A Note of a Unique Inland, Saline Water Fishery: Brine Flies (Diptera: Ephydridae) of Lake Cuitzeo, Mexico
Abstract
:1. Introduction
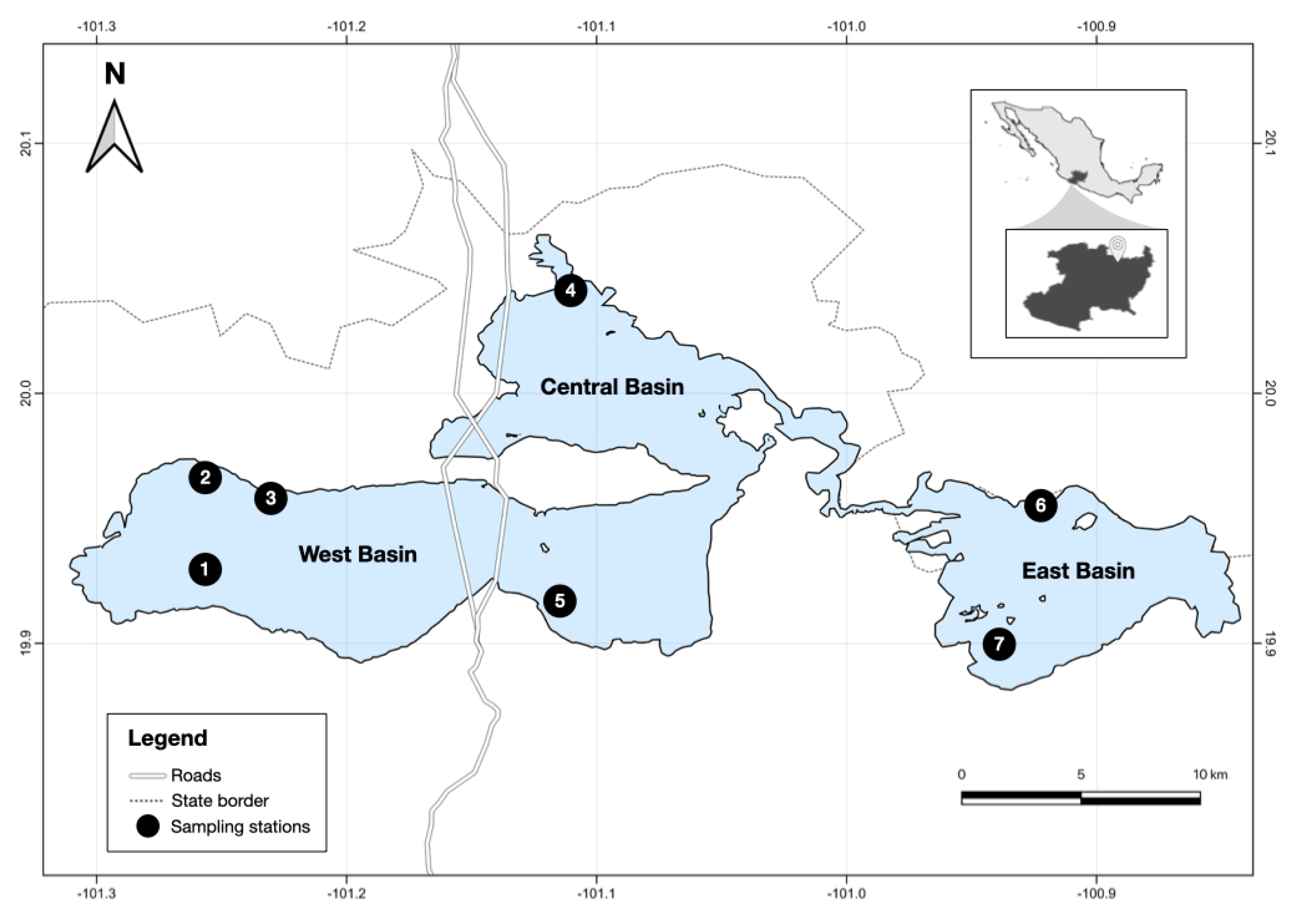
2. Materials and Methods
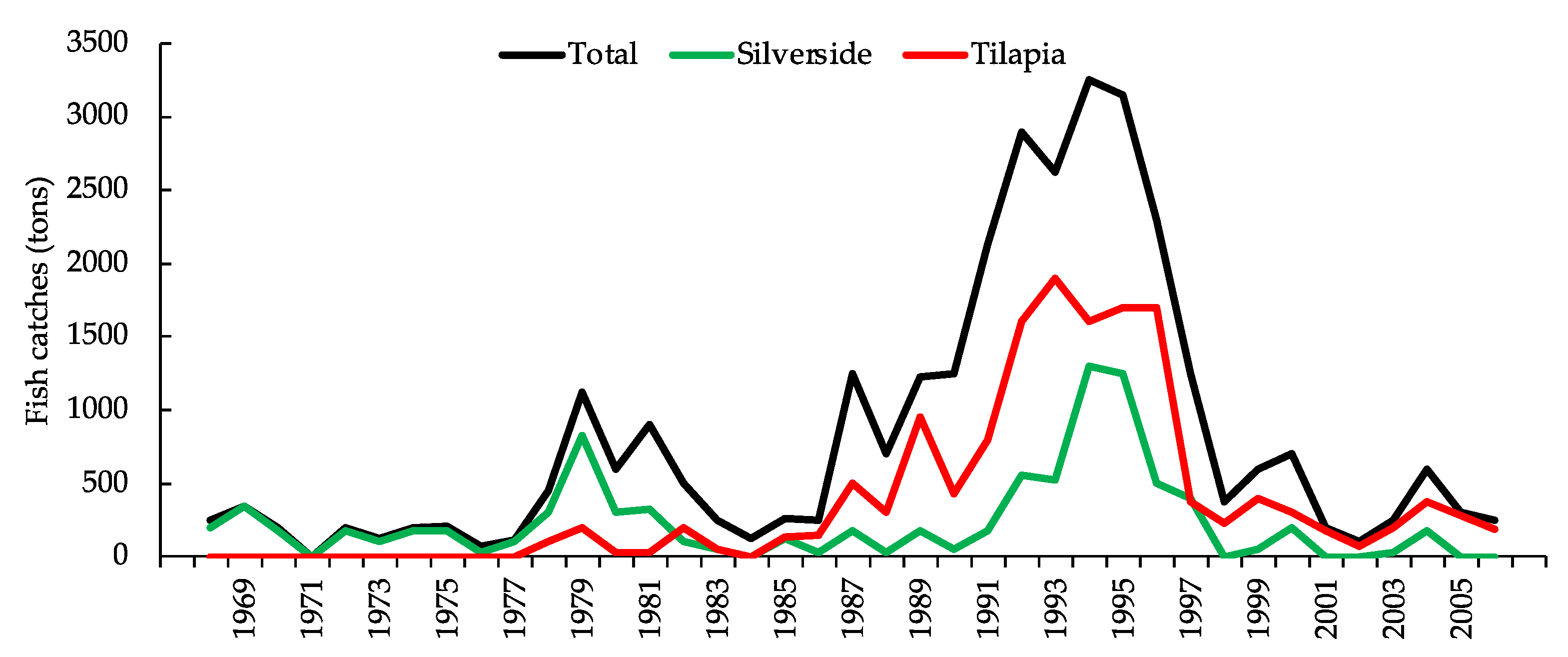
3. Results
3.1. The Habitat
3.2. The Ephydrids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcocer, J. Saline lake ecosystems of Mexico. Aquat. Ecosyst. Heal. Manag. 1999, 1, 291–315. [Google Scholar] [CrossRef]
- Alcocer Durand, J.; Bernal-Brooks, F.W. Long-term ecological research in epicontinental aquatic bodies. Rev. Dig. Univ. 2009, 10. Available online: http://www.revista.unam.mx/vol.10/num8/art52/int52.htm (accessed on 6 February 2022).
- Arellano Torres, A.; Meléndez Galicia, C.; Hernández Montaño, D.; Hernández Zárate, N. Ordenamiento pesquero del Lago de Cuitzeo, Michoacán-Guanajuato. In Centro Regional de Investigación Pesquera Pátzcuaro; INP, SAGARPA: Pátzcuaro, Mexico, 2011; p. 31. [Google Scholar]
- Franco, C.; Galicia, L.; Durand, L.; Cram, S. Análisis del impacto de las políticas ambientales en el lago de Cuitzeo (1940–2010). Inv. Geog. 2011, 75, 7–22. [Google Scholar] [CrossRef]
- Pompa, L.I.Y. Deterioro Ambiental en la Cuenca del Lago de Cuitzeo y su Impacto en Organizaciones de Pescadores de la Ribera. Master’s Thesis, Universidad Autónoma de Chapingo, Estado de México, Mexico, 1995. [Google Scholar]
- Alvarado Díaz, J.; Zubieta Rojas, T.; Ortega Murillo, R.; Chacón Torres, A.; Espinoza Gómez, R. Hipertroficación en un lago tropical somero (Lago de Cuitzeo, Michoacan, Mexico). Biológicas 1985, 1, 1–22. [Google Scholar]
- Martínez-Pantoja, M.A.; Alcocer, J.; Maeda-Martínez, A.M. On the Spinicaudata (Branchiopoda) from Lake Cuitzeo, Michoacán, México: First report of a clam shrimp fishery. Hydrobiologia 2002, 486, 207–213. [Google Scholar] [CrossRef]
- González-Santoyo, S.; Alcocer, J.; Oseguera, L.A. The “mosco” (Hemiptera: Corixidae and Notonectidae) of Lake Cuitzeo, Mexico: An unusual inland water fishery. Limnology 2020, 21, 119–127. [Google Scholar] [CrossRef]
- Adler, P.H.; Courtney, G.W. Ecological and Societal Services of Aquatic Diptera. Insects 2019, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Aldrich, J.M. The biology of some Western species of the dipterous genus Ephydra. J. N. Y. Entomol. Soc. 1912, 20, 77–99. [Google Scholar]
- Wirth, W.W. The Brine Flies of the Genus Ephydra in North America (Diptera: Ephydridae). Ann. Èntomol. Soc. Am. 1971, 64, 357–377. [Google Scholar] [CrossRef]
- Chacón Torres, A.; Rosas-Monge, C.; Alvarado-Díaz, J. The effects of hypereutrophycation in a tropical Mexican lake. In Aquatic Ecosystems of Mexico: Status and Scope; Munawar, M., Lawrence, S.G., Munawar, I.F., Malley, D.F., Eds.; Backhuys: Kerkwerve, The Netherlands, 2000; pp. 89–101. [Google Scholar]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 1989; p. 1193. [Google Scholar]
- Folk, R.L. A Review of Grain-Size Parameters. Sedimentology 1966, 6, 73–93. [Google Scholar] [CrossRef]
- Dean, J.W.E. Determination of Carbonate and Organic Matter in Calcareous Sediments and Sedimentary Rocks by Loss on Ignition: Comparison with Other Methods. J. Sediment. Res. 1974, 44, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.R.; Retana, A.N. Flora y vegetación acuáticas del Lago de Cuitzeo, Michoacán, México. Acta Bot. Mex. 1995, 1–17. [Google Scholar] [CrossRef]
- Lehmkuhl, D.M. How to know the aquatic insects. In The Pictured Key Nature Series; Wm C. Brown Co.: Dubuque, IA, USA, 1979; p. 168. [Google Scholar]
- Merrit, R.W.; Cummins, K.W.; Berg, M.B. An Introduction to the Aquatic Insects of North America; Kendall Hunt: Dubuque, IA, USA, 1978; p. 1158. [Google Scholar]
- Usinger, R.L. Aquatic insects of California. With Keys to North American Genera and California Species; University of California Press: Berkeley, CA, USA, 1956; p. 508. [Google Scholar]
- Pompa, L.I.Y. Composición y Estructura del Perifiton Animal del Lago de Cuitzeo, Michoacán, Mexico. Bachelor’s Thesis, Escuela de Biologia, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mexico, 1990. [Google Scholar]
- Green, J. Associations of zooplankton in six crater lakes in Arizona, Mexico and New Mexico. J. Zool. 1986, 208, 135–159. [Google Scholar] [CrossRef]
- Alcocer, J.; Lugo, A.; Oliva, M.G. The crater lakes of Valle de Santiago, Guanajuato. In Lagos y Presas Mexicanos; de la Lanza, G., García-Calderón, J.L., Eds.; Centro de Ecología y Desarrollo: Mexico City, Mexico, 2002; pp. 193–212. [Google Scholar]
- Alcocer, J.; Escobar, E.; Lugo, A.; Peralta, L. Littoral benthos of the saline crater lakes of the basin of Oriental, Mexico. Int. J. Salt Lake Res. 1998, 7, 87–108. [Google Scholar] [CrossRef]
- Alcocer, J.; Escobar, E.G.; Lugo, A.; Oseguera, L.A. Benthos of a perennially-astatic, saline, soda lake in Mexico. Int. J. Salt Lake Res. 1999, 8, 113–126. [Google Scholar] [CrossRef]
- Alcocer, J.; Lugo, A.; Escobar, E.; Sánchez, M. The macrobenthic fauna of a former perennial and now episodically filled mexican saline lake. Int. J. Salt Lake Res. 1996, 5, 261–274. [Google Scholar] [CrossRef]
- Ramos Elorduy, J.; Pino, J.M.; Conconi, M. Ausencia de una reglamentación y normalización de la explotación y comercia-lización de insectos comestibles en México. Folia Entomológica Mex. 2006, 45, 291–318. [Google Scholar]
- Alcocer, J.; Williams, W.D. Historical and recent changes in Lake Texcoco, a saline lake in Mexico. Int. J. Salt Lake Res. 1996, 5, 45–61. [Google Scholar] [CrossRef]
- Geddes, M.C.; De Deckker, P.; Williams, W.D.; Morton, D.W.; Topping, M. On the chemistry and biota of some saline lakes in Western Australia. Hydrobiologia 1981, 81, 201–222. [Google Scholar] [CrossRef]
- Collins, N. Population ecology of Ephydra cinerea Jones (Diptera: Ephydridae), the only benthic metazoan of the Great Salt Lake, USA. Hydrobiologia 1980, 68, 99–112. [Google Scholar] [CrossRef]
- Herbst, D.B. Distribution and abundance of the alkali fly (Ephydra hians) Say at Mono Lake, California (USA) in relation to physical habitat. Hydrobiologia 1990, 197, 193–205. [Google Scholar] [CrossRef]
- Herbst, D.B. Salinity controls on trophic interactions among invertebrates and algae of solar evaporation ponds in the Mojave Desert and relation to shorebird foraging and selenium risk. Wetlands 2006, 26, 475–485. [Google Scholar] [CrossRef]
- Collins, N.C. Mechanisms Determining the Relative Abundance of Brine Flies (Diptera: Ephydridae) in Yellowstone Thermal Spring Effluents. Can. Entomol. 1977, 109, 415–422. [Google Scholar] [CrossRef]
- Herbst, D.B. Biogeography and physiological adaptations of the brine fly genus Ephydra (Diptera: Ephydridae) in saline waters of the Great Basin. Great Basin Nat. 1999, 59, 127–135. [Google Scholar]
- Scheiring, J.F.; Foote, B.A. Habitat distribution of the shore flies of Northeastern Ohio (Diptera: Ephydridae). Ohio J. Sci. 1973, 73, 152–166. [Google Scholar]
- Hammer, U.T.; Sheard, J.S.; Kranabetter, J. Distribution and abundance of littoral benthic fauna in Canadian prairie saline lakes. Hydrobiologia 1990, 197, 173–192. [Google Scholar] [CrossRef]
- Herbst, D.B. Comparative population ecology of Ephydra hians Say (Diptera: Ephydridae) at Mono Lake (California) and Abert Lake (Oregon). Hydrobiologia 1988, 158, 145–166. [Google Scholar] [CrossRef]
- Herbst, D.B. Gradients of salinity stress, environmental stability and water chemistry as a templet for defining habitat types and physiological strategies in inland salt waters. Hydrobiologia 2001, 466, 209–219. [Google Scholar] [CrossRef]
- Herbst, D.B.; Bradley, T.J. A population model for the alkali fly at Mono Lake: Depth distribution and changing habitat availability. Hydrobiologia 1993, 267, 191–201. [Google Scholar] [CrossRef]
- Bradley, T.J.; Herbst, D.B. Growth and Survival of Larvae of Ephydra hians Say (Diptera: Ephydridae) on Unialgal Diets. Environ. Entomol. 1994, 23, 276–281. [Google Scholar] [CrossRef]
- Roberts, A.J. Avian diets in a saline ecosystem: Great Salt Lake, Utah, USA. Hum. Wildl. Interact. 2013, 7, 158–168. [Google Scholar] [CrossRef]
- Senner, N.R.; Moore, J.N.; Seager, S.T.; Dougill, S.; Kreuz, K.; Senner, S.E. A salt lake under stress: Relationships among birds, water levels, and invertebrates at a Great Basin saline lake. Biol. Conserv. 2018, 220, 320–329. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Arreola, J.B.; Alcocer, J.; Oseguera, L.A. A Note of a Unique Inland, Saline Water Fishery: Brine Flies (Diptera: Ephydridae) of Lake Cuitzeo, Mexico. Water 2022, 14, 900. https://doi.org/10.3390/w14060900
Calderón-Arreola JB, Alcocer J, Oseguera LA. A Note of a Unique Inland, Saline Water Fishery: Brine Flies (Diptera: Ephydridae) of Lake Cuitzeo, Mexico. Water. 2022; 14(6):900. https://doi.org/10.3390/w14060900
Chicago/Turabian StyleCalderón-Arreola, Jaquelina Beatríz, Javier Alcocer, and Luis A. Oseguera. 2022. "A Note of a Unique Inland, Saline Water Fishery: Brine Flies (Diptera: Ephydridae) of Lake Cuitzeo, Mexico" Water 14, no. 6: 900. https://doi.org/10.3390/w14060900
APA StyleCalderón-Arreola, J. B., Alcocer, J., & Oseguera, L. A. (2022). A Note of a Unique Inland, Saline Water Fishery: Brine Flies (Diptera: Ephydridae) of Lake Cuitzeo, Mexico. Water, 14(6), 900. https://doi.org/10.3390/w14060900







