Multivariate Analysis of Heavy Metals and Human Health Risk Implications Associated with Fish Consumption from the Yangtze River in Zhenjiang City, China
Abstract
1. Introduction
2. Materials and Methods
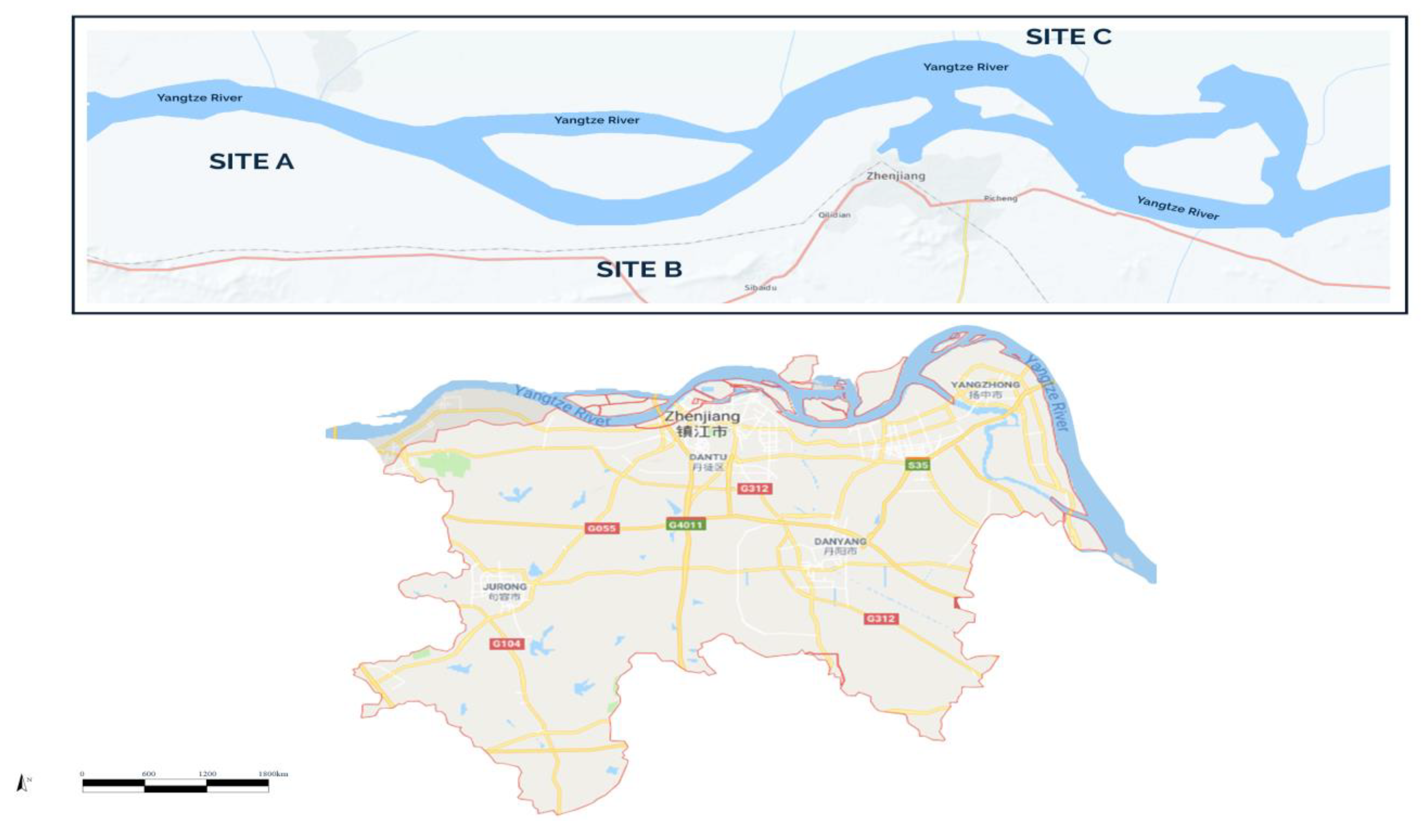
2.1. The Study Area
2.2. Sampling and Sample Preparation
Fish Sampling
2.3. Digestion of Fish Samples
2.4. Digestion of Water Samples
2.5. Heavy Metal Analysis
Quality Control and Quality Assurance Procedures
2.6. The Bioaccumulation Factor (BAF) of Fish Species
2.7. Health Risk Assessment of Fish Species
2.8. Target Hazard Quotient
2.9. The Target Cancer Risk
2.10. Statistical Analysis
3. Results and Discussion
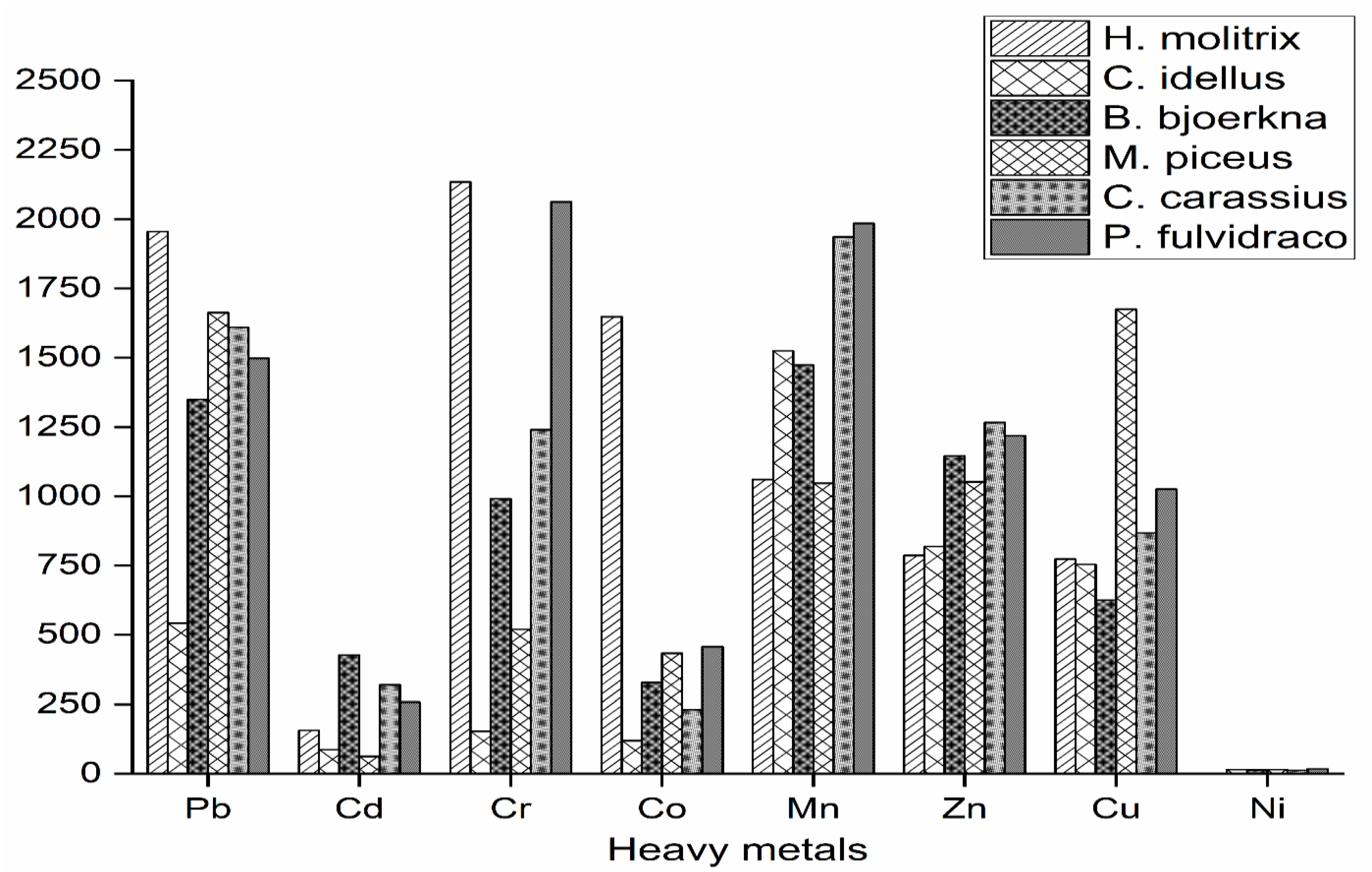
3.1. Fish Species as Bioindicators of Trace Metal Contamination
3.2. Coefficient of Condition of the Selected Fish Species
3.3. The Concentration of Metals in Various Fish Species
3.4. Biological Accumulation Factor (BAF) of Selected Fish Species
3.5. Non-Carcinogenic Risk Assessment of Heavy Metals in Fish Species
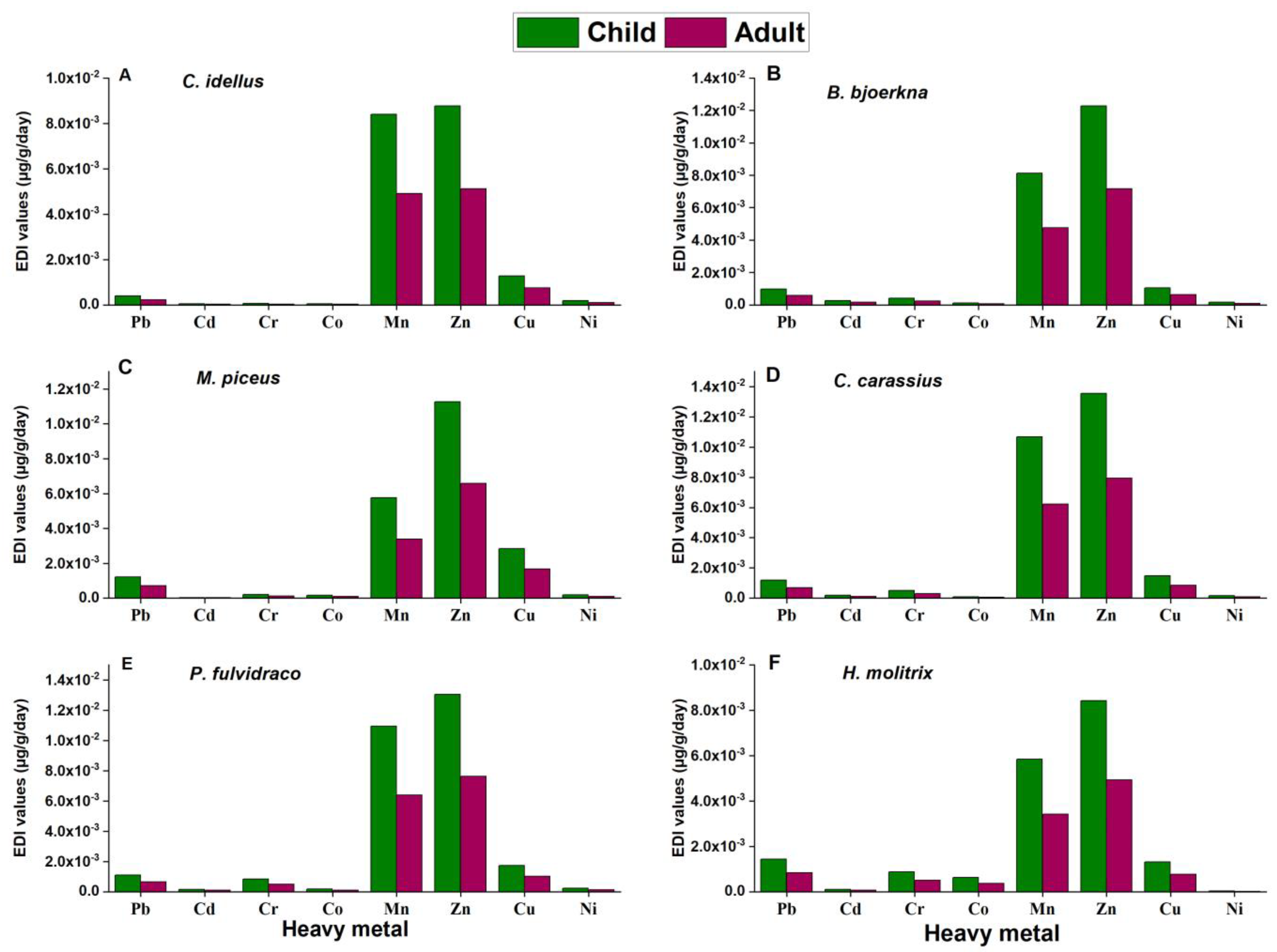
3.5.1. Estimated Daily Intake (EDI)
3.5.2. The Target Hazard Quotient (THQ)
3.5.3. Carcinogenic Risk Assessment of Heavy Metals in Fish Species from the Yangtze River
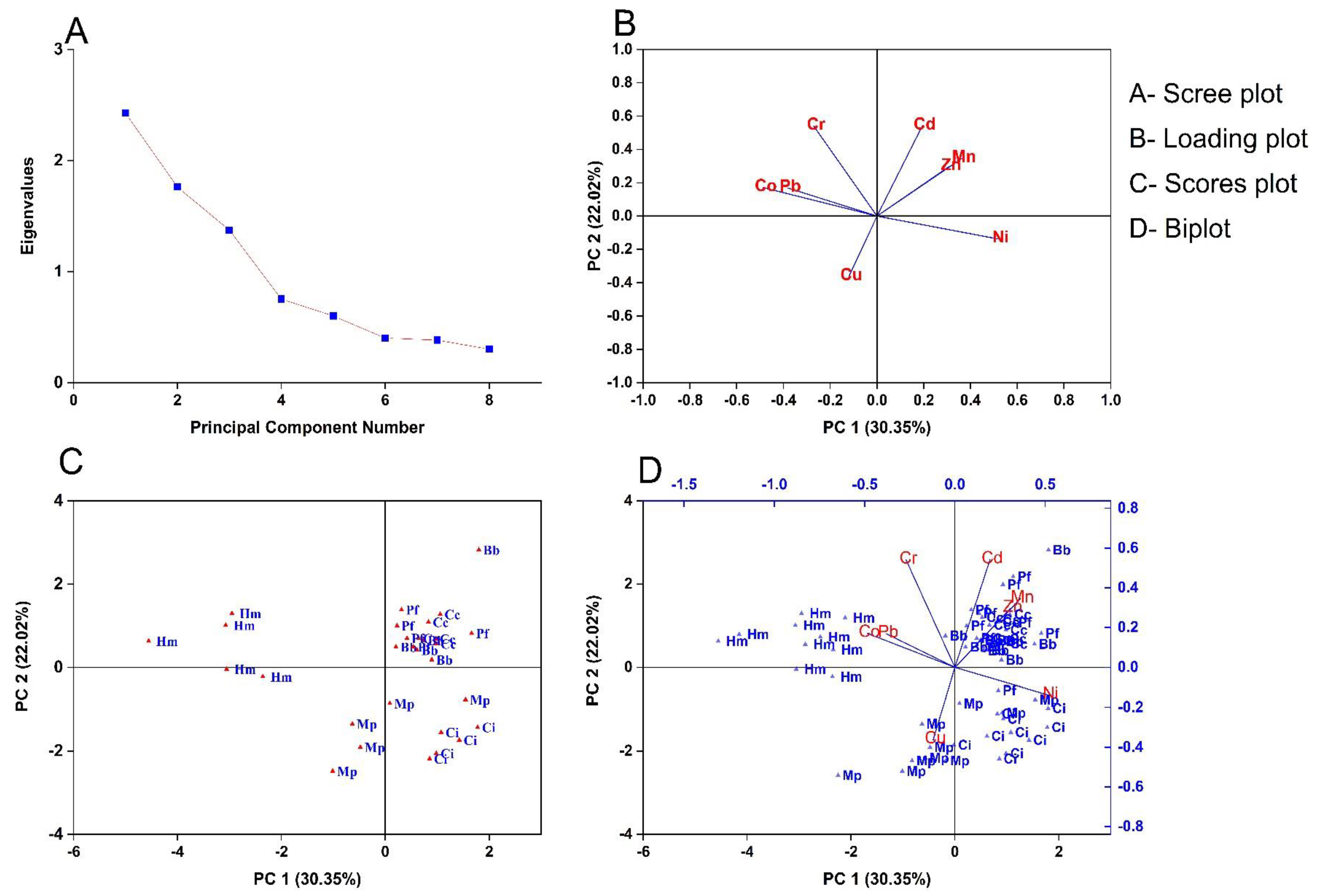
3.6. Multivariate Analysis of Heavy Metals with Fish Species
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Huang, J.; Almutairi, A.W.; Lan, X.; Zheng, L.; Lin, Y.; Chen, L.; Fu, N.; Lin, Z.; Abomohra, A.E.-F. Integrated approach for enhanced bio-oil recovery from disposed face masks through co-hydrothermal liquefaction with Spirulina platensis grown in wastewater. Biomass-Convers. Biorefinery 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abomohra, A.E.-F.; El-Hefnawy, M.E.; Wang, Q.; Huang, J.; Li, L.; Tang, J.; Mohammed, S. Sequential bioethanol and biogas production coupled with heavy metal removal using dry seaweeds: Towards enhanced economic feasibility. J. Clean. Prod. 2021, 316, 128341. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.A.; Khan, M.B. Status of heavy metal pollution of water and fishes in Balu and Brahmaputra rivers. Progress. Agric. 2016, 27, 444–452. [Google Scholar] [CrossRef]
- Lokhande, R.S.; Singare, P.U.; Pimple, D.S. Toxicity study of heavy metals pollutants in waste water effluent samples collected from Taloja Industrial Estate of Mumbai, India. Resour. Environ. 2011, 1, 13–19. [Google Scholar]
- Cáceres-Saez, I.; Haro, D.; Blank, O.; Aguayo Lobo, A.; Dougnac, C.; Arredondo, C.; Cappozzo, H.L.; Guevara, S.R. High status of mercury and selenium in false killer whales (Pseudorca crassidens, Owen 1846) stranded on Southern South America: A possible toxicological concern? Chemosphere 2018, 199, 637–646. [Google Scholar] [CrossRef]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Kumar, B.; Mukherjee, D.; Kumar, S.; Mishra, M.; Prakash, D.; Singh, S.; Sharma, C. Bioaccumulation of heavy metals in muscle tissue of fishes from selected aquaculture ponds in east Kolkata wetlands. Ann. Biol. Res. 2011, 2, 125–134. [Google Scholar]
- Hohle, T.H.; O’Brian, M.R. Manganese is required for oxidative metabolism in unstressed Bradyrhizobium japonicum cells. Mol. Microbiol. 2012, 84, 766–777. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Ali, S.S.; Shaaban, M.T.; Abomohra, A.E.-F.; El-Safity, K. Macroalgal activity against multiple drug resistant Aeromonas hydrophila: A novel treatment study towards enhancement of fish growth performance. Microb. Pathog. 2016, 101, 89–95. [Google Scholar] [CrossRef]
- Gyimah, E.; Akoto, O.; Mensah, J.K.; Bortey-Sam, N. Bioaccumulation factors and multivariate analysis of heavy metals of three edible fish species from the Barekese reservoir in Kumasi, Ghana. Environ. Monit. Assess. 2018, 190, 553. [Google Scholar] [CrossRef]
- Alamdar, A.; Eqani, S.A.M.A.S.; Hanif, N.; Ali, S.M.; Fasola, M.; Bokhari, H.; Katsoyiannis, I.A.; Shen, H.J.C. Human exposure to trace metals and arsenic via consumption of fish from river Chenab, Pakistan and associated health risks. Chemosphere 2017, 168, 1004–1012. [Google Scholar] [CrossRef]
- Renieri, E.A.; Safenkova, I.V.; Alegakis, A.Κ.; Slutskaya, E.S.; Kokaraki, V.; Kentouri, M.; Dzantiev, B.B.; Tsatsakis, A.M. Cadmium, lead and mercury in muscle tissue of gilthead seabream and seabass: Risk evaluation for consumers. Food Chem. Toxicol. 2019, 124, 439–449. [Google Scholar] [CrossRef]
- Ramos-Miras, J.J.; Sanchez-Muros, M.J.; Morote, E.; Torrijos, M.; Gil, C.; Zamani-Ahmadmahmoodi, R.; Rodríguez Martin, J.A. Potentially toxic elements in commonly consumed fish species from the western Mediterranean Sea (Almería Bay): Bioaccumulation in liver and muscle tissues in relation to biometric parameters. Sci. Total Environ. 2019, 671, 280–287. [Google Scholar] [CrossRef]
- Copat, C.; Bella, F.; Castaing, M.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy metals concentrations in fish from Sicily (Mediterranean Sea) and evaluation of possible health risks to consumers. Bull. Environ. Contam. Toxicol. 2012, 88, 78–83. [Google Scholar] [CrossRef]
- El-Moselhy, K.M.; Othman, A.; Abd El-Azem, H.; El-Metwally, M. Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt. J. Basic Appl. Sci. 2014, 1, 97–105. [Google Scholar] [CrossRef]
- Murtala, B.A.; Abdul, W.O.; Akinyemi, A.A. Bioaccumulation of heavy metals in fish (Hydrocynus forskahlii, Hyperopisus bebe occidentalis and Clarias gariepinus) organs in downstream Ogun coastal water, Nigeria. J. Agric. Sci. 2012, 4, 51. [Google Scholar] [CrossRef]
- Malakootian, M.; Tahergorabi, M.; Daneshpajooh, M.; Amirtaheri, K. Determination of Pb, Cd, Ni, and Zn concentrations in canned fish in Southern Iran. Sacha J. Environ. Stud. 2011, 1, 94–100. [Google Scholar]
- Sun, J.; Pan, L.; Zhan, Y.; Lu, H.; Tsang, D.C.; Liu, W.; Wang, X.; Li, X.; Zhu, L. Contamination of phthalate esters, organochlorine pesticides and polybrominated diphenyl ethers in agricultural soils from the Yangtze River Delta of China. Sci. Total. Environ. 2016, 544, 670–676. [Google Scholar] [CrossRef]
- Yi, Y.; Yang, Z.; Zhang, S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Q.; Hu, W.; Huang, B.; Dong, L.; Liu, G. Using multi-medium factors analysis to assess heavy metal health risks along the Yangtze River in Nanjing, Southeast China. Environ. Pollut. 2018, 243, 1047–1056. [Google Scholar] [CrossRef]
- Xiong, X.; Qian, Z.; Mei, Z.; Wu, J.; Hao, Y.; Wang, K.; Wu, C.; Wang, D. Trace elements accumulation in the Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis)—A threat to the endangered freshwater cetacean. Sci. Total Environ. 2019, 686, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gong, D.; Zhao, W.; Lin, L.; Yang, W.; Guo, W.; Tang, X.; Li, Q. Spatial-temporal distribution characteristics and health risk assessment of heavy metals in surface water of the Three Gorges Reservoir, China. Sci. Total Environ. 2020, 704, 134883. [Google Scholar] [CrossRef] [PubMed]
- Zhenjiang People’s Government. Available online: http://szb.zhenjiang.gov.cn/zjgk/201905/t20190528_2142453.htm (accessed on 28 May 2019).
- Li, L.; Jiang, M.; Liu, Y.; Shen, X. Heavy metals inter-annual variability and distribution in the Yangtze River estuary sediment, China. Mar. Pollut. Bull. 2019, 141, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.K.; Malik, D.S.; Yadav, K.K.; Kumar, A.; Kumar, S.; Kamyab, H. Bioaccumulation and potential sources of heavy metal contamination in fish species in River Ganga basin: Possible human health risks evaluation. Toxicol. Rep. 2019, 6, 472–481. [Google Scholar] [CrossRef] [PubMed]
- USEPA Human Health Risk Assessment; Regional Screening Level (RSL) Fish ingestion Table. Washington, DC. March 2019. Available online: http://www.epa.gov/reg3hwmd/risk/human/pdf/NOV2012FISH.pdf (accessed on 28 May 2019).
- Borgå, K. Ecotoxicology: Bioaccumulation; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Bhupander, K.; Mukherjee, D.P. Assessment of human health risk for arsenic, copper, nickel, mercury and zinc in fish collected from tropical wetlands in India. Adv. Life Sci. Technol. 2011, 2, 13–24. [Google Scholar]
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef]
- Bo, S.O.N.G.; Mei, L.E.I.; Tongbin, C.H.E.N.; Zheng, Y.; Yunfeng, X.I.E.; Xiaoyan, L.I.; Ding, G.A.O. Assessing the health risk of heavy metals in vegetables to the general population in Beijing, China. J. Environ. Sci. 2009, 21, 1702–1709. [Google Scholar]
- Li, Z.Y.; Ma, Z.W.; van der Kuijp, T.J.; Yuan, Z.W.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef]
- Gutiérrez, A.J.; Rubio, C.; Moreno, I.M.; González, A.G.; Gonzalez-Weller, D.; Bencharki, N.; Hardisson, A.; Revert, C. Estimation of dietary intake and target hazard quotients for metals by consumption of wines from the Canary Islands. Food Chem. Toxicol. 2017, 108, 10–18. [Google Scholar] [CrossRef]
- Proshad, R.; Rahman, M.M.; Kumar, T.; Islam, M.S.; Mursheed, N.; Howladar, R. Human health hazard implications of heavy metals in agricultural foodstuff grown around brick kiln areas in Bangladesh. J. Environ. Sci. Comput. Sci. Eng. Technol. 2017, 6, 138–156. [Google Scholar]
- Kundu, G.K.; Alauddin, M.; Akter, M.S.; Khan, M.S.; Islam, M.M.; Mondal, G.; Islam, D.; Mohanta, L.C.; Huque, A. Metal contamination of commercial fish feed and quality aspects of farmed tilapia (Oreochromis niloticus) in Bangladesh. Bioresearch Commun.-BRC 2017, 3, 345–353. [Google Scholar]
- Yi, Y.; Tang, C.; Yi, T.; Yang, Z.; Zhang, S. Health risk assessment of heavy metals in fish and accumulation patterns in food web in the upper Yangtze River, China. Ecotoxicol. Environ. Saf. 2017, 145, 295–302. [Google Scholar] [CrossRef] [PubMed]
- USEPA. USEPA Regional Screening Level (RSL) Summary Table: November 2011; United States Environmental Protection Agency: Washington, DC, USA, 2011.
- Du, Z.-Y.; Zhang, J.; Wang, C.; Li, L.; Man, Q.; Lundebye, A.-K.; Frøyland, L. Risk–benefit evaluation of fish from Chinese markets: Nutrients and contaminants in 24 fish species from five big cities and related assessment for human health. Sci. Total. Environ. 2012, 416, 187–199. [Google Scholar] [CrossRef] [PubMed]
- GB2762. National Food Safety Standards Contaminant Limit in Food; 2017. Available online: https://food.chemlinked.com/database/view/803 (accessed on 14 March 2019).
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M. Determination of heavy metals in fish and vegetables in Bangladesh and health implications. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 986–1006. [Google Scholar] [CrossRef]
- Chien, L.-C.; Hung, T.-C.; Choang, K.-Y.; Yeh, C.-Y.; Meng, P.-J.; Shieh, M.-J.; Han, B.-C. Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci. Total Environ. 2002, 285, 177–185. [Google Scholar] [CrossRef]
- Council, N.R. Review of EPA’s Integrated Risk Information System (IRIS) Process; National Academies Press: Washington, DC, USA, 2014. [Google Scholar]
- Madden, E.F. The role of combined metal interactions in metal carcinogenesis: A review. Rev. Environ. Health 2003, 18, 91–110. [Google Scholar] [CrossRef]
- Castaldo, G.; Pillet, M.; Slootmaekers, B.; Bervoets, L.; Town, R.; Blust, R.; De Boeck, G. Investigating the effects of a sub-lethal metal mixture of Cu, Zn and Cd on bioaccumulation and ionoregulation in common carp, Cyprinus carpio. Aquat. Toxicol. 2020, 218, 105363. [Google Scholar] [CrossRef]
- Chi, Q.-Q.; Zhu, G.-W.; Langdon, A. Bioaccumulation of heavy metals in fishes from Taihu Lake, China. J. Environ. Sci. 2007, 19, 1500–1504. [Google Scholar] [CrossRef]
- Jiang, Q.; Lee, T.; Chen, K.; Wong, H.; Zheng, J.; Giesy, J.; Lo, K.; Yamashita, N.; Lam, P. Human health risk assessment of organochlorines associated with fish consumption in a coastal city in China. Environ. Pollut. 2005, 136, 155–165. [Google Scholar] [CrossRef]
- Zaidi, J.; Pal, A. Chapter-6 Research in India on Aquatic Plants and Their Role in Remediation of Metal Pollution. Recent Trends 2019, 87. [Google Scholar]
- Sauliutė, G.; Svecevičius, G. Environmental Protection Engineering. Siesarties ir Vilnios upių ekotoksikologinės būklės įvertinimas pagal atlantinės lašišos (Salmo salar L.) jauniklių morfologinius rodiklius. Moksl.–Liet. Ateitis/Sci.–Future Lith. Environ. Prot. Eng. 2015, 7, 424–429. [Google Scholar]
- Anene, A. Condition factor of four Cichlid species of a man-made lake in Imo State, Southeastern Nigeria. Turk. J. Fish. Aquat. Sci. 2005, 5, 43–47. [Google Scholar]
- Shakir, H.A.; Qazi, J.I. Impact of industrial and municipal discharges on growth coefficient and condition factor of major carps from Lahore stretch of River Ravi. J. Anim. Plant Sci. 2013, 23, 167–173. [Google Scholar]
- Lizama, M.D.L.A.P.; Ambrósio, A.M. Condition factor in nine species of fish of the Characidae family in the upper Paraná river floodplain, Brazil. Braz. J. Biol. 2002, 62, 113–124. [Google Scholar] [CrossRef]
- Casado-Martinez, M.C.; Smith, B.D.; Luoma, S.N.; Rainbow, P.S. Bioaccumulation of arsenic from water and sediment by a deposit-feeding polychaete (Arenicola marina): A biodynamic modelling approach. Aquat. Toxicol. 2010, 98, 34–43. [Google Scholar] [CrossRef]
- Mahboob, S.; Al-Balawi, H.F.A.; Al-Misned, F.; Al-Quraishy, S.; Ahmad, Z. Tissue metal distribution and risk assessment for important fish species from Saudi Arabia. Bull. Environ. Contam. Toxicol. 2014, 92, 61–66. [Google Scholar] [CrossRef]
- Majnoni, F.; Rezaei, M.; Mansouri, B.; Hamidian, A.H. Metal concentrations in tissues of common carp, cyprinus carpio, and silver carp, hypophthalmichthys molitrix from the Zarivar Wetland in Western Iran. Arch. Pol. Fish. 2013, 21, 11–18. [Google Scholar] [CrossRef]
- Hellberg, R.S.; DeWitt, C.A.M.; Morrissey, M.T. Risk-benefit analysis of seafood consumption: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 490–517. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Xiang, Y.; Liang, X. An investigation of outpatient children’s blood lead level in Wuhan China. PLoS ONE 2014, 9, e95284. [Google Scholar] [CrossRef]
- Zhang, Z.; He, L.; Li, J.; Wu, Z.-B. Analysis of heavy metals of muscle and intestine tissue in fish-in Banan section of Chongqing from three gorges reservoir, China. Pol. J. Environ. Stud. 2007, 16, 949. [Google Scholar]
- Pham, N.T.T.; Pulkownik, A.; Buckney, R.T. Assessment of heavy metals in sediments and aquatic organisms in West Lake (Ho Tay), Hanoi, Vietnam. Lakes Reserv. Res. Manag. 2007, 12, 285–294. [Google Scholar] [CrossRef]
- GB 2736-94; Healthy Standard for Fresh Water Fish. Chinese National Criterion Press: Beijing, China, 1994; Volume 32.
- Porea, T.J.; Belmont, J.W.; Mahoney, D.H.J. Zinc-induced anemia and neutropenia in an adolescent. J. Pediatr. 2000, 136, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Kamal, A.; Malik, R.N. Human health risk from organ-specific accumulation of toxic metals and response of antioxidants in edible fish species from Chenab River, Pakistan. Environ. Sci. Pollut. Res. 2014, 21, 4409–4417. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Li, X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018, 5, 288–295. [Google Scholar] [CrossRef]
- Fang, T.; Lu, W.; Cui, K.; Li, J.; Yang, K.; Zhao, X.; Liang, Y.; Li, H. Distribution, bioaccumulation and trophic transfer of trace metals in the food web of Chaohu Lake, Anhui, China. Chemosphere 2019, 218, 1122–1130. [Google Scholar] [CrossRef]
- ATSDR, U. Toxicological Profile for Polybrominated Biphenyls and Polybrominated Diphenyl Ethers (PBBs and PBDEs); ATSDR: Atlanta, GA, USA, 2004.
- Safiur Rahman, M.; Solaiman Hossain, M.; Ahmed, M.K.; Akther, S.; Jolly, Y.N.; Akhter, S.; Jamiul Kabir, M.; Choudhury, T.R. Assessment of heavy metals contamination in selected tropical marine fish species in Bangladesh and their impact on human health. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100210. [Google Scholar] [CrossRef]
- NYSDOH. Hopewell Precision Area Contamination: Appendix C-NYS DOH. Procedure for Evaluating Potential Health Risks for Contaminants of Concern; New York State Department of Health: New York, NY, USA, 2007.
- Javed, M.; Usmani, N. Accumulation of heavy metals and human health risk assessment via the consumption of freshwater fish Mastacembelus armatus inhabiting, thermal power plant effluent loaded canal. SpringerPlus 2016, 5, 776. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, L.; Wang, F.; Pan, B.; Song, N.; Li, F.; Lu, M. Phosphorus in the catchment of high sediment load river: A case of the Yellow River, China. Sci. Total Environ. 2016, 572, 660–670. [Google Scholar] [CrossRef]
- Singh, U.K.; Kumar, B. Pathways of heavy metals contamination and associated human health risk in Ajay River basin, India. Chemosphere 2017, 174, 183–199. [Google Scholar] [CrossRef]
- Soliman, N.F.; El Zokm, G.M.; Okbah, M.A. Risk assessment and chemical fractionation of selected elements in surface sediments from Lake Qarun, Egypt using modified BCR technique. Chemosphere 2018, 191, 262–271. [Google Scholar] [CrossRef]
- Cai, L.; Xu, Z.; Bao, P.; He, M.; Dou, L.; Chen, L.; Zhou, Y.; Zhu, Y.-G. Multivariate and geostatistical analyses of the spatial distribution and source of arsenic and heavy metals in the agricultural soils in Shunde, Southeast China. J. Geochem. Explor. 2015, 148, 189–195. [Google Scholar] [CrossRef]
- Pulles, T.; van der Gon, H.D.; Appelman, W.; Verheul, M. Emission factors for heavy metals from diesel and petrol used in European vehicles. Atmos. Environ. 2012, 61, 641–651. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kumar, R.; Bhardwaj, R.; Kumar Thukral, A.; Rodrigo-Comino, J. Assessment of heavy-metal pollution in three different Indian water bodies by combination of multivariate analysis and water pollution indices. Hum. Ecol. Risk Assess. Int. J. 2018, 26, 1–16. [Google Scholar] [CrossRef]

| Fish Species | Common Name | Number of Samples | Range of Body Length (cm) | Range of Body Weight (g) | Coefficient of Condition/gcm−3 (K) |
|---|---|---|---|---|---|
| Hypophthalmichthys molitrix | Silver crap | 10 | 26.10–28.60 (27.33 ± 0.79) | 270–330 (304.40 ± 23.98) | 1.18–1.59 (1.49 ± 0.12) |
| Ctenopharyngodon idellus | Grass crap | 10 | 24.30–31.20 (26.09 ± 2.22) | 250–320 (273.80 ± 25.66) | 1.05–1.76 (1.57 ± 0.23) |
| Blicca bjoerkna | White bream | 10 | 21.50–24.30 (22.65 ± 0.91) | 124–186 (149.70 ± 19.60) | 1.15–1.35 (1.28 ± 0.63) |
| Mylopharyngodon piceus | Black crap | 10 | 20.50–23.70 (22.18 ± 0.86) | 173–216 (196.60 ± 14.30) | 1.60–2.01 (1.80 ± 0.11) |
| Carassius carassius | Crucian crap | 10 | 22.80–24.50 (23.52 ± 0.67) | 178–206 (193 ± 9.75) | 1.27–1.63 (1.49 ± 0.10) |
| Pelteobagrus fulvidraco | Yellow catfish | 10 | 18.40–21.20 (20.29 ± 0.92) | 75–108 (95.00 ± 11.05) | 1.04–1.22 (1.13 ± 0.06) |
| Element | Certified Value | Measured Value (n = 5) | Recovery (%) | Detection Limits (mg/L) |
|---|---|---|---|---|
| Cu | 380.01 ± 3.10 | 379.13 ± 5.19 | 100.22 | 0.002 |
| Pb | 330.00 ± 48.00 | 329.07 ± 3.92 | 100.28 | 0.001 |
| Cd | 16.02 ± 2.31 | 15.63 ± 4.12 | 102.50 | 0.004 |
| Mn | 500.23 ± 87.11 | 498.02 ± 87.05 | 100.44 | 0.001 |
| Zn | 670.23 ± 98.32 | 656.00 ± 75.00 | 97.87 | 0.046 |
| Ni | 70.21 ± 13.02 | 72.31 ± 12.01 | 97.09 | 0.001 |
| Cr | 70.02 ± 12.61 | 82.01 ± 11.00 | 85.31 | 0.007 |
| Co | 50.23 ± 10.53 | 54.35 ± 12.08 | 101.61 | 0.003 |
| Heavy Metal | Reference Dose (mg/kg-day) | Cancer Slope Factor | Reference |
|---|---|---|---|
| Pb | 3.0 × 10−3 | 8.5 × 10−3 | [27] |
| Ni | 2.0 × 10−2 | 1.7 | [27] |
| Cd | 5.0 × 10−4 | NA | [27] |
| Co | 3.0 × 10−4 | NA | [27] |
| Zn | 3.0 × 10−1 | NA | [27] |
| Mn | 1.4 × 10−1 | NA | [27] |
| Cr | 3.0 × 10−3 | 0.5 | [27] |
| Cu | 4.0 × 10−2 | NA | [27] |
| Fish Species | Trace Metals Muscle Tissue | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Statistics | Pb | Ni | Cd | Co | Zn | Mn | Cr | Cu | |
| Hypophthalmichthys molitrix | Range Mean ± STD | 0.52–1.44 (1.04 ± 0.39) | 0.01–0.05 (0.02 ± 0.10) | 0.03–0.1 (0.07 ± 0.02) | 0.01–0.81 (0.46 ± 0.29) | 4.74–8.45 (6.13 ± 1.32) | 2.25–7.43 (4.25 ± 1.59) | 0.45–0.86 (0.63 ± 0.16) | 0.79–1.48 (0.97 ± 0.19) |
| Ctenopharyngodon idellus | Range Mean ± STD | 0.17–0.39 (0.29 ± 0.09) | 0.08–0.19 (0.14 ± 0.04) | 0.01–0.07 (0.04 ± 0.02) | 0.01–0.06 (0.03 ± 0.02) | 4.24–11.87 (6.38 ± 2.55) | 3.45–9.25 (6.12 ± 2.01) | 0.01–0.1 (0.05 ± 0.03) | 0.62–1.22 (0.93 ± 0.23) |
| Blicca bjoerkna | Range Mean ± STD | 0.62–0.81 (0.72 ± 0.08) | 0.04–0.19 (0.12 ± 0.04) | 0.13–0.53 (0.19 ± 0.12) | 0.07–0.12 (0.09 ± 0.01) | 7.55–10.85 (8.93 ± 0.96) | 4.35–7.82 (5.91 ± 1.15) | 0.27–0.31 (0.29 ± 0.01) | 0.58–0.89 (0.77 ± 0.09) |
| Mylopharyngodon piceus | Range Mean ± STD | 0.32–1.51 (0.89 ± 0.40) | 0.08–0.17 (0.13 ± 0.03) | 0.01–0.08 (0.03 ± 0.02) | 0.09–0.15 (0.12 ± 0.01) | 5.33–9.87 (8.20 ± 1.56) | 2.08–6.24 (4.20 ± 1.19) | 0.12–0.18 (0.15 ± 0.02) | 0.58–3.22 (2.07 ± 1.01) |
| Carassius carassius | Range Mean ± STD | 0.64–0.92 (0.86 ± 0.08) | 0.09–0.14 (0.11 ± 0.02) | 0.06–0.21 (0.15 ± 0.04) | 0.04–0.08 (0.06 ± 0.01) | 9.05–10.31 (9.87 ± 0.39) | 4.95–9.47 (7.77 ± 1.34) | 0.28–0.46 (0.37 ± 0.07) | 1.01–1.14 (1.07 ± 0.04) |
| Pelteobagrus fulvidraco | Range Mean ± STD | 0.23–1.27 (0.79 ± 0.29) | 0.12–0.18 (0.16 ± 0.02) | 0.03–0.18 (0.12 ± 0.05) | 0.1–0.15 (0.13 ± 0.01) | 8.85–10.03 (9.49 ± 0.42) | 5.73–9.59 (7.97 ± 1.44) | 0.18–0.92 (0.61 ± 0.20) | 1.19–1.34 (1.27 ± 0.05) |
| All samples | Range Mean ± STD | 0.17—1.51 (0.77 ± 0.34) | 0.01–0.19 (0.11 ± 0.01) | 0.01–0.53 (0.10 ± 0.08) | 0.01–0.81 (0.15 ± 0.18) | 4.24–11.87 8.17 ± 1.99 | 2.08–9.59 (6.04 ± 2.06) | 0.18–0.92 (0.35 ± 0.24) | 0.58–3.22 1.18 ± 0.59 |
| Area | Heavy Metals | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pb | Ni | Cd | Co | Zn | Mn | Cr | Cu | ||
| Yangtze River(China) a | 1.04 | 0.02 | 0.07 | 0.46 | 6.13 | 4.25 | 0.63 | 0.97 | Present study |
| Yangtze River, (China) a | 0.117 | NA | 0.062 | NA | 12.193 | NA | 0.420 | 1.02 | [39] |
| Chenab River, (Pakistan) a | 0.1 | 0.9 | NA | 0.1 | 16 | 1.98 | 1.3 | 1.1 | [63] |
| Wadi Hanifah, (Saudi Arabia) b | 5.12 | 5.77 | 2.48 | NA | 301.42 | 43.61 | 2.11 | NA | [53] |
| Taihu Lake, (China) a | 0.61 | NA | 0.12 | NA | NA | NA | 0.34 | 0.21 | [64] |
| ZarivarWetland (Western Iran) b | 1.4 | 0.3 | 0.1 | NA | NA | NA | NA | 2.1 | [54] |
| Chaohu Lake, (China) b | 0.13 | NA | 0.007 | NA | 42.54 | NA | 0.92 | 1.65 | [65] |
| Criterion | 0.5 d | 10 d | 0.1 c | 0.02 | 50 c | 0.1 | 0.5 c | 40 d | |
| Heavy Metals | CSF | Child (Bw = 32.7 kg) | Fish Species | Adult (Bw = 55.9 kg) | Fish Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H. molitrix | C. idellus | B. bjoerkna | M. piceus | C. carassius | P. fulvidraco | H. molitrix | C. idellus | B. bjoerkna | M. piceus | C. carassius | P. fulvidraco | ||
| Pb | 0.0085 | 1.22 × 10−5 | 3.37 × 10−6 | 8.39 × 10−6 | 1.03 × 10−5 | 1.00 × 10−5 | 9.32 × 10−6 | 7.11 × 10−6 | 1.97 × 10−6 | 4.91 × 10−6 | 6.05 × 10−6 | 5.86 × 10−6 | 5.45 × 10−6 |
| Cr | 0.5 | 4.34 × 10−4 | 3.11 × 10−5 | 2.02 × 10−4 | 1.06 × 10−4 | 2.52 × 10−4 | 4.19 × 10−4 | 2.54 × 10−4 | 1.82 × 10−5 | 1.18 × 10−4 | 6.18 × 10−5 | 1.48 × 10−4 | 2.45 × 10−4 |
| Ni | 1.7 | 4.37 × 10−5 | 3.16 × 10−4 | 2.85 × 10−4 | 3.06 × 10−4 | 2.65 × 10−4 | 3.77 × 10−4 | 2.55 × 10−5 | 1.85 × 10−4 | 1.67 × 10−4 | 1.79 × 10−4 | 1.55 × 10−4 | 2.21 × 10−4 |
| Pb | Cd | Cr | Co | Mn | Zn | Cu | Ni | |
|---|---|---|---|---|---|---|---|---|
| Pb | 1 | 0.04029 | 0.39321 | 0.31756 | −0.22217 | 0.02883 | 0.38017 | −0.33448 |
| Cd | 1 | 0.22772 | −0.09784 | 0.25401 | 0.36339 | −0.3314 | 0.12108 | |
| Cr | 1 | 0.36396 | 0.13868 | 0.04868 | −0.13303 | −0.35649 | ||
| Co | 1 | −0.2851 | −0.18781 | −0.02192 | −0.57229 | |||
| Mn | 1 | 0.3008 | −0.24814 | 0.30827 | ||||
| Zn | 1 | 0.05698 | 0.38415 | |||||
| Cu | 1 | 0.12149 | ||||||
| Ni | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaba, P.; Shushi, S.; Gyimah, E.; Husein, M.; Abomohra, A. Multivariate Analysis of Heavy Metals and Human Health Risk Implications Associated with Fish Consumption from the Yangtze River in Zhenjiang City, China. Water 2023, 15, 1999. https://doi.org/10.3390/w15111999
Kaba P, Shushi S, Gyimah E, Husein M, Abomohra A. Multivariate Analysis of Heavy Metals and Human Health Risk Implications Associated with Fish Consumption from the Yangtze River in Zhenjiang City, China. Water. 2023; 15(11):1999. https://doi.org/10.3390/w15111999
Chicago/Turabian StyleKaba, Peter, Sato Shushi, Eric Gyimah, Mansuur Husein, and Abdelfatah Abomohra. 2023. "Multivariate Analysis of Heavy Metals and Human Health Risk Implications Associated with Fish Consumption from the Yangtze River in Zhenjiang City, China" Water 15, no. 11: 1999. https://doi.org/10.3390/w15111999
APA StyleKaba, P., Shushi, S., Gyimah, E., Husein, M., & Abomohra, A. (2023). Multivariate Analysis of Heavy Metals and Human Health Risk Implications Associated with Fish Consumption from the Yangtze River in Zhenjiang City, China. Water, 15(11), 1999. https://doi.org/10.3390/w15111999









