Bioscorodite Production from As(III) and Fe(II) Salts under Oxidizing and Acidic Conditions of Trichoderma atroviride Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Cultivation
2.2. Evaluation of Fungi for Acidic and Oxidizing Culture Conditions
2.3. Effect of As(III) and As(V) on the Culture Parameters of Fungi
2.4. Selection of Fungal Cultivation Conditions for Acidic and Oxidizing Parameters Using an Experiment with a 23−1 Fractional Factorial Design (FFD)
2.5. Scorodite Seed and Bioscorodite Production
2.6. Scorodite Characterization
2.7. Statistical Analysis
3. Results and Discussion
3.1. Determination of Fungal Culture Parameters
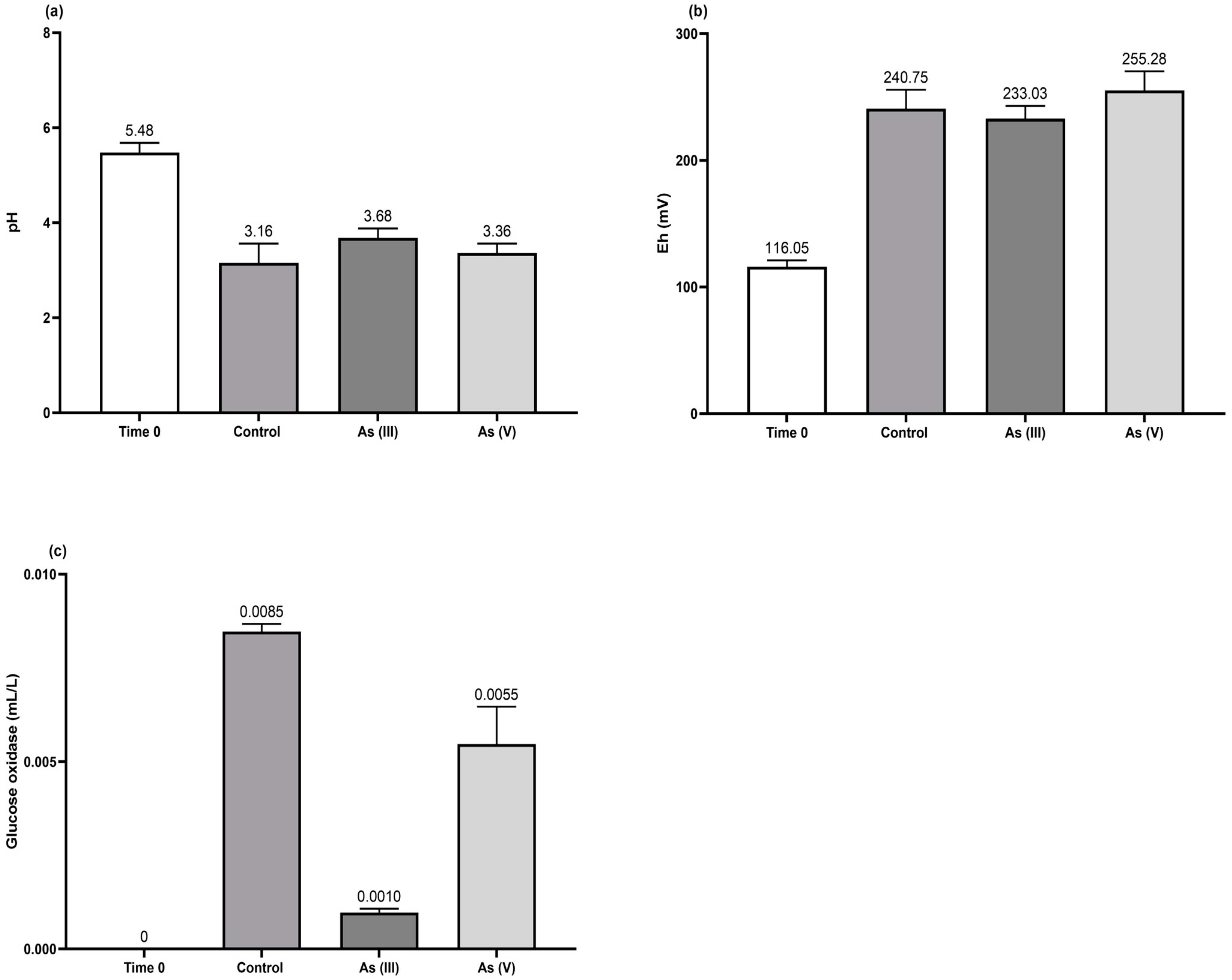
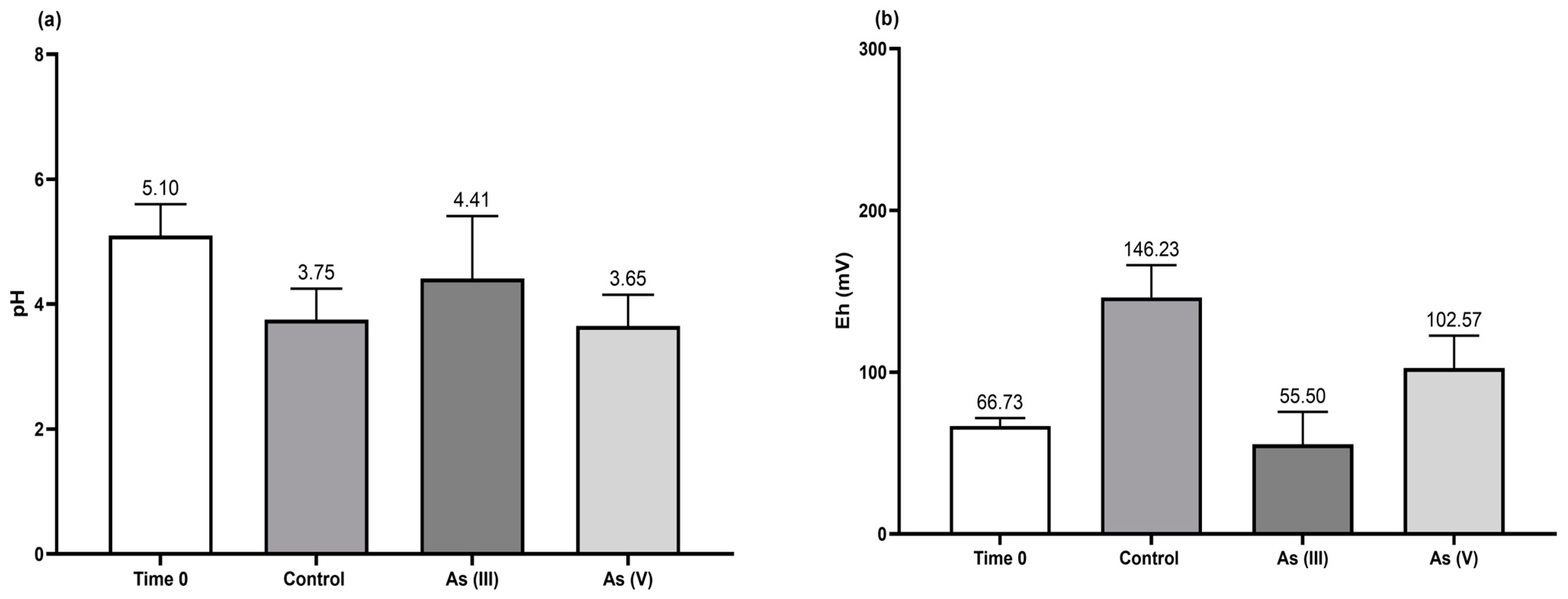
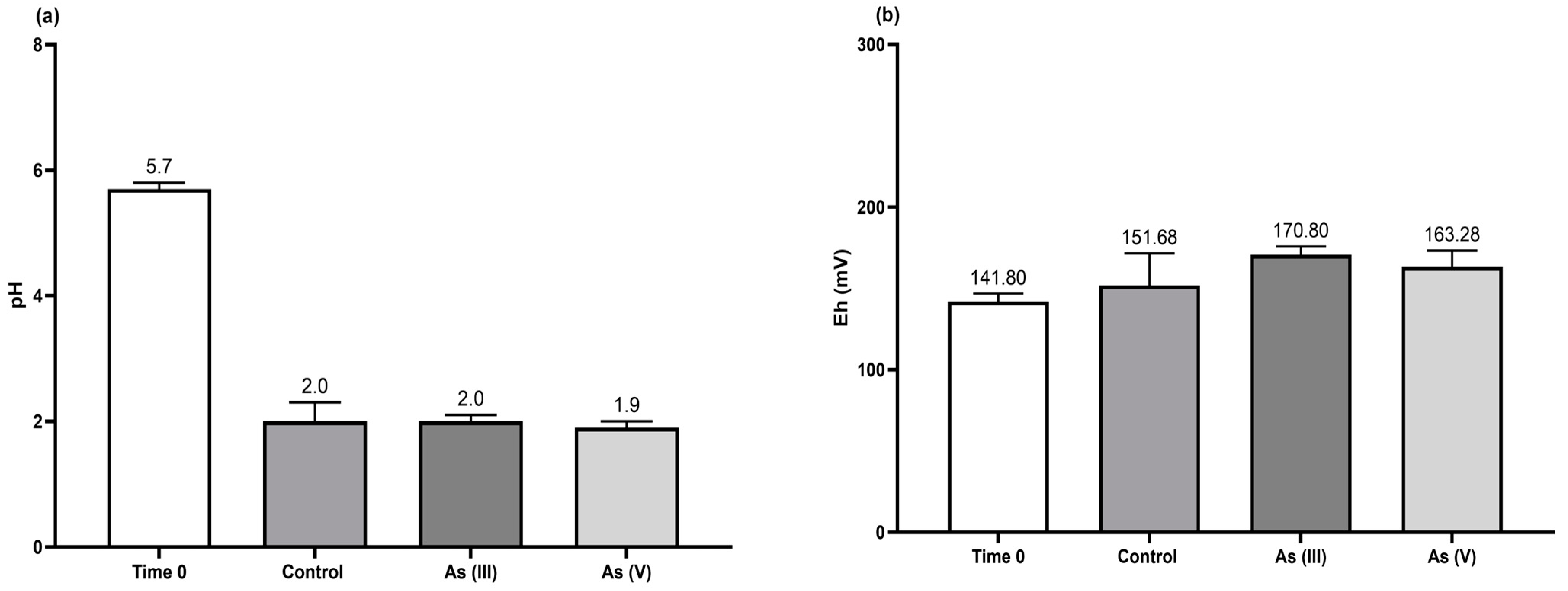
3.2. Effect of As(III) and As(V) on Culture Parameters
3.3. Improvement in the Oxidizing and Acidic Conditions of T. atroviride Using a 23−1 FFD
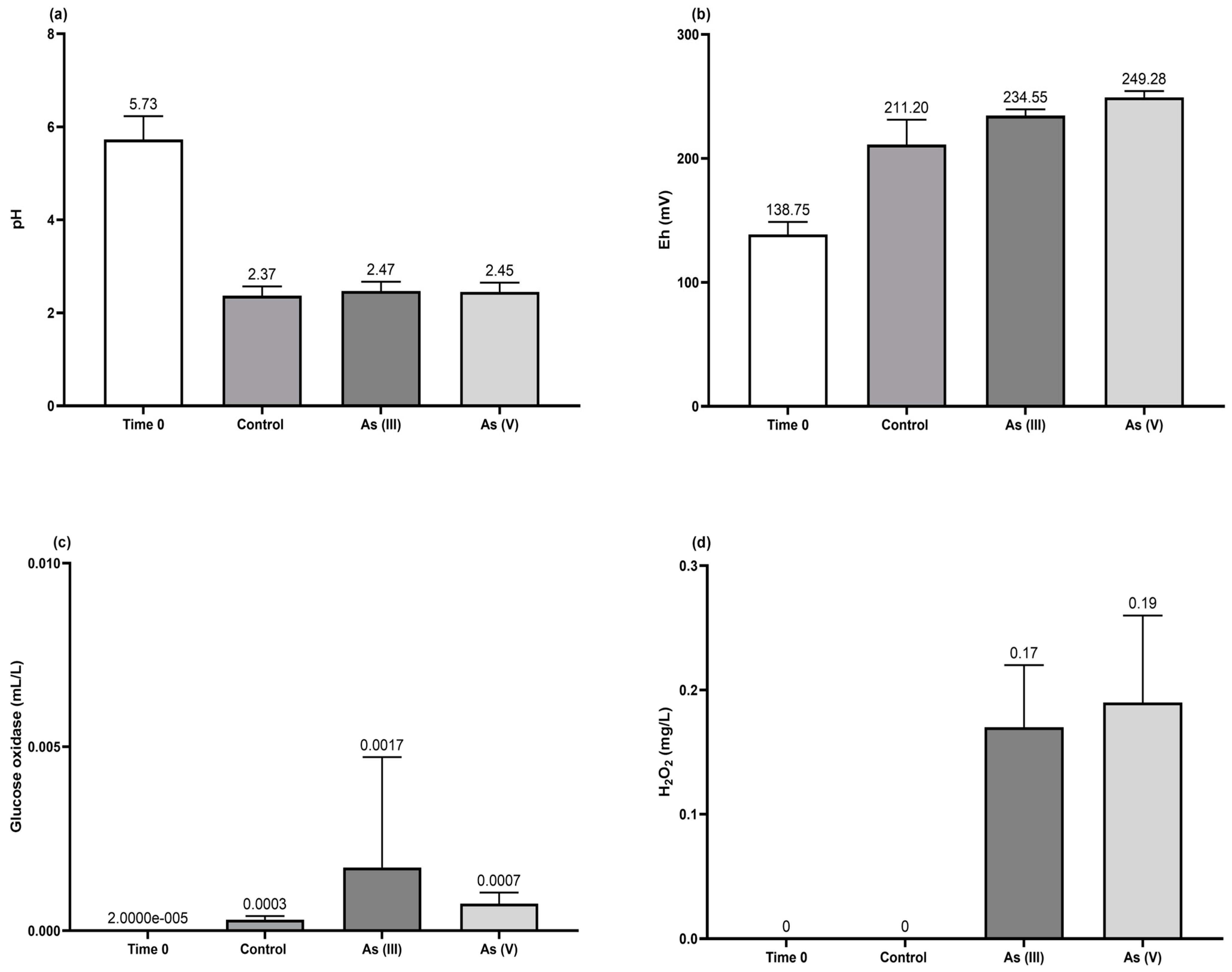
3.4. Bioscorodite Production under the Selected Culture Conditions
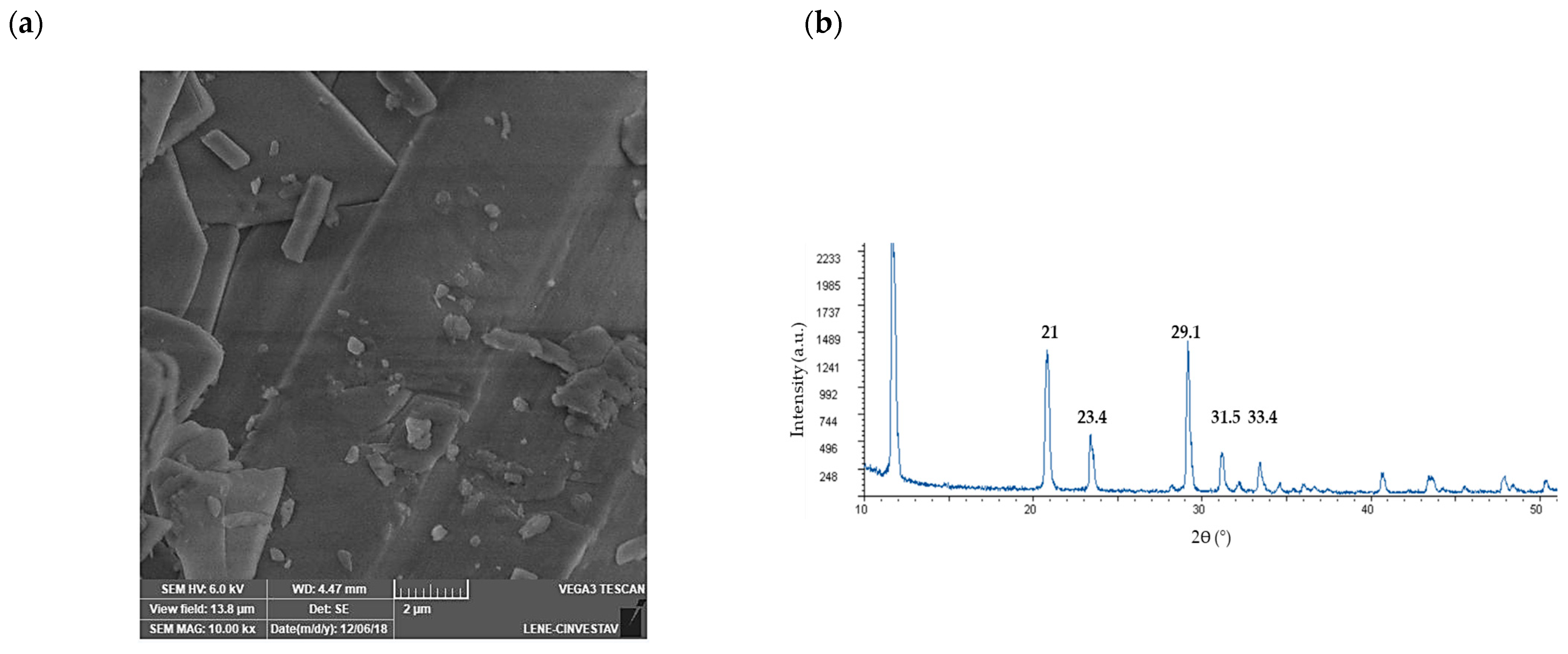
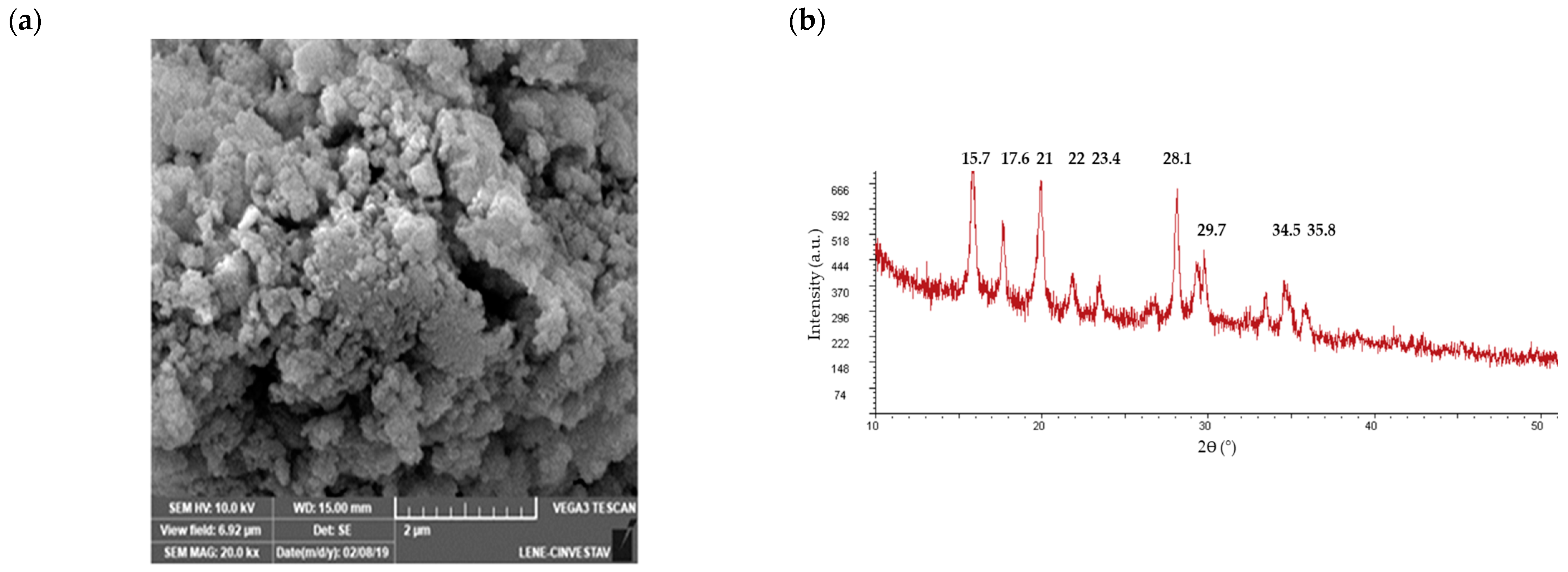
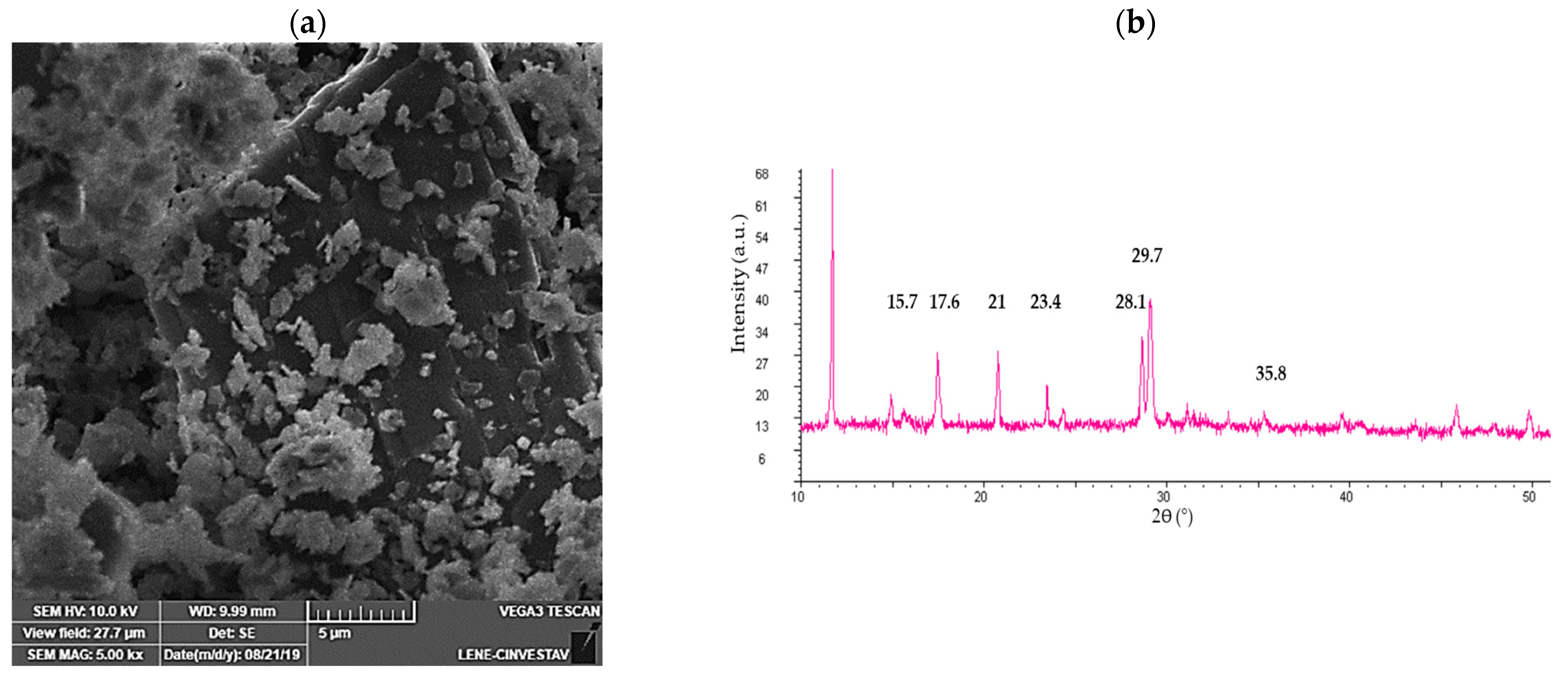
3.5. Characterization of the Calcium Sulfate Scorodite Seeds
- (a)
- The As(III) oxidation was proceeded by the H2O2 generated (Figure 5c) from the glucose oxidase enzyme detected in the broth culture of T. atroviride (Figure 5b). This implies the activation of a microbial detoxification mechanism when the fungus is exposed to trivalent As (see Equations (7)–(12)) (modified from [35]).
- (b)
- The initiating reaction, Fe(III) with H2O2, produces Fe(II) and the anion radical superoxide (O2•-) (Equation (7)).
- (c)
- Fe(II) is oxidized by H2O2, which correlates positively (p < 0.0006) with Eh, making it the final electron acceptor in the respiratory chain to form hydroxylate Fe (Equation (8)).
- (d)
- Further, Fe(III)•OH reacts with As(III) to produce As(IV) (Equation (9)).
- (e)
- Once As(IV) is generated by H2O2 and Fe(III), it forms Fe(III)•OH and the oxidized As(V) (Equation (10)).
- (f)
- As(IV) reacts with Fe(III)•OH to produce As(V) (Equation (11)).
- (g)
- Fe(III) reacts with As(V) and precipitates as bioscorodite crystals (Equation (12)):
3.6. Bioscorodite Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Yadav, R.; Sharma, S.; Narain, S.A. Arsenic contamination in the food chain: A threat to food security and human health. J. Appl. Biol. Biotech. 2023, 11, 1–10. [Google Scholar] [CrossRef]
- Bowell, R.J.; Alpers, C.N.; Jamieson, H.E.; Nordstrom, D.K.; Majzlan, J. The environmental geochemistry of arsenic—An Overview. Rev. Miner. Geochem. 2014, 79, 1–16. [Google Scholar] [CrossRef]
- Morgada, M.E. Metodologías Analíticas para la Determinación y Especiación de Arsénico en Aguas y Suelos. In Formas Arsenicales en Agua Y Suelos (Analytical Methodologies for the Determination and Speciation of Arsenic in Water and Soil. In Arsenical Forms in Water and Soils); Litter, M.I., Armienta, M.A., Farías, S.S., Eds.; Iberoarsen, CYTED: Buenos Aires, Argentina, 2009; pp. 19–27. ISBN 978-84-96023-71-0. [Google Scholar]
- Northstrom, D.K. Worldwide occurrences of arsenic in ground water. Sci. Compas 2002, 296, 2143–2145. [Google Scholar] [CrossRef] [PubMed]
- Arreguín Cortés, F.I.; Chávez Guillén, R.; Soto Navarro, P.R. Una Revisión de la Presencia de Arsénico en el Agua Subterránea en México (A Review of the Presence of Arsenic in Groundwater in Mexico). Available online: https://docplayer.es/23322870-Una-revision-de-la-presencia-de-arsenico-en-el-agua-subterranea-en-mexico.html (accessed on 8 March 2022).
- Alarcón-Herrera, M.T.; Bunduschuh, J.; Nath, B.; Nicolli, H.B.; Gutiérrez, M.; Reyes-Gómez, V.M.; Nuñez, D.; Martín-Domínguez, I.R.; Sracek, O. Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: Genesis, mobility and remediation. J. Hazard. Mater. 2013, 262, 960–969. [Google Scholar] [CrossRef]
- Armienta, M.A.; Segovia, N. Arsenic and fluoride in the groundwater of Mexico. Environ. Geochem. Health 2008, 30, 345–353. [Google Scholar] [CrossRef]
- Basu, A.; Saha, D.; Saha, R.; Ghosh, T.; Saha, B. A review on sources, toxicity and remediation technologies for removing arsenic from drinking water. Res. Chem. Intermed. 2014, 40, 447–485. [Google Scholar] [CrossRef]
- Abejón, R.; Garea, A. A bibliometric analysis of research on arsenic in drinking water during the 1992-2012 period: An outlook to treatment alternatives for arsenic removal. J. Water Process. Eng. 2015, 6, 105–119. [Google Scholar] [CrossRef]
- Jadhav, S.; Bringas, E.; Yadav, G.; Rathod, V.; Ortiz, I.; Marathe, K. Arsenic and fluoride contaminated ground waters: A Review of current technologies for contaminants removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef]
- Mohd, S.; Singh, K.A.; Shukla, J.; Mandrah, K.; Shankar, J.; Arjaria, N.; Narain, S.P.; Khare, P.; Narayan, R.; Dixit, S.; et al. Fungal mediated biotransformation reduces toxicity of arsenic to soil dwelling microorganism and plant. Ecotoxicol. Environ. Saf. 2019, 176, 108–118. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.H.; Chung, H.; An, J.; Nam, K. Effect of organic substrate and Fe oxides transformation on the mobility of arsenic by biotic reductive dissolution under repetitive redox conditions. Chemosphere 2022, 305, 135–431. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Shi, J.; Yan, G.; Wang, H.; Zhang, A. Removal of arsenic from smelting wastewater using Fe3O4 as an in situ Fe source: The effect of predissolution and the evolution process of scorodite. Environ. Sci. Water Res. Technol. 2022, 8, 2796–2806. [Google Scholar] [CrossRef]
- Ehrlich, H.; Bailey, E.; Wysokowski, M.; Jesionowski, T. Forced Biomineralization: A Review. Biomimetics 2021, 6, 46. [Google Scholar] [CrossRef]
- Nam, I.H.; Murugesan, K.; Jungho, R.R.; Hwan, K.J. Arsenic (As) removal using Talaromyces sp. KM-31 isolated from As-contaminated mine soil. Minerals 2019, 9, 568. [Google Scholar] [CrossRef]
- Say, R.; Denizli, A.; Arica, Y. Biosorption of cadmium (II), lead (II) and copper(II) with the filamentous fungus Phanerochaete chrysosporium. Bioresour. Technol. 2001, 76, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Silva, J.R.; Chan-Cupul, W.; Loera, O.; Aguilar-López, R.; Xoconostle-Cázares, B.; Rodríguez-Vázquez, R. In vitro toxic effects of heavy metals on fungal growth and phosphate-solubilizing abilities of isolates obtained from Phragmites australis rhizosphere. Chem. Ecol. 2016, 32, 49–67. [Google Scholar] [CrossRef]
- Cortés-Espinosa, D.; Fernández, F.J.; Ainhoa-Arana, A.; Rodríguez-Vázquez, R. Selection and identification of fungi isolated from sugarcane bagasse and their application for phenanthrene removal from soil. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2006, 41, 475–486. [Google Scholar] [CrossRef]
- Chan-Cupul, W.; Heredia-Abarca, G.; Martínez-Carrera, D.; Rodríguez-Vázquez, R. Enhancement of ligninolytic enzyme activities in a Trametes maxima-Paecilomyces carneus co-culture: Key factors revealed after screening using a Plackett-Burman experimental design. Electron. J. Biotechnol. 2014, 17, 114–121. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicilyc acid reagent for determination of reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Titrations (2009–2022). Available online: http://www.titrations.info/permanganate-titration-hydrogen-peroxide (accessed on 5 July 2022).
- Acosta-Rubí, S.; Tomasini-Campocosio, A.; Montes-Horcasitas, M.C.; Quintanar-Vera, L.; Esparza-García, F.; Rodríguez-Vázquez, R. Production of a halotolerant biofilm from green coffee beans immobilized on loofah fiber (Luffa cylindrical) and its effect on phenanthrene degradation in sea-water. J Env. Sci. Health 2017, 52, 632–640. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 1118214714/9781118214718. [Google Scholar]
- Lira-Pérez, J.; Rodríguez-Vázquez, R. Removal of orange G dye by Aspergillus niger and its effect on organic acid production. Prep. Biochem. Biotechnol. 2022, 17, 1–12. [Google Scholar] [CrossRef]
- Ritschkoff, A.; Rättö, M.; Buchert, J.; Viikari, L. Effect of carbon source on the production of oxalic acid and hydrogen peroxide by brown-rot fungus Poria placenta. J. Biotechnol. 1995, 40, 179–186. [Google Scholar] [CrossRef]
- Li, Z.; Bai, T.; Dai, L.; Wang, F.; Tao, J.; Meng, S.; Hu, Y.; Wang, S.; Hu, S. A study of organic acid production in contrasts between two phosphate solubilizing fungi: Penicillium oxalicum and Aspergillus niger. Sci. Rep. 2016, 6, 25313. [Google Scholar] [CrossRef] [PubMed]
- González-Contreras, P.; Weijma, J.; van der Weijden, R.; Buisman, C.J.N. Biogenic scorodite crystallization by Acidianus sulfidivorans for arsenic removal. Environ. Sci. Technol. 2010, 44, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Caetano, M.L.; Ciminelli, V.S.T.; Rocha, S.D.F.; Spitale, M.C.; Caldeira, C.L. Batch and continuous precipitation of scorodite from dilute industrial solutions. Hydrometallurgy 2009, 95, 44–52. [Google Scholar] [CrossRef]
- Bissaro, B.; Várnai, A.; Rohr, A.K.; Eijsink, V.G.H. Oxidoreductasas y especies reactivas de oxígeno en la conversión de bio-masa lignocelulósica (Oxidoreductases and reactive oxygen species in the conversion of lignocellulosic biomass). Microbiol. Mol. Biol. Rev. 2018, 82, E00029-18. [Google Scholar] [CrossRef]
- Berkani, M.; Vasseghian, Y.; Huan-Le, V.Y.; Dragoi, E.N.; Khaneghah, A.M. The Fenton-like reaction for arsenic removal from groundwater: Health risk assessment. Environ. Res. 2021, 202, 111698. [Google Scholar] [CrossRef]
- Izcapa-Treviño, C.; Loera, O.; Tomasini-Campocosio, A.; Esparza-García, F.; Salazar-Montoya, J.A.; Díaz-Cervantes, M.D.; Rodríguez-Vázquez, R. Fenton (H2O2/Fe) reaction involved in Penicillium sp culture for DDT [1,1,1–2,2-bis(p-chlorophenyl)ethane)] degradation. J. Environ. Sci. Health 2009, 44, 798–804. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L. Kinetic study of hydroxyl radical formation in a continuous hydroxyl generation system. RSC Adv. 2018, 8, 40632–40638. [Google Scholar] [CrossRef]
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Pereira-Mira, N.; Cetecioglu, Z.; Noriega-Fernández, E.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding how microorganisms respond to acid pH is central to their control and successful exploi-tation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef]
- González-Contreras, P.; Weijma, J.; Buisman, C.J.N. Continuous bioscorodite crystallization in CSTRs for arsenic removal and disposal. Water Res. 2012, 46, 5883–5892. [Google Scholar] [CrossRef]
- Voegelin, A.; Hug, S.J. Catalyzed oxidation of arsenic(III) by hydrogen peroxide on the surface of ferrihydrite: An in Situ ATR-FTIR Study. Environ. Sci. Technol. 2003, 37, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Okibe, N. Factors to enable crystallization of environmentally stable bioscorodite from dilute As(III)-contaminated waters. Minerals 2018, 8, 23. [Google Scholar] [CrossRef]
 , T. atroviride
, T. atroviride  , P. placenta
, P. placenta  , and P. chrysosporium
, and P. chrysosporium  . T = 28 °C for all fungi except P. chrysosporium, which was cultivated at 39 °C and 125 rpm.
. T = 28 °C for all fungi except P. chrysosporium, which was cultivated at 39 °C and 125 rpm.
 , T. atroviride
, T. atroviride  , P. placenta
, P. placenta  , and P. chrysosporium
, and P. chrysosporium  . T = 28 °C for all fungi except P. chrysosporium, which was cultivated at 39 °C and 125 rpm.
. T = 28 °C for all fungi except P. chrysosporium, which was cultivated at 39 °C and 125 rpm.
| Variable | |||
|---|---|---|---|
| T | C | Fe | P |
| T1 | Lactose (−1) | FeCl3 (−1) | KH2PO4 (+1) |
| T2 | Dextrose (+1) | FeCl3 (−1) | K2HPO4 (−1) |
| T3 | Lactose (−1) | FeSO4 (+1) | K2HPO4 (−1) |
| T4 | Dextrose (+1) | FeSO4 (+1) | KH2PO4 (+1) |
| Treatment | Biomass (g/L) | pH | Eh (mV) | H2O2 (mg/L) |
|---|---|---|---|---|
| T1 | 2.9 ± 0.07 | 2.50 ± 0.04 | 286.20 ± 1.58 | 0.40 ± 0.13 |
| T2 | 3.2 ± 0.05 | 2.30 ± 0.06 | 312.50 ± 1.88 | 0.65 ± 0.09 |
| T3 | 4.4 ± 0.04 | 2.20 ± 0.02 | 324.80 ± 2.49 | 0.31 ± 0.03 |
| T4 | 3.6 ± 0.30 | 2.40 ± 0.03 | 301.50 ± 0.99 | 0.12 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Castillo, J.A.; Rodríguez-Vázquez, R.; Aguilar-López, R.; Zúñiga-Silva, J.R. Bioscorodite Production from As(III) and Fe(II) Salts under Oxidizing and Acidic Conditions of Trichoderma atroviride Culture. Water 2023, 15, 1905. https://doi.org/10.3390/w15101905
Ramírez-Castillo JA, Rodríguez-Vázquez R, Aguilar-López R, Zúñiga-Silva JR. Bioscorodite Production from As(III) and Fe(II) Salts under Oxidizing and Acidic Conditions of Trichoderma atroviride Culture. Water. 2023; 15(10):1905. https://doi.org/10.3390/w15101905
Chicago/Turabian StyleRamírez-Castillo, Jesús Adriana, Refugio Rodríguez-Vázquez, Ricardo Aguilar-López, and José Roberto Zúñiga-Silva. 2023. "Bioscorodite Production from As(III) and Fe(II) Salts under Oxidizing and Acidic Conditions of Trichoderma atroviride Culture" Water 15, no. 10: 1905. https://doi.org/10.3390/w15101905





