Abstract
The EU Water Framework Directive stipulates that all EU waterways shall have good chemical and ecological status by 2027. Methodologies are described for how to assess and classify waterbodies and make 7-year management plans. Aquatic risk assessment methodologies and environmental quality standards are defined and a biotic ligand model methodology is available to assess the influence of water chemistry on the ability of aquatic organisms to take up metals. Aquatic status classification practices of naturally occurring river basin-specific metals are discussed, specifically how Cu and Zn water quality criteria guideline values have been adopted and defined for Swedish coastal and estuarine waters and how well they represent possible ecological risks. Calculations of bioavailability and ecotoxicity are conducted using recognised models for the Strömmen-Saltsjön water body in Stockholm, in which naturally occurring metals, especially Cu, have among the highest background concentrations of Sweden. Proposals are made to improve risk assessment methodologies to better reflect the vitality of living organisms, and to what extent current levels of these metals in Swedish waterways may influence their welfare. The study concludes that a more local assessment including, e.g., studies of the benthic fauna would be relevant for ecological status classification.
1. Introduction
Copper (Cu) and Zinc (Zn) are abundant elements in the earth’s crust and soil and cannot be either generated or destroyed. The metals also naturally occur in natural waters at ambient concentrations. Both metals readily form complexes and solids with both organic and inorganic ligands at pH- and redox conditions relevant for such waters, and the aqueous free metal ions are thermodynamically disfavoured at these conditions. As a result, only minor fractions of their total concentrations exist in any bioavailable form, i.e., accessible for organism uptake. Numerous studies show that when metal bioavailability is considered on a larger spatial scale in fresh- and saltwater, neither Cu nor Zn can be concluded to pose any considerable threat to the aquatic environment [1,2,3,4,5]. However, there may occasionally be exceptions due to very specific water chemistry conditions in terms of concentrations of organic matter and dissolved organic carbon (DOC), water pH and ionic strength (salinity) and/or total metal concentrations [6,7]. This makes conditions for metal exposure extra sensitive. Both Cu and Zn are essential elements to all aquatic animals and their uptake is homeostatically regulated [6,7], though governed by different mechanisms depending on water chemistry (e.g., salt water compared to freshwater) [8]. In freshwater, fish are expected to absorb waterborne metals mainly by the gills, while the pathway in saltwater can include both gills and the gut [9]. Other organisms, such as phytoplankton, which exist in both fresh- and saltwater can take up considerable amounts of, e.g., Cu from the water column and transfer metals to other aquatic organisms in the food chain [10]. Both minerogenic (of mineral origin) and biogenic (produced by living organisms) particles of the water compartment can further act as both carriers and sinks of Cu and Zn via different processes including, e.g., heteroagglomeration, sorption, sedimentation and desorption [8].
Potential adverse toxicological effects on aquatic organisms are closely dependent on the chemical form of the metal, which is related to the chemistry of the water setting. This can be predicted for both Cu and Zn using different biotic ligand models, such as the BLM and Bio-Met models, discussed below. From variations in water chemistry, such as typically the case for estuarian low-salinity waters, follow changes in metal chemical speciation due to a lower buffering capacity compared to intermediate and high salinity waters [11]. Toxic effects are generally lower in high compared to low salinity waters, though toxicological effects of Cu and Zn in marine waters (high salinity) are less explored [6,7,8,12]. These effects are mainly attributed to higher competition for available sites on aquatic organisms combined with an even higher extent of strongly bonded metal complexes in high-compared with low salinity waters [13,14,15]. Since the physico-chemical conditions of coastal waters and marine estuaries are continuously varying and therefore also the speciation of metals, it is difficult to define and predict the toxicity based on certain parameters, such as salinity, ion composition and/or ionic strength, Figure 1 [8,16].
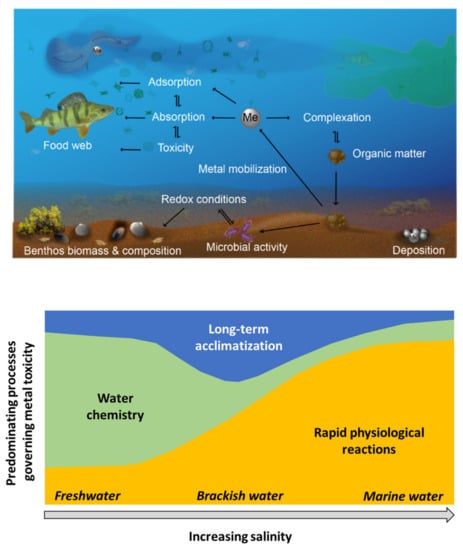
Figure 1.
Schematic illustrations of selected processes influencing and governing metal toxicity in the aquatic and sediment compartments influenced by water salinity, adapted from ref. [8].
The uptake and excretion of metal ions from the water compartment are regulated by several enzymes and ion channels in, e.g., fish and other aquatic organisms [9,10,13]. Active uptake is high in order to counteract ion diffusive loss while excretion is minor. The internal concentration of a given ion over time is relatively constant in freshwater organisms and higher compared with the surrounding water, particularly in low salinity waters [6]. Increased salinity increases the ion diffusive gain and therefore also ion excretion. In order to maintain homeostasis, the organisms can change their membrane properties [13]. Toxic effects on aquatic organisms in brackish water and in estuarine environments will hence not only be governed by the chemical speciation but also by effects on the homeostatic processes induced by differences in salinity [11,13,17]. The transient chemical nature of these environments means that the parameters that govern metal bioavailability and toxicity to aquatic life vary in both space and time [8].
Acclimatisation to metal exposure was shown for various marine and freshwater organisms [18] including Daphnids [19]. However, lack of acclimatisation was also reported, both for a fresh water green algae and a marine diatom [20]. Any generalisation regarding acclimatisation in risk assessment is hence doubtful.
In order to monitor and protect aquatic life according to the water policy of the EU member states, the chemical and ecological status of all water bodies, including brackish and marine waters, should be monitored. Predictions of toxicity for different waters with different chemistry can be made using the biotic ligand model (BLM), initially developed for Cu, later on for Zn, Nickel (Ni) and Lead (Pb) to facilitate risk assessment for freshwater settings [21,22,23]. The models relate chemical speciation calculations with toxicological information to predict the toxicity and consider metal bioavailability (the fraction of the bioaccessible metal concentration that can be taken up by an organism). The full BLMs all target freshwater conditions and consider pH, DOC, hardness (calcium concentrations, Ca2+), alkalinity and competing and complexing ions (e.g., K+, Mg2+, Na+, Cl−, SO42−). The models are validated and adopted into the EU guidelines for metal risk assessment of freshwater and for setting environmental quality standards [24]. However, there are currently no models validated for marine or estuarine environments, although the freshwater BLM has successfully been applied for, e.g., Cu [25]. Studies have shown difficulties to model the more complex marine environment and conditions of high salinity [26,27].
The Bio-Met tool, a free online simplified and user-friendly tool (www.bio-met.net, accessed 5 November 2020), is based on the BLM model and recommended by the EU Water Framework Directive (EU WFD) [28] to be used for bioavailability-based assessments of risks related to metals (Cu, Zn, Ni, Pb) in freshwater settings. The tool considers spatial and temporal variations at ambient water quality using dissolved metal concentrations and hardness (dissolved Ca concentration), pH, and DOC as input parameters, all with relevance when predicting both acute and chronic effects on aquatic life. Bio-Met is a conservative tool in its design and is able to predict effects on the most sensitive aquatic species. It includes both evidence-based data as well as case studies. Bio-Met predictions likely overestimate, rather than underestimate, environmental risks [29]. The inclusion of site-specific biological analyses to complement chemical-based assays can preferably be used for improved precision in environmental risk assessment. The reliability of the Bio-Met tool is well proven and established [30]. Validation was performed at pH levels between 6.0 and 8.5 for both Cu and Zn, and for Ca2+ concentrations between 3.1 and 129.0 mg/L for Cu and between 5.0 and 160.0 mg/L for Zn. The water chemistry of most freshwater bodies in Sweden falls within the boundaries of the validated BLM models with only some exceptions with lower pH and Ca levels [31].
DOC is one of the most important factors together with pH and salinity in risk assessment related to toxicity induced by Cu and Zn to aquatic organisms. Except for concentration variations, the physical properties vary, in particular in estuarine environments, which in turn influence the chemical interactions and the toxic effects [8,16,27]. When applied to marine or estuarine environments, these differences in physical properties and speciation can, for a given setting, result in less accurate metal toxicity predictions [25]. For calculations in Bio-Met, DOC is set to 0.8×TOC (total organic carbon).
Classifications of coastal waters are in current regulations from the water authorities based on marine PNEC (predicted no effect concentration) values set in the voluntary risk assessment report for Cu [1] and in the European risk assessment report for Zn [2]. These values are derived from vast datasets of the highest validity and quality in relation to available ecotoxicological findings. After additional mesocosm studies [3,32] and the verification of derived PNEC values based on species sensitivity distributions (SSD), the EU has, via their Committees on Health and Environmental Risks (SCHER), accepted an assessment factor (AF) of 1 for Cu [33]. The risk assessment report of Zn [2] presents NOEC values for marine organisms spanning from 10 to 2700 µg/L dissolved Zn with the lowest marine NOEC of 10 µg/L. A PNECadd value of 6.1 µg/L was proposed based on the SCHER dataset [34]. Analogous to Cu, a supplementary marine mesocosm study performed for Zn [32] verified that the derived HC550/PNEC value from species sensitivity distributions constitutes a safe level when used with AF = 1. The same study did not show any measurable negative effects in the mesocosm for dissolved Zn concentrations up to 12 μg/L. However, the Swedish environmental quality criteria for Cu and Zn used for classification of coastal waters have implemented higher AF levels for the Swedish west coast (AF = 2 for Cu, AF = 1.8 for Zn) and for the east coast and the Baltic Sea (AF = 6 for Cu, AF = 5.5 for Zn) due to extra sensitiveness of the brackish Baltic water of continuously changing water chemistry in terms of pH, salinity and DOC, both temporally and spatially, and possible adverse effects on sensitive aquatic organisms [35]. Based on the lowest NOEC in the Zn risk assessment report, the Swedish EQS for the Baltic sea would correspond to an AF of approximately 9.
The aim of this paper is to address how generic water quality criteria can be applied to Cu and Zn in dynamic aquatic environments since the water chemistry of several Swedish coastal water bodies are partially (spatially and/or temporally) outside validated ranges for aquatic risk assessments using either the BLM or the Bio-Met prediction models. There is currently no specific regulation that is adapted to estuary/varying salinity conditions for status classifications in coastal water bodies. This is problematic since surface waters of such mixing zones can consist of freshwater during some periods, whereas the deeper water has increasingly higher salt content. According to the current status classification regulation of metals, this cannot be considered despite its relevance when assessing how the risk varies both spatially and with time [8]. Instead of introducing uncertainties using AFs based on marine PNECs of brackish water, it can be argued that classifications should rather be based on freshwater BLM modelling to strengthen their validity in estuary waters of low salinity.
Different approaches for assessing risks related to Cu and Zn in the aquatic environment are discussed in this study using the water body Strömmen-Saltsjön in central Stockholm, Sweden as a case study. This coastal water body (including its surrounding water bodies) was selected since it is classified as marine water according to Swedish regulations even though its environmental chemistry of very low salinity (mixed fresh- and salt water) rather reflects freshwater characteristics. Differences in derived risk characterisation ratios when applying methodologies for status classification for a water body both as coastal and fresh water are presented and discussed in terms of water chemistry, site-specific and urban factors, as well as aquatic species sensitivity, proposing a best practice way forward. The possibility to use sedimentation data on Cu and Zn to classify risks is discussed.
2. Strömmen-Saltsjön Water Body—A Case Study
2.1. Site Description
The location of the coastal water body Strömmen (in this paper denoted Strömmen-Saltsjön) in central Stockholm, Sweden, is part of the brackish Baltic Sea, Figure 2. It has a surface area of 4 km2, a depth varying between 20 and 35 m and a volume of approximately 0.05 km3 [36]. Its coastline is to a large extent made up of quays and shores with man-made protections against erosion. It has intense ship traffic in the area with passenger ferries of varying sizes. The water body is characterised by the inflow of fresh water from Lake Mälaren (1140 km2, average depth: 13 m) through a very short river (Norrström) and two sluices (Hammarby and Söderström). Two discharge channels exist next to these sluices to regulate the lake level. Lake Mälaren was separated from the Baltic Sea through post-glacial isostasy around 1000 A.D. Its inflow is highly seasonal with pronounced peaks during winter and spring, and low flows during summer and autumn. The lake water level is generally higher than the Baltic Sea water (mean value of 0.68 m during 1990–2015), even though small opposite flows can occur on rare occasions. The average annual discharge between Mälaren and Strömmen-Saltsjön was during the period from 1968 to 2018 4900 Mm3 [37] with seasonal variations (1968–2015: 2000 mm3 (March–May); 600 mm3 (June–August); 1200 Mm3 (September–December)). Its main discharge is at the natural topographical boundary at Norrström characterised by high flows (200 to 300 m3/s). The flow at the Söderström sluice is substantially lower (0 and 10 m3/s).
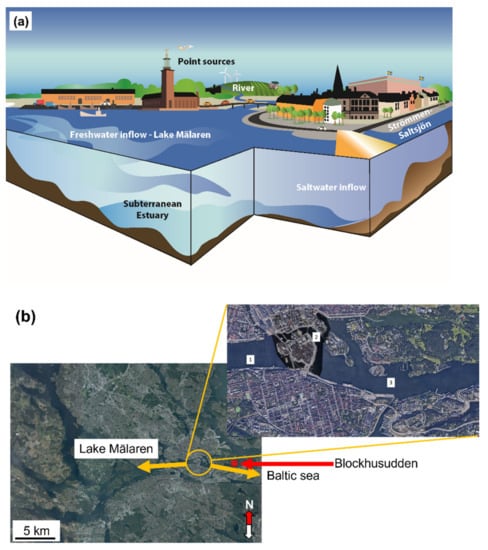
Figure 2.
Schematic (a) and overview pictures (b) of the Strömmen-Saltsjön water body where 1-3 denotes locations of Lake Mälaren (Riddarfjärden) (1); Strömmen (2) and Saltsjön (3)—area. The Strömmen-Saltsjön water body includes areas 2 and 3. The red circle marks the Blockhusudden water monitoring station.
Since the Strömmen-Saltsjön water body receives a substantial inflow of, e.g., nutrients and other species from the wastewater treatment plants (Henriksdal and Bromma) which influence the water quality, and the major part of the inflow from Lake Mälaren, several stations exist within the water body that monitor its ecological and chemical status [37,38].
2.2. Water Chemistry and Flows of Cu and Zn to the Strömmen-Saltsjön Water Body
The water temperature varies at the surface between 0 °C in the winter and approximately 20 °C in the summer. Because of the inflow of freshwater from Lake Mälaren, the Strömmen-Saltsjön water body has distinct seasonal variations in salinity. This is observed with PSU (Practical Salinity Unit, ≈ppt) levels at the surface between 0 during the summer and 4 during autumn, whereas the practical salinity is stable in the bottom water (4–5 PSU) [39]. This results in a stable stratification of the water column during the period between January and July with a salinity difference of approximately 2 PSU between the surface and the bottom water. During autumn, the water column becomes mixed and the stratification disappears. Density variations of the water depend on both salinity and temperature and are the greatest at 10 m (the pycnocline level).
The oxygen levels of the water reach a minimum from October to November with concentrations varying between 1 and 3 mg/L in the bottom waters.
The content of total organic carbon (TOC) varies between 4 and 7 mg/L. A total of 1200 tons TOC and 440 ton suspended solids were discharged during 2017 into the water body from the wastewater treatment plant at Henriksdal [39]. The calculated dissolved organic content (DOC) equals approximately 0.8 times the TOC content. The corresponding TOC numbers from the plant in Bromma were 460 and 81 tons, respectively. In total, 30 times more TOC is received from Lake Mälaren [36], though most TOC is transported with the general water flow to the Stockholm Archipelago and the Baltic Sea. A large fraction, 30–50% of all TOC, which is buried in the sediments in the Strömmen-Saltsjön water body, originates from Lake Mälaren [36].
A total of 870 kg Cu and 3124 kg Zn were discharged from the wastewater treatments plants (Henriksdal and Bromma) during 2017 [39]. These numbers are small compared to annual natural discharges of Cu (8,900,000 to 37,000,000 kg) and Zn (7,500,000 to 77,000,000 kg) from Lake Mälaren to the water body Strömmen-Saltsjön, primarily originating from mineral erosion [40,41]. There are also drain and storm water flows directly to Strömmen-Saltsjön that do not pass the wastewater treatment plants. These untreated water flows originate from storm water of natural surfaces (6 mm3), streets and roofs (7 mm3) [39] and are estimated to contribute annually with approximately 500 kg Cu and 500 kg Zn [41], i.e., 1–7% of the natural annual discharge of the same metals from Lake Mälaren.
Discharged Cu from Lake Mälaren results in a sediment burial of approximately 6% Cu in Strömmen-Saltsjön, i.e., close to the corresponding number for the discharge of TOC (4%) [36,41]. In accordance with the expected aqueous chemistry of Cu in natural waters, the environmental fate of Cu is, to a large extent, governed by organic matter dynamics [42]. The removal of the fraction of Cu (as organic complexes heteroagglomerated with Fe–oxy–hydroxy complexes, or adsorbed to inorganic particles) from the water column to the sediment occurs at the turbidity maximum where fresh- and salt waters mix [27]. The fraction of inorganic complexes becomes more important in marine waters [8]. Since the ability of Cu to form organic complexes is stronger than its adsorption to particles, the main sedimentation mechanism of Cu is connected to its interaction with either organic matter or inorganic particles with adsorbed organic matter [42]. The importance of the sedimentation of organic matter due to aggregation on the sedimentation rate of Cu can be indicated by comparing sediment burial rates of Cu in the area of Lake Mälaren closest to Strömmen-Saltsjön, Riddarfjärden (see Figure 2), with corresponding rates in Strömmen-Saltsjön [41]. The sediment burial rate of dry matter is almost three times as high at Strömmen-Saltsjön compared to Riddarfjärden. A ratio of 0.6 is obtained if the sediment burial rate of Cu at Riddarfjärden is multiplied by three and divided by the corresponding sedimentation rate at Strömmen-Saltsjön. This ratio can be interpreted as the additional sediment load caused by aggregation and sedimentation of organic matter, including Fe–Mn-organic complexes [41].
A higher binding of Cu to suspended solids in marine/estuarine waters compared to freshwater is also concluded from comparing sorption coefficients (Kd) for freshwater, estuarine waters, and marine waters, respectively [1], Table 1.

Table 1.
Sorption coefficients between Cu and suspended solids in freshwater, estuarine waters and marine waters [1].
Complexation between Zn and organic matter is intermediate compared with Cu, which forms the strongest complexes, and Ni the weakest, with organic matter [21]. Zn can hence in the presence of organic matter be expected to exist in a larger portion as labile (weakly bonded complexes) and free ions compared to Cu. Effects of heteroagglomeration with particles will hence be more prevailing for Zn compared with Cu [16,42]. Indeed, such differences in the chemical speciation of Cu and Zn will influence their environmental fate. Aggregation and sedimentation of organic matter in Strömmen-Saltsjön are hence not equally important removal mechanisms for Zn as observed for Cu.
Riddarfjärden and Strömmen-Saltsjön show similar sediment fluxes of Zn (ratio equals 1) as both water bodies are expected to have a significant fraction of labile complexes and free Zn ions [16,42]. As salinity increases, the concentrations of cations increase, which compete for sorption sites on particles [16,43]. Increased concentrations of chloride ions (Cl−) result in increased formation of Zn-Cl complexes. These processes can hence result in reduced sedimentation rates of Zn since they reduce the capability of Zn to sorb to particles able to sediment [43]. However, the ratio between the sediment fluxes in Strömmen-Saltsjön and the discharge of Zn and Cu from Lake Mälaren is actually higher for Zn (13%) than for Cu (6%) [41]. This might be explained by a higher sorption rate of Zn compared to Cu to particles.
2.3. Aquatic Life of the Strömmen-Saltsjön Water Body
Brackish water environments generally have low biodiversity, a condition that is also the case for the Strömmen-Saltsjön water body [37]. Such environments are in general considered to be moderately disturbed according to the AAB-index initiated by the Swedish EPA [37]. This index classifies the soft-bottom fauna in the Baltic Sea using a ranked scale of number of species, biomass and abundance [44]. The benthic fauna (organisms present on and in the seabed) consists of a few species that are tolerant to discharges from the wastewater treatments plants (see above). Areas of the Baltic Sea that do not receive any direct impact from wastewater discharges, Lake Mälaren or larger ships, lack benthic fauna altogether [37].
Two planktonic blooms occur in Strömmen-Saltsjön and the Baltic Sea, one in spring (April to May) and one in autumn, during which the former produces the largest biovolume. Based on chlorophyll and biovolume data, the ecological status is moderate except for the innermost station in Strömmen-Saltsjön showing a poor status [37]. There is also a cyanobacterium bloom from June to September.
The zooplankton community in the outermost part of Strömmen-Saltsjön is dominated by copepods (small crustaceans) between January and May, which can be explained by the abundance of dinoflagellates diatoms and other taxa [37]. From June to August, Cladocera is the dominating zooplankton. Both phyto- and zooplankton communities are important as food for fish in their early stages of development [37].
The dominating species among fish with respect to the number of individuals are European perch (Perca fluviatilis) and common roach (Rutilus rutilus). Considering biomass, the common bream (Abramis brama) is almost equally abundant [37]. Salmon and sea trout are very popular species for sport fishing in Strömmen. Since sea trout frequently spawn in several local streams in the Stockholm area, it constitutes a valuable fish resource in the area.
3. Results and Discussion
3.1. Current Status Classification
According to the most recent assessment, the Strömmen-Saltsjön water body does not comply with the criteria for good chemical status with respect to sediment concentrations of Cu [45]. The criterion for a good chemical status of Cu corresponds to 52 mg/kg dwt (deadweight tonnage) when normalised for organic content and background values are subtracted [33,45]. Measurements of sediment concentrations of Cu in the top 2 cm at 38 different locations in Strömmen-Saltsjön revealed that this criterium was surpassed for 35 out of 38 samples with a mean value of 269.4 mg/kg dwt (max 1076.5 mg/kg dwt; min 12.8 mg/kg dwt). It was, however, not reported if the concentrations were normalised to the organic carbon concentration and/or if background concentrations were subtracted (if yes, no values were reported) [38]. It is likely that the data are rather log-normally than normally distributed, which is often the case with sediment concentrations of elements that are associated with sedimented organic matter, and where there is an important point source [34]. It is evident from the data used to classify the chemical status of Cu in Strömmen-Saltsjön that there is a large difference (two orders of magnitude) between the minimum and the maximum values. No classification based on sediment concentrations is available for Zn.
The Swedish authorities recommend that status classification of these freshwaters should be performed using the Bio-Met tool [46]. The tool applies an assessment factor of 1 (AF1) in the water quality criteria to calculate local environmental quality standards (EQS), a factor that is based on EU acceptance of the risk assessment reports of both Cu and Ni in freshwater [47]. Sweden has stated their water quality criteria to be based on bioavailable concentrations but has applied an assessment factor of 2 (AF2) for both Cu and Zn [45]. Since the Bio-Met tool applies AF1, this means that the calculated local EQS-values from Bio-Met cannot be used directly and be compared with measured dissolved concentrations of each metal for deriving risk characterisation ratios (RCRs) of relevance for Swedish conditions. Instead, compliance assessment should be based on calculated bioavailable concentrations derived from Bio-Met by considering AFs of 2.
Classification of Swedish coastal waters follows current regulations from the water authorities [45]. The quality criteria for Cu and Zn are based on the marine PNEC (predicted no effect concentration) value for Cu (5.2 µg/L) proposed in the voluntary risk assessment report for Cu and the lowest NOEC value (no observed effect concentration, 10 µg/L) in the EU risk assessment report for Zn [1,2]. These PNEC or NOEC values are derived from vast datasets of the highest validity and quality in relation to available ecotoxicological reference databases. For both Cu [3] and Zn [32], additional marine mesocosm studies were performed which verify and support these values. For Zn, no marine PNEC value was derived in the risk assessment report [2]. However, a generic PNEC of 7.8 µg/L (AF2) was proposed for freshwater during water hardness conditions exceeding 24 mg CaCO3/L, which also could be used in some local marine scenarios. A marine Zn PNEC of 6.1 µg/L was reported using statistical extrapolations from species sensitivity distributions [48].
The Swedish environmental quality criteria for Cu and Zn for status classification of coastal waters are based on the proposed PNEC/NOEC values with additional AFs. For the Swedish west coast, the Cu criteria include AF2 which corresponds to an EQS annual average concentration of 2.6 µg bioavailable Cu/L. Motivated by the extra sensitiveness of the aquatic life in the brackish Baltic Sea water [46], the Swedish authorities apply an additional safety factor AF3 (2.6 µg/L divided by 3) for the east coast and the Baltic Sea, which corresponds to AF6 compared to the PNEC proposed in the voluntary risk assessment for Cu [1]. No considerations of ambient background concentrations should be made for Cu according to Swedish regulation HVMFS 2019:25 [45], an approach that in 2017 was challenged when setting regulatory guideline values.
Ambient background concentrations should be considered for Zn in both freshwater and marine water before a compliance check and status classification. Marine water quality criteria differ for Zn between the Swedish west coast (3.4 µg/L) and the east coast and Baltic Sea (1.1 µg/L). Compared to the proposed PNECadd [48] of 6.1 µg/L, these criteria correspond to AF1.8 and AF5.5. When compared to the marine NOEC for Zn accepted from the EU accepted risk assessment (10 µg/L) [2], the criteria correspond to AF 3 for the west coast and AF9 for the east coast/Baltic Sea.
These differences motivate the importance of highlighting the outcome of different approaches in assessing risks for an estuary using the freshwater methodology with the Bio-Met tool compared to the outcome using limit values proposed in current regulations for Swedish coastal waters.
3.2. Water Classification Based on Bioavailability of Cu and Zn Is Different from Predictions Made Using Current National Classification Employing Generic Safety Factors
Speciation calculations of Cu and Zn for the Strömmen-Saltsjön water body were made using the Bio-Met model, as well as Visual Minteq, a chemical equilibrium model for predictions of metal speciation, solubility and sorption in natural waters [49]. The Bio-Met software uses only the most important input parameters for modelling metal bioavailability, namely pH, DOC and concentration Ca2+. Due to its extensive validation, the software has gained acceptance for deriving environmental quality standard threshold values. The BLM methodology was validated for Ni, Pb, Zn and Cu for modelling chronic toxicity, and the Bio-Met tool was validated against the full BLM of each metal. For further information, see www.bio-met.net (accessed on 5 November 2020) and the cited literature within.
Total dissolved concentrations of Cu and Zn measured in the brackish water body of Strömmen-Saltsjön [39,50] were used to calculate the chemical speciation, i.e., fractions of free ions, labile complexes, solids, and/or formation of complexes with the organic matter with the intention to implement these calculated bioavailable concentrations into risk classification for the specific water body of this study.
Generated results were compared with current risk classification numbers for defined coastal waters corrected for saltwater following current Swedish regulations [45]. Local background concentrations of Zn were set to 2 µg/L, as used by the Stockholm city for status classification since the background is not already integrated into the threshold value [51]. For Cu, the background levels are integrated into the threshold value as it was derived using a total risk approach where the ambient background was considered in the risk limit value as an average value for European datasets. However, when high AFs are used it can be questioned whether the ambient background concentration is actually considered, especially when the local natural background is high. In the PNEC derivation for the marine compartment, the voluntary risk assessment report on Cu [1] stresses that it is very important to use AFs with caution since the background concentrations in test media for unexposed controls had a median value of 2.4 µg Cu/L, i.e., in the same range as the proposed marine PNEC value for Cu (5.2 µg/L). Hence, regardless of whether background concentrations are sufficiently considered or not in the Swedish general limit value, the use of AF6 for Cu in the Baltic Sea is doubtful. This is especially the case for the Strömmen-Saltsjön water body, in which ambient background concentrations are considered high. The average monthly concentration of dissolved Cu in the closest upstream sampling location (Stockholm Centralbron, EU ID SE658065-162841) was 2.3 µg/L during the period January 2017 to August 2021.
For Zn, the EQS was derived using the added risk approach. The added risk approach was developed to enable consideration of the ambient background concentration of naturally occurring substances, such as Zn, in environmental risk limits derivations [52].
Data of dissolved Cu and Zn and water chemistry (pH, DOC, alkalinity—as Ca2+) for one of the monitoring stations (Blockhusudden) in Strömmen-Saltsjön were provided by the County Administrative Board of Stockholm [50], Table 2. In addition to concentrations of dissolved metals, information on pH, DOC and Ca2+ concentrations are minimum data requirements for compliance assessment using the Bio-Met tool. The water pH of the water body is slightly alkaline and both the DOC (0.8 × TOC) and hardness levels can be considered as medium-to-high.

Table 2.
Basic water chemistry including dissolved (diss.) Cu and Zn concentrations, pH DOC and Ca2+ in the Strömmen-Saltsjön water body (data from 2018 [50]) at the Blockhusudden monitoring station (see Figure 2).
Speciation calculations were made using the Visual Minteq software to elucidate differences in chemical speciation for two salinity levels mimicking the top (0.25 PSU) and the bottom (5.23 PSU) of the water column (composition given in Table 2 [39]), as well as chemical data for the Strömmen-Saltsjön water body, Table 3 [39].

Table 3.
Salinity data for two PSU levels (0.25 and 5.23 PSU) of the water column used for Visual Minteq speciation modelling. Further general input data were T = 10 °C, pH = 7.93, DOC = 5.74 mg/L, Cutot = 1.70 µg/L, Zntot = 3.65 µg/L [39].
Consistent with the literature findings (see introduction), the modelling results show Cu, at both salinity conditions, to be completely bound in non-available organic complexes, regardless of whether the origin of DOC was either humic or fulvic, Figure 3. No free Cu ions or labile complexes are predicted stable at these conditions [53]. Similar results were obtained for Zn with the exception of small fractions of free Zn ions (2–13%) and some labile complexes (3–4% in the case for the highest water salinity conditions). The speciation modelling hence indicates a low and very low bioavailable fraction for Zn and Cu (<17% for Zn, i.e., <0.42 µg/L; <0.1% for Cu, i.e., <0.15 µg/L) at prevailing conditions in the Strömmen-Saltsjön water body (mean total dissolved values in Table 2).
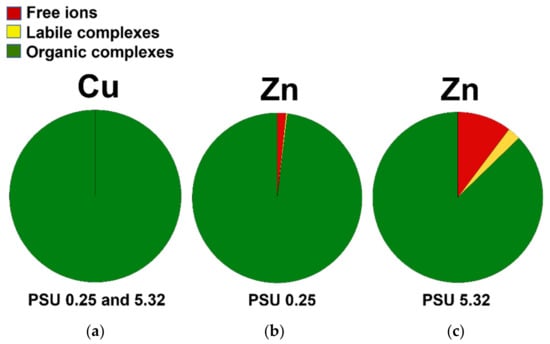
Figure 3.
Visual MINTEQ modelling of Cu (a) and Zn (b,c) speciation for brackish water of two different PSU values representative of the Strömmen-Saltsjön water body. Labile complexes denote weakly bound Cu and Zn inorganic complexes.
These observations are in line with findings for Cu investigating levels of compliance for European fresh waters using EQS based on the bioavailable fraction of Cu rather than the dissolved fraction [1]. When based on regionally relevant water chemistry data, only sites with very low DOC concentrations (in some alpine regions) showed compliance issues. In addition, the same study reported low sensitivity to Cu in water bodies of the Baltic Sea due to high DOC concentrations in surface waters [54].
Calculations of bioavailable concentrations of Cu and Zn (Table 4) to assess local EQS were performed using the Visual Minteq and the Bio-Met software from which corresponding risk characterisation ratios (RCR = PEC/PNEC) were determined. The results are presented in Table 4 together with existing data for status classification according to Swedish regulations [45].

Table 4.
Bioavailability (BA) and speciation calculations for Cu (a) and Zn (b) using Visual Minteq (VM) and Bio-Met v.4.0 (BM) based on mean data of dissolved (diss) Cu (data from 2018, Table 1) for the Strömmen-Saltsjön water body and resulting status classification and derivation of risk characterisation ratios (RCRs) according to different approaches (AF—assessment factor, EQS—environmental quality standard, SWE—Sweden, DOC—dissolved organic carbon).
The results for Cu show a risk characterisation ratio (RCR) of 0.93 when calculated based on the Swedish Agency for Marine and Water Management Regulation (2019:25) [45]. Even though this value implies good status (a ratio >1 implies a risk), it is close to the threshold value. However, when using the bioavailable fraction of Cu calculated using Visual Minteq and comparing data with the Swedish general EQS (0.87 µg bioavailable Cu/L), the RCR is much lower, 0.13, which is far below 1. A considerably lower ratio (an RCR of 0.22) was also obtained when applying the freshwater methodology in Bio-Met to the brackish water conditions of Strömmen-Saltsjön.
The Cu voluntary risk assessment report [1] stresses that it is very important to use AFs with caution. Their concern was based on the findings when deriving PNEC values for the marine compartment, which showed a median concentration of Cu in unexposed controls of 2.4 µg Cu/L, i.e., in the same range as the proposed marine PNEC value for Cu (5.2 µg/L). The use of an AF6, as in the Swedish general limit value for Cu in the Baltic Sea, is hence questionable for a water body such as Stockholm-Strömmen, where the ambient natural background concentrations of Cu are high. The average monthly concentration of dissolved Cu in the closest upstream sampling location for Strömmen (Stockholm Centralbron, EU ID SE658065-162841) was 2.3 µg/L (2017 August 2021).
For Zn, the calculated RCR is highly dependent on which ambient background is stipulated in the Swedish regulations [45] to establish a local Zn EQS used in the status classification. Applying an ambient background concentration of 2 µg/L, as previously used by the local municipality in Stockholm, the resulting RCR is 0.81 (RCR < 1, good status). As local authorities have set ambient background concentration for Zn to 2 µg/L based on local specific datasets, this is considered a relevant measure of Zn background although it may be difficult to establish a fixed ambient background concentration due to natural seasonal and spatial variations.
Calculations of bioavailable Zn using Visual Minteq and Bio-Met were performed to derive RCRs. However, as for Cu, no EQS for Zn based on bioavailable concentrations has, in the Swedish regulations, been established for coastal waters [45]. Therefore, the bioavailable concentrations derived using Visual Minteq and Bio-Met were, in both cases, compared to freshwater EQS from the Swedish regulations. This approach for comparative compliance checks resulted in considerably lower RCRs for Zn (0.08 and 0.10, respectively), Table 5.

Table 5.
Calculated RCRs for Cu and Zn in fresh water and coastal water formations in Stockholm city monitoring program 2015–2018. n = number of samples.
The results clearly show that RCRs calculated based on bioavailability data of Cu and Zn calculated for the specific water chemistry of Strömmen-Saltsjön determined using the Visual Minteq and the Bio-Met software (the latter an EU validated methodology) were 4–7 times lower for Cu, and 8–10 times lower for Zn, respectively, than using the methodology including AF6 stipulated in Swedish regulations for coastal waters [45], Table 4.
Large differences in RCR values between fresh and coastal waters for Cu and Zn are also indicated by the latest data reported for Stockholm city and regional water bodies that were status classified [55,56]. Calculated RCRs based on the average of monitoring data between 2015 and 2018 for all fresh and coastal waters in the Stockholm area monitoring program are presented in Table 5. The fact that differences in RCR values between fresh and coastal water bodies for both Cu and Zn are in the same range (7–8 times higher RCR for Cu for coastal water compared to fresh water (2015–2018), and 10–16 times higher RCR for Zn) reflects the influence of the higher AFs used for coastal water formations according to the Swedish regulations compared to freshwater conditions of lower AF.
In all, the results clearly show that status classification in coastal waters, here exemplified by the estuary characterised by Strömmen-Saltsjön, profoundly differs in calculated RCRs if bioavailability data using, e.g., Bio-Met predictions of Cu and Zn in fresh water are used compared with current Swedish regulations using high AFs.
For coastal water formations, AFs used in the Swedish water quality criteria shift from AF2, as used in freshwater formations, to AF6, in the defined coastal water formation. The results of this study clearly show that the risks of Cu and Zn at estuary conditions in the Strömmen-Saltsjön waterbody are overestimated due to overly conservative assessment factors. This could lead to inaccurate risk assessments and unnecessary preventative measures.
3.3. Sediment Concentrations of Cu and Zn Cannot Be Used as a Basis for Chemical and Ecological Status in Water Formations
It was estimated that the background concentrations of Cu and Zn in sediments in Strömmen-Saltsjön are 50% to 79% and 48% to 84% of their total concentrations, respectively [40]. The method employed by Lindström et al. [40] was to use Ni metal, a metal without any specific point source in the Stockholm area and with similar chemical properties as Cu and Zn in the aquatic environment, as a proxy to derive background concentrations of Cu and Zn. Even though it can be argued about the similarity between these three metals with respect to physical, chemical, and biological processes and properties, which affect their environmental fates in the aquatic environment, it is interesting to note that the study derived similar estimates of the anthropogenic sources of Cu and Zn as in the study by Jönsson [41], in which a totally different methodology was employed. Even if a 50% background concentration of Cu and Zn of their total sediment concentrations is assumed, it is unclear if this would change the current classification of Strömmen-Saltsjön. The reason is partly due to a lack of data and partly related to the fact that a correction for background concentrations involving a reduction by the observed concentration by, e.g., 50% will not change the skewed and probably log-normal sediment concentration distribution of metals (here Cu and Zn). There will still be a two-order of magnitude difference between the maximum and minimum metal concentration in the sediment. Another possible approach would be to assign a fixed background concentration based on available information. However, this will be an arbitrary choice and may result in negative concentrations in certain sediments for which the derived background concentration is higher than the observed concentration. However, even if consistently employing the lowest observed sediment concentration as a background concentration for a given water formation will resolve the issue of negative concentrations, it cannot be considered properly scientifically justified.
The seabed bottom is made up of coarser material, such as sand and stones and exposed bedrock. In principle, metals accumulate locally where fine particles (clay and silt) and organic matter are deposited on the bottom, so-called accumulation areas [57,58]. These areas are surrounded by bottom locations. In glaciated areas, erosional areas also include glacial clay and till. Actual locations of these accumulation areas vary widely and depend on topography and water depth. Since all types of bottom material exist in the Strömmen-Saltsjön water body, [59] and the locations of the accumulation areas are unknown, the current very limited information of sediment concentrations of Cu and Zn currently available cannot be used to determine background concentrations.
The bioavailability of Cu and Zn via respiration through the gills of sediment-dwelling organisms are, as for surface water, determined by the combination of physical and chemical properties of the sediment pore water with respect to redox potential (dissolved oxygen concentration), pH, alkalinity and salinity and the amount of inorganic and organic ligands. In addition, the metals can form solid phases. Sediment-dwelling organisms are also exposed to Cu and Zn through dietary uptake through indiscriminate feeding of sediment and suspended particles and targeted feeding on, e.g., detritus and algae, see Figure 1. According to the determined SEM/AVS (simultaneously extracted metals/acid-volatile sulphides) ratios, sediment accumulation areas in the Strömmen-Saltsjön water formation show constantly very low bioavailability of Cu and Zn with ratios of less than 0.1, which is at least 10 times lower than the threshold ratio of one for which a toxic effect due to bioavailability may be expected [60,61]. The main reasons for the low SEM/AVS ratios are the large amounts of AVS (i.e., sulphides), organic matter and inorganic ligands present in these anoxic and sub-oxic sediments [62]. The importance of the different binding forms, sulphides, organic matter, Fe–Mn-(hydr)oxides, vary depending on the metal [63]. Metal phases in the sediment can also transform upon oxidation of AVS due to bioturbation, e.g., the formation of carbonates [59]. Due to the high concentrations of TOC and inorganic ligands in the sediment of the Strömmen-Saltsjön water body, these sediments show low toxicity in terms of mortality and embryonic malformations of amphipods towards Cu and Zn, even after oxygenation and bioturbation for several months [62].
The concentration of AVS typically decreases close to the sediment surface with increasing redox potential, but can in the top 10 cm vary further down in the sediment [62,64,65,66]. As an operational definition, AVS can be divided into different fractions (e.g., phases) of different kinetics and stability to oxygen [53]. Considering the high concentrations of TOC in the sediments with the highest Cu and Zn concentrations in the Strömmen-Saltsjön, all top 10 cm of sediments should be considered to have low toxicity towards these metals [41,63,67]. For deposit feeders, such as the bivalve (Mytilus edulis), synthetic humic acid was shown to reduce the uptake of Cu over the gills but increase its uptake via the gut [68]. However, regardless of exposure route, respiratory or dietary, the exposure of Cu and Zn to the benthic fauna is dependent on abiotic factors of the sediment matrix itself, such as organic matter content and particle size distribution, which determines the sorption coefficient (Kd) for the metals [69,70].
The variation in concentrations of Cu and Zn in sediments and the toxicity governing properties (TOC and particle size) in a water formation is far larger than for water concentrations. For a given water body, the biomass and species composition of the benthic community will vary both spatially and temporally as a function of salinity, oxygenation and sediment type including organic carbon content [57,58,71,72,73]. This means that every species of benthic fauna will occupy its ecological niche and the benthic fauna will respond to changes in these variables. The variables determining the ecological niches for a given body of water are, to a large degree, dependent on the water depth [74]. Therefore, to reflect spatial variations in sediment properties when assessing the risk of Cu and Zn toxicity to benthic organisms based on sediment concentrations in a given water body, a large number of samples should be collected [55,69]. In a study by Jönsson (2011) [41], there was a clear reduction for both filtrated and non-filtrated surface and bottom water concentrations of Cu with increasing distance from the Lake Mälaren input. For the three corresponding surface sediment samples, the maximum concentration was observed at the second location. This can be explained by sedimentological processes which determine the spatial distribution of organic matter and metals associated with it [57,58].
In all, it is evident that the assessment of exposure and bioavailability of Cu and Zn to the benthic fauna requires a large number of sediment samples and chemical and sedimentological analyses for every water formation. Sediment samples are, as a consequence, unsuitable as a means of determining chemical or ecological status for Cu and Zn in water formations.
3.4. Background Concentrations, Bioavailability and Organism Sensitivity in Waters of Dynamic Salinity Need to Be Considered in Risk Assessment
Surface waters within the EU show large variations in both concentrations of metals related to geology as well as metal bioavailability. Member-state specific environmental quality standard (EQS) values for Cu range according to JRC (Joint Research Centre) between 1 and 120 µg Cu/L [1]. Plausible explanations to variations in PNEC values in different countries are connected to different water chemistry and hence different bioavailability, as well as different ambient background levels. The Bio-Met tool was recently used to assess differences in bioavailability of Cu in a few European countries (Austria, Belgium, Denmark, France, Germany (River Rhine), Sweden, the Netherlands and the United Kingdom) [30]. The study concluded that freshwaters in Sweden were not among the most sensitive waters in Europe due to high levels of DOC and hence low bioavailability of Cu. Calculated hazardous concentrations (HC550) of Cu for Sweden ranged between 5.2 and 47.4 µg dissolved Cu/L (5th to 95th percentile). A total of 97% of the Swedish waters (134 in total) showed calculated RCR values of less than 0.20. As the RCRs were considerably lower than 1, it was concluded that concentration levels of Cu in Sweden do not pose any risk to the aquatic environment. Calculated RCR values based on bioavailability calculations using Visual Minteq and Bio-Met for the given water chemistry of the coastal water body of Strömmen-Saltsjön showed similar results. The results elucidate that the brackish coastal conditions of the Baltic Sea can be assessed by assuming freshwater conditions.
Ambient background concentrations of Cu and Zn in a large number of Swedish surface waters were estimated from larger datasets compiled from the national monitoring activities [75]. Dissolved concentrations of Cu and Zn in Swedish lakes and rivers are compiled in Table 6. Based on these datasets, bioavailable concentrations were calculated for Cu and Zn in the Swedish lakes and rivers based on the Swedish water quality criteria applying an AF of 2 [76]. These calculations resulted in similar findings as in the European study [54], with RCRs for both Cu and Zn less than 1 for 98% of the Swedish lakes and rivers, which means that in general, neither Cu nor Zn poses any high risk in Swedish surface waters.

Table 6.
Dissolved Cu and Zn concentrations in Swedish lakes and rivers. The 5th, 50th (median), 95th and 98th percentiles [75].
As indicated, large variations in ambient levels of metals occur in different regions in Sweden. The catchment area of Lake Mälaren is a well-known water body with elevated ambient background levels of several metals including Cu and Zn. The largest individual water formation, Lake Ekoln-Vreta, in the northernmost Gulf of Lake Mälaren is characterised by higher median concentrations of Cu (measured from 2017 to 2020) compared with the average national values in lakes and rivers, Table 6 [55,75]. The comparatively high ambient background concentrations of Cu in Lake Mälaren are also supported by higher background concentrations of Cu in soils in the Lake Mälaren catchment area [55]. Naturally caused releases of metals including Cu and Zn can further be attributed to a naturally high abundance of soils and bedrock rich in sulphide minerals (including Cu and Zn) [77,78]. These minerals can potentially be oxidised and thereby lower the acidic conditions, which subsequently can result in increased metal leaching from soil och rock materials to surface waters. Cu and Zn are also naturally eroded from these minerals [79].
The difficulties in establishing relevant ambient background concentrations of Cu can partly explain why sediment concentrations for many water formations in different parts of Lake Mälaren were classified as moderate due to Cu levels exceeding stipulated sediment criteria (36 mg Cu/kg dwt) [43]. Unless reliable and representative local data for ambient background concentrations are used when performing status classification, classification based on sediment concentrations will result in skewed classification patterns for Cu.
Other aspects that need to be considered are differences in organism-specific physiology. One example is the effect of the osmoregulatory mechanism exemplified by the Zn sensitivity of two species of killifish [80]. The two species show a linear correlation in water with a practical salinity up to 10 PSU, whereas differences in the Ca2+ osmoregulatory mechanisms resulted in a higher sensitivity to Zn for one of the species in the water of higher PSU levels. For these species, such effects mean that the use of BLM/Bio-Met is valid in waters of low to intermediate salinity for Zn as long as Ca2+-concentrations are within the validated boundaries.
Acute toxicity data for Cu towards killifish reported across the full salinity range show that freshwater and high salinity water result in higher toxicity than the water of intermediate salinity [11]. However, differences in chemical speciation could only partly explain the toxicity, whereas physiology related to osmoregulation explained almost all remaining variations in toxicity. This indicates that osmoregulation is a critical mechanism behind Cu sensitivity in marine and estuarine environments, and is attributed to Na+ gradients [6,11]. Available freshwater BLMs consider the effects of water chemistry on metal speciation and bioavailability when used in risk assessment, however, they do not fully cover the influence of water chemistry on the physiology of the organisms [12].
The applicability of BLMs for marine waters into the EU regulatory framework has been discussed, though no models are yet available. The common view is that the use of BLM in estuarine and marine environments requires careful evaluation both regarding salinity and species-dependent sensitivity (such as differences in osmoregulatory responses between species). Variations in dissolved organic matter (DOM) characteristics from riverine, marine and estuarian sources should also be considered [12], as well as effects of energy level and food status on organism sensitivity at different salinities [15]. The latter study suggests that food is a protective factor against acute waterborne Cu toxicity indicating that the nourishing status of organisms possibly should be included in a marine BLM to reflect the ability to cope with osmotic stress and metal exposure at changing salinity.
A future BLM for marine water must therefore consider these additional factors discussed above as compared to freshwater BLMs, in order to accurately assess the toxicity of Cu under dynamic salinity conditions. The complexity in modelling estuaries with intermediate salinity likely increases compared to both freshwater and marine water due to diverse variations in water chemistry and physiological strategies [11].
In all, BLMs/Bio-Met bioavailability predictions could, in low salt waters, potentially have high validity, however, such studies need to be further explored for estuaries and brackish water conditions. In risk assessment, it is important to identify deleterious effects that any pollutant might exert on aquatic organisms both on their own, as well as in combination with others. However, this is challenging when only using computational model tools as every exposure situation is unique in terms of chemical composition, physico-chemical conditions and presence of essential nutrients and organisms. This emphasises the importance of generating site-specific water quality criteria when defining safe levels of exposure. If uncertainties remain when only using chemical methods, the additional use of bioanalytical tools on representative test organisms for an estuary by targeting metal-specific toxicity in authentic water at local sites can provide valuable guidance in risk assessment. For example, the use of metal-sensitive specific biomarkers to address whether metal stress is present or not has successfully been employed elsewhere [81,82,83].
BLMs/Bio-Met are very cost-effective and beneficial to use in early tiers in risk assessment for regulatory purposes and compliance checks in order to identify situations that potentially require further investigations. As shown in Table 6, levels of Cu and Zn are, from a national perspective, elevated in both lake Mälaren and in Strömmen-Saltsjön. However, according to the low salinity circumstances and indications from Bio-Met modelling suggesting low bioavailability conditions, the measured Cu- and Zn levels in Strömmen-Saltsjön will likely not cause any toxic effects under such conditions. Thus, the RCRs presented in Table 4 and Table 5, just above or just below one are a result of the overly conservative AFs set in the current Swedish regulations rather than indicating any potential threat from Cu and Zn at these locations. Due to species diversity in the brackish environment of the Baltic Sea, it is unlikely that for example, a lower abundance of a certain species would be a result of metal stress but rather a result of poor species adaption to the low salinity conditions and ability to cope with osmotic pressure.
3.5. The Prevailing Benthic Fauna Should Be Considered in Risk Assessment
The current Swedish methodology for Cu and sediment quality acknowledges that geochemical and physical factors, such as water depth and particle size, directly or indirectly influence the bioavailability of Cu [33]. It does not, however, provide any other means of evaluating the uncertainty than the employment of safety factors. A much more scientifically correct, cost-effective and robust method is to remove all of these confounding factors and directly study the organisms concerned, namely the benthic fauna.
The benthic fauna in several water formations in Stockholm is assessed every second year following standardised methods [37]. Several of these water formations, including Strömmen-Saltsjön, suffers from low dissolved oxygen concentrations in the sediments. Despite the low oxygen levels, there is an abundance of species extremely tolerant to low oxygen conditions.
In all, if Cu and Zn toxicity were the main factor behind low biodiversity this would not be expected. Assessment of the benthic fauna should therefore be considered if the assessment based on bioavailability by, e.g., using Bio-Met is uncertain.
4. Conclusions
The EU Water Framework Directive stipulates that all EU waterways shall have good chemical and ecological status by 2027. Cu and Zn were targeted metals for this study because in Sweden they are chosen as national river basin-specific pollutants (RBSP) as defined in the Water Framework Directive, and there are no harmonised Environmental Quality Standards for these metals in the EU. This paper discusses how Cu and Zn water quality criteria guideline values are adopted and defined in Swedish coastal and estuarine waters, and implemented in a case water body, Strömmen-Saltsjön, and how this approach can be improved to reach the ecological status of water bodies in Sweden. The following main conclusions were drawn:
- It is possible to improve ecological status classification by making more locally suitable assessments of the ecotoxicity of Cu and Zn in low salinity brackish waters in Swedish estuaries.
- Suitable assessment grounds for fresh waters versus marine waters shall be chosen according to water characteristics rather than geographical location.
- Where metal variations are great, especially in sediments, monitoring the organism vitality and reproduction makes more sense.
- Background concentrations and acclimatisation are important aspects to consider for metals such as Cu and Zn, which are naturally occurring and essential nutrients for organisms.
- Overly conservative assessment factors and lack of assessment of the ability of biological uptake of metals will make risk assessments less reliable. This will result in higher administrative costs and difficulties for society to comply, while no improvement in the real status of the environment will be achieved.
Author Contributions
Conceptualisation, D.R., G.H., A.J. and I.O.; methodology, D.R., G.H., A.J. and I.O.; software, D.R. and G.H.; validation, D.R., G.H., A.J. and I.O.; formal analysis, D.R., G.H., A.J. and I.O.; investigation, D.R. and A.J.; resources, D.R., G.H., A.J. and I.O.; data curation, D.R., G.H., A.J. and I.O.; writing—original draft preparation, D.R., G.H., A.J. and I.O.; writing—review and editing, D.R., G.H., A.J. and I.O.; visualisation, D.R., G.H., A.J. and I.O.; supervision, I.O.; project administration, I.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge Amanda Kessler (KTH) and My Olausson (My Olausson AB) for the graphical illustration of Figure 1 and Figure 2, respectively. Valuable comments from Pia Voutilainen (Scandinavian Copper Development Association, SCDA), and Annikki Hirn (Nordic Galvanizers Association) are highly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Copper Institute Voluntary Risk Assessment Report (VRAR) for Copper and Copper Compound Submitted to ECHA Based on Industry Initiative to Follow the Risk Assessment Procedures of Existing Substance Regulation (EEC) No 793/93. Available online: https://echa.europa.eu/copper-voluntary-risk-assessment-reports (accessed on 10 December 2021).
- Available online: http://echa.europa.eu/documents/10162/d7248de0-eb5b-4a9b-83b9-042c4fd66998 (accessed on 1 January 2021).
- Foekema, E.M.; Kaag, N.H.B.M.; Kramer, K.J.M.; Long, K. Mesocosm validation of the marine No Effect Concentration of dissolved copper derived from a species sensitivity distribution. Sci. Total Environ. 2015, 521–522, 173–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arnold, W.R.; Cotsifas, J.S.; Smith, D.S.; Le Page, S.; Gruenthal, K.M. A comparison of the copper sensitivity of two economically important saltwater mussel species and a review of previously reported copper toxicity data for mussels: Important implications for determining future ambient copper saltwater criteria in the USA. Environ. Toxicol. 2009, 24, 618–628. [Google Scholar] [CrossRef]
- Hall, L.W.; Anderson, R.D.; Lewis, B.L.; Arnold, W.R. The influence of salinity and dissolved organic carbon on the toxicity of copper to the estuarine copepod, Eurytemora affinis. Arch. Environ. Contam. Toxicol. 2008, 54, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M.; Nielsen, C.; Bianchini, A. Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2002, 133, 287–303. [Google Scholar] [CrossRef]
- Khan, F.R.; Bury, N.R.; Hogstrand, C. Copper and zinc detoxification in Gammarus pulex (L.). J. Exp. Biol. 2012, 215, 822–832. [Google Scholar] [CrossRef] [PubMed]
- De Souza Machado, A.A.; Spencer, K.; Kloas, W.; Toffolon, M.; Zarfl, C. Metal fate and effects in estuaries: A review and conceptual model for better understanding of toxicity. Sci. Total Environ. 2016, 541, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.M.; Farrell, A.P.; Brauner, C.J. Homeostasis and Toxicology of Essential Metals; Elsevier: London, UK, 2012. [Google Scholar]
- Sunda, W.G.; Huntsman, S.A. Processes regulating cellular metal accumulation and physiological effects: Phytoplankton as model systems. Sci. Total Environ. 1998, 219, 165–181. [Google Scholar] [CrossRef]
- Grosell, M.; Blanchard, J.; Brix, K.V.; Gerdes, R. Physiology is pivotal for interactions between salinity and acute copper toxicity to fish and invertebrates. Aquat. Toxicol. 2007, 84, 162–172. [Google Scholar] [CrossRef]
- De Polo, A.; Scrimshaw, M.D. Challenges for the development of a biotic ligand model predicting copper toxicity in estuaries and seas. Environ. Toxicol. Chem. 2012, 31, 230–238. [Google Scholar] [CrossRef]
- Wright, D.A. Trace metal and major ion interactions in aquatic animals. Mar. Pollut. Bull. 1995, 31, 8–18. [Google Scholar] [CrossRef]
- Pinho, G.L.L.; Pedroso, M.S.; Rodrigues, S.C.; Souza, S.S.D.; Bianchini, A. Physiological effects of copper in the euryhaline copepod Acartia tonsa: Waterborne versus waterborne plus dietborne exposure. Aquat. Toxicol. 2007, 84, 62–70. [Google Scholar] [CrossRef]
- Pinho, G.L.L.; Bianchini, A. Acute copper toxicity in the euryhaline copepod Acartia tonsa: Implications for the development of an estuarine and marine biotic ligand model. Environ. Toxicol. Chem. 2010, 29, 1834–1840. [Google Scholar] [CrossRef]
- Pearson, H.B.C.; Comber, S.D.W.; Braungardt, C.B.; Worsfold, P.; Stockdale, A.; Lofts, S. Determination and Prediction of Zinc Speciation in Estuaries. Environ. Sci. Technol. 2018, 52, 14245–14255. [Google Scholar] [CrossRef]
- Monserrat, J.M.; Martínez, P.E.; Geracitano, L.A.; Lund Amado, L.; Martinez Gaspar Martins, C.; Lopes Leães Pinho, G.; Soares Chaves, I.; Ferreira-Cravo, M.; Ventura-Lima, J.; Bianchini, A. Pollution biomarkers in estuarine animals: Critical review and new perspectives. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 221–234. [Google Scholar] [CrossRef]
- Klerks, P.L.; Weis, J.S. Genetic adaptation to heavy metals in aquatic organisms: A review. Environ. Pollut. 1987, 45, 173–205. [Google Scholar] [CrossRef]
- Muyssen, B. Tolerance and acclimation to zinc of field-collected Daphnia magna populations. Aquat. Toxicol. 2002, 56, 69–79. [Google Scholar] [CrossRef]
- Johnson, H.L.; Stauber, J.L.; Adams, M.S.; Jolley, D.F. Copper and zinc tolerance of two tropical microalgae after copper acclimation. Environ. Toxicol. 2007, 22, 234–244. [Google Scholar] [CrossRef]
- Paquin, P.R.; Gorsuch, J.W.; Apte, S.; Batley, G.E.; Bowles, K.C.; Campbell, P.G.C.; Delos, C.G.; Di Toro, D.M.; Dwyer, R.L.; Galvez, F.; et al. The biotic ligand model: A historical overview. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 3–35. [Google Scholar] [CrossRef]
- Niyogi, S.; Wood, C.M. Biotic Ligand Model, a flexible tool for developing site-specific water quality guidelines for metals. Environ. Sci. Technol. 2004, 38, 6177–6192. [Google Scholar] [CrossRef]
- Nys, C.; Van Regenmortel, T.; Janssen, C.R.; Oorts, K.; Smolders, E.; De Schamphelaere, K.A.C. A framework for ecological risk assessment of metal mixtures in aquatic systems. Environ. Toxicol. Chem. 2018, 37, 623–642. [Google Scholar] [CrossRef]
- European Union. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council. Off. J. Eur. Union 2018, L 348, 84–97. [Google Scholar]
- Arnold, W.R.; Santore, R.C.; Cotsifas, J.S. Predicting copper toxicity in estuarine and marine waters using the Biotic Ligand Model. Mar. Pollut. Bull. 2005, 50, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Guidance document n.o 5. In Transitional and Coastal Waters, Typology, Reference Conditions and Classification Systems; Commission, European Communities: Luxembourg, Luxembourg, 2003. [Google Scholar]
- Pearson, H.B.C.; Comber, S.D.W.; Braungardt, C.; Worsfold, P.J. Predicting copper speciation in estuarine waters—Is dissolved organic carbon a good proxy for the presence of organic ligands? Environ. Sci. Technol. 2017, 51, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. The EU Water Framework Directive—Integrated River Basin Management for Europe. Available online: https://ec.europa.eu/environment/water/water-framework/index_en.html (accessed on 17 January 2021).
- Pradhan, A.; Ivarsson, P.; Ragnvaldsson, D.; Berg, H.; Jass, J.; Olsson, P.-E. Transcriptional responses of zebrafish to complex metal mixtures in laboratory studies overestimates the responses observed with environmental water. Sci. Total Environ. 2017, 584–585, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Nys, C.; Merrington, G.; Verdonck, F.; Baken, S.; Cooper, C.A.; Van Assche, F.; Schlekat, C.; Garman, E. Demonstrating the reliability of bio-met for determining compliance with environmental quality standards for etals in Europe. Environ. Toxicol. Chem. 2020, 39, 2361–2377. [Google Scholar] [CrossRef] [PubMed]
- Palm Cousins, A.; Jönsson, A.; Iverfeldt, Å. Testing the Biotic Ligand Model for Swedish Surface Water Conditions—A Pilot Study to Investigate the Applicability of BLM in Sweden; IVL Report B1858; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2009. [Google Scholar]
- Foekema, E.M.; Kramer, K.J.M.; Kaag, N.H.B.M.; Sneekes, A.C.; Bierman, S.; Hoornsman, G.; Koelemij, E. Determination of the Biological Effects and Fate of Dissolved Zinc in Outdoor Marine Mesocosms; C108/12; Institute for Marine Resources & Ecosystem Studies: Yerseke, The Netherlands, 2012. [Google Scholar]
- Available online: https://www.aces.su.se/aces/wp-content/uploads/2018/10/Copper-EQS-data-overview-2018.pdf (accessed on 1 January 2021).
- Jönsson, A.; Gustafsson, Ö.; Axelman, J.; Sundberg, H. Global Accounting of PCBs in the Continental Shelf Sediments. Environ. Sci. Technol. 2003, 37, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://havochvatten.diva-portal.org/smash/record.jsf?pid=diva2%3A1368668&dswid=2186 (accessed on 1 January 2021).
- Jönsson, A.; Lindström, M.; Carman, R.; Mörth, C.-M.; Meili, M.; Gustafsson, Ö. Evaluation of the Stockholm archipelago sediments, northwestern Baltic Sea proper, as a trap for freshwater runoff organic carbon. J. Mar. Syst. 2005, 56, 167–178. [Google Scholar] [CrossRef]
- Available online: https://www.stockholmvattenochavfall.se/globalassets/pdf1/rapporter/svoa/svoa-mr-2019_avlopp_20mb658.pdf (accessed on 1 January 2021).
- Havs-och Vattenmyndigheten; Vattenmyndigheterna. Länsstyrelserna VISS Strömmen—WA79755821/SE591920-180800. Available online: https://viss.lansstyrelsen.se/Waters.aspx?waterMSCD=WA79755821 (accessed on 13 January 2022). (In Swedish).
- Lücke, J. Undersökningar i Stockholms skärgård 2018. In Vattenkemi, Plankton och Bottenfauna; Stockholm Vatten och Avfall: Stockholm, Sweden, 2019. (In Swedish) [Google Scholar]
- Lindström, M.; Jonsson, A.; Brolin, A.A.; Håkanson, L. Heavy metal sediment load from the city of Stockholm. Water Air Soil Pollut. Focus 2001, 1, 103–118. [Google Scholar] [CrossRef]
- Jönsson, A. Ni, Cu, Zn, Cd and Pb in Sediments in the Citycentre of Stockholm, Sweden Origins, Deposition Rates and Bioavailability; IVl Report B2013; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2011. [Google Scholar]
- Benjamin, M.M.; Honeyman, B.D. Global biogeochemical cycle. In Global Biogeochemical Cycle; Butcher, S.S., Charlson, R.J., Orians, G.H., Wolfe, G.V., Eds.; Academic Press: London, UK, 1998. [Google Scholar]
- Turner, A. Trace-metal partitioning in estuaries: Importance of salinity and particle concentration. Mar. Chem. 1996, 54, 27–39. [Google Scholar] [CrossRef]
- European Commision. Revision of EC Sludge Directive Challenges Landspreading; ENDS Report 299; European Commision: Brussels, Belgium, 1999. [Google Scholar]
- Havs- och Vattenmyndigheten. Havs- och Vattenmyndighetens Föreskrifter om Klassificering och Miljökvalitetsnormer Avseende Ytvatten; HVMFS; Swedish Agency for Marine and Water Management: Gothenburg, Sweden, 2019. (In Swedish) [Google Scholar]
- Havs- och Vattenmyndigheten. Miljögifter i Vatten—Klassificering av Ytvattenstatus; Vägledning för Tillämpning av HVMFS; Swedish Agency for Marine and Water Management: Gothenburg, Sweden, 2013. (In Swedish) [Google Scholar]
- Available online: https://ec.europa.eu/health/ph_risk/committees/04_scher/docs/scher_o_115.pdf (accessed on 1 January 2021).
- Bodar, C.W.M. Environmental Risk Limits for Zinc; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2007; Available online: https//www.rivm.nl/bibliotheek/rapporten/601782004.pdf (accessed on 1 January 2021).
- Visual Minteq, v.3.1; KTH Royal Institute of Technology: Stockholm, Sweden, 2013.
- County Administrative Board of Stockholm, Stockholm, Sweden, Monitoring Data for 2018 for Water Formation Strömmen, Blockhusudden, Part of Saltsjön. Available online: https://miljobarometern.stockholm.se/vatten/kustvatten/strommen/ (accessed on 23 January 2021).
- Miljödata-MVM Datavärdskap Sjöar Och Vattendrag, Samt Datavärdskap Jordbruksmark. Available online: https://miljodata.slu.se/MVM/Search (accessed on 5 November 2021). (In Swedish).
- Crommentuijn, T.; Polder, M.; Sijm, D.; De Bruijn, J.; Van De Plassche, E. Evaluation of the Dutch environmental risk limits for metals by application of the added risk approach. Environ. Toxicol. Chem. 2000, 19, 1692–1701. [Google Scholar] [CrossRef]
- Di Toro, D.M.; Mahony, J.D.; Hansen, D.J.; Berry, W.J. A model of the oxidation of iron and cadmium sulfide in sediments. Environ. Toxicol. Chem. 1996, 15, 2168–2186. [Google Scholar] [CrossRef]
- Peters, A.; Wilson, I.; Merrington, G.; Heijerick, D.; Baken, S. Assessing Compliance of European Fresh Waters for Copper: Accounting for Bioavailability. Bull. Environ. Contam. Toxicol. 2019, 102, 153–159. [Google Scholar] [CrossRef]
- Miljödata-MVM Datavärdskap Sjöar Och Vattendrag, Samt Datavärdskap Jordbruksmark. Available online: https://miljodata.slu.se/MVM/Search (accessed on 5 November 2020). (In Swedish).
- Stockholms Stad. Miljögifter i Ytvatten. Available online: https://miljobarometern.stockholm.se/vatten/kemisk-status-och-miljogifter/miljogifter-i-ytvatten (accessed on 5 November 2021). (In Swedish).
- Kumke, T.; Schoonderwaldt, A.; Kienel, U. Spatial variability of sedimentological properties in a large Siberian lake. Aquat. Sci. 2005, 67, 86–96. [Google Scholar] [CrossRef]
- Håkanson, L.; Jansson, M. Principles of Lake Sedimentology; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Marin Miljöanalys. Rapport Ytgeologi Slussen; Stockholm City Council: Stockholm, Sweden, 2009. (In Swedish) [Google Scholar]
- Ankley, G.T. Evaluation of metal/acid-volatile sulfide relationships in the prediction of metal bioaccumulation by benthic macroinvertebrates. Environ. Toxicol. Chem. 1996, 15, 2138–2146. [Google Scholar] [CrossRef]
- Pesch, C.E.; Hansen, D.J.; Boothman, W.S.; Berry, W.J.; Mahony, J.D. The role of acid-volatile sulfide and interstitial water metal concentrations in determining bioavailability of cadmium and nickel from contaminated sediments to the marine polychaete Neanthes arenaceodentata. Environ. Toxicol. Chem. 1995, 14, 129–141. [Google Scholar] [CrossRef]
- Sundelin, B.; Eriksson, A.-K. Mobility and bioavailability of trace metals in sulfidic coastal sediments. Environ. Toxicol. Chem. 2001, 20, 748–756. [Google Scholar] [CrossRef]
- Kelderman, P.; Osman, A.A. Effect of redox potential on heavy metal binding forms in polluted canal sediments in Delft (The Netherlands). Water Res. 2007, 41, 4251–4261. [Google Scholar] [CrossRef]
- Van Den Berg, G.A.; Meijers, G.G.A.; Van Der Heijdt, L.M.; Zwolsman, J.J.G. Dredging-related mobilisation of trace metals: A case study in The Netherlands. Water Res. 2001, 35, 1979–1986. [Google Scholar] [CrossRef]
- Van Den Berg, G.A.; Loch, J.P.G.; Winkels, H.J. Effect of fluctuating hydrological conditions on the mobility of heavy metals in soils of a freshwater estuary in The Netherlands. Water Air Soil Pollut. 1998, 102, 377–388. [Google Scholar] [CrossRef]
- Van Den Berg, G.A.; Loch, J.P.G.; Zwolsman, J.J.G.; Van Der Heijdt, L.M. Non-steady state behaviour of heavy metals in contaminated freshwater sediments. Water Sci. Technol. 1998, 37, 39–46. [Google Scholar] [CrossRef]
- Mahony, J.D.; Di Toro, D.M.; Gonzalez, A.M.; Curto, M.; Dilg, M.; De Rosa, L.D.; Sparrow, L.A. Partitioning of metals to sediment organic carbon. Environ. Toxicol. Chem. 1996, 15, 2187–2197. [Google Scholar] [CrossRef]
- Sánchez-Marín, P.; Aierbe, E.; Lorenzo, J.I.; Mubiana, V.K.; Beiras, R.; Blust, R. Dynamic modeling of copper bioaccumulation by Mytilus edulis in the presence of humic acid aggregates. Aquat. Toxicol. 2016, 178, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.L.; King, C.K. Exposure-Pathway Models Explain Causality in Whole-Sediment Toxicity Tests. Environ. Sci. Technol. 2005, 39, 837–843. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Simpson, S.; Smith, S.; Stauber, J.; Batley, G. Short-term accumulation of Cd and Cu from water, sediment and algae by the amphipod Melita plumulosa and the bivalve Tellina deltoidalis. Mar. Ecol. Prog. Ser. 2005, 287, 177–188. [Google Scholar] [CrossRef]
- Persson, J.; Jonsson, P. Historical Development of Laminated Sediments—An Approach to Detect Soft Sediment Ecosystem Changes in the Baltic Sea. Mar. Pollut. Bull. 2000, 40, 122–134. [Google Scholar] [CrossRef]
- Eckhéll, J.; Jonsson, P.; Meili, M.; Carman, R. Storm influence on the accumulation and lamination of sediments in deep areas of the Northwestern Baltic proper. AMBIO A J. Hum. Environ. 2000, 29, 238–245. [Google Scholar] [CrossRef]
- Jönsson, A.; Carman, R. Distribution of PCBs in sediment from different bottom types and water depths in Stockholm archipelago, Baltic Sea. AMBIO A J. Hum. Environ. 2000, 29, 277–281. [Google Scholar] [CrossRef]
- Leonardsson, K.; Blomqvist, M.; Rosenberg, R. Reducing spatial variation in environmental assessment of marine benthic fauna. Mar. Pollut. Bull. 2016, 104, 129–138. [Google Scholar] [CrossRef]
- Available online: https://pub.epsilon.slu.se/12590/7/herbert_r_etal_gamla_pb_150908.pdf (accessed on 1 December 2021).
- Ragnvaldsson, D. Bakgrundshalter av Metaller i Svenska Sjöar Och Vattendrag—Sammanställning av Halter för Ett Urval av Metaller Jämfört Med Bedömningsgrunder i HaVs Föreskrift; Report 2015:4; Envix: Umeå, Sweden, 2017. (In Swedish) [Google Scholar]
- Wahlgren, C.H.; Schoning, K.; Tenne, M.; Hansen, L.M. Stockholmsområdets Berggrund, Jordarter, Geologiska Utveckling och Erfarenheter Från Infrastrukturprojekt; Report 2018:8; SGU Geological Survey of Sweden: Uppsala, Sweden, 2018. [Google Scholar]
- Schoning, K.; Lundqvist, L. Hållbar Ballastförsörjning–förutsättningar i Stockholms och Uppsala Län; Report 2018:09; SGU Geological Survey of Sweden: Uppsala, Sweden, 2018. (In Swedish) [Google Scholar]
- Landner, L.; Reuther, R. Metals in Society and in the Environment. In A Critical Review of Current Knowledge on Fluxes, Speciation, Bioavailability and Risk for Adverse Effects of Copper, Chromium, Nickel and Zinc; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Bielmyer, G.K.; Bullington, J.B.; Decarlo, C.A.; Chalk, S.J.; Smith, K. The effects of salinity on acute toxicity of zinc to two euryhaline species of fish, Fundulus heteroclitus and Kryptolebias marmoratus. Integr. Comp. Biol. 2012, 52, 753–760. [Google Scholar] [CrossRef]
- Brown, R.J.; Galloway, T.S.; Lowe, D.; Browne, M.A.; Dissanayake, A.; Jones, M.B.; Depledge, M.H. Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat. Toxicol. 2004, 66, 267–278. [Google Scholar] [CrossRef]
- Figueira, E.; Branco, D.; Antunes, S.C.; Gonçalves, F.; Freitas, R. Are metallothioneins equally good biomarkers of metal and oxidative stress? Ecotoxicol. Environ. Saf. 2012, 84, 185–190. [Google Scholar] [CrossRef]
- Kumar, R.; Pradhan, A.; Khan, F.A.; Lindström, P.; Ragnvaldsson, D.; Ivarsson, P.; Olsson, P.-E.; Jass, J. Comparative analysis of stress induced gene expression in Caenorhabditis elegans following exposure to environmental and lab reconstituted complex metal mixture. PLoS ONE 2015, 10, e0132896. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).