooghly and Matla Estuaries Sundarban West Bengal. J. Bombay Nat. Hist. Soc. 1991, 88, 167–171. [Google Scholar]
- Alberts-Hubatsch, H.; Lee, S.Y.; Meynecke, J.-O.; Diele, K.; Nordhaus, I.; Wolff, M. Life-history, movement, and habitat use of Scylla serrata (Decapoda, Portunidae): Current knowledge and future challenges. Hydrobiologia 2016, 763, 5–21. [Google Scholar] [CrossRef]
- Sayeed, Z.; Sugino, H.; Sakai, Y.; Yagi, N. Consumer Preferences and Willingness to Pay for Mud Crabs in Southeast Asian Countries: A Discrete Choice Experiment. Foods 2021, 10, 2873. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Spence, A.R.; Tingley, M.W.; Spence, A.R. The challenge of novel abiotic conditions for species undergoing climate-induced range shifts. Ecography 2020, 43, 1571–1590. [Google Scholar] [CrossRef]
- Pati, S.; Sujila, P.; Ng, P.K. On the collection of marine crabs (Decapoda: Brachyura) in the Zoological Survey of India, Western Regional Centre, Pune, with a note on the taxonomy of Sphaerozius scaber (Fabricius, 1798) (Menippidae). Zootaxa 2022, 5094, 501–552. [Google Scholar] [CrossRef]
- Webley, J.A.C.; Connolly, R.M.; Young, R.A. Habitat Selectivity of Megalopae and Juvenile Mud Crabs (Scylla serrata): Im-plications for Recruitment Mechanism. Mar. Biol. 2009, 156, 891–899. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Mohapatra, A.; Mohanty, R.K.; Bhatta, K.S.; Pattnaik, A.K. Occurrence and Biological Outlines of Two Speciesof Scylla (De Haan) in Chilika Lagoon, India. Indian J. Fish. 2006, 53, 191–202. [Google Scholar]
- Chen, J.; Chia, P. Hemolymph ammonia and urea and nitrogenous excretions of Scylla serrata at different temperature and salinity levels. Mar. Ecol. Prog. Ser. 1996, 139, 119–125. [Google Scholar] [CrossRef]
- Hamasaki, K. Effects of temperature on the egg incubation period, survival and developmental period of larvae of the mud crab Scylla serrata (Forskål) (Brachyura: Portunidae) reared in the laboratory. Aquaculture 2003, 219, 561–572. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G. Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhang, Y.; Jiang, R.; Zhong, M.; Hu, Z.; Du, H.; Lun, J.; Chen, J.; Li, Y. Identification and agglutination properties of hemocyanin from the mud crab (Scylla serrata). Fish Shellfish Immunol. 2011, 30, 354–360. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Leng, B.; Huang, Q.-S.; Yan, Y.-W.; Liu, X.; Wang, Q.; Chen, Q.-X. Inactivation kinetics of ?-N-acetyl-D-glucosaminidase from green crab (Scylla serrata) by guanidinium chloride. Protein Pept. Lett. 2012, 19, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Paital, B.; Sablok, G.; Kumar, S.; Singh, S.K.; Chainy, G.B.N. Investigating the Conformational Structure and Potential Site Interactions of SOD Inhibitors on Ec-SOD in Marine Mud Crab Scylla serrata: A Molecular Modeling Approach. Interdiscip. Sci. Comput. Life Sci. 2016, 8, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Wang, H.P. Transcriptional stress responses to environmental and husbandry stressors in aquaculture species. Rev. Aquac. 2016, 8, 61–88. [Google Scholar] [CrossRef]
- Panda, F.; Pati, S.G.; Bal, A.; Das, K.; Samanta, L.; Paital, B. Control of invasive apple snails and their use as pollutant ecotoxic indicators: A review. Environ. Chem. Lett. 2021, 19, 4627–4653. [Google Scholar] [CrossRef]
- Pourmozaffar, S.; Jahromi, S.T.; Rameshi, H.; Sadeghi, A.; Bagheri, T.; Behzadi, S.; Gozari, M.; Zahedi, M.R.; Lazarjani, S.A. The role of salinity in physiological responses of bivalves. Rev. Aquac. 2020, 12, 1548–1566. [Google Scholar] [CrossRef]
- Azra, M.N.; Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi J. Biol. Sci. 2016, 23, 257–267. [Google Scholar] [CrossRef]
- Koch, V.; Nordhaus, I. Feeding Ecology and Ecological Role of North Brazilian Mangrove Crabs. In Sustainable Fisheries and Aquaculture View Project Mangrove Fauna Ecology View Project; Springer: Berlin/Heidelberg, Germany, 2010; Volume 211, pp. 265–273. [Google Scholar] [CrossRef]
- Sarower, M.G.; Bilkis, S.; Rauf, M.A.; Khanom, M.; Islam, M.S. Comparative Biochemical Composition of Natural and Fattened Mud Crab Scylla serrata. J. Sci. Res. 2013, 5, 545–553. [Google Scholar] [CrossRef]
- Mohapatra, A.; Rautray, T.; Patra, A.K.; Vijayan, V.; Mohanty, R.K. Trace element-based food value evaluation in soft and hard shelled mud crabs. Food Chem. Toxicol. 2009, 47, 2730–2734. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.-K.; Huang, J.-P. Chemical constituents in the abdominal muscle of cultured mud crab Scylla serrata in relation to seasonal variation and maturation. Fish. Sci. 2003, 69, 597–604. [Google Scholar] [CrossRef]
- Mohapatra, A.; Mohanty, R.K.; Mohanty, S.K.; Bhatta, K.S.; Das, N.R. Fisheries enhancement and biodiversity assessment of fish, prawn and mud crab in Chilika lagoon through hydrological intervention. Wetl. Ecol. Manag. 2007, 15, 229–251. [Google Scholar] [CrossRef]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A. Utilization of Waste Crab Shell (Scylla serrata) as a Catalyst in Palm Olein Transesterification. J. Oleo Sci. 2009, 58, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Haryati, E.; Dahlan, K.; Togibasa, O.; Dahlan, K. Protein and Minerals Analyses of Mangrove Crab Shells (Scylla serrata) from Merauke as a Foundation on Bio-ceramic Components. J. Phys. Conf. Ser. 2019, 1204, 012031. [Google Scholar] [CrossRef]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A. Biodiesel production via transesterification of palm olein using waste mud crab (Scylla serrata) shell as a heterogeneous catalyst. Bioresour. Technol. 2009, 100, 6362–6368. [Google Scholar] [CrossRef]
- Thakur, S.; Mathur, S.; Patel, S.; Paital, B. Microplastic Accumulation and Degradation in Environment via Biotechnological Approaches. Water 2022, 14, 4053. [Google Scholar] [CrossRef]
- Cadano, J.R.; Jose, M.; Lubi, A.G.; Maling, J.N.; Moraga, J.S.; Shi, Q.Y.; Vegafria, H.M.; VinceCruz-Abeledo, C.C. A comparative study on the raw chitin and chitosan yields of common bio-waste from Philippine seafood. Environ. Sci. Pollut. Res. 2021, 28, 11954–11961. [Google Scholar] [CrossRef]
- Divya, M.; Vaseeharan, B.; Anjugam, M.; Iswarya, A.; Karthikeyan, S.; Velusamy, P.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; et al. Phenoloxidase activation, antimicrobial, and antibiofilm properties of β-glucan binding protein from Scylla serrata crab hemolymph. Int. J. Biol. Macromol. 2018, 114, 864–873. [Google Scholar] [CrossRef]
- Anju, A.; Smitha, C.; Preetha, K.; Boobal, R.; Rosamma, P. Molecular characterization, recombinant expression and bioactivity profile of an antimicrobial peptide, Ss-arasin from the Indian mud crab, Scylla serrata. Fish Shellfish Immunol. 2019, 88, 352–358. [Google Scholar] [CrossRef]
- Akbar, N.; Siddiqui, R.; Sagathevan, K.; Khan, N.A. Gut bacteria of animals living in polluted environments exhibit broad-spectrum antibacterial activities. Int. Microbiol. 2020, 23, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Yedery, R.D.; Reddy, K.V.R. Purification and characterization of antibacterial proteins from granular hemocytes of Indian mud crab, Scylla serrata. Acta Biochim. Pol. 2009, 56, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Yedery, R.D.; Reddy, K.V.R. Identification, cloning, characterization and recombinant expression of an anti-lipopolysaccharide factor from the hemocytes of Indian mud crab, Scylla serrata. Fish Shellfish Immunol. 2009, 27, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Schauber, J.; Dorschner, R.A.; Yamasaki, K.; Brouha, B.; Gallo, R.L. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 2006, 118, 509–519. [Google Scholar] [CrossRef]
- Afsal, V.; Antony, S.P.; Sathyan, N.; Philip, R. Molecular characterization and phylogenetic analysis of two antimicrobial peptides: Anti-lipopolysaccharide factor and crustin from the brown mud crab, Scylla serrata. Results Immunol. 2011, 1, 6–10. [Google Scholar] [CrossRef]
- Vidya, R.; Paria, A.; Deepika, A.; Sreedharan, K.; Makesh, M.; Purushothaman, C.S.; Chaudhari, A.; Babu, P.G.; Rajendran, K.V. Toll-like receptor of mud crab, Scylla serrata: Molecular characterisation, ontogeny and functional expression analysis following ligand exposure, and bacterial and viral infections. Mol. Biol. Rep. 2014, 41, 6865–6877. [Google Scholar] [CrossRef]
- Vidya, R.; Makesh, M.; Purushothaman, C.; Chaudhari, A.; Gireesh-Babu, P.; Rajendran, K. Report of leucine-rich repeats (LRRs) from Scylla serrata: Ontogeny, molecular cloning, characterization and expression analysis following ligand stimulation, and upon bacterial and viral infections. Gene 2016, 590, 159–168. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, B.; Bao, C.; Huang, H.; Ye, H. Vitellogenin2: Spermatozoon specificity and immunoprotection in mud crabs. Reproduction 2016, 152, 235–243. [Google Scholar] [CrossRef]
- Deepika, A.; Makesh, M.; Rajendran, K.V. Development of primary cell cultures from mud crab, Scylla serrata, and their potential as an in vitro model for the replication of white spot syndrome virus. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 406–416. [Google Scholar] [CrossRef]
- Sharma, S.; Yedery, R.; Patgaonkar, M.; Selvaakumar, C.; Reddy, K. Antibacterial activity of a synthetic peptide that mimics the LPS binding domain of Indian mud crab, Scylla serrata Anti-lipopolysaccharide Factor (SsALF) also involved in the modulation of vaginal immune functions through NF-kB signaling. Microb. Pathog. 2011, 50, 179–191. [Google Scholar] [CrossRef]
- Srisapoome, P.; Klongklaew, N.; Areechon, N.; Wongpanya, R. Molecular and functional analyses of novel anti-lipopolysaccharide factors in giant river prawn (Macrobrachium rosenbergii, De Man) and their expression responses under pathogen and temperature exposure. Fish Shellfish Immunol. 2018, 80, 357–375. [Google Scholar] [CrossRef]
- Afsal, V.; Antony, S.P.; Bright, A.R.; Philip, R. Molecular identification and characterization of Type I crustin isoforms from the hemocytes of portunid crabs, Scylla tranquebarica and Portunus pelagicus. Cell. Immunol. 2013, 284, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Brockton, V.; Smith, V.J. Crustin expression following bacterial injection and temperature change in the shore crab, Carcinus maenas. Dev. Comp. Immunol. 2008, 32, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- van de Braak, C.B.T.; Faber, R.; Boon, J.H. Cellular and humoral characteristics of Penaeus monodon (Fabricius, 1798) haemolymph. Comp. Clin. Pathol. 1996, 6, 194–203. [Google Scholar] [CrossRef]
- Kumar, B.; Deepika, A.; Makesh, M.; Purushothaman, C.; Rajendran, K. Production and characterization of monoclonal antibodies to the hemocytes of mud crab, Scylla serrata. J. Invertebr. Pathol. 2012, 111, 86–89. [Google Scholar] [CrossRef]
- Durairaj, K.R.; Saravanan, K.; Mohan, K.; Ravichandran, S. Purification, characterization and biological functions of metalloprotein isolated from haemolymph of mud crab Scylla serrata (Forskal, 1775). Int. J. Biol. Macromol. 2020, 164, 3901–3908. [Google Scholar] [CrossRef]
- Asthana, M.; Ahamed, M.; Shanthi, C. First mass spectrometric report of cryptocyanin, a moulting protein from the mud crab Scylla serrata (Forskål, 1775) (Decapoda: Brachyura: Portunidae) in India. J. Crustac. Biol. 2021, 41, ruaa094. [Google Scholar] [CrossRef]
- Wang, G.-Z.; Kong, X.-H.; Wang, K.-J.; Li, S.-J. Variation of specific proteins, mitochondria and fatty acid composition in gill of Scylla serrata (Crustacea, Decapoda) under low temperature adaptation. J. Exp. Mar. Biol. Ecol. 2007, 352, 129–138. [Google Scholar] [CrossRef]
- Terwilliger, N.B.; Dumler, K. Ontogeny of Decapod Crustacean Hemocyanin: Effects of Temperature and Nutrition. J. Exp. Biol. 2001, 204, 1013–1020. [Google Scholar] [CrossRef]
- Jeyachandran, S.; Chandrabose, S.; Singh, S.K.; Baskaralingam, V.; Park, K.; Kwak, I.-S. Characterization and structural analysis of prophenoloxidase in mud crab Scylla serrata and discovering novel chemical inhibitors through virtual screening. Struct. Chem. 2020, 31, 1563–1584. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, F.; Li, S.; Peng, H.; Wang, K.-J. A Novel Antimicrobial Peptide Sparanegtin Identified in Scylla paramamosain Showing Antimicrobial Activity and Immunoprotective Role In Vitro and Vivo. Int. J. Mol. Sci. 2022, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, F.; Chen, H.-Y.; Peng, H.; Hao, H.; Wang, K.-J. A Novel Antimicrobial Peptide Scyreprocin from Mud Crab Scylla paramamosain Showing Potent Antifungal and Anti-biofilm Activity. Front. Microbiol. 2020, 11, 1589. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-H.; Zhou, Z.-K.; Tu, D.-D.; Zhou, Y.-L.; Wang, C.; Liu, Z.-P.; Gu, W.-B.; Chen, Y.-Y.; Shu, M.-A. Effect of cadmium exposure on hepatopancreas and gills of the estuary mud crab (Scylla paramamosain): Histopathological changes and expression characterization of stress response genes. Aquat. Toxicol. 2018, 195, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pandiammal, S.; Rajalakshmi, E.; Senthilkumaar, P.; Bashini, J.M.; Pandiammal, S.; Rajalakshmi, E.; Senthilkumaar, P.; Bashini, J.M. Impact of toxicants in mud crab (Scylla serrata) muscle. JETIR 2019, 6, 221–224. [Google Scholar]
- Yu, Y.; Wang, P.; Bian, L.; Hong, S. Rare Death Via Histamine Poisoning Following Crab Consumption: A Case Report. J. Forensic Sci. 2017, 63, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Shuqing, G.; Zhen, F.; Bing, N.; Xiaojun, D.; Dehua, G.; Jian, Z.; Fang, H. Purification, cloning, expression and immunological analysis of Scylla serrata arginine kinase, the crab allergen. J. Sci. Food Agric. 2011, 91, 1326–1335. [Google Scholar] [CrossRef]
- Meng, J.; Gu, S.; Fang, Z.; Niu, B.; Deng, X.; Guo, D.; Zhu, J.; Han, F. Detection of seven kinds of aquatic product allergens in meat products and seasoning by liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2019, 37, 712–722. [Google Scholar] [CrossRef]
- Divya, M.; Govindarajan, M.; Karthikeyan, S.; Preetham, E.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Vaseeharan, B. Antibiofilm and anticancer potential of β-glucan-binding protein-encrusted zinc oxide nanoparticles. Microb. Pathog. 2020, 141, 103992. [Google Scholar] [CrossRef]
- Xie, J.-J.; Chen, C.-Q.; Yan, Y.-W.; Zhang, J.-P.; Lin, J.-C.; Wang, Q.; Zhou, H.-T.; Chen, Q.-X. Inactivation Kinetics of β-N-Acetyl-D-glucosaminidase from Green Crab (Scylla serrata) in Dioxane Solution. J. Biomol. Struct. Dyn. 2012, 26, 509–515. [Google Scholar] [CrossRef]
- Annual Reports—MPEDA. Available online: https://mpeda.gov.in/?page_id=2365 (accessed on 21 April 2023).
- Myanmar Country Environmental Analysis. Sustainability, Peace, and Prosperity: Forests, Fisheries, and Environmental Management. 2019. Available online: https://documents.worldbank.org/en/publication/documents-reports/documentdetail/288491560183163331/myanmar-country-environmental-analysis-sustainability-peace-and-prosperity-forests-fisheries-and-environmental-management-fisheries-sector-report (accessed on 21 April 2023).
- Quinitio, E.T. Status of Mud Crab Industry in the Philippines. In Proceedings of the International Seminar-Workshop on Mud Crab Aquaculture and Fisheries Management, Tamil Nadu, India, 10–12 April 2013; Rajiv Gandhi Centre for Aquaculture: Tamil Nadu, India, 2015; pp. 27–35. [Google Scholar]
- Fisheries, Cultivation and Research Aspects of Mud Crab (Genus scylla) in China. Available online: https://repository.seafdec.org.ph/handle/10862/3205 (accessed on 21 April 2023).
- Nooseng, S. Status of Mud Crab Industry in Thailand. In Proceedings of the International Seminar-Workshop on Mud Crab Aquaculture and Fisheries Management, Tamil Nadu, India, 10–12 April 2013; Rajiv Gandhi Centre for Aquaculture: Tamil Nadu, India, 2015; pp. 37–43. [Google Scholar]
- Bhuiyan, S.; Shamsuzzaman, M.; Hossain, M.M.; Mitu, S.J.; Mozumder, M.M.H. Mud crab (Scylla serrata Forsskal 1775) value chain analysis in the Khulna region of Bangladesh. Aquac. Fish. 2021, 6, 330–336. [Google Scholar] [CrossRef]
- Mulya, M.B.; Harahap, Z.A. Abundance and Growth Parameter of Mangrove Crab (Scylla serrata) in Estuary Water of Karang Gading, District Deli Serdang. IOP Conf. Ser. Earth Environ. Sci. 2019, 305, 012034. [Google Scholar] [CrossRef]
- Leoville, A.; Lagarde, R.; Grondin, H.; Faivre, L.; Rasoanirina, E.; Teichert, N. Influence of environmental conditions on the distribution of burrows of the mud crab, Scylla serrata, in a fringing mangrove ecosystem. Reg. Stud. Mar. Sci. 2021, 43, 101684. [Google Scholar] [CrossRef]
- Chaudhuri, A.B.; Choudhury, A. Mangroves of the Sundarbans. India. In Mangroves of the Sundarbans. Volume 1: India; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 1994. [Google Scholar]
- Biology and Conservation of the Genus Scylla in India Subcontinent—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23734453/ (accessed on 15 May 2023).
- Srinivasagam, S.; Kathiravel, M.; Kulasekarapandain, S. Captive Broodstock Development Induced Breeding and Larval Stages of Mud Crabs; Indian Council of Agricultural Research: New Delhi, India, 2000. [Google Scholar]
- LE Vay, L. Ecology and Management of Mud Crab Scylla spp. Asian Fish. Sci. 2001, 14, 101–111. [Google Scholar] [CrossRef]
- Oshiro, N. Aquaculture in Tropical; 1988, Undefined Mangrove Crabs (Scylla spp.). 2023. Available online: https://cir.nii.ac.jp/ (accessed on 15 May 2023).
- Panigrahi, S.; Acharya, B.C.; Panigrahy, R.C.; Nayak, B.K.; Banarjee, K.; Sarkar, S.K. Anthropogenic impact on water quality of Chilika lagoon RAMSAR site: A statistical approach. Wetl. Ecol. Manag. 2006, 15, 113–126. [Google Scholar] [CrossRef]
- Kamaruddin, E.; Siregar, Y.I.; Saam, Z.S.S. Diversity and abundance of scylla spp in mangrove habitat at Sungai Pinang village, Lingga. Biodivers. Int. J. 2019, 3, 235–239. [Google Scholar] [CrossRef]
- Thangal, S.H.; Muralisankar, T.; Anandhan, K.; Gayathri, V.; Yogeshwaran, A. Effect of CO2 driven ocean acidification on the mud crab Scylla serrata instars. Environ. Pollut. 2022, 312, 119995. [Google Scholar] [CrossRef]
- Pratoomchat, B.; Sawangwong, P.; Machado, J. Effects of controlled pH on organic and inorganic composition in haemolymph, epidermal tissue and cuticle of mud crab Scylla serrata. J. Exp. Zool. Part A Comp. Exp. Biol. 2003, 295A, 47–56. [Google Scholar] [CrossRef]
- Syafaat, M.N.; Azra, M.N.; Mohamad, F.; Che-Ismail, C.Z.; Amin-Safwan, A.; Asmat-Ullah, M.; Syahnon, M.; Ghazali, A.; Abol-Munafi, A.B.; Ma, H.; et al. Thermal Tolerance and Physiological Changes in Mud Crab, Scylla paramamosain Crablet at Different Water Temperatures. Animals 2021, 11, 1146. [Google Scholar] [CrossRef]
- Ruscoe, I.M.; Shelley, C.C.; Williams, G.R. The combined effects of temperature and salinity on growth and survival of juvenile mud crabs (Scylla serrata Forskål). Aquaculture 2004, 238, 239–247. [Google Scholar] [CrossRef]
- Romano, N.; Wu, X.; Zeng, C.; Genodepa, J.; Elliman, J. Growth, osmoregulatory responses and changes to the lipid and fatty acid composition of organs from the mud crab, Scylla serrata, over a broad salinity range. Mar. Biol. Res. 2014, 10, 460–471. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Acute toxicity of ammonia and its effects on the haemolymph osmolality, ammonia-N, pH and ionic composition of early juvenile mud crabs, Scylla serrata (Forskål). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Paital, B.; Chainy, G. Effects of salinity on O2 consumption, ROS generation and oxidative stress status of gill mitochondria of the mud crab Scylla serrata. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 228–237. [Google Scholar] [CrossRef] [PubMed]
- (PDF) Food and Feeding Habits of Mud Crab Scylla serrata (Forssakal) from Chilika Lagoon. Available online: https://www.researchgate.net/publication/260843535_Food_and_Feeding_habits_of_mud_crab_Scylla_serrata_Forssakal_from_Chilika_lagoon (accessed on 27 January 2023).
- Davis, J.A.; Wille, M.; Hecht, T.; Sorgeloos, P. Optimal first feed organism for South African mud crab Scylla serrata (Forskål) larvae. Aquac. Int. 2005, 13, 187–201. [Google Scholar] [CrossRef]
- Suprayudi, M.; Takeuchi, T.; Hamasaki, K. Essential fatty acids for larval mud crab Scylla serrata: Implications of lack of the ability to bioconvert C18 unsaturated fatty acids to highly unsaturated fatty acids. Aquaculture 2004, 231, 403–416. [Google Scholar] [CrossRef]
- Ayaz, K.S.M.; Vadher, K.H. Evaluation of the nutritional quality of selected dietary ingredients for mud crab Scylla serrata of Suarashtra region in Gujarat, India. J. Appl. Nat. Sci. 2020, 12, 288–291. [Google Scholar] [CrossRef]
- Hill, B.J. Activity, track and speed of movement of the crab Scylla serrata in an estuary. Mar. Biol. 1978, 47, 135–141. [Google Scholar] [CrossRef]
- Ragunathan, M. Vicissitudes of oxidative stress biomarkers in the estuarine crab Scylla serrata with reference to dry and wet weather conditions in Ennore estuary, Tamil Nadu, India. Mar. Pollut. Bull. 2017, 116, 113–120. [Google Scholar] [CrossRef]
- Dennis, M.; Diggles, B.; Faulder, R.; Olyott, L.; Pyecroft, S.; Gilbert, G.; Landos, M. Pathology of finfish and mud crabs Scylla serrata during a mortality event associated with a harbour development project in Port Curtis, Australia. Dis. Aquat. Org. 2016, 121, 173–188. [Google Scholar] [CrossRef]
- van Oosterom, J.; King, S.C.; Negri, A.; Humphrey, C.; Mondon, J. Investigation of the mud crab (Scylla serrata) as a potential bio-monitoring species for tropical coastal marine environments of Australia. Mar. Pollut. Bull. 2010, 60, 283–290. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G. Seasonal variability of antioxidant biomarkers in mud crabs (Scylla serrata). Ecotoxicol. Environ. Saf. 2013, 87, 33–41. [Google Scholar] [CrossRef]
- Pati, S.G.; Panda, F.; Jena, S.; Sahoo, D.K.; Paital, B. Effects of soil trace metals, organic carbon load and physicochemical stressors on active oxygen species metabolism in Scylla serrata sampled along the Bay of Bengal in Odisha state, India. Front. Environ. Sci. 2022, 10, 1733. [Google Scholar] [CrossRef]
- Anderson, N.J. Miniview: Diatoms, temperature and climatic change. Eur. J. Phycol. 2000, 35, 307–314. [Google Scholar] [CrossRef]
- Saha, N.; Koner, D.; Sharma, R. Environmental hypoxia: A threat to the gonadal development and reproduction in bony fishes. Aquac. Fish. 2022, 7, 572–582. [Google Scholar] [CrossRef]
- De Lucena, I.C.; Nascimento, W.M.D.; Pinheiro, A.P.; Cascon, P. Ecological responses of two shrimp populations (Palaemonidae) to seasonal abiotic factor variations in a Brazilian semiarid reservoir. Ethol. Ecol. Evol. 2020, 32, 409–432. [Google Scholar] [CrossRef]
- Dehnel, P.A. Effect of Temperature and Salinity on The Oxygen Consumption of Two Intertidal Crabs. Biol. Bull. 1960, 118, 215–249. [Google Scholar] [CrossRef]
- Newell, R.C. Factors Affecting the Respiration of Intertidal Invertebrates. Am. Zool. 1973, 13, 513–528. [Google Scholar] [CrossRef]
- Rojas, N.E.T.; Marins, M.A.; Rocha, O. The effect of abiotic factors on the hatching of Moina micrura Kurz, 1874 (Crustacea: Cladocera) ephippial eggs. Braz. J. Biol. 2001, 61, 371–376. [Google Scholar] [CrossRef]
- Ewel, K.C.; Rowe, S.; McNaughton, B.; Bonine, K.M. Characteristics of Scylla spp. (Decapoda: Portunidae) and Their Mangrove Forest Habitat in Ngaremeduu Bay, Republic of Palau1. Pac. Sci. 2009, 63, 15–26. [Google Scholar] [CrossRef]
- Koolkalya, S.; Thapanand, T.; Tunkijjanujij, S.; Havanont, V.; Jutagate, T. Aspects in spawning biology and migration of the mud crab Scylla olivacea in the Andaman Sea, Thailand. Fish. Manag. Ecol. 2006, 13, 391–397. [Google Scholar] [CrossRef]
- Weng, S.-P.; Guo, Z.-X.; Sun, J.-J.; Chan, S.-M.; He, J.-G. A reovirus disease in cultured mud crab, Scylla serrata, in southern China. J. Fish Dis. 2007, 30, 133–139. [Google Scholar] [CrossRef]
- Robertson, W.; Kruger, A. Size at Maturity, Mating and Spawning in the Portunid Crab Scylla serrata (Forskål) in Natal, South Africa. Estuarine, Coast. Shelf Sci. 1994, 39, 185–200. [Google Scholar] [CrossRef]
- Bhavanishankar, S.; Subramoniam, T. Cryopreservation of spermatozoa of the edible mud crabScylla serrata (Forskal). J. Exp. Zool. 1997, 277, 326–336. [Google Scholar] [CrossRef]
- Hill, B.J. Offshore spawning by the portunid crab Scylla serrata (Crustacea: Decapoda). Mar. Biol. 1994, 120, 379–384. [Google Scholar] [CrossRef]
- Unnikrishnan, U.; Paulraj, R. Dietary protein requirement of giant mud crab Scylla serrata juveniles fed iso-energetic formulated diets having graded protein levels. Aquac. Res. 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Onyango, S.D. The Breeding Cycle of Scylla serrata (Forskål, 1755) at Ramisi River Estuary, Kenya. Wetl. Ecol. Manag. 2002, 10, 257–263. [Google Scholar] [CrossRef]
- Davis, J.A.; Churchill, G.J.; Hecht, T.; Sorgeloos, P. Spawning Characteristics of the South African Mudcrab Scylla serrata (Forskål) in Captivity. J. World Aquac. Soc. 2004, 35, 121–133. [Google Scholar] [CrossRef]
- Millamena, O.M.; Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture 2000, 181, 81–90. [Google Scholar] [CrossRef]
- Allayie, S.A.; Ravichandran, S.; Bhat, B.A. Hormonal regulatory role of eyestalk factors on growth of heart in mud crab, Scylla serrata. Saudi J. Biol. Sci. 2011, 18, 283–286. [Google Scholar] [CrossRef]
- Ikhwanuddi, M.; Adnan, M.-F.; Mohamad, S.; Abol-Munaf, A.B. Effect of Eyestalk Ablation on the Ovarian Maturation Stages of Blue Swimming Crab, Portunus pelagicus. Asian J. Biol. Sci. 2019, 12, 437–441. [Google Scholar] [CrossRef]
- Hill, B.J. Abundance, breeding and growth of the crab Scylla serrata in two South African estuaries. Mar. Biol. 1975, 32, 119–126. [Google Scholar] [CrossRef]
- Huang, H.; Huang, C.; Guo, L.; Zeng, C.; Ye, H. Profiles of calreticulin and Ca2+ concentration under low temperature and salinity stress in the mud crab, Scylla paramamosain. PLoS ONE 2019, 14, e0220405. [Google Scholar] [CrossRef] [PubMed]
- Sroyraya, M.; Hanna, P.J.; Changklungmoa, N.; Senarai, T.; Siangcham, T.; Tinikul, Y.; Sobhon, P. Expression of the male reproduction-related gene in spermatic ducts of the blue swimming crab, Portunus pelagicus, and transfer of modified protein to the sperm acrosome. Microsc. Res. Tech. 2013, 76, 102–112. [Google Scholar] [CrossRef]
- Shrestha, A.M.S.; Crissa, C.A.; Joyce, J.E.; Maria, M.R.; Ablan Lagman, M.C. Comparative Transcriptome Profiling of Heat Stress Response of the Mangrove Crab Scylla serrata across Sites of Varying Climate Profiles. BMC Genomics 2021, 22, 580. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.; Mamun, A.-A. Feasibility study on the culture of mud crab Scylla serrata in the mid coast region of Bangladesh. Pak. J. Biol. Sci. 2012, 15, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Baticados, D.B.; Agbayani, R.F.; Quinitio, E.T. Community-Based Technology Transfer in Rural Aquaculture: The Case of Mudcrab Scylla serrata Nursery in Ponds in Northern Samar, Central Philippines. AMBIO 2014, 43, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Manduzio, H.; Rocher, B.; Durand, F.; Galap, C.; Leboulenger, F.; Manduzio, H. The Point about Oxidative Stress in Molluscs. Invertebrate Survival Journal 2005.
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and Physiological Ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef]
- Chen, J.-C.; Chia, P.-G. Oxygen Uptake and Nitrogen Excretion of Juvenile Scylla serrata at Different Temperature and Salinity Levels. J. Crustac. Biol. 1996, 16, 437. [Google Scholar] [CrossRef]
- Hill, B.J. Salinity and temperature tolerance of zoeae of the portunid crab Scylla serrata. Mar. Biol. 1974, 25, 21–24. [Google Scholar] [CrossRef]
- Kong, X.; Wang, G.; Li, S. Antioxidation and ATPase activity in the gill of mud crab Scylla serrata under cold stress. Chin. J. Oceanol. Limnol. 2007, 25, 221–226. [Google Scholar] [CrossRef]
- Kong, X.; Wang, G.; Li, S.; Ai, C. Antioxidant Effects and ATPase Activity Changes in Hepatopancreas of Mud Crab Scylla serrata under Low Temperature Acclimation. J. Fish. Sci. China 2005, 12, 708–713. [Google Scholar]
- Fang, W.H.; Hu, L.L.; Yang, X.L.; Hu, K.; Liang, S.C.; Zhou, S. Effect of temperature on pharmacokinetics of enrofloxacin in mud crab, Scylla serrata (Forsskål), following oral administration. J. Fish Dis. 2008, 31, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Chutia, P.; Saha, N.; Das, M.; Goswami, L.M. Differential expression of aquaporin genes and the influence of environmental hypertonicity on their expression in juveniles of air-breathing stinging catfish (Heteropneustes fossilis). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 274, 111314. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Bert, T.M.; Tweedale, W.A.; Torres, J.J.; Lindberg, W.J. The effects of temperature and salinity on survival and development of early life stage Florida stone crabs Menippe mercenaria (Say). J. Exp. Mar. Biol. Ecol. 1992, 157, 115–136. [Google Scholar] [CrossRef]
- Evenset, A.; Hallanger, I.; Tessmann, M.; Warner, N.; Ruus, A.; Borgå, K.; Gabrielsen, G.; Christensen, G.; Renaud, P. Seasonal variation in accumulation of persistent organic pollutants in an Arctic marine benthic food web. Sci. Total. Environ. 2016, 542, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Rumisha, C.; Huyghe, F.; Rapanoel, D.; Mascaux, N.; Kochzius, M. Genetic diversity and connectivity in the East African giant mud crab Scylla serrata: Implications for fisheries management. PLoS ONE 2017, 12, e0186817. [Google Scholar] [CrossRef]
- Batvari, B.P.D.; Sivakumar, S.; Shanthi, K.; Lee, K.-J.; Oh, B.-T.; Krishnamoorthy, R.R.; Kamala-Kannan, S. Heavy metals accumulation in crab and shrimps from Pulicat lake, north Chennai coastal region, southeast coast of India. Toxicol. Ind. Health 2013, 32, 1–6. [Google Scholar] [CrossRef]
- Harris, J.M.; Vinobaba, P.; Kularatne, R.K.A.; Kankanamge, C.E. Heavy metal bioaccumulation and Fulton’s K condition indices in Scylla serrata (Forskål) in relation to sex. Int. J. Environ. Sci. Technol. 2019, 16, 201–210. [Google Scholar] [CrossRef]
- Hudspith, M.; Reichelt-Brushett, A.; Harrison, P.L. Factors affecting the toxicity of trace metals to fertilization success in broadcast spawning marine invertebrates: A review. Aquat. Toxicol. 2017, 184, 1–13. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, H.; Wang, Y.; Wang, S.; Xie, F.; Li, S. Acrosome Reaction of Sperm in the Mud Crab Scylla serrata as a Sensitive Toxicity Test for Metal Exposures. Arch. Environ. Contam. Toxicol. 2010, 58, 96–104. [Google Scholar] [CrossRef]
- Enhanced Levels of Metallothionein in Scylla serrata Exposed to Cadmium. Available online: http://www.envirobiotechjournals.com/article_abstract.php?aid=1460&iid=59&jid=1 (accessed on 6 May 2023).
- Vasanthi, L.A.; Muruganandam, A.; Revathi, P.; Baskar, B.; Jayapriyan, K.; Baburajendran, R.; Munuswamy, N. The application of histo-cytopathological biomarkers in the mud crab Scylla serrata (Forskal) to assess heavy metal toxicity in Pulicat Lake, Chennai. Mar. Pollut. Bull. 2014, 81, 85–93. [Google Scholar] [CrossRef]
- Vijayavel, K.; Balasubramanian, M. Fluctuations of biochemical constituents and marker enzymes as a consequence of naphthalene toxicity in the edible estuarine crab Scylla serrata. Ecotoxicol. Environ. Saf. 2006, 63, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Vijayavel, K.; Balasubramanian, M. Reproductive dysfunction induced by naphthalene in an estuarine crab Scylla serrata with reference to vitellogenesis. Ecotoxicol. Environ. Saf. 2008, 69, 89–94. [Google Scholar] [CrossRef]
- Vijayavel, K.; Balasubramanian, M.P. DNA damage and cell necrosis induced by naphthalene due to the modulation of biotransformation enzymes in an estuarine crabScylla serrata. J. Biochem. Mol. Toxicol. 2008, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vijayavel, K.; Gopalakrishnan, S.; Thiagarajan, R.; Thilagam, H. Immunotoxic effects of nickel in the mud crab Scylla serrata. Fish Shellfish Immunol. 2009, 26, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Bowles, K.C.; Johnson, D.D.; Moltschaniwskyj, N.A. Depuration of perfluoroalkyl substances from the edible tissues of wild-caught invertebrate species. Sci. Total. Environ. 2017, 581–582, 258–267. [Google Scholar] [CrossRef]
- Sujitha, V.; Murugan, K.; Dinesh, D.; Pandiyan, A.; Aruliah, R.; Hwang, J.-S.; Kalimuthu, K.; Panneerselvam, C.; Higuchi, A.; Aziz, A.T.; et al. Green-synthesized CdS nano-pesticides: Toxicity on young instars of malaria vectors and impact on enzymatic activities of the non-target mud crab Scylla serrata. Aquat. Toxicol. 2017, 188, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Nikapitiya, C.; Kwak, T.S.; Kwak, I.S. Antioxidative-Related Genes Expression Following Perfluorooctane Sul-fonate (PFOS) Exposure in the Intertidal Mud Crab, Macrophthalmus japonicus. Ocean. Sci. J. 2015, 50, 547–556. [Google Scholar] [CrossRef]
- Yang, X.; Shi, A.; Song, Y.; Niu, C.; Yu, X.; Shi, X.; Pang, Y.; Ma, X.; Cheng, Y. The effects of ammonia-N stress on immune parameters, antioxidant capacity, digestive function, and intestinal microflora of Chinese mitten crab, Eriocheir sinensis, and the protective effect of dietary supplement of melatonin. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 250, 109127. [Google Scholar] [CrossRef]
- Sainath, S.; Reddy, P.S. Evidence for the involvement of selected biogenic amines (serotonin and melatonin) in the regulation of molting of the edible crab, Oziotelphusa senex senex Fabricius. Aquaculture 2010, 302, 261–264. [Google Scholar] [CrossRef]
- Girish, B.; Swetha, C.; Reddy, P.S. Induction of ecdysteroidogenesis, methyl farnesoate synthesis and expression of ecdysteroid receptor and retinoid X receptor in the hepatopancreas and ovary of the giant mud crab, Scylla serrata by melatonin. Gen. Comp. Endocrinol. 2015, 217–218, 37–42. [Google Scholar] [CrossRef]
- Girish, B.; Swetha, C.; Reddy, P.S. Serotonin induces ecdysteroidogenesis and methyl farnesoate synthesis in the mud crab, Scylla serrata. Biochem. Biophys. Res. Commun. 2017, 490, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Therisa, K.K.; Desai, P.V. Study of epileptiform activity in cerebral ganglion of mud crab Scylla serrata. Invertebr. Neurosci. 2011, 11, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Singaram, G.; Harikrishnan, T.; Chen, F.-Y.; Bo, J.; Giesy, J.P. Modulation of immune-associated parameters and antioxidant responses in the crab (Scylla serrata) exposed to mercury. Chemosphere 2013, 90, 917–928. [Google Scholar] [CrossRef]
- Stomeo, F.; Makhalanyane, T.; Valverde, A.; Pointing, S.B.; Stevens, M.I.; Cary, S.; Tuffin, M.; Cowan, D.A. Abiotic factors influence microbial diversity in permanently cold soil horizons of a maritime-associated Antarctic Dry Valley. FEMS Microbiol. Ecol. 2012, 82, 326–340. [Google Scholar] [CrossRef]
- Rajendran, K.V.; Vijayan, K.K.; Santiago, T.C.; Krol, R.M. Experimental host range and histopathology of white spot syndrome virus (WSSV) infection in shrimp, prawns, crabs and lobsters from India. J. Fish Dis. 1999, 22, 183–191. [Google Scholar] [CrossRef]
- Supamattaya, K.; Hoffmann, R.; Boonyaratpalin, S.; Kanchanaphum, P. Experimental transmission of white spot syndrome virus (WSSV) from black tiger shrimp Penaeus monodon to the sand crab Portunus pelagicus, mud crab Scylla serrata and krill Acetes sp. Dis. Aquat. Org. 1998, 32, 79–85. [Google Scholar] [CrossRef]
- Andersen, L.; Norton, J.; Levy, N. A new shell disease in the mud crab Scylla serrata from Port Curtis, Queensland (Australia). Dis. Aquat. Org. 2000, 43, 233–239. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.; Wu, Q.; Liu, W.; Lin, Y. Infection with Hematodinium sp. in mud crabs Scylla serrata cultured in low salinity water in southern China. Dis. Aquat. Org. 2008, 82, 145–150. [Google Scholar] [CrossRef]
- Chen, J.; Xiong, J.; Cui, B.; Yang, J.; Li, W.; Mao, Z. Molecular characterization of eight segments of Scylla serrata reovirus (SsRV) provides the complete genome sequence. Arch. Virol. 2012, 157, 1551–1557. [Google Scholar] [CrossRef]
- Yuan, Y.; Fan, D.; Zhang, Z.; Yang, J.; Liu, J.; Chen, J. Identification and RNA segment assignment of six structural proteins of Scylla serrata reovirus. Virus Genes 2016, 52, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Midorikawa, Y.; Shimizu, T.; Sanda, T.; Hamasaki, K.; Dan, S.; Lal, M.T.B.M.; Kato, G.; Sano, M. Characterization of Aquimarina hainanensis isolated from diseased mud crab Scylla serrata larvae in a hatchery. J. Fish Dis. 2020, 43, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Burhanuddin; Saru, A.; Rantetondok, A.; Zainuddin, E.N. MIC (Minimum Inhibition Concentration) test of metanol extract on rhizophora stylosa and chloroform Avicennia marina against vibriosis in mangrove crab larvae (Scylla serrata forsskal). IOP Conf. Ser. Earth Environ. Sci. 2020, 473, 012010. [Google Scholar] [CrossRef]
- Gopikrishna, G.; Shekhar, M.S. Karyological and PCR-RFLP Studies of the Mud Crabs-Scylla serrata and Scylla tranquebarica. J. Fish. Soc. 2003, 30, 315–320. [Google Scholar] [CrossRef]
- Niiyama, H. A Comparative Study of the Chromosomes in Decapods, Isopods and Amphipods, with Some Remarks on Cytotaxonomy and Sex-Determination in the Crustacea; Memoirs of the Faculty of Fisheries Hokkaido University; Hokkaido University Collection of Scholarly and Academic Papers: Sapporo, Japan, 1959; Volume 7, pp. 1–60. [Google Scholar]
- Vishnoi, D.N. Studies on the Chromosomes of Some Indian Crustacea. Cytologia 1972, 37, 43–51. [Google Scholar] [CrossRef]
- Jondeung, A.; Karinthanyakit, W.; Kaewkhumsan, J. The Complete Mitochondrial Genome of the Black Mud Crab, Scylla serrata (Crustacea: Brachyura: Portunidae) and Its Phylogenetic Position among (Pan) Crustaceans. Mol. Biol. Rep. 2012, 39, 10921–10937. [Google Scholar] [CrossRef]
- Shi, X.; Waiho, K.; Li, X.; Ikhwanuddin, M.; Miao, G.; Lin, F.; Zhang, Y.; Li, S.; Zheng, H.; Liu, W.; et al. Female-specific SNP markers provide insights into a WZ/ZZ sex determination system for mud crabs Scylla paramamosain, S. tranquebarica and S. serrata with a rapid method for genetic sex identification. BMC Genom. 2018, 19, 981. [Google Scholar] [CrossRef]
- Waiho, K.; Fazhan, H.; Ikhwanuddin, M.; Quinitio, E.T.; Baylon, J.C.; Shu-Chien, A.C.; Liew, H.J.; Afiqah-Aleng, N.; Ma, H. Chromosomal sex determination system in brachyurans and its potential application in aquaculture. Aquaculture 2021, 543, 736990. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, H.; Huang, H.; Li, S.; Liu, X.; Zeng, X.; Gong, J. Expression of Hsp70 in the mud crab, Scylla paramamosain in response to bacterial, osmotic, and thermal stress. Cell Stress Chaperon 2013, 18, 475–482. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, F.; Liao, M.; Liu, M.; Zheng, B.; Yang, H.; Zhong, M. Molecular cloning and expression analysis of a cytosolic heat shock protein 70 gene from mud crab Scylla serrata. Fish Shellfish Immunol. 2013, 34, 1306–1314. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, K.; Sun, M.; Zhang, D.; Ma, L. Multiplex immune-related genes expression analysis response to bacterial challenge in mud crab, Scylla paramamosain. Fish Shellfish Immunol. 2013, 34, 712–716. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Zhu, X.-L.; Lu, J.; Cai, W.-J.; Ye, Y.-P.; Lv, Y.-P. Effect of high temperature stress on heat shock protein expression and antioxidant enzyme activity of two morphs of the mud crab Scylla paramamosain. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 223, 10–17. [Google Scholar] [CrossRef]
- Rumisha, C.; Leermakers, M.; Mdegela, R.H.; Kochzius, M.; Elskens, M. Bioaccumulation and public health implications of trace metals in edible tissues of the crustaceans Scylla serrata and Penaeus monodon from the Tanzanian coast. Environ. Monit. Assess. 2017, 189, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Botsford, L.W.; Brumbaugh, D.R.; Grimes, C.; Kellner, J.B.; Largier, J.; O’farrell, M.R.; Ralston, S.; Soulanille, E.; Wespestad, V. Connectivity, sustainability, and yield: Bridging the gap between conventional fisheries management and marine protected areas. Rev. Fish Biol. Fish. 2008, 19, 69–95. [Google Scholar] [CrossRef]
- McLeod, E.; Salm, R.; Green, A.; Almany, J. Designing marine protected area networks to address the impacts of climate change. Front. Ecol. Environ. 2009, 7, 362–370. [Google Scholar] [CrossRef]
- Bal, A.; Pati, S.G.; Panda, F.; Mohanty, L.; Paital, B. Low salinity induced challenges in the hardy fish Heteropneustes fossilis; future prospective of aquaculture in near coastal zones. Aquaculture 2021, 543, 737007. [Google Scholar] [CrossRef]
- Bal, A.; Panda, F.; Pati, S.G.; Das, K.; Agrawal, P.K.; Paital, B. Modulation of physiological oxidative stress and antioxidant status by abiotic factors especially salinity in aquatic organisms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 241, 108971. [Google Scholar] [CrossRef]
- Paital, B.; Kumar, S.; Farmer, R.; Chainy, G.B.N. In silico prediction of 3D structure of Mn superoxide dismutase of Scylla serrata and its binding properties with inhibitors. Interdiscip. Sci. Comput. Life Sci. 2013, 5, 69–76. [Google Scholar] [CrossRef]
- Paital, B.; Kumar, S.; Farmer, R.; Tripathy, N.K.; Chainy, G.B.N. In silico prediction and characterization of 3D structure and binding properties of catalase from the commercially important crab, Scylla serrata. Interdiscip. Sci. Comput. Life Sci. 2011, 3, 110–120. [Google Scholar] [CrossRef]
- Oersted Mirera, D.; Mtile, A. A Preliminary Study on the Response of Mangrove Mud Crab (Scylla serrata) to Different Feed Types under Drive-in Cage Culture System. J. Ecol. Nat. Environ. 2009, 1, 7–14. [Google Scholar]
- Holme, M.-H.; Brock, I.; Southgate, P.C.; Zeng, C. Effects of Starvation and Feeding on Lipid Class and Fatty Acid Profile of Late Stage Mud Crab, Scylla serrata, larvae. J. World Aquac. Soc. 2009, 40, 493–504. [Google Scholar] [CrossRef]
- Nurdiani, R.; Zeng, C. Effects of temperature and salinity on the survival and development of mud crab, Scylla serrata (Forsskål), larvae. Aquac. Res. 2007, 38, 1529–1538. [Google Scholar] [CrossRef]
- Kurkute, S.L.; Pawase, A.S.; Dey, S.; Pathan, D.I.; Sawant, M.S.; Swain, S. Effect of Some Minerals on Shell Hardening of Mud Crab, Scylla serrata (Forskal, 1775). Int. J. Fish. Aquat. Stud. 2019, 7, 44–47. [Google Scholar]
- Hastuti, Y.P.; Nadeak, H.; Affandi, R.; Faturrohman, K. Penentuan pH optimum untuk pertumbuhan kepiting bakau Scylla serrata dalam wadah terkontrol. J. Akuakultur Indones. 2016, 15, 171–179. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Lin, F.-Y.; Hu, Y.; Wei, Z.-J.; Yeh, S.-L. Integration of Independent Box Culture of Mud Crab, Scylla serrata, and Solar Power System. J. Fish. Soc. 2020, 47, 73–85. [Google Scholar] [CrossRef]
- Deshimaru, O.; Kuroki, K.; Sakamoto, S.; Yone, Y. Absorption of labelled calcium-45Ca by prawn from sea water. Nippon. Suisan Gakkaishi 1978, 44, 975–977. [Google Scholar] [CrossRef]
- Aaqillah-Amr, M.A.; Hidir, A.; Ahmad-Ideris, A.R.; Muhamad-Zulhilmi, R.; Peng, T.H.; Abualreesh, M.H.; Noordiyana, M.N.; Ma, H.; Ikhwanuddin, M. The effect of lipid level on the growth and reproductive performance of female orange mud crab, Scylla olivacea (Herbst, 1796), during the fattening period. Aquac. Nutr. 2021, 27, 2497–2513. [Google Scholar] [CrossRef]
- Ur Rahman, A.; Kumar, P.; Khan, A. All of a Piece: Identification of the Different Life Stages of Scylla serrata (Forsskål, 1775) Using DNA Barcodes. J. Aquat. Biol. Fish. 2020, 8, 12–17. [Google Scholar]
- Hastuti, Y.P.; Wicaksono, P.H.; Nurusallam, W.; Tridesianti, S.; Fatma, Y.S.; Nirmala, K.; Rusmana, I.; Affandi, R. Addition of shelters to control the physiological responses and production of mud crab Scylla serrata in recirculation aquaculture system. J. Ilmu dan Teknol. Kelaut. Trop. 2020, 12, 299–310. [Google Scholar] [CrossRef]
- Islam, T.; Saha, D.; Bhowmik, S.; Nordin, N.; Islam, S.; Nur, A.-A.U.; Begum, M. Nutritional properties of wild and fattening mud crab (Scylla serrata) in the south-eastern district of Bangladesh. Heliyon 2022, 8, e12806. [Google Scholar] [CrossRef]
- Lin, W.-C.; He, Y.-M.; Shi, C.; Mu, C.-K.; Wang, C.-L.; Li, R.-H.; Ye, Y.-F. ATP catabolism and bacterial succession in postmortem tissues of mud crab (Scylla paramamosain) and their roles in freshness. Food Res. Int. 2022, 155, 110992. [Google Scholar] [CrossRef] [PubMed]
- Baag, S.; Mandal, S. Do global environmental drivers’ ocean acidification and warming exacerbate the effects of oil pollution on the physiological energetics of Scylla serrata? Environ. Sci. Pollut. Res. 2023, 30, 23213–23224. [Google Scholar] [CrossRef]
- Barathkumar, S.; Padhi, R.; Parida, P.; Marigoudar, S. In vivo appraisal of oxidative stress response, cell ultrastructural aberration and accumulation in Juvenile Scylla serrata exposed to uranium. Chemosphere 2022, 300, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lin, S.; Du, Y.; Gong, Y.; Li, S. SpBAG3 assisted WSSV infection in mud crab (Scylla paramamosain) by inhibiting apoptosis. Dev. Comp. Immunol. 2022, 129, 104349. [Google Scholar] [CrossRef]
- Tran, N.T.; Zhou, Y.; Chen, L.; Sun, Z.; Li, S. SpBNIP3 regulates apoptosis and autophagy in mud crab (Scylla paramamosain) during white spot syndrome virus infection. Dev. Comp. Immunol. 2022, 135, 104465. [Google Scholar] [CrossRef]
- Cheng, C.H.; Ma, H.L.; Liu, G.X.; Deng, Y.Q.; Jiang, J.J.; Feng, J.; Guo, Z.X. Biochemical, Metabolic, and Immune Responses of Mud Crab (Scylla paramamosain) after Mud Crab Reovirus Infection. Fish Shellfish Immunol. 2022, 127, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Liao, J.; Zhang, Z.; Li, Y.; Jia, X.; Zeng, X.; Wang, Y. Regulation of Vtg and VtgR in Mud Crab Scylla paramamosain by MiR-34. Mol. Biol. Rep. 2022, 49, 7367–7376. [Google Scholar] [CrossRef]
- Fazhan, H.; Waiho, K.; Shu-Chien, A.C.; Wang, Y.; Ikhwanuddin, M.; Abualreesh, M.H.; Kasan, N.A.; Wu, Q.; Muda, S.; Sor, C.S.; et al. Fine Sand Facilitates Egg Extrusion and Improves Reproductive Output in Female Mud Crab Genus Scylla. PeerJ 2022, 10, e13961. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J.; Shi, C.; Migaud, H.; Ye, Y.; Song, C.; Mu, C.; Ren, Z.; Wang, C. Effect of Photoperiod on Growth, Survival, and Lipid Metabolism of Mud Crab Scylla paramamosain Juveniles. Aquaculture 2023, 567, 739279. [Google Scholar] [CrossRef]








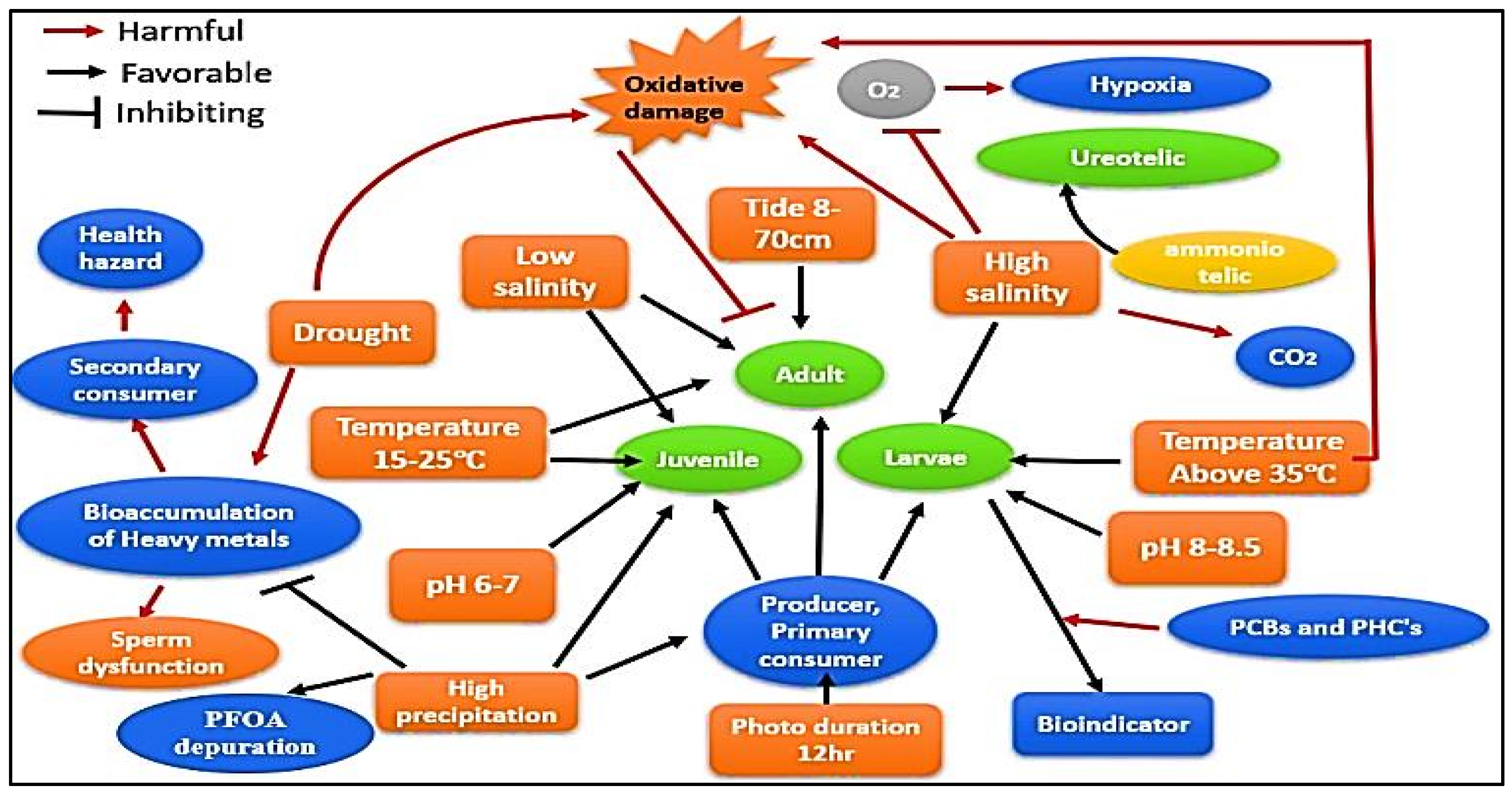
| Pollutants Level in the Water | Temperature and Salinity | Effect on Biomolecule | Levels of Biomarker | Mortality % | Reference |
|---|---|---|---|---|---|
| Naphthalene (10 µg mL−1) | 28 °C, 30 ppt | DNA-16%, RNA-20% | ADP-5.38% ↓, | 50% | [145] |
| ACP-30% ↓, | |||||
| ALP-38% ↓, | |||||
| AST-35% ↓, and | |||||
| ALT-13% ↓ | |||||
| Perfluorooctyl sulfonate (30 µg mL−1) | 21 °C, 30 ppt | NA | SOD-73%, CAT-71%, and | 66% | [151] |
| GPx-50% | |||||
| Carbon dots (8 µg mL−1) | 25 °C, 30 ppt | NA | AChE-12% and GST-50% | 55% | [150] |
| Water Physicochemical Factors | Zoea-II | Zoea-III | Zoea-IV | Zoea-V | Megalopa | First Crab Stage | Adult Crab | Reference |
|---|---|---|---|---|---|---|---|---|
| Temperature | 25–28 °C | 25–28 °C | 25–28 °C | 25–28 °C | 28–34 °C | 22–25 °C | 22–25 °C | [186] |
| Salinity (ppt) | 30 | 35 | 30–34 | 30–34 | 34 | 24 | 20–21 | [186] |
| pH | 8.1–7.8 | 8.1–7.8 | 8.1–7.8 | 8.1–7.8 | 8.1–7.8 | 7.8–7.5 | 7.3–6.4 | [188] |
| Light condition | Low light | Low light | Low light | Low light | Full light | Full light | Full light | [189] |
| Survivability | 86% | 85% | 82% | 78% | 60% | 85% | 90% | [186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pati, S.G.; Paital, B.; Panda, F.; Jena, S.; Sahoo, D.K. Impacts of Habitat Quality on the Physiology, Ecology, and Economical Value of Mud Crab Scylla sp.: A Comprehensive Review. Water 2023, 15, 2029. https://doi.org/10.3390/w15112029
Pati SG, Paital B, Panda F, Jena S, Sahoo DK. Impacts of Habitat Quality on the Physiology, Ecology, and Economical Value of Mud Crab Scylla sp.: A Comprehensive Review. Water. 2023; 15(11):2029. https://doi.org/10.3390/w15112029
Chicago/Turabian StylePati, Samar Gourav, Biswaranjan Paital, Falguni Panda, Srikanta Jena, and Dipak Kumar Sahoo. 2023. "Impacts of Habitat Quality on the Physiology, Ecology, and Economical Value of Mud Crab Scylla sp.: A Comprehensive Review" Water 15, no. 11: 2029. https://doi.org/10.3390/w15112029
APA StylePati, S. G., Paital, B., Panda, F., Jena, S., & Sahoo, D. K. (2023). Impacts of Habitat Quality on the Physiology, Ecology, and Economical Value of Mud Crab Scylla sp.: A Comprehensive Review. Water, 15(11), 2029. https://doi.org/10.3390/w15112029









