Spatiotemporal Variation in Phytoplankton and Physiochemical Factors during Phaeocystis globosa Red-Tide Blooms in the Northern Beibu Gulf of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Strategy and Physico-Chemical Variables
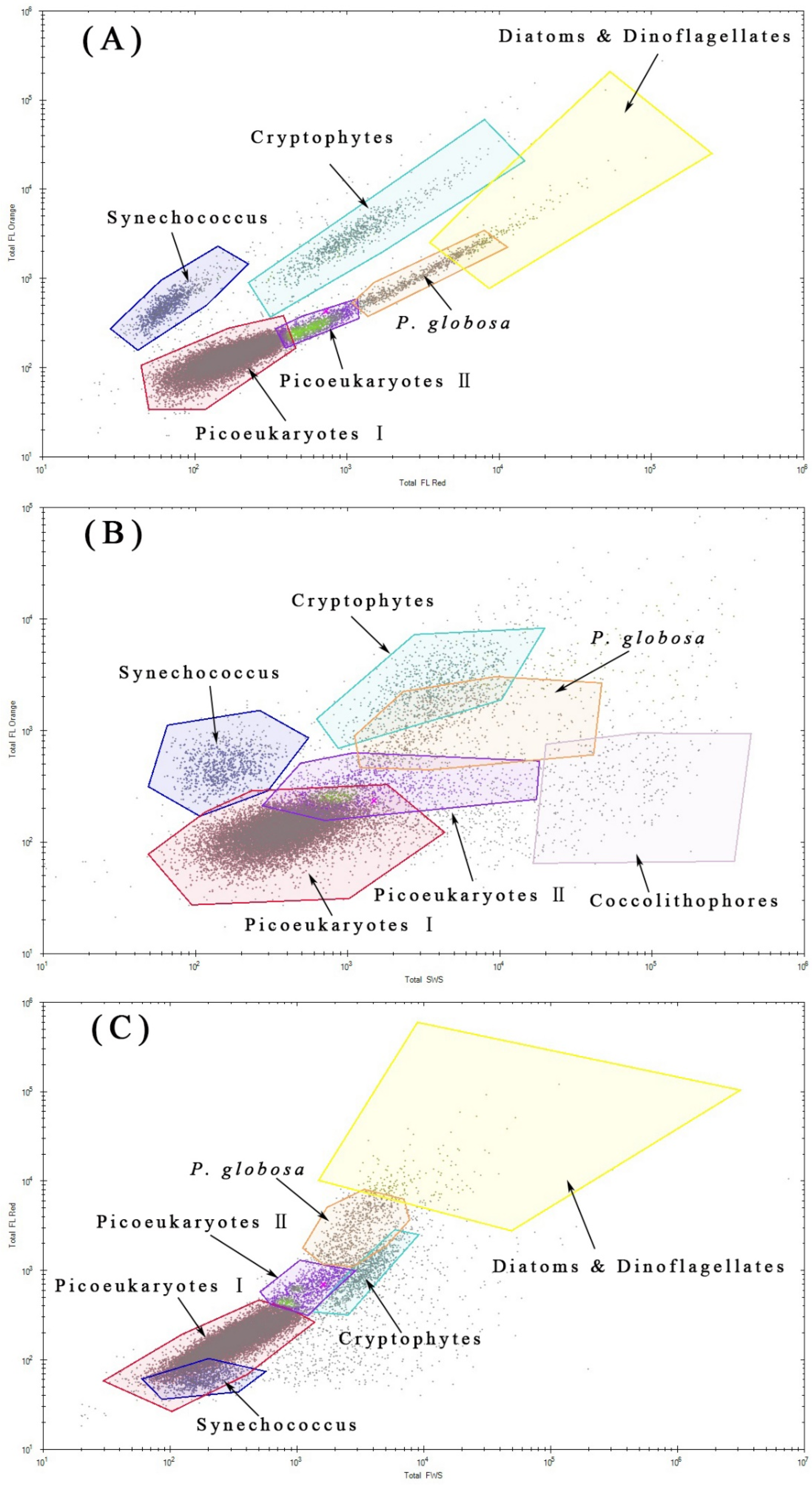
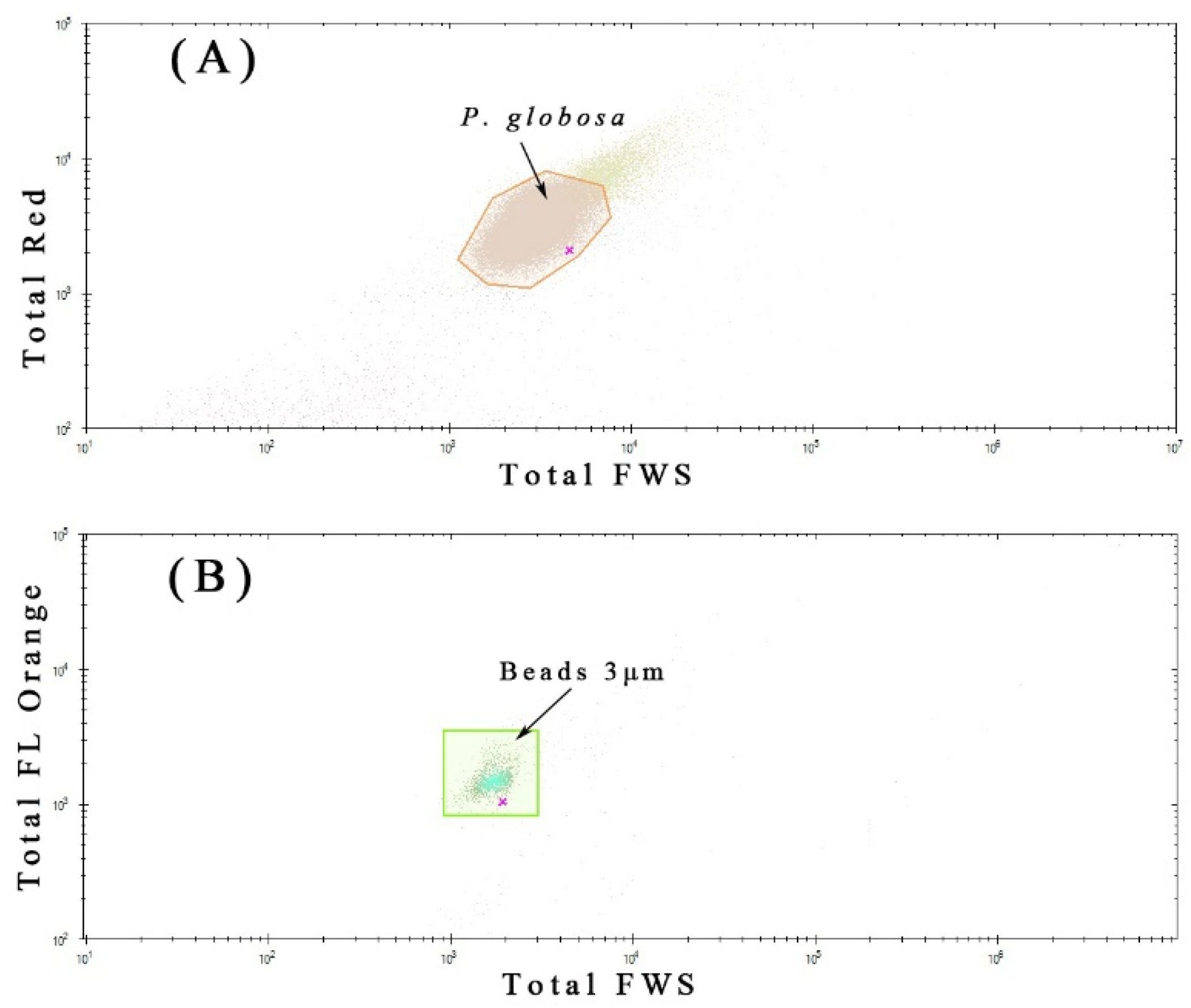
2.2. Phytoplankton Discrimination by Flow Cytometry
2.3. Data Treatments and Analysis
3. Results
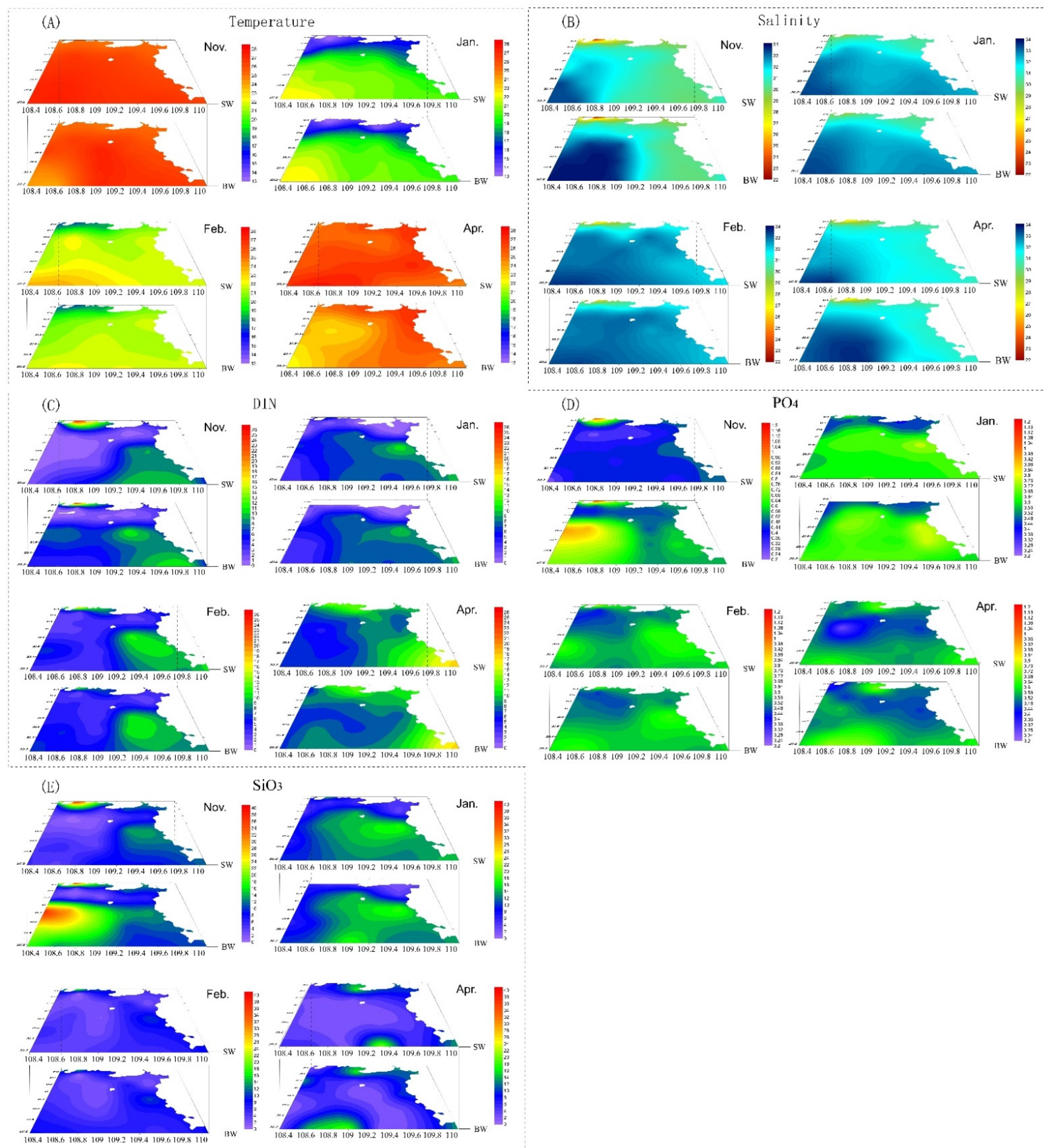
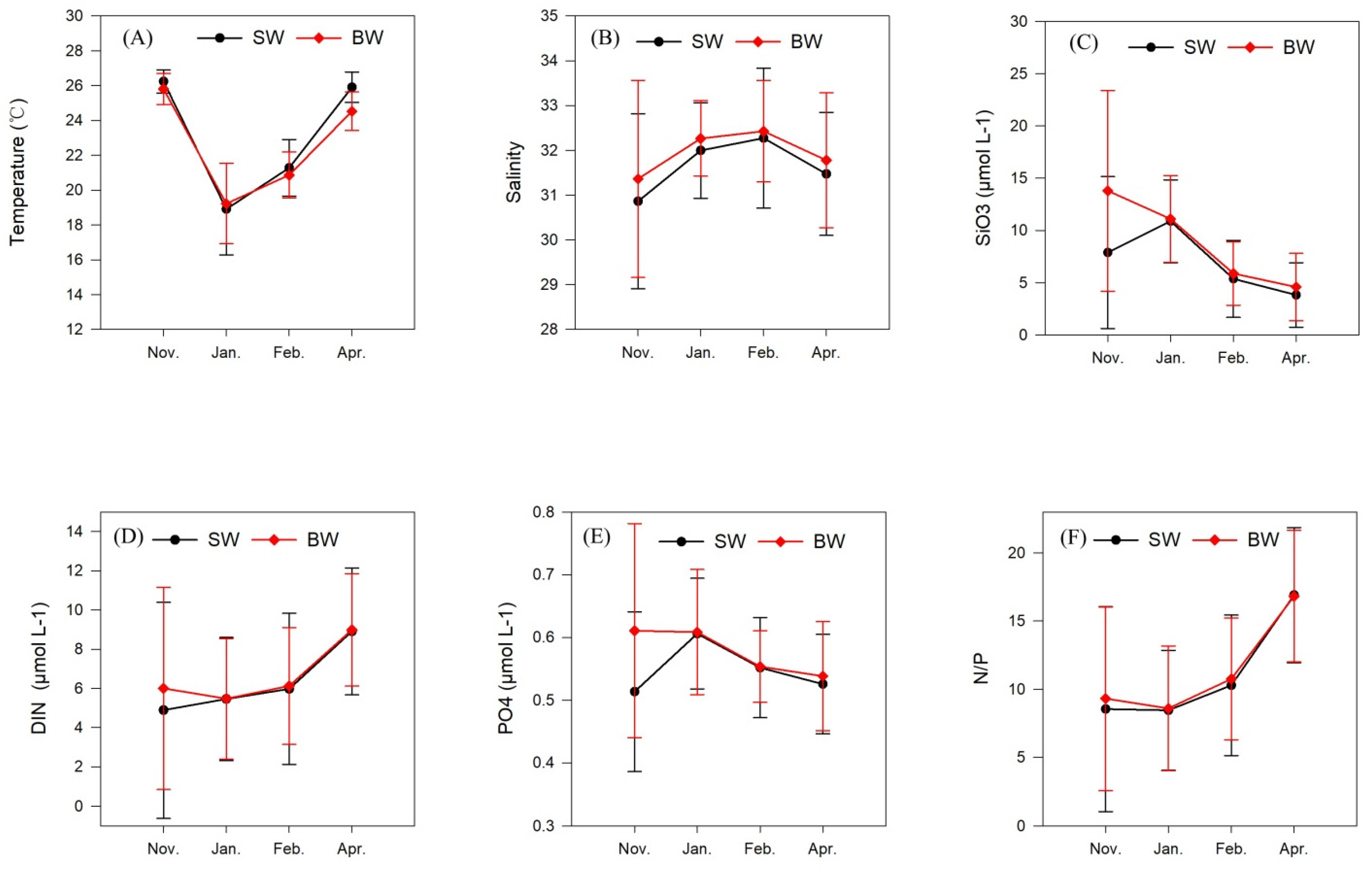
3.1. Temperature and Salinity
3.2. Nutrients
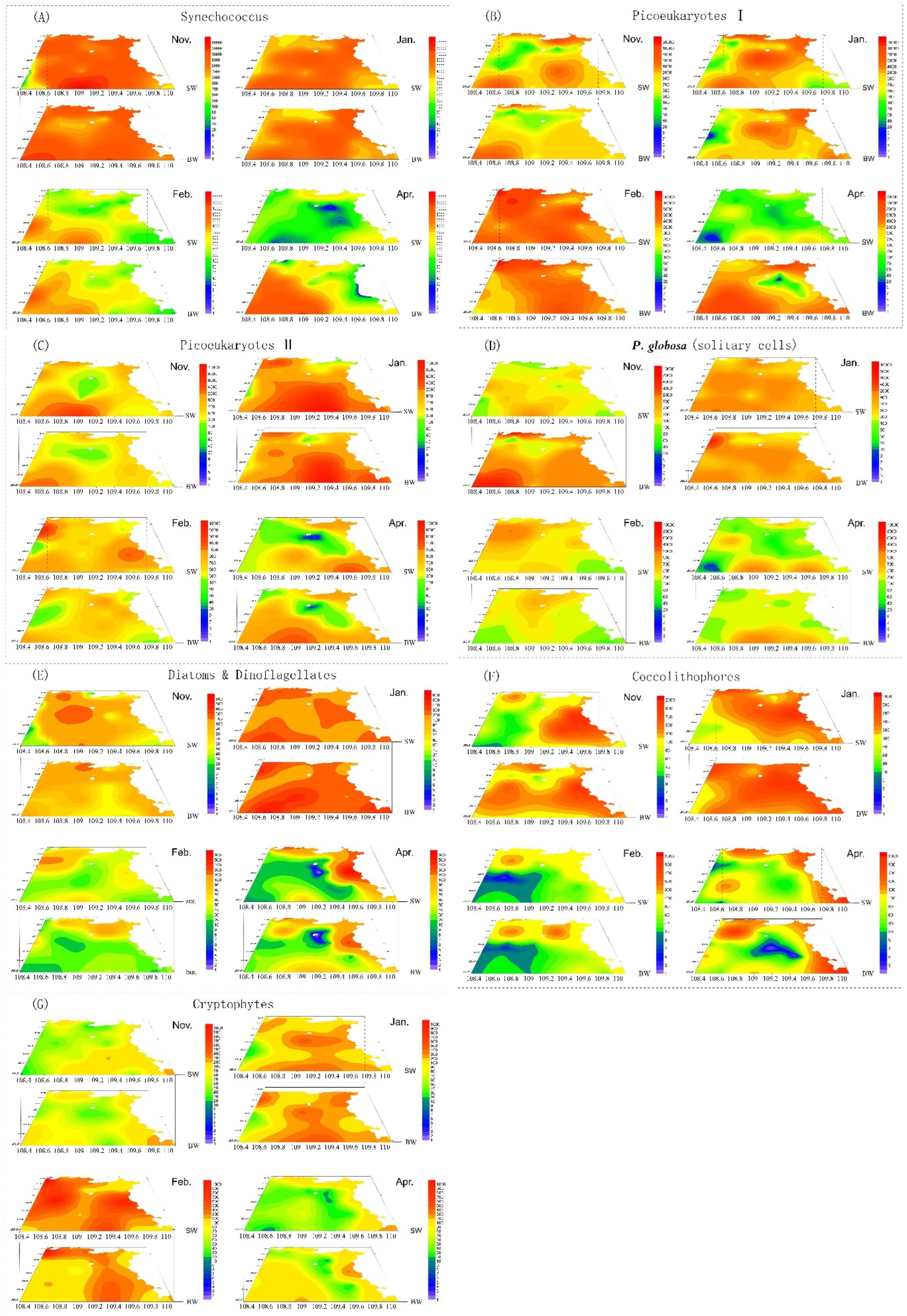
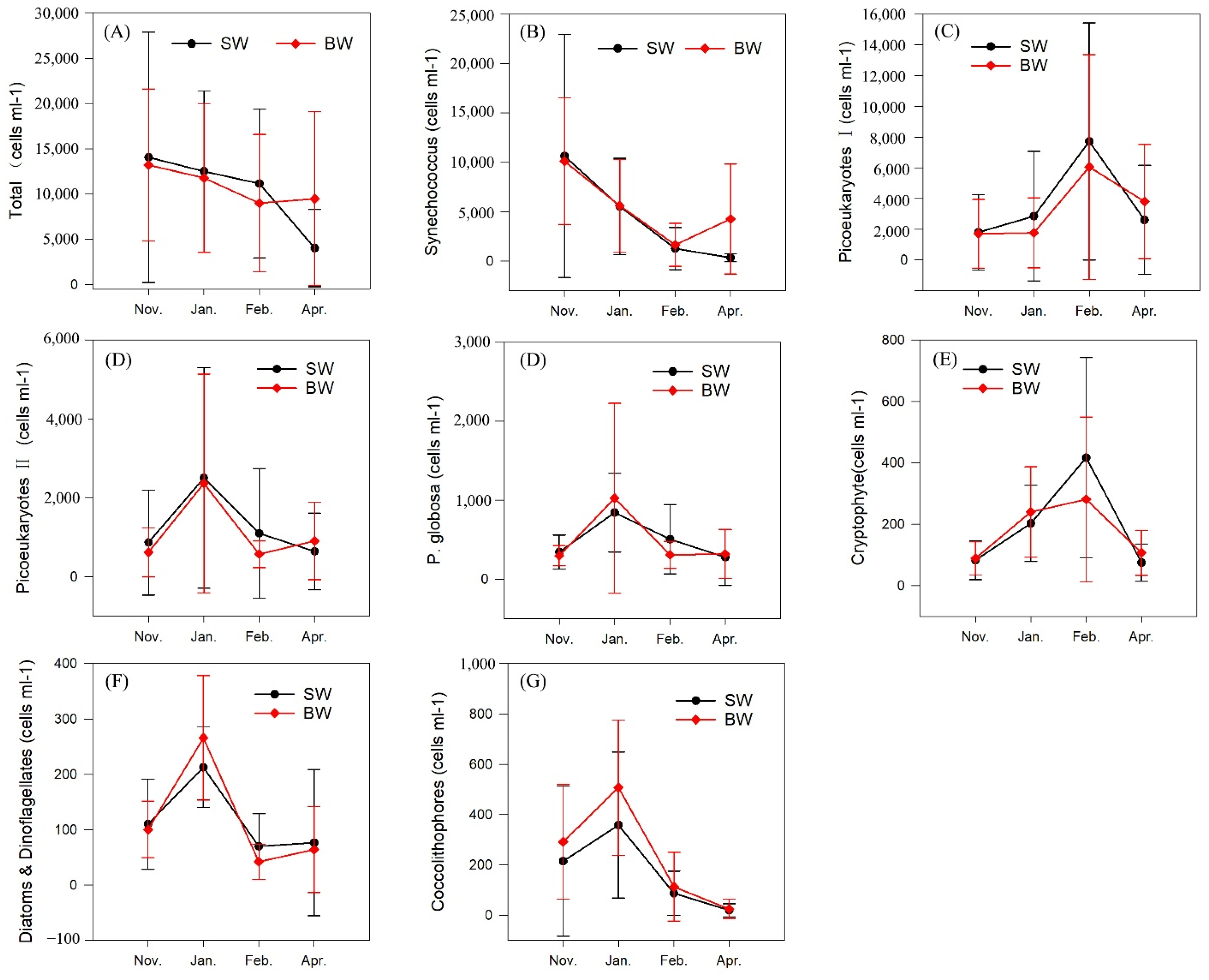
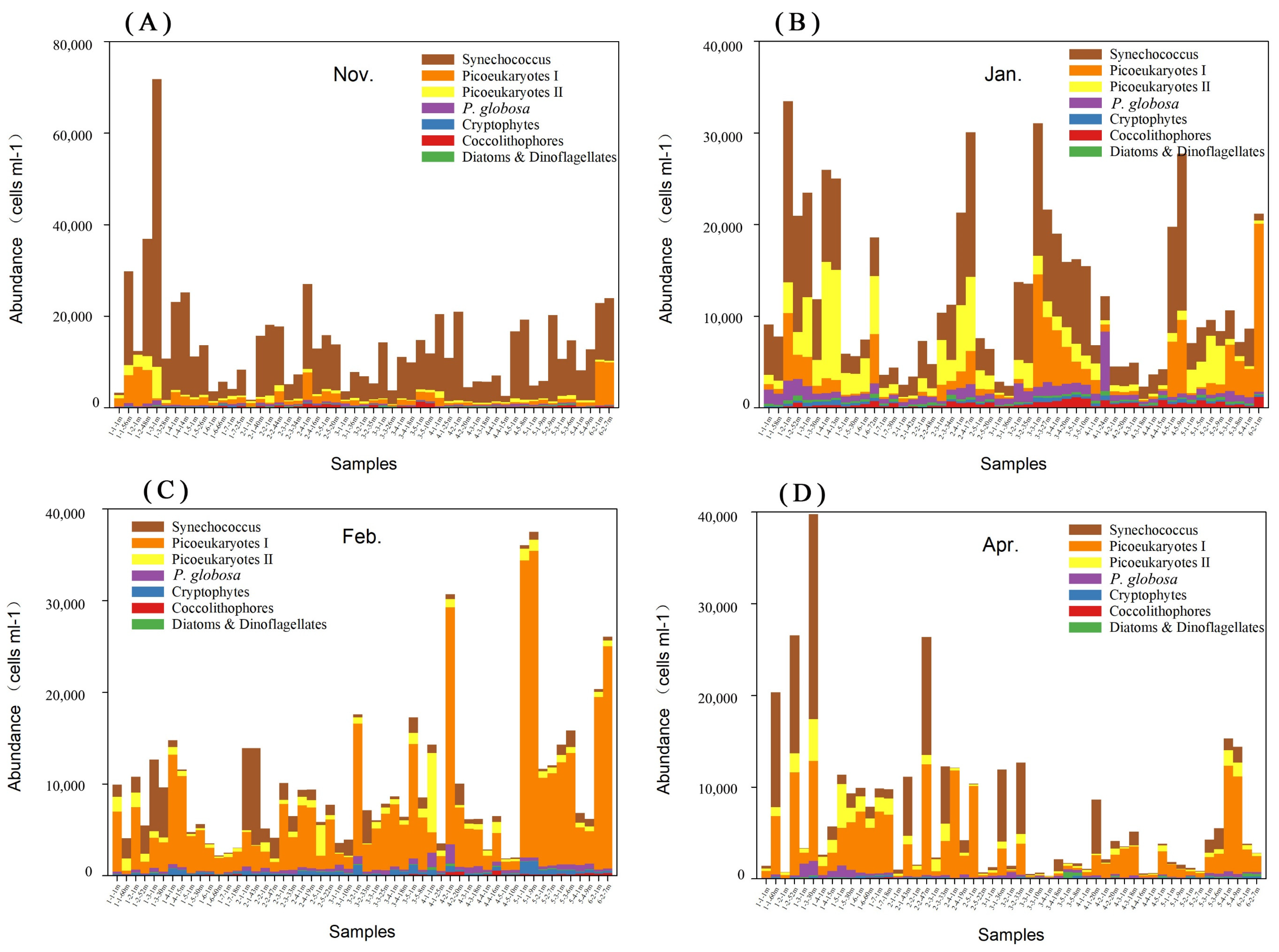
3.3. Phytoplankton Composition and Spatial and Temporal Patterns
3.4. Phaeocystis Globosa Colonies
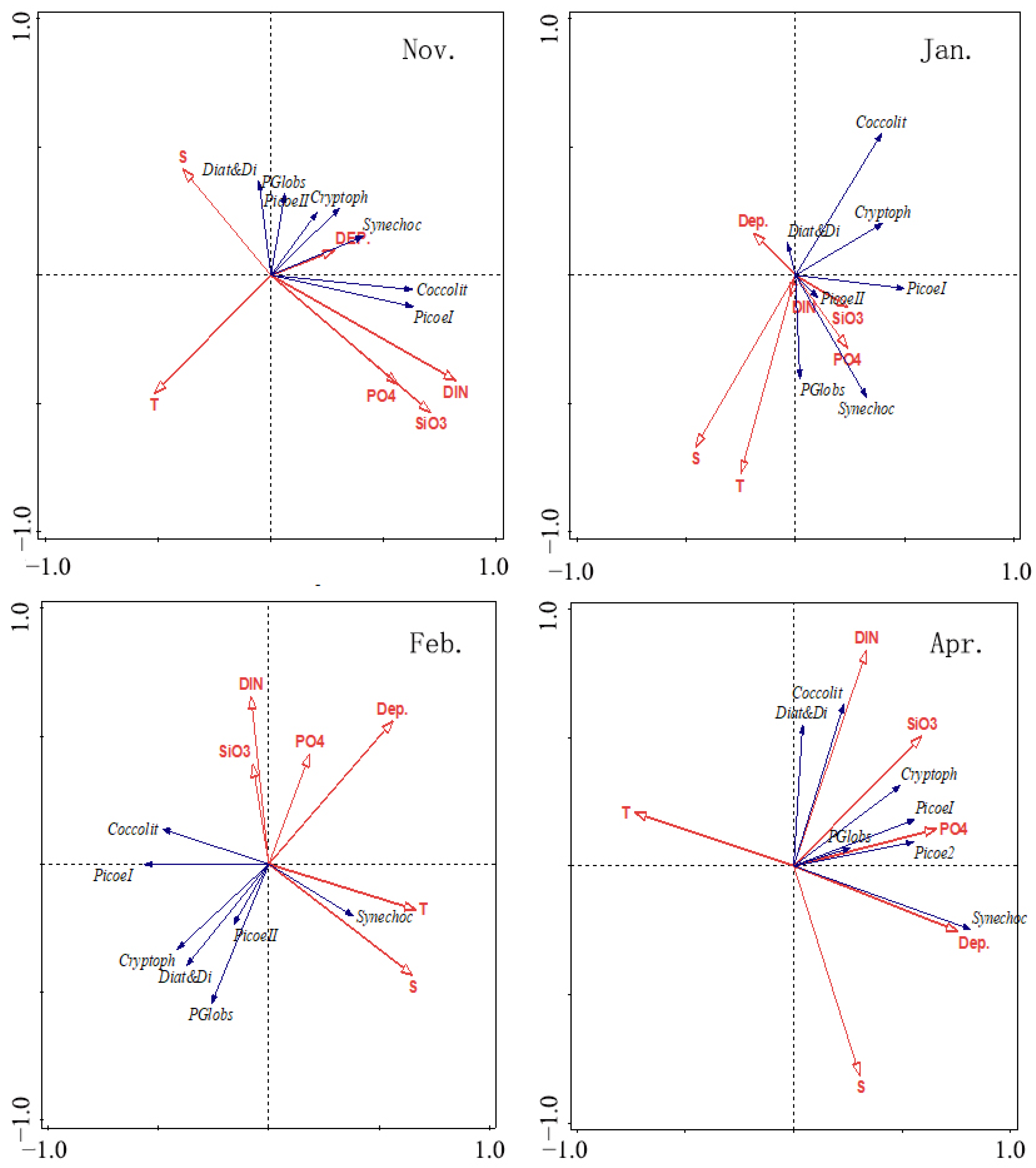
3.5. RDA Results
4. Discussion
4.1. Hydrology
4.2. Nutrients Spatiotemporal Distribution and Sources
4.3. Phytoplankton Abundance and Composition
4.4. Relationships between Environmental Variables and Phytoplankton Assemblages
4.5. P. globosa Solitary Cells and Colonies
4.6. Relationships between Environmental Variables and P. globosa Colony Formation
4.7. Suggestion for Eutrophication Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, M.-C.; Chen, C.-S.; Xu, Q.-C.; Lin, H.-C.; Liu, G.-M.; Wang, H.; Wang, F.; Yan, J.-H. The role of qiongzhou strait in the seasonal variation of the south China Sea circulation. J. Phys. Oceanogr. 2002, 32, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, R.-D.; Zhao, Z.-M.; Weng, S.-C.; Li, F.-H. Temporal and spatial distribution characteristics of nutrients in the coastal seawater of Guangxi Beibu Gulf during the past 25 years. Ecol. Environ. Sci. 2015, 24, 1493–1498. (In Chinese) [Google Scholar] [CrossRef]
- Lan, W.-L.; Peng, X.-Y. Variation characteristics of nutrient concentrations in the Tieshangang bay. Guangxi Sci. 2011, 18, 380–384. (In Chinese) [Google Scholar] [CrossRef]
- Xu, Y.-X.; Zhang, T.; Zhou, J. Historical occurrence of algal blooms in the Northern Beibu Gulf of China and implications for future trends. Front. Microbiol. 2019, 10, 451–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.-M.; Wei, M.-X. Reason and mechanism caused red tide in Beihai Bay. Mar. Environ. Sci. 2009, 28, 62–66. (In Chinese) [Google Scholar] [CrossRef]
- He, C.; Song, S.-Q.; Li, C.-W. The spatial-temporal distribution of Phaeocystis globosa colonies and related affecting factors in Guangxi Beibu Gulf. Oceanol. Et Limnol. Sin. 2019, 50, 630–643. (In Chinese) [Google Scholar] [CrossRef]
- Liu, C.-L.; Tang, D.-L. Spatial and temporal variations in algal blooms in the coastal waters of the western South China Sea. J. Hydroenviron. Res. 2012, 6, 239–247. [Google Scholar] [CrossRef]
- Wang, X.-D.; Song, H.-Y.; Wang, Y.; Chen, N.-S. Research on the biology and ecology of the harmful algal bloom species Phaeocystis globosa in China: Progresses in the last 20 years. Harmful Algae 2021, 107, 102057. [Google Scholar] [CrossRef]
- Whipple, S.J.; Patten, B.C.; Verity, P.G. Life cycle of the marine alga Phaeocystis: A conceptual model to summarize literature and guide research. J. Mar. Syst. 2005, 57, 83–110. [Google Scholar] [CrossRef]
- Rousseau, V.; Chrétiennot-Dinet, M.; Jacobsen, A.; Verity, P.; Whipple, S. The life cycle of Phaeocystis: State of knowledge and presumptive role in ecology. Biogeochemistry 2007, 83, 29–47. [Google Scholar] [CrossRef]
- Rousseau, V.; Vaulot, D.; Casotti, R.; Cariou, V.; Lenz, J.; Gunkel, J.; Ba Umann, M. The life cycle of Phaeocystis (Prymnesiophycaea): Evidence and hypotheses. J. Mar. Syst. 1994, 5, 23–39. [Google Scholar] [CrossRef]
- Peperzak, L.; Gäbler-Schwarz, S. Current knowledge of the life cycles of Phaeocystis globosa and Phaeocystis antarctica (Prymnesiophyceae). J. Phycol. 2012, 48, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, V.; Lantoine, F.; Rodriguez, F.; LeGall, F.; Chrétiennot-Dinet, M.-J.; Lancelot, C. Characterization of Phaeocystis globosa (Prymnesiophyceae), the blooming species in the Southern North Sea. J. Sea Res. 2013, 76, 105–113. [Google Scholar] [CrossRef]
- Cariou, V.; Casotti, R.; Birrien, J.-L.; Vaulot, D. The initiation of Phaeocystis colonies. J. Plankton Res. 1994, 16, 457–470. [Google Scholar] [CrossRef]
- Vaulot, D.; Birrien, J.L.; Marie, D.; Casotti, R.; Chrétiennot-Dinet, M. Morphology, ploidy, pigment composition, and genome size of cultured strains of Phaeocystis (Prymnesiophyceae). J. Phycol. 1994, 30, 1022–1035. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Wang, N.; Zhang, P.; Zhou, H.; Qu, L.-H. Molecular evidence identifies bloom-forming Phaeocystis (Prymnesiophyta) from coastal waters of southeast China as Phaeocystis globosa. Biochem. Syst. Ecol. 2002, 30, 15–22. [Google Scholar] [CrossRef]
- Smith, W.O.; Liu, X.; Tang, K.W.; DeLizo, L.M.; Doan, N.H.; Nguyen, N.L.; Wang, X. Giantism and its role in the harmful algal bloom species Phaeocystis globosa. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 101, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Hamm, C.E.; Simson, D.A.; Merkel, R.; Smetacek, V. Colonies of Phaeocystis globosa are protected by a thin but tough skin. Mar. Ecol. Prog. Ser. 1999, 187, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Kang, Z.; Yang, B.; Lai, J.; Ning, Y.; Zhong, Q.; Dongliang, L.U.; Liao, R.; Wang, P.; Dan, S.F.; She, Z. Phaeocystis globosa bloom monitoring: Based on P. globosa induced seawater viscosity modification adjacent to a nuclear power plant in Qinzhou Bay, China. J. Ocean Uni. China 2020, 19, 14. [Google Scholar] [CrossRef]
- Turner, S.M.; Nightingale, P.D.; Broadgate, W.; Liss, P.S. The distribution of dimethyl sulphide and dimethylsulphoniopropionate in Antarctic waters and sea ice. Deep Sea Res. Part II Top. Stud. Oceanogr. 1995, 42, 1059–1080. [Google Scholar] [CrossRef]
- Verity, P.G.; Brussaard, C.P.; Nejstgaard, J.C.; Leeuwe, M.; Lancelot, C.; Medlin, L.K. Current understanding of Phaeocystis ecology and biogeochemistry, and perspectives for future research. Biogeochemistry 2007, 83, 311–330. [Google Scholar] [CrossRef] [Green Version]
- Peperzak, L.; Duin, R.N.M.; Colijn, F.; Gieskes, W.W.C. Growth and mortality of flagellates and non-flagellate cells of Phaeocystis globosa (Prymnesiophyceae). J. Plankton Res. 2000, 22, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Dubelaar, G.; Jonker, R.R. Flow cytometry as a tool for the study of phytoplankton. Sci. Mar. 2000, 64, 135–156. [Google Scholar] [CrossRef] [Green Version]
- Veldhuis, M.; Kraay, G.W. Application of flow cytometry in marine phytoplankton research: Current applications and future perspectives. Sci. Mar. 2000, 64, 121–134. [Google Scholar] [CrossRef]
- Leroux, R.; Gregori, G.; Leblanc, K.; Carlotti, F.; Thyssen, M.; Dugenne, M.; Pujo-Pay, M.; Conan, P.; Jouandet, M.P.; Bhairy, N.; et al. Combining laser diffraction, flow cytometry and optical microscopy to characterize a nanophytoplankton bloom in the Northwestern Mediterranean. Prog. Oceanogr. 2018, 163, 248–259. [Google Scholar] [CrossRef]
- Boucher, N.; Vaulot, D.; Partensky, F. Flow cytometric determination of phytoplankton DNA in cultures and oceanic populations. Mar. Ecol. Prog. Ser. 1991, 71, 75–84. [Google Scholar] [CrossRef]
- Marrec, P.; Grégori, G.; Doglioli, A.M.; Dugenne, M.; Penna, A.D.; Bhairy, N.; Cariou, T.; Nunige, S.H.; Lahbib, S.; Rougier, G. Coupling physics and biogeochemistry thanks to high-resolution observations of the phytoplankton community structure in the northwestern Mediterranean Sea. Biogeosciences 2018, 15, 1579–1606. [Google Scholar] [CrossRef] [Green Version]
- Bonato, S.; Breton, E.; Didry, M.; Lizon, F.; Cornille, V.; Lécuyer, E.; Christaki, U.; Artigas, L.F. Spatio-temporal patterns in phytoplankton assemblages in inshore–offshore gradients using flow cytometry: A case study in the eastern English Channel. J. Mar. Syst. 2016, 156, 76–85. [Google Scholar] [CrossRef]
- Bonato, S.; Christaki, U.; Lefebvre, A.; Lizon, F.; Thyssen, M.; Artigas, L.F. High spatial variability of phytoplankton assessed by flow cytometry, in a dynamic productive coastal area, in spring: The eastern English Channel. Estuar. Coast. Shelf Sci. 2015, 154, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Louchart, A.; Lizon, F.; Lefebvre, A.; Didry, M.; Schmitt, F.G.; Artigas, L.F. Phytoplankton distribution from Western to Central English Channel, revealed by automated flow cytometry during the summer-fall transition. Cont. Shelf Res. 2020, 195, 104056. [Google Scholar] [CrossRef]
- Dubelaar, G.; Gerritzen, P.L.; Beeker, A.; Jonker, R.R.; Tangen, K. Design and first results of CytoBuoy: A wireless flow cytometer for in situ analysis of marine and fresh waters. Cytometry 1999, 37, 247–254. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, R.-C.; Zhang, Q.-C.; Kong, F.-Z.; Kang, Z.-J.; Cao, Z.-Y.; Geng, H.-X.; Guo, W.; Zhou, M.-J. Relationship between seasonal variation of pico-and nano-phytoplankton assemblages and phaeocystis red tides in Beibu Gulf. Oceanol. Limnol. Sin. 2019, 50, 590–600. (In Chinese) [Google Scholar] [CrossRef]
- China National Standardization Management Committee. The Specification of Oceanographic Survey. In Part 4: Survey of Chemical Parameters in Sea Water; China Standards Press: Beijing, China, 2007. [Google Scholar]
- Zhang, P.; Chen, Y.; Peng, C.-H.; Dai, P.-D.; Lai, J.-Y.; Zhao, L.-R.; Zhang, J.-B. Spatiotemporal variation, composition of DIN and its contribution to eutrophication in coastal waters adjacent to Hainan Island, China. Reg. Stud. Mar. Sci. 2020, 37, 101332. [Google Scholar] [CrossRef]
- Yang, B.; Kang, Z.-J.; Lu, D.-L.; Dan, S.; Ning, Z.-M.; Lan, W.-L.; Zhong, Q.-P. Spatial variations in the abundance and chemical speciation of phosphorus across the river–sea interface in the Northern Beibu Gulf. Water 2018, 10, 1103. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.-Q.; Lu, X.-N.; Wu, Z.-X.; He, C.; Song, X.-X.; Cao, X.-H.; Yu, Z.-M. Spatial-temperal distribution of Phaeocystis globosa colonies and related affecting factors in Guangxi Beibu Gulf. Oceanol. Limnol. Sin. 2020, 50, 579–589. (In Chinese) [Google Scholar] [CrossRef]
- Hu, S.; Xing, R.; Wang, H.; Chen, L. Comparing spatial-temporal characteristics of dissolved nitrogen and phosphorus in water of sea cucumber Apostichopus japonicas culture ponds between sandy and muddy sediments. Aquaculture 2022, 552, 737990. [Google Scholar] [CrossRef]
- Seitzinger, S.P.; Harrison, J.A. Land-Based Nitrogen Sources and Their Delivery to Coastal Systems. In Nitrogen in the Marine Environment; Capone, D.G., Bronk, D.A., Mulholland, M.R., Carpenter, E.J., Eds.; Academic: Burlington, MA, USA, 2008; pp. 469–510. [Google Scholar]
- Lovejoy, C.; Thomas, M.K.; Fontana, S.; Reyes, M.; Pomati, F. Quantifying cell densities and biovolumes of phytoplankton communities and functional groups using scanning flow cytometry, machine learning and unsupervised clustering. PLoS ONE 2018, 13, e0196225. [Google Scholar] [CrossRef]
- Thyssen, M.; Alvain, S.; Lefèbvre, A.; Dessailly, D.; Rijkeboer, M.; Guiselin, N.; Creach, V.; Artigas, L.-F. High-resolution analysis of a North Sea phytoplankton community structure based on in situ flow cytometry observations and potential implication for remote sensing. Biogeosciences 2015, 12, 4051–4066. [Google Scholar] [CrossRef] [Green Version]
- Olson, R.J.; Chisholm, S.W.; Zettler, E.R.; Altabet, M.A.; Dusenberry, J.A. Spatial and temporal distributions of prochlorophyte picoplankton in the North Atlantic Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 1990, 37, 1033–1051. [Google Scholar] [CrossRef]
- Jiao, N.-Z.; Yang, Y.-H.; Koshikawa, H.; Harada, S.; Watanabe, M. Responses of picoplankton to nutrient perturbation in the South China Sea, with special reference to the coast-wards distritution of prochlorococcus. Acta Bot. Sin. 2002, 44, 731–739. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, J.-Q.; Wang, X.-C.C.; Zhang, Y.; Liu, H.L.; Zhou, B.; Pan, P.; Liu, Y.-Z.; Ding, F.-Y. Relationship between phytoplankton community and environmental factors in landscape water with high salinity in a coastal city of China. Environ. Sci. Pollut. Res. 2018, 25, 28460–28470. [Google Scholar] [CrossRef] [PubMed]
- López-Flores, R.; Quintana, X.D.; Romaní, A.M.; Bañeras, L.; Ruiz-Rueda, O.; Compte, J.; Green, A.J.; Egozcue, J.J. A compositional analysis approach to phytoplankton composition in coastal Mediterranean wetlands: Influence of salinity and nutrient availability. Estuar. Coast. Shelf Sci. 2014, 136, 72–81. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Zheng, B.-H.; Liu, L.-S.; Wang, L.-P.; Huang, M.-S.; Wu, G.-Y. Seasonal variation of phytoplankton in the DaNing River and its relationships with environmental factors after impounding of the Three Gorges Reservoir: A four-year study. Procedia Environ. Sci. 2010, 2, 1479–1490. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Oh, Y. Different roles of top-down and bottom-up processes in the distribution of size-fractionated phytoplankton in Gwangyang Bay. Water 2021, 13, 1682. [Google Scholar] [CrossRef]
- Gao, J.-S.; Xue, H.-J.; Chai, F.; Shi, M.-C. Modeling the circulation in the Gulf of Tonkin, South China Sea. Ocean Dyn. 2013, 63, 979–993. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Qiao, F.-L.; Xia, C.-S.; Wang, G. The numerical investigation of seasonal variation of the cold water mass in the Beibu Gulf and its mechanisms. Acta Oceanol. Sin. 2015, 34, 44–54. [Google Scholar] [CrossRef]
- Cao, Z.-Y.; Bao, M.; Guan, W.-B.; Chen, Q. Water-mass evolution and seasonal change in northeast of the Beibu Gulf, China. Oceanol. Limnol. Sin. 2019, 50, 532–542. (In Chinese) [Google Scholar] [CrossRef]
- Le, T.P.Q.; Gilles, B.; Garnier, J.; Sylvain, T.; Denis, R.; Anh, N.X.; Minh, C.V. Nutrient (N, P, Si) transfers in the subtropical Red River system (China and Vietnam): Modelling and budget of nutrient sources and sinks. J. Asian Earth Sci. 2010, 37, 259–274. [Google Scholar] [CrossRef]
- Shi, M.-C.; Chen, B.; Ding, Y.; Wu, L.-Y.; Zheng, B.-X. Wind effects on spread of runoffs in Beibu Bay. Guangxi Sci. 2016, 23, 485–491. (In Chinese) [Google Scholar] [CrossRef]
- Dong-Qiao, X.; Dong-Wang, B.; Sun, X.; Kang-Liang, S. Numerical simulation of nutrient and phytoplankton dynamics in Guangxi coastal bays, China. J. Ocean Univ. China 2013, 13, 338–346. [Google Scholar] [CrossRef]
- Zhang, P.; Dai, P.-D.; Zhang, J.-B.; Li, J.-X.; Zhao, H.; Song, Z.-G. Spatiotemporal variation, speciation, and transport flux of TDP in Leizhou Peninsula coastal waters, South China Sea. Mar. Pollut. Bull. 2021, 167, 112284. [Google Scholar] [CrossRef] [PubMed]
- Stockner, J.G. Phototrophic picoplankton: An overview from marine and freshwater ecosystems. Limnol. Oceanogr. 1988, 33, 765–775. [Google Scholar] [CrossRef]
- Geider, R.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Hodgkiss, I.J.; Ho, K.C. Are changes in N-P ratios in coastal waters the key to increased red tide blooms. Hydrobiologia 1997, 352, 141–147. [Google Scholar] [CrossRef]
- Fang, T.; Li, D.-J.; Yu, L.-H.; Gao, L.; Zhang, L.-H. Effects of irradiance and phosphate on growth of nanophytoplankton and picophytoplankton. Acta Ecol. Sin. 2006, 26, 2783–2789. [Google Scholar] [CrossRef]
- Majumder, A.; Adak, D.; Bairagi, N. Phytoplankton-zooplankton interaction under environmental stochasticity: Survival, extinction and stability. Appl. Math. Model. 2021, 89, 1382–1404. [Google Scholar] [CrossRef]
- Parke, M.; Green, J.C.; Manton, I. Observations on the fine structure of zoids of the genus Phaeocystis [Haptophyceae]. J. Mar. Biol. Assoc. UK 1971, 51, 927–941. [Google Scholar] [CrossRef]
- Escaravage, V.; Peperzak, L.; Prins, T.C.; Peeters, J.; Joordens, J. The development of a Phaeocystis bloom in a mesocosm experiment in relation to nutrients, irradiance and coexisting algae. Ophelia 1995, 42, 55–74. [Google Scholar] [CrossRef]
- Hai, D.-N.; Lam, N.-N.; Dippner, J.W. Development of Phaeocystis globosa blooms in the upwelling waters of the South Central coast of Viet Nam. J. Mar. Syst. 2010, 83, 253–261. [Google Scholar] [CrossRef]
- Cadée, G.C.; Hegeman, J. Phytoplankton in the Marsdiep at the end of the 20th century; 30 years monitoring biomass, primary production, and Phaeocystis blooms. J. Sea Res. 2002, 48, 97–110. [Google Scholar] [CrossRef]
- Lancelot, C.; Billen, G.; Sournia, A.; Weisse, T.; Colijn, F.; Veldhuis, M.; Davies, A.; Wassman, P. Phaeocystis blooms and nutrient enrichment in the continental coastal zones of the North Sea. Ambio 1987, 16, 38–46. [Google Scholar] [CrossRef]
- Riegman, R.; Noordeloos, A.A.M.; Cadée, G.C. Phaeocystis blooms and eutrophication of the continental coastal zones of the North Sea. Mar. Biol. 1992, 112, 479–484. [Google Scholar] [CrossRef]
- Booth, B.C.; Lewin, J.; Norris, R.E. Nanoplankton species predominant in the subarctic Pacific in May and June 1978. Deep Sea Res. Part I Oceanogr. Res. Pap. 1982, 29, 185–200. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Scale-bearing and Loricate Nanoplankton from the East Australian Current. Bot. Mar. 1983, 26, 493–516. [Google Scholar] [CrossRef]
- Reid, P.C.; Lancelot, C.; Gieskes, W.; Hagmeier, E.; Weichart, G. Phytoplankton of the North Sea and its dynamics: A review. N. J. Sea Res. 1990, 26, 295–331. [Google Scholar] [CrossRef] [Green Version]
- Peperzak, L.; Colijn, F.; Gieskes, W.; Peeters, J. Development of the diatom-Phaeocystis spring bloom in the Dutch coastal zone of the North Sea: The silicon depletion versus the daily irradiance threshold hypothesis. J. Plankton Res. 1998, 20, 517–537. [Google Scholar] [CrossRef] [Green Version]
- Koski, M.; Dutz, J.; Klein Breteler, W.C.M. Selective grazing of Temora longicornis in different stages of a Phaeocystis globosa bloom—A mesocosm study. Harmful Algae 2005, 4, 915–927. [Google Scholar] [CrossRef]
- Verity, P.G.; Medlin, L.K. Observations on colony formation by the cosmopolitan phytoplankton genus Phaeocystis. J. Mar. Syst. 2003, 43, 153–164. [Google Scholar] [CrossRef]
- Schapira, M.; Seuront, L.; Gentilhomme, V. Effects of small-scale turbulence on Phaeocystis globosa (Prymnesiophyceae) growth and life cycle. J. Exp. Mar. Biol. Ecol. 2006, 335, 27–38. [Google Scholar] [CrossRef]
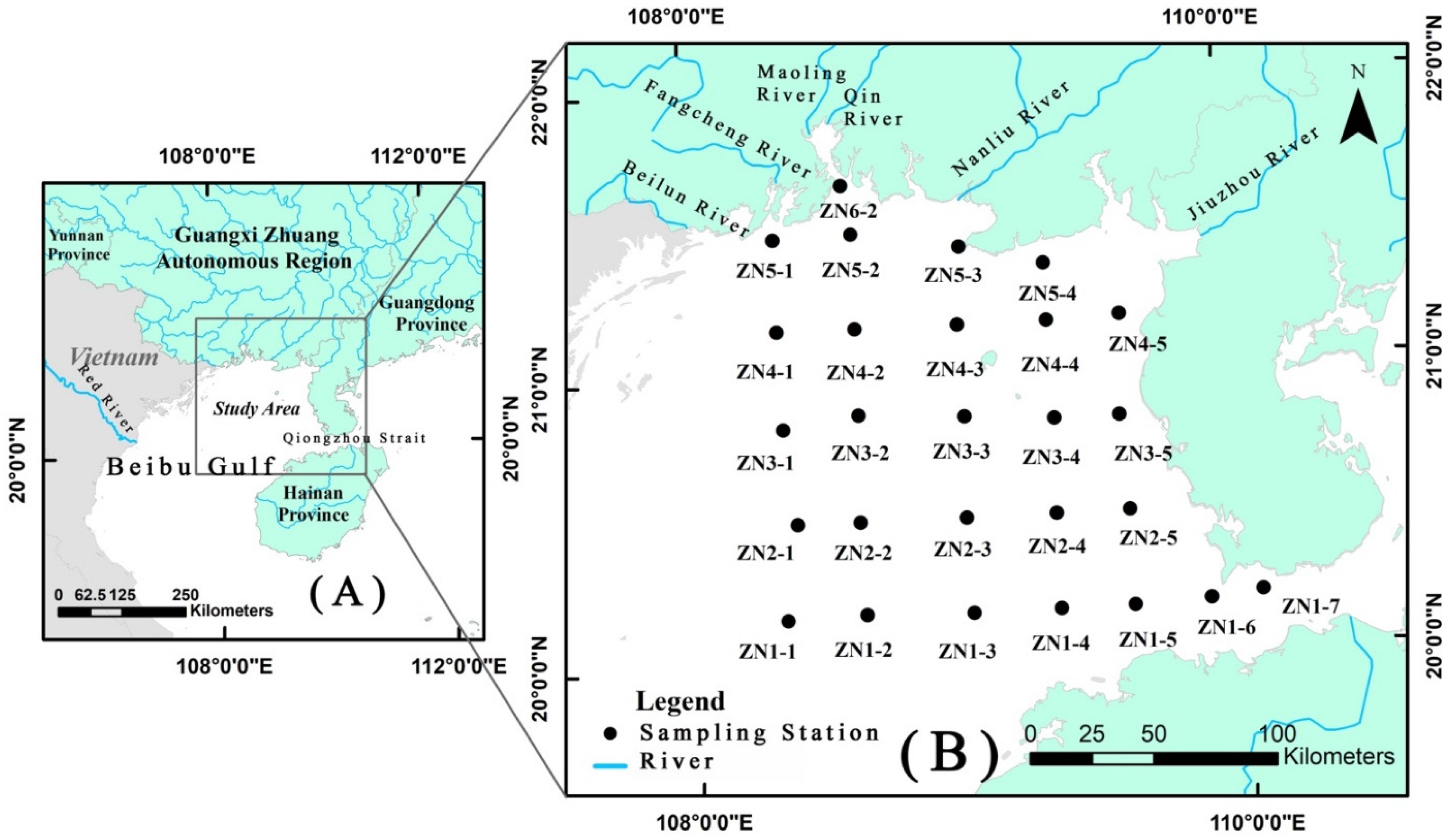

| Station Number | Location | |
|---|---|---|
| Longitude | Latitude | |
| ZN1-1 | 108°20′ E | 20°10′ N |
| ZN1-2 | 108°37′ E | 20°11′ N |
| ZN1-3 | 109°01′ E | 20°10′ N |
| ZN1-4 | 109°20′ E | 20°10′ N |
| ZN1-5 | 109°36′ E | 20°10′ N |
| ZN1-6 | 109°53′ E | 20°11′ N |
| ZN1-7 | 110°04′ E | 20°12′ N |
| ZN2-1 | 108°23′ E | 20°30′ N |
| ZN2-2 | 108°37′ E | 20°30′ N |
| ZN2-3 | 109°00′ E | 20°30′ N |
| ZN2-4 | 109°20′ E | 20°30′ N |
| ZN2-5 | 109°36′ E | 20°30′ N |
| ZN3-1 | 108°20′ E | 20°50′ N |
| ZN3-2 | 108°37′ E | 20°52′ N |
| ZN3-3 | 109°01′ E | 20°51′ N |
| ZN3-4 | 109°20′ E | 20°50′ N |
| ZN3-5 | 109°35′ E | 20°50′ N |
| ZN4-1 | 108°20′ E | 21°10′ N |
| ZN4-2 | 108°37′ E | 21°10′ N |
| ZN4-3 | 109°00′ E | 21°10′ N |
| ZN4-4 | 109°20′ E | 21°10′ N |
| ZN4-5 | 109°36′ E | 21°11′ N |
| ZN5-1 | 108°20′ E | 21°29′ N |
| ZN5-2 | 108°37′ E | 21°30′ N |
| ZN5-3 | 109°01′ E | 21°26′ N |
| ZN5-4 | 109°20′ E | 21°22′ N |
| ZN6-2 | 108°35′ E | 21°40′ N |
| Cruise Time | Start Date | End Date | Depth Range | Station Number | Phytoplankton Sample Number |
|---|---|---|---|---|---|
| November | 4 November 2018 | 11 November 2018 | 5 m–69 m | 27 | 53 |
| January | 6 January 2019 | 14 January 2019 | 5 m–75 m | 27 | 52 |
| February | 22 February 2019 | 28 February 2019 | 7 m–75 m | 27 | 54 |
| April | 2 April 2019 | 10 April 2019 | 7 m–72 m | 27 | 54 |
| Physiochemical Factors | Average Values ± Standard Deviation | |||||||
|---|---|---|---|---|---|---|---|---|
| November | January | February | April | |||||
| SW | BW | SW | BW | SW | BW | SW | BW | |
| Temperature, °C | 26.24 ± 0.64 | 25.81 ± 0.88 | 18.91 ± 2.59 | 19.22 ± 2.26 | 21.28 ± 1.59 | 20.86 ± 1.30 | 25.91 ± 0.85 | 24.53 ± 1.08 |
| Salinity | 30.86 ± 1.91 | 33.81 ± 2.16 | 32.00 ± 1.05 | 32.26 ± 0.83 | 32.27 ± 1.53 | 32.43 ± 1.11 | 31.47 ± 1.35 | 31.78 ± 1.48 |
| DIN, μmol L−1 | 4.89 ± 5.40 | 6.00 ± 5.05 | 5.46 ± 3.08 | 5.47 ± 3.02 | 5.98 ± 3.78 | 6.12 ± 2.93 | 8.91 ± 3.18 | 8.99 ± 2.81 |
| PO4, μmol L−1 | 0.51 ± 0.13 | 0.61 ± 0.17 | 0.61 ± 0.09 | 0.61 ± 0.10 | 0.55 ± 0.08 | 0.55 ± 0.06 | 0.53 ± 0.08 | 0.54 ± 0.09 |
| SiO3, μmol L−1 | 7.89 ± 7.13 | 9.43 ± 9.43 | 10.89 ± 3.89 | 11.11 ± 4.06 | 5.37 ± 3.62 | 5.89 ± 2.98 | 3.83 ± 3.03 | 4.59 ± 3.18 |
| N/P | 8.6 ± 7.4 | 9.3 ± 6.6 | 8.5 ± 4.3 | 8.6 ± 4.5 | 10.3 ± 5.1 | 10.8 ± 4.4 | 16.9 ± 4.9 | 16.8 ± 4.7 |
| Phytoplankton Groups | Average Values ± Standard Deviation (Cells mL−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| November | January | February | April | |||||
| SW | BW | SW | BW | SW | BW | SW | BW | |
| Total | 14,053 ± 13,536 | 13,210 ± 8235 | 12,176 ± 8714 | 11,783 ± 8039 | 10,818 ± 8033 | 9006 ± 7434 | 4058 ± 4208 | 9490 ± 9415 |
| Synechococcus | 10,633 ± 12,063 | 10,105 ± 6298 | 5720 ± 4794 | 5608 ± 4582 | 1310 ± 2092 | 1641 ± 2141 | 358 ± 399 | 4256 ± 5470 |
| Picoeukaryote I | 1801 ± 2414 | 1707 ± 2195 | 2255 ± 4136 | 1769 ± 2233 | 7291 ± 7568 | 6051 ± 7184 | 2617 ± 3482 | 3811 ± 3631 |
| Picoeukaryote II | 868 ± 1301 | 620 ± 612 | 2589 ± 2740 | 2368 ± 2711 | 1120 ± 1612 | 571 ± 332 | 647 ± 950 | 908 ± 962 |
| Phaeocystis globosa | 343 ± 212 | 297 ± 124 | 866 ± 490 | 1025 ± 1178 | 516 ± 429 | 307 ± 168 | 281 ± 348 | 319 ± 303 |
| Cryptophytes | 83 ± 62 | 89 ± 53 | 204 ± 122 | 240 ± 145 | 425 ± 320 | 281 ± 264 | 71 ± 9 | 107 ± 72 |
| Diatoms and dinoflagellates | 110 ± 80 | 100 ± 50 | 216 ± 71 | 266 ± 110 | 71 ± 58 | 42 ± 31 | 66 ± 130 | 64 ± 76 |
| Coccolithophores | 215 ± 292 | 292 ± 224 | 327 ± 285 | 508 ± 264 | 83 ± 86 | 113 ± 134 | 18 ± 25 | 25 ± 38 |
| RDA Results | November | January | February | April | ||||
|---|---|---|---|---|---|---|---|---|
| Axis 1 | Axis 2 | Axis 1 | Axis 2 | Axis 1 | Axis 2 | Axis 1 | Axis 2 | |
| Eigenvalues | 0.225 | 0.039 | 0.135 | 0.082 | 0.180 | 0.064 | 0.328 | 0.116 |
| Explained variation (%) | 22.5 | 26.4 | 13.5 | 21.6 | 18.0 | 24.3 | 32.8 | 44.4 |
| Pseudo-canonical correlation | 0.80 | 0.43 | 0.53 | 0.79 | 0.67 | 0.56 | 0.80 | 0.72 |
| Explained fitted variation (%) | 73.3 | 86.1 | 47.9 | 76.9 | 60.0 | 81.2 | 68.9 | 93.1 |
| Physiochemical Factors | November | January | February | April | ||||
|---|---|---|---|---|---|---|---|---|
| Contribution % | p | Contribution % | p | Contribution % | p | Contribution % | p | |
| DIN | 51.7 | 0.002 | 19.9 | 0.02 | 13.3 | 0.032 | 25.4 | 0.002 |
| PO4 | 4.6 | 0.464 | 9.4 | 0.174 | 11.9 | 0.064 | 15.8 | 0.002 |
| SiO3 | 7.1 | 0.208 | 18.9 | 0.03 | 6.7 | 0.264 | 5.5 | 0.048 |
| S | 5.5 | 0.308 | 26.7 | 0.01 | 10.2 | 0.126 | 5.4 | 0.066 |
| T | 25.4 | 0.002 | 18.2 | 0.03 | 30.8 | 0.002 | 6.5 | 0.05 |
| Depth | 5.6 | 0.3 | 6.9 | 0.292 | 27.2 | 0.004 | 41.3 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.-B.; Zhang, R.-C.; Jiang, F.-J.; Pan, H.-Z.; Li, J.; Yu, K.-F.; Lai, J.-X. Spatiotemporal Variation in Phytoplankton and Physiochemical Factors during Phaeocystis globosa Red-Tide Blooms in the Northern Beibu Gulf of China. Water 2022, 14, 1099. https://doi.org/10.3390/w14071099
Xu M-B, Zhang R-C, Jiang F-J, Pan H-Z, Li J, Yu K-F, Lai J-X. Spatiotemporal Variation in Phytoplankton and Physiochemical Factors during Phaeocystis globosa Red-Tide Blooms in the Northern Beibu Gulf of China. Water. 2022; 14(7):1099. https://doi.org/10.3390/w14071099
Chicago/Turabian StyleXu, Ming-Ben, Rong-Can Zhang, Fa-Jun Jiang, Hui-Zhu Pan, Jie Li, Ke-Fu Yu, and Jun-Xiang Lai. 2022. "Spatiotemporal Variation in Phytoplankton and Physiochemical Factors during Phaeocystis globosa Red-Tide Blooms in the Northern Beibu Gulf of China" Water 14, no. 7: 1099. https://doi.org/10.3390/w14071099
APA StyleXu, M.-B., Zhang, R.-C., Jiang, F.-J., Pan, H.-Z., Li, J., Yu, K.-F., & Lai, J.-X. (2022). Spatiotemporal Variation in Phytoplankton and Physiochemical Factors during Phaeocystis globosa Red-Tide Blooms in the Northern Beibu Gulf of China. Water, 14(7), 1099. https://doi.org/10.3390/w14071099





