Advance Oxidation Process (AOP) of Bisphenol A Using a Novel Surface-Functionalised Polyacrylonitrile (PAN) Fibre Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Chromatography
2.3. Procedure
3. Results
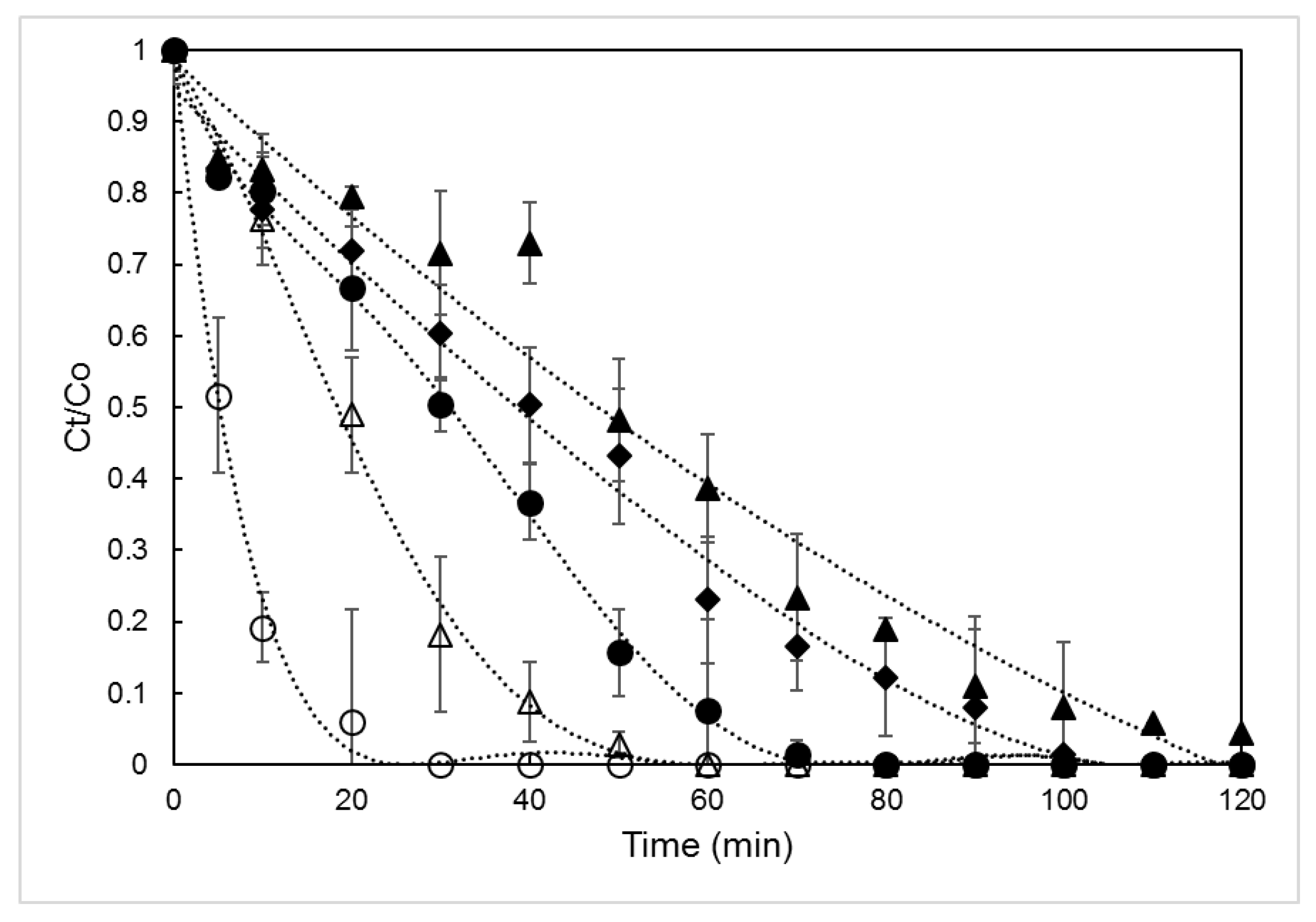
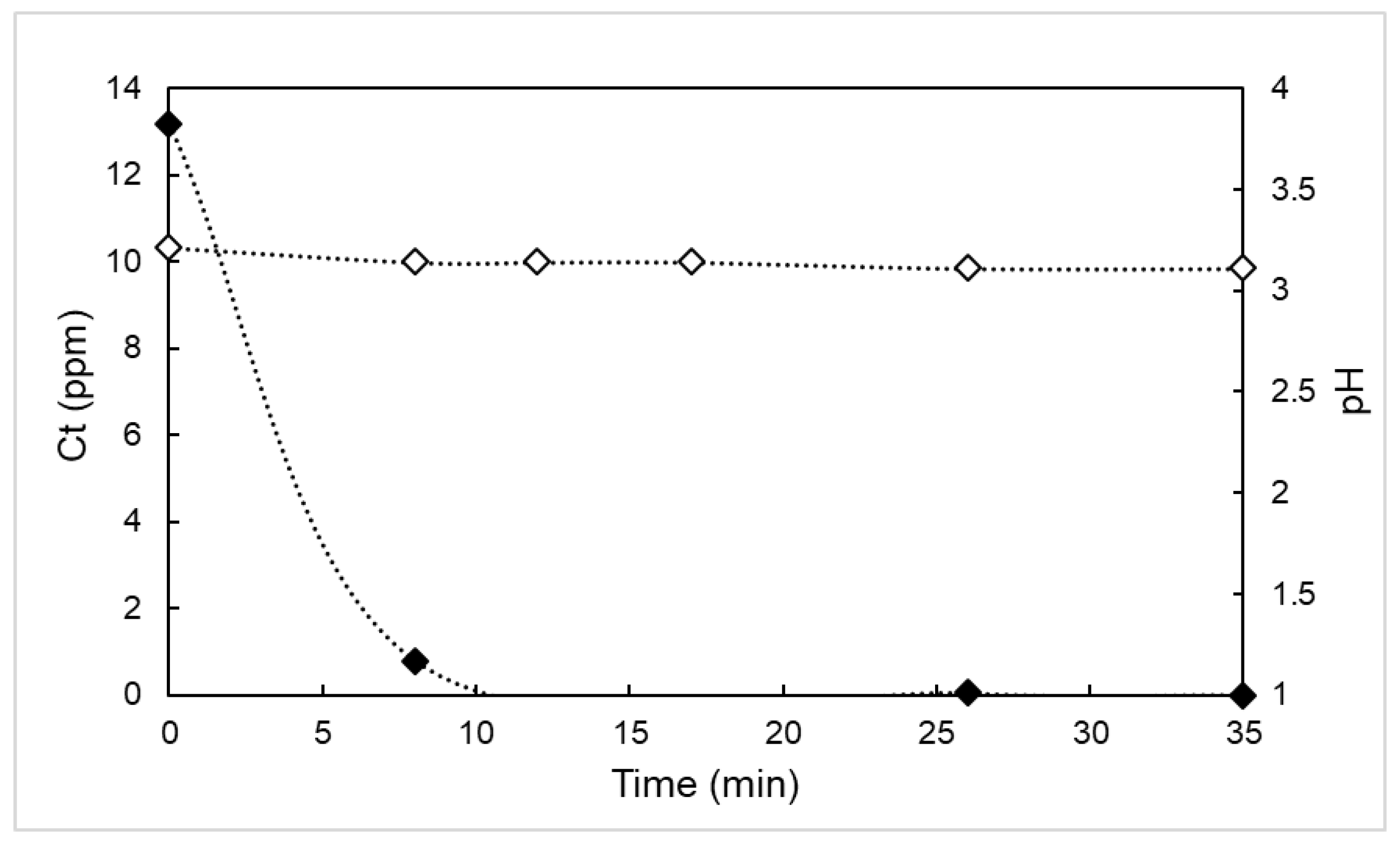
3.1. BPA Degradation
3.2. Effect of the Temperature
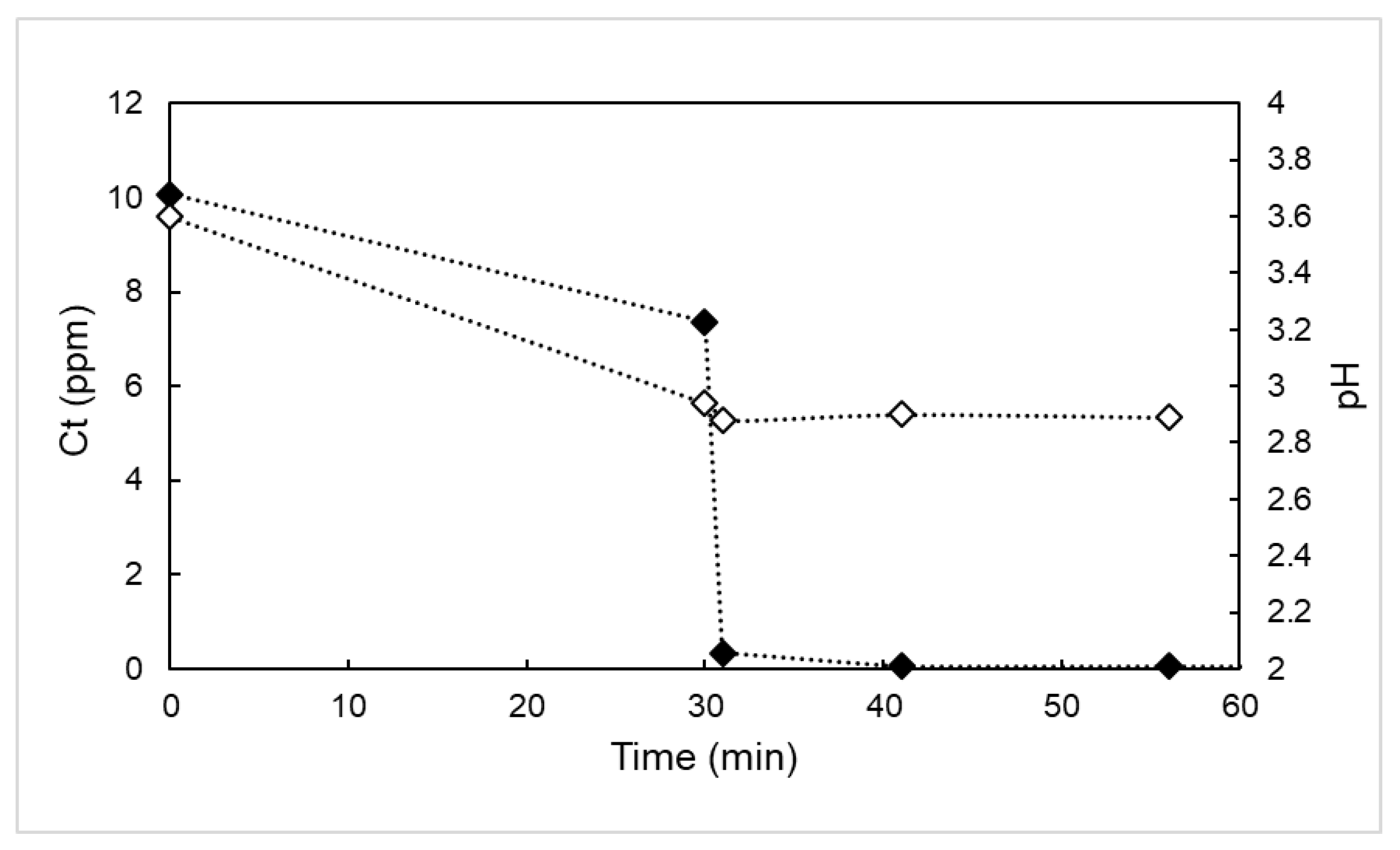
3.3. Effect of the pH
3.4. Catalyst Reuse
3.5. Rotating Catalytic Reactor
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katsumata, H.; Kawabe, S.; Knaeco, S.; Suzuki, T. Degradation of bisphenol A in water by photo-Fenton reaction. J. Photochem. Photobiol. A Chem. 2004, 162, 297–305. [Google Scholar] [CrossRef]
- Crain, D.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef]
- Kulesza, K.; Pielichowski, K.; German, K. Thermal decomposition of bisphenol A-based polyetherurethanes blown with pentane: Part I- Thermal and pyrolytical studies. J. Anal. Appl. Pyrolysis 2006, 76, 243–248. [Google Scholar] [CrossRef]
- Xie, Y.; Li, X. Degradation of bisphenol A in aqueous solution by H2O2-assisted photoelectrocatalytic oxidation. J. Hazard Mater. 2006, 138, 526–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadzadeh, S.; Dolatabadi, M. Modeling and kinetics study of electrochemical peroxidation process for mineralization of bisphenol A; a new paradigm for groundwater treatment. J. Mol. Liq. 2018, 254, 76–82. [Google Scholar] [CrossRef]
- Wang, G.; Wu, F.; Zhang, X.; Luo, M.; Deng, N. Enhanced TiO2 photocatalytic degradation of bisphenol A by β-cyclodextrin in suspended solutions. J. Photochem. Photobiol. A: Chem. 2005, 179, 49–56. [Google Scholar] [CrossRef]
- Irmak, S.; Erbatur, O.; Akgerman, A. Degradation of 17β-estradiol and bisphenol A in aqueous medium by using ozone and ozone/UV techniques. J. Hazard. Mater. 2005, 126, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wan, Y.; Huang, Y.; He, C.; Zhang, Z.; He, Z.; Hu, L.; Zeng, J.; Shu, D. Three-dimensional MnO2 porous hollow microspheres for enhanced activity as ozonation catalysts in degradation of bisphenol A. J. Hazard. Mate. 2017, 321, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Nadejde, C.; Neamtu, M.; Hodoroaba, V.; Schneider, R.; Paul, A.; Ababei, G.; Panne, U. Green Fenton-like magnetic nanocatalysts: Synthesis, characterization and catalytic application. Appl. Catal. B Environ. 2015, 176, 667–677. [Google Scholar] [CrossRef]
- Yu, Q.; Fenga, L.; Chai, X.; Qiu, X.; Ouyang, H.; Deng, G. Enhanced surface Fenton degradation of BPA in soil with a high pH. Chemosphere 2019, 220, 335–343. [Google Scholar] [CrossRef]
- Michizoe, J.; Ichinose, H.; Kamiya, N.; Maruyama, T.; Goto, M. Biodegradation of phenolic environmental pollutants by a surfactant-laccase complex in organic media. J. Biosci. Bioeng. 2005, 99, 642–647. [Google Scholar] [CrossRef]
- Heidari, H.; Sedighi, M.; Zamir, S.; Shojaosadati, S. Bisphenol A degradation by Ralstonia eutropha in the absence and presence of phenol. Int. Biodeter. Biodegr. 2017, 119, 37–42. [Google Scholar] [CrossRef]
- Grelska, A.; Noszczyńska, M. White rot fungi can be a promising tool for removal of bisphenol A, bisphenol S, and nonylphenol from wastewater. Environ. Sci. Pollut. Res. 2020, 27, 39958–39976. [Google Scholar] [CrossRef]
- Bai, X.; Acharya, K. Removal of seven endocrine disrupting chemicals (EDCs) from municipal wastewater effluents by a freshwater green alga. Environ. Pollut. 2019, 247, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceutical, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Catrinescu, C.; Teodosiu, C.; Macoveanu, M.; Miehe-Brendle, J.; Le Dred, R. Catalytic wet peroxide of phenol over Fe-exchanged pillared beidellite. Water Res. 2003, 37, 1154–1160. [Google Scholar] [CrossRef]
- Liou, R.; Chen, S.; Hung, M.; Hsu, C.; Lai, J. Fe (III) supported on resin as effective catalyst for the heterogeneous oxidation of phenol in aqueous solution. Chemosphere 2005, 59, 117–125. [Google Scholar] [CrossRef]
- Noorjahan, M.; Kumari, V.; Subrahmanyam, M.; Panda, L. Immobilized Fe(III)-HY: As efficient and stable photo-Fenton catalyst. Appl. Catal. B: Environ. 2005, 57, 291–298. [Google Scholar] [CrossRef]
- Flores, Y.; Flores, R.; Gallegos, A. Heterogeneous catalysis in the Fenton-type system reactive black 5/H2O2. J. Mol. Catal. A Chem. 2008, 281, 184–191. [Google Scholar] [CrossRef]
- Muthuvel, I.; Swaminathan, M. Highly solar active Fe (III) immobilized alumina for the degradation of Acid Violet 7. Sol. Energy Mater. Sol. Cells 2008, 92, 857–863. [Google Scholar] [CrossRef]
- Ishtchenko, V.; Huddersman, K.; Vitkovskaya, R. Part 1. Production of a modified PAN fibrous catalyst and its optimization towards the decomposition of hydrogen peroxide. Appl. Catal. A Gen. 2003, 242, 123–137. [Google Scholar] [CrossRef]
- Umar, K.; Yaqoob, A.; Ibrahim, M.; Parveen, T.; Safian, M. Environmental applications of smart polymer composites. Smart Polym. Nanocompos. Biomed. Environ. Appl. 2020, 15, 295–320. [Google Scholar]
- Ishtchenko, V.; Vitkovskaya, R.; Huddersman, K. Investigation of the mechanical and physico-chemical properties of a modified PAN fibrous catalyst. Appl. Catal. A Gen. 2003, 242, 221–231. [Google Scholar] [CrossRef]
- Chi, G.; Huddersman, K. Maleic acid oxidation using A heterogeneous modified polyacrylonitrile (PAN) fibrous catalyst. J. Adv. Oxid. Technol. 2011, 14, 235–243. [Google Scholar] [CrossRef]
- Chi, G.; Nagy, Z.; Huddersman, K. Kinetic modelling of the Fenton-like oxidation of maleic acid using a heterogeneous modified polyacrylonitrile (PAN) catalyst. Prog. React. Kinet. Mech. 2011, 36, 189–214. [Google Scholar] [CrossRef]
- Kiran, M.G.; Das, R.; Behera, S.K.; Pakshirajan, K.; Das, G. Modelling a rotating biological contactor treating heavy metal contaminated wastewater using artificial neural network. Water Supply 2021, 21, 1895–1912. [Google Scholar] [CrossRef]
- Hamill, N.A.; Weatherley, L.R.; Hardacre, C. Use of a batch rotating photocatalytic contactor for the degradation of organic pollutants in wastewater. Appl. Catal. B Environ. 2001, 30, 49–60. [Google Scholar] [CrossRef]
- Montalvo-Romero, C.; Aguilar-Ucán, C.; Alcocer-Delahoz, R.; Ramirez-Elias, M.; Cordova-Quiroz, V. A Semi-Pilot Photocatalytic Rotating Reactor (RFR) with Supported TiO2/Ag Catalysts for Water Treatment. Molecules 2018, 23, 224. [Google Scholar] [CrossRef] [Green Version]
- Huddersman, K.; Chitangyie, G. Rotating Contactor Reactor. U.S. Patent 10,689,275, 23 June 2020. [Google Scholar]
- Chiang, K.; Lim, T.; Tsen, L.; Lee, C. Photocatalytic degradation and mineralization of bisphenol A by platinized TiO2. Appl. Catal. A Gen. 2004, 261, 225–237. [Google Scholar] [CrossRef]
- Zhou, D.; Wu, F.; Deng, N.; Xiang, W. Photooxidation of bisphenol A (BPA) in water in the presence of ferric and carboxylate salts. Water Res. 2004, 38, 4107–4116. [Google Scholar] [CrossRef]
- Wadley, S.; Waite, T.D. Fenton Process–Advanced Oxidation Processes for Water and Wastewater Treatment; Parson, S., Ed.; IWA Publishing: London, UK, 2004; pp. 111–136. ISBN 9781780403076. [Google Scholar]
- Li, X.; Xiao, B.; Wu, M.; Wang, L.; Chen, R.; Wei, Y.; Liu, H. In-situ generation of multi-homogeneous/heterogeneous Fe-based Fenton catalysts toward rapid degradation of organic pollutants at near neutral pH. Chemosphere 2020, 245, 125663. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Miller, C.J.; Waite, T.D. pH Dependence of Hydroxyl Radical, Ferryl, and/or Ferric Peroxo Species Generation in the Heterogeneous Fenton Process. Environ. Sci. Technol. 2022, 56, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Xiaoa, J.; Wangb, C.; Liuc, H. Fenton-like degradation of dimethyl phthalate enhanced by quinone species. J. Hazard. Mater. 2020, 382, 121007. [Google Scholar] [CrossRef] [PubMed]
- Chi, G.; Churchley, J.; Huddersman, K. Pilot-Scale removal of trace steroid hormones and pharmaceuticals and personal care products from municipal wastewater using a heterogenous Fenton’s catalytic process. Int. J. Chem. Eng. 2013, 2013, 760915. [Google Scholar] [CrossRef] [Green Version]
- Pushpa, U. Remediation of Textile and Mining Influence Effluents Using Novel Heterogenous PAN Catalyst and Modified PAN Mesh. Ph.D. Thesis, Environmental Technology. De Montfort University, Leicester, UK, 2018. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Farias, J.; Tiwary, A.; Tangyie, G.C.; Huddersman, K. Advance Oxidation Process (AOP) of Bisphenol A Using a Novel Surface-Functionalised Polyacrylonitrile (PAN) Fibre Catalyst. Water 2022, 14, 640. https://doi.org/10.3390/w14040640
Wang J, Farias J, Tiwary A, Tangyie GC, Huddersman K. Advance Oxidation Process (AOP) of Bisphenol A Using a Novel Surface-Functionalised Polyacrylonitrile (PAN) Fibre Catalyst. Water. 2022; 14(4):640. https://doi.org/10.3390/w14040640
Chicago/Turabian StyleWang, Jiafan, Jorgelina Farias, Abhishek Tiwary, George Chi Tangyie, and Katherine Huddersman. 2022. "Advance Oxidation Process (AOP) of Bisphenol A Using a Novel Surface-Functionalised Polyacrylonitrile (PAN) Fibre Catalyst" Water 14, no. 4: 640. https://doi.org/10.3390/w14040640
APA StyleWang, J., Farias, J., Tiwary, A., Tangyie, G. C., & Huddersman, K. (2022). Advance Oxidation Process (AOP) of Bisphenol A Using a Novel Surface-Functionalised Polyacrylonitrile (PAN) Fibre Catalyst. Water, 14(4), 640. https://doi.org/10.3390/w14040640






