Detection of Eight Cannabinoids and One Tracer in Wastewater and River Water by SPE-UPLC–ESI-MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
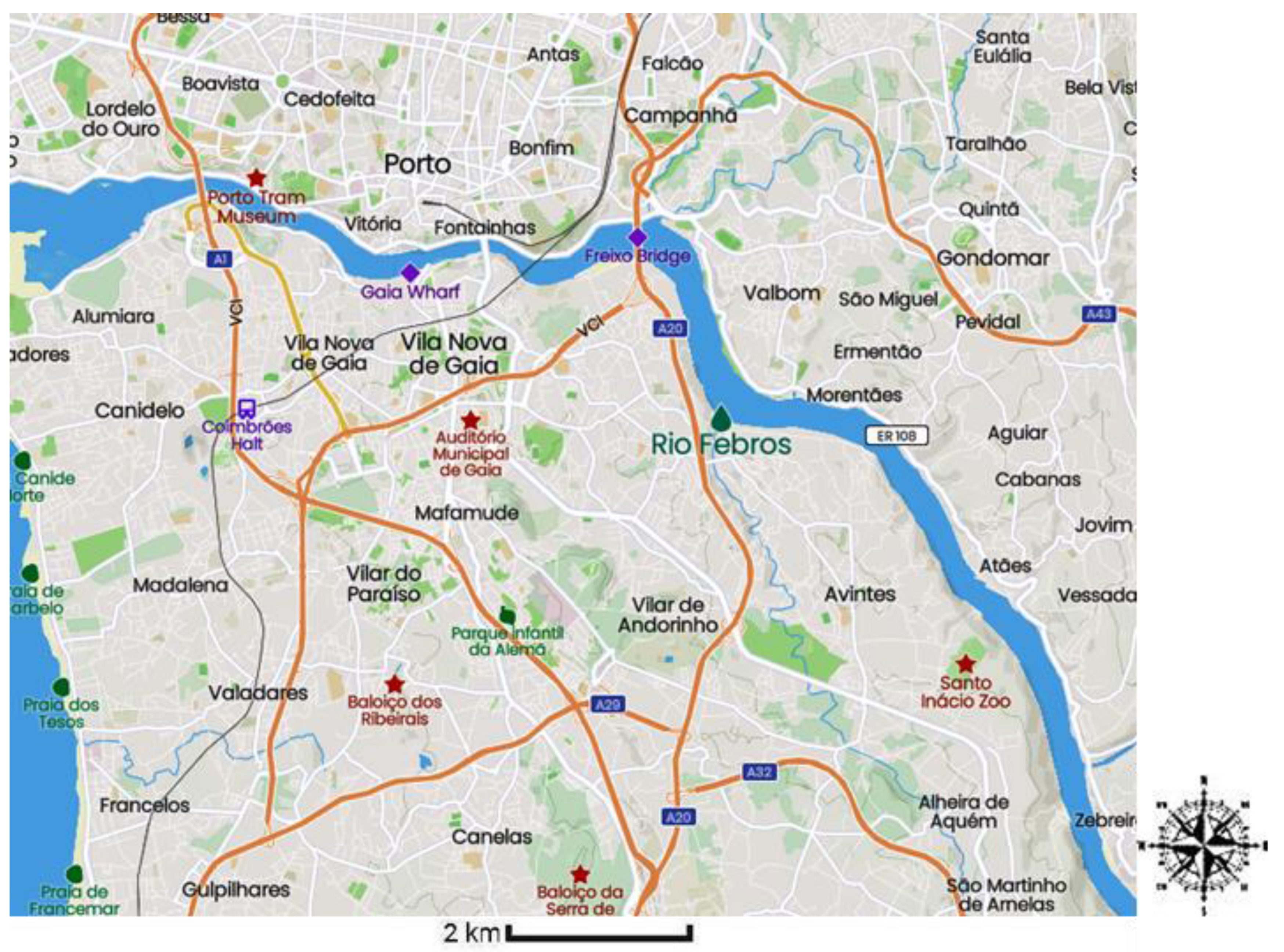
2.2. Description of the Sampling Site
2.3. Sample Pretreatment and Solid-Phase Extraction (SPE)
2.4. Ultra-Performance Liquid Chromatography–Electrospray Ionization-Tandem Mass Spectrometry (UPLC–ESI-MS/MS) Analysis
2.5. Method Validation
3. Results and Discussion
3.1. SPE Procedure
3.2. UPLC Optimization
3.3. MS/MS Parameter Optimization
3.4. Method Validation
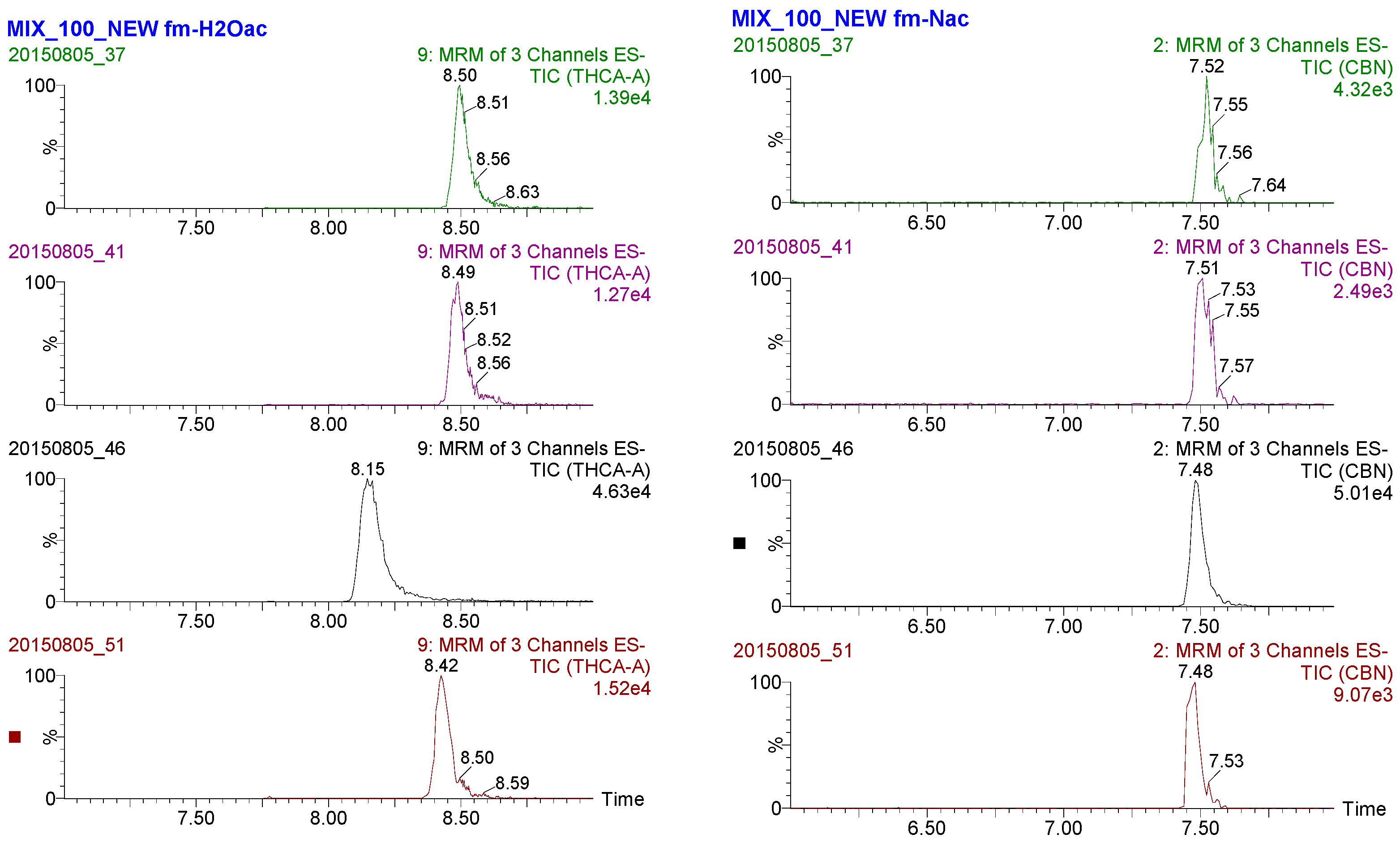
3.5. Application to Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breijyeh, Z.; Jubeh, B.; Bufo, S.A.; Karaman, R.; Scrano, L. Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses. Toxins 2021, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Thomaidis, N.S.; Asimakopoulos, A.G.; Bletsou, A.A. Emerging contaminants: A tutorial mini-review. Glob. NEST J. 2012, 14, 72–79. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Annual Report on the State of the Drugs Problem in Europe. Lisbon 2021. Available online: https://www.emcdda.europa.eu/publications/edr/trends-developments/2021_en (accessed on 1 September 2021).
- United Nations Office on Drugs and Crime (UNODC). World Drug Report. United Nations Publication, 2021. Available online: https://www.unodc.org/unodc/data-and-analysis/wdr2021.html (accessed on 1 September 2021).
- Van Laar, M.W.; Oomen, P.E.; Van Miltenburg, C.J.; Vercoulen, E.; Freeman, T.P.; Hall, W.D. Cannabis and COVID-19: Reasons for concern. Front. Psychiatry 2020, 11, 1419. [Google Scholar] [CrossRef] [PubMed]
- Vanderbruggen, N.; Matthys, F.; Van Laere, S.; Zeeuws, D.; Santermans, L.; Van den Ameele, S.; Crunelle, C.L. Self-reported alcohol, tobacco, and cannabis use during COVID-19 lockdown measures: Results from a web-based survey. Eur. Addict. Res. 2020, 26, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Bufo, S.A.; Milan, S.; Milella, L.; Scrano, L.; Varallo, C. Cannabis sativa L. in Foodstuffs: The Italian case and the need for EU harmonised limits for THC unavoidable contamination. Eur. Food Feed Law Rev. 2016, 11, 52–57. [Google Scholar]
- Pinto, I.C.; Pereira, O.R.; Fernandes, A.; Nogueira, J.; Costa, R. Psychoactive substance consumption: A Portuguese cross-sectional study. Work 2021, 70, 335–344. [Google Scholar] [CrossRef]
- Eurobarometer, F. Youth Attitudes on Drugs; Analytical; The European Commission and The GALLUP Organization: Luxembourg, 2011. [Google Scholar]
- Molinaro, S.; Vicente, J. (Espad Group) ESPAD Report 2019: Results from European School Survey Project on Alcohol and Other Drugs; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Burns, N.K.; Theakstone, A.G.; Zhu, H.; O’Dell, L.A.; Pearson, J.R.; Ashton, T.D.; Pfeffer, F.M.; Conlan, X.A. The identification of synthetic cannabinoids surface coated on herbal substrates using solid-state nuclear magnetic resonance spectroscopy. Anal. Chim. Acta 2021, 1104, 105–109. [Google Scholar] [CrossRef]
- Peacock, A.; Bruno, R.; Gisev, N.; Degenhardt, L.; Hall, W.; Sedefov, R.; White, J.; Thomas, K.V.; Farrell, M.; Griffiths, P. New psychoactive substances: Challenges for drug surveillance, control, and public health responses. Lancet 2019, 394, 1668–1684. [Google Scholar] [CrossRef]
- Potts, A.J.; Cano, C.; Thomas, S.H.L.; Hill, S.L. Synthetic cannabinoid receptor agonists: Classification and nomenclature. Clin. Toxicol. 2020, 58, 82–98. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; Van der Kamp, H.J.; Van Eeuwijk, F.A. Characterisation of cannabis accessions with regard to cannabinoid content in relation to other plant characters. Euphytica 1992, 62, 187–200. [Google Scholar] [CrossRef]
- Casano, S.; Grassi, G.; Martini, V.; Michelozzi, M. Variations in terpene profiles of different strains of Cannabis sativa L. Acta Hortic. 2011, 925, 115–121. [Google Scholar] [CrossRef]
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and medicinal chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Aliferis, K.A.; Bernard-Perron, D. Cannabinomics: Application of metabolomics in Cannabis (Cannabis sativa L.) research and development. Front. Plant Sci. 2020, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sanchez, I.J.; Choi, Y.H.; Verpoorte, R. Metabolite analysis of Cannabis sativa L. by NMR spectroscopy. In Functional Genomics; Springer: New York, NY, USA, 2012; pp. 363–375. [Google Scholar]
- Fontes, M.K.; Maranho, L.A.; Pereira, C.D.S. Review on the occurrence and biological effects of illicit drugs in aquatic ecosystems. Environ. Sci. Pollut. Res. 2020, 27, 30998–31034. [Google Scholar] [CrossRef]
- Huerta-Fontela, M.; Galceran, M.T.; Martin-Alonso, J.; Ventura, F. Occurrence of psychoactive stimulatory drugs in wastewaters in north-eastern Spain. Sci. Total Environ. 2008, 397, 31–40. [Google Scholar] [CrossRef]
- Adams, P.J.; Rychert, M.; Wilkins, C. Policy influence and the legalized cannabis industry: Learnings from other addictive consumption industries. Addiction 2021, 116, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, P.V.; Carapinha, L. Drug decriminalisation in Portugal. BMJ 2010, 341, c4554. [Google Scholar] [CrossRef]
- Laqueur, H. Uses and abuses of drug decriminalization in Portugal. Law Soc. Inq. 2015, 40, 746–781. [Google Scholar] [CrossRef] [Green Version]
- Ximene, R.Ê.G.O.; Oliveira, M.J.; Lameira, C. 20 years of Portuguese drug policy-developments, challenges and the quest for human rights. Subst. Abus. Treat. Prev. Policy 2021, 16, 59. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Statistical Bulletin 2020—Prevalence of Drug Use 2020. Available online: https://www.emcdda.europa.eu/data/stats2020/gps_en. (accessed on 22 December 2020).
- European Commission, Directorate-General for Communication. Flash Eurobarometer 401: Young People and Drugs Report, Version v1.00. 2015. Available online: https://data.europa.eu/data/datasets/s2029_401?locale=en (accessed on 22 December 2020).
- Schuster, W.M.; Bird, R.C. Legal Strategy during Legal Uncertainty: The Case of Cannabis Regulation. Stanf. J. Law Bus. Financ. 2021, 26, 362–412. [Google Scholar]
- Bridgeman, M.B.; Abazia, D.T. Medicinal cannabis: History, pharmacology, and implications for the acute care setting. Pharm. Ther. 2017, 42, 180. [Google Scholar]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Sajdeya, R.; Goodin, A.J.; Tighe, P.J. Cannabis use assessment and documentation in healthcare: Priorities for closing the gap. Prev. Med. 2021, 153, 106798. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [Green Version]
- Abazia, D.T.; Bridgeman, M.B. Reefer madness or real medicine? A plea for incorporating medicinal cannabis in pharmacy curricula. Curr. Pharm. Teach. Learn. 2018, 10, 1165–1167. [Google Scholar] [CrossRef]
- Davoli, E.; Zuccato, E.; Castiglioni, S. Illicit drugs in drinking water. Curr. Opin. Environ. Sci. Health 2019, 7, 92–97. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Arcella, D.; Cascio, C.; Mackay, K. Acute human exposure assessment to tetrahydrocannabinol (Δ9-THC). EFSA J. 2020, 18, e05953. [Google Scholar]
- Castaneto, M.S.; Wohlfarth, A.; Desrosiers, N.A.; Hartman, R.L.; Gorelick, D.A.; Huestis, M.A. Synthetic cannabinoids pharmacokinetics and detection methods in biological matrices. Drug Metab. Rev. 2015, 47, 124–174. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Uddin, S.J.; Alam, M.A.; Sarker, S.D. Extraction of naturally occurring cannabinoids: An update. Phytochem. Anal. 2021, 32, 228–241. [Google Scholar] [CrossRef]
- Lelario, F.; Scrano, L.; De Franchi, S.; Bonomo, M.G.; Salzano, G.; Milan, S.; Milella, L.; Bufo, S.A. Identification and antimicrobial activity of most representative secondary metabolites from different plant species. Chem. Biol. Technol. Agric. 2018, 5, 13. [Google Scholar] [CrossRef]
- Zarei, S.; Salimi, Y.; Repo, E.; Daglioglu, N.; Safaei, Z.; Güzel, E.; Asadi, A. A global systematic review and meta-analysis on illicit drug consumption rate through wastewater-based epidemiology. Environ. Sci. Pollut. Res. 2020, 278, 36037–36051. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, L.; Burgard, D.A.; Been, F.; Ort, C.; Matias, J.; Yargeau, V. The estimation of cannabis consumption through wastewater analysis. Compr. Anal. Chem. 2020, 90, 453–482. [Google Scholar]
- Sulej-Suchomska, A.M.; Klupczynska, A.; Dereziński, P.; Matysiak, J.; Przybyłowski, P.; Kokot, Z.J. Urban wastewater analysis as an effective tool for monitoring illegal drugs, including new psychoactive substances, in the Eastern European region. Sci. Rep. 2020, 10, 4885. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yuan, M.; Wang, D.; Zhao, Y.; Hu, K.; Qian, Z.; Gao, L.; Zheng, H. Sewage Epidemiology for Drug Situation Assessment. Asian J. Ecotoxicol. 2020, 4, 79–87. [Google Scholar]
- Devault, D.A.; Néfau, T.; Levi, Y.; Karolak, S. The removal of illicit drugs and morphine in two waste water treatment plants (WWTPs) under tropical conditions. Environ. Sci. Pollut. Res. 2017, 24, 25645–25655. [Google Scholar] [CrossRef]
- Daughton, C.G. Illicit drugs: Contaminants in the environment and utility in forensic epidemiology. Rev. Environ. Contam. Toxicol. 2011, 210, 59–110. [Google Scholar]
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Estimating community drug abuse by wastewater analysis. Environ. Health Perspect. 2008, 116, 1027–1032. [Google Scholar] [CrossRef] [Green Version]
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Calamari, D.; Bagnati, R.; Schiarea, S.; Fanelli, R. Cocaine in surface waters: A new evidence-based tool to monitor community drug abuse. Environ. Health 2005, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Bijlsma, L.; Celma, A.; López, F.J.; Hernández, F.E.L.I.X. Monitoring new psychoactive substances use through wastewater analysis: Current situation, challenges and limitations. Curr. Opin. Environ. Sci. Health 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Citti, C.; Braghiroli, D.; Vandelli, M.A.; Cannazza, G. Pharmaceutical and biomedical analysis of cannabinoids: A critical review. J. Pharm. Biomed. Anal. 2018, 147, 565–579. [Google Scholar] [CrossRef]
- Nicolaou, A.G.; Christodoulou, M.C.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Analysis of cannabinoids in conventional and alternative biological matrices by liquid chromatography: Applications and challenges. J. Chromatogr. A 2021, 1651, 462277. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Marchei, E.; Papaseit, E.; Farré, M.; Zaami, S. UHPLC-HRMS and GC-MS Screening of a Selection of Synthetic Cannabinoids and Metabolites in Urine of Consumers. Medicina 2020, 56, 408. [Google Scholar] [CrossRef]
- Gorziza, R.P.; Duarte, J.A.; González, M.; Arroyo-Mora, L.E.; Limberger, R.P. A systematic review of quantitative analysis of cannabinoids in oral fluid. J. Forensic Sci. 2021, 66, 2104–2112. [Google Scholar] [CrossRef]
- Krizman-Matasic, I.; Senta, I.; Kostanjevecki, P.; Ahel, M.; Terzic, S. Long-term monitoring of drug consumption patterns in a large-sized European city using wastewater-based epidemiology: Comparison of two sampling schemes for the assessment of multiannual trends. Sci. Total Environ. 2019, 647, 474–485. [Google Scholar] [CrossRef]
- Van Nuijs, A.L.N.; Castiglioni, S.; Tarcomnicu, I.; Postigo, C.; de Alda, M.L.; Neels, H.; Zuccato, E.; Barcelo, D.; Covaci, A. Illicit drug consumption estimations derived from wastewater analysis: A critical review. Sci. Total Environ. 2011, 409, 3564–3577. [Google Scholar] [CrossRef]
- Postigo, C.; de Alda, M.L.; Barceló, D. Analysis of drugs of abuse and their human metabolites in water by LC-MS2: A non-intrusive tool for drug abuse estimation at the community level. Trends Anal. Chem. 2008, 27, 1053–1069. [Google Scholar] [CrossRef]
- Castiglioni, S.; Bijlsma, L.; Covaci, A.; Emke, E.; Hernández, F.; Reid, M.; Ort, C.; Thomas, K.V.; van Nuijs, A.L.N.; de Voogt, P. Evaluation of uncertainties associated with the determination of community drug use through the measurement of sewage drug biomarkers. Environ. Sci. Technol. 2013, 47, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Postigo, C.; Lopez de Alda, M.; Barceló, D. Fully automated determination in the low nanogram per liter level of different classes of drugs of abuse in sewage water by on-line solid-phase extraction-liquid chromatography–electrospray-tandem mass spectrometry. Anal. Chem. 2008, 80, 3123–3134. [Google Scholar] [CrossRef]
- Lelario, F.; Pascale, R.; Bianco, G.; Scrano, L.; Bufo, S.A. Hemp Chemotype Definition by Cannabinoids Characterization Using LC-ESI(+)-LTQ-FTICRMS and Infrared Multiphoton Dissociation. Separations 2021, 8, 245. [Google Scholar] [CrossRef]
- Baker, D.R.; Kasprzyk-Hordern, B. Critical evaluation of methodology commonly used in sample collection, storage and preparation for the analysis of pharmaceuticals and illicit drugs in surface water and wastewater by solid phase extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 8036–8059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; González-Díez, M.; Cela, R. Screening and selective quantification of illicit drugs in wastewater by mixed-mode solid-phase extraction and quadrupole-time-of-flight liquid chromatography-mass spectrometry. Anal. Chem. 2012, 84, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Mohan, H.; Rajput, S.S.; Jadhav, E.B.; Sankhla, M.S.; Sonone, S.S.; Jadhav, S.; Kumar, R. Ecotoxicity, Occurrence, and Removal of Pharmaceuticals and Illicit Drugs from Aquatic Systems. Biointerface Res. Appl. Chem. 2021, 11, 12530–12546. [Google Scholar]
- Hernández, F.; Ibáñez, M.; Botero-Coy, A.M.; Bade, R.; Bustos-López, M.C.; Rincón, J.; Moncayo, A.; Bijlsma, L. LC-QTOF MS screening of more than 1000 licit and illicit drugs and their metabolites in wastewater and surface waters from the area of Bogotá, Colombia. Anal. Bioanal. Chem. 2015, 407, 6405–6416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelario, F.; Brienza, M.; Bufo, S.A.; Scrano, L. Effectiveness of different advanced oxidation processes (AOPs) on the abatement of the model compound mepanipyrim in water. J. Photochem. Photobiol. Chem. 2016, 321, 187–201. [Google Scholar] [CrossRef]
- Benaglia, L.; Udrisard, R.; Bannwarth, A.; Gibson, A.; Béen, F.; Lai, F.Y.; Esseiva, P.; Delémont, O. Testing wastewater from a music festival in Switzerland to assess illicit drug use. Forensic Sci. Int. 2020, 309, 110148. [Google Scholar] [CrossRef]
- Lai, F.Y.; Ort, C.; Gartner, C.; Carter, S.; Prichard, J.; Kirkbride, P.; Bruno, R.; Hall, W.; Eaglesham, G.; Mueller, J.F. Refining the estimation of illicit drug consumptions from wastewater analysis: Co-analysis of prescription pharmaceuticals and uncertainty assessment. Water Res. 2011, 45, 4437–4448. [Google Scholar] [CrossRef]
- Bijlsma, L.; Beltrán, E.; Boix, C.; Sancho, J.V.; Hernández, F. Improvements in analytical methodology for the determination of frequently consumed illicit drugs in urban wastewater. Anal. Bioanal. Chem. 2014, 406, 4261–4272. [Google Scholar] [CrossRef]
- Mendoza, A.; Rodríguez-Gil, J.L.; González-Alonso, S.; Mastroianni, N.; De Alda, M.L.; Barceló, D.; Valcárcel, Y. Drugs of abuse and benzodiazepines in the Madrid Region (Central Spain): Seasonal variation in river waters, occurrence in tap water and potential environmental and human risk. Environ. Int. 2014, 70, 76–87. [Google Scholar] [CrossRef]
- Prichard, J.; Hall, W.; de Voogt, P.; Zuccato, E. Sewage epidemiology and illicit drug research: The development of ethical research guidelines. Sci. Total Environ. 2014, 472, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Bijlsma, L.; Emke, E.; Hernández, F.; De Voogt, P. Investigation of drugs of abuse and relevant metabolites in Dutch sewage water by liquid chromatography coupled to high resolution mass spectrometry. Chemosphere 2012, 89, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Nefau, T.; Karolak, S.; Castillo, L.; Boireau, V.; Levi, Y. Presence of illicit drugs and metabolites in influents and effluents of 25 sewage water treatment plants and map of drug consumption in France. Sci. Total Environ. 2013, 461, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.V.; Bijlsma, L.; Castiglioni, S.; Covaci, A.; Emke, E.; Grabic, R.; Hernández, F.; Karolak, S.; Kasprzyk-Hordern, B.; Lindberg, R.H.; et al. Comparing illicit drug use in 19 European cities through sewage analysis. Sci. Total Environ. 2012, 432, 432–439. [Google Scholar] [CrossRef] [Green Version]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Annual Report on the State of the Drugs Problem in Europe; EMCDDA: Lisbon, Portugal, 2012; ISSN 1609-6150.
- Huestis, M.A. Pharmacokinetics and metabolism of the plant cannabinoids, Δ 9-tetrahydrocannibinol, cannabidiol and cannabinol. Cannabinoids 2005, 168, 657–690. [Google Scholar]
- Desrosiers, N.A. Pharmacodynamics, Pharmacokinetics, and Cannabinoid Stability Following Smoked Cannabis in Occasional and Frequent Cannabis Smokers. Ph.D. Thesis, University of Maryland, Baltimore, ML, USA, 2014. [Google Scholar]
- Jurado, A.; Mastroianni, N.; Vàzquez-Suñé, E.; Carrera, J.; Tubau, I.; Pujades, E.; Postigo, C.; López de Alda, M.; Barceló, D. Drugs of abuse in urban groundwater. A case study: Barcelona. Sci. Total Environ. 2012, 424, 280–288. [Google Scholar] [CrossRef]
- Jurado, A.; Walther, M.; Díaz-Cruz, M.S. Occurrence, fate and environmental risk assessment of the organic microcontaminants included in the Watch Lists set by EU Decisions 2015/495 and 2018/840 in the groundwater of Spain. Sci. Total Environ. 2019, 663, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, N.; Postigo, C.; de Alda, M.L.; Viana, M.; Rodríguez, A.; Alastuey, A.; Querol, X.; Barceló, D. Comprehensive monitoring of the occurrence of 22 drugs of abuse and transformation products in airborne particulate matter in the city of Barcelona. Sci. Total Environ. 2015, 532, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Senta, I.; Krizman, I.; Ahel, M.; Terzic, S. Integrated procedure for multiresidue analysis of dissolved and particulate drugs in municipal wastewater by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 3255–3268. [Google Scholar] [CrossRef]
- Vazquez-Roig, P.; Andreu, V.; Blasco, C.; Picó, Y. SPE and LC-MS/MS determination of 14 illicit drugs in surface waters from the Natural Park of L’Albufera (València, Spain). Anal. Bioanal. Chem. 2010, 397, 2851–2864. [Google Scholar] [CrossRef]
- Been, F.; Rossi, L.; Ort, C.; Rudaz, S.; Delémont, O.; Esseiva, P. Population normalisation with ammonium in wastewater-based epidemiology: Application to illicit drug monitoring. Environ. Sci. Technol. 2014, 48, 8162–8169. [Google Scholar] [CrossRef]
- Postigo, C.; de Alda, M.J.L.; Barceló, D. Drugs of abuse and their metabolites in the Ebro River basin: Occurrence in sewage and surface water, sewage treatment plants removal efficiency, and collective drug usage estimation. Environ. Int. 2010, 36, 75–84. [Google Scholar] [CrossRef]
- Baker, D.R.; Očenášková, V.; Kvicalova, M.; Kasprzyk-Hordern, B. Drugs of abuse in wastewater and suspended particulate matter—Further developments in sewage epidemiology. Environ. Int. 2012, 48, 28–38. [Google Scholar] [CrossRef]
- Irvine, R.J.; Kostakis, C.; Felgate, P.D.; Jaehne, E.J.; Chen, C.; White, J.M. Population drug use in Australia: A wastewater analysis. Forensic Sci. Int. 2011, 210, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasprzyk-Hordern, B. Testing wastewater in public health epidemiology: Pharmaceuticals, illicit drugs and the phenomenon of chirality. In The SfAM Meeting Control of Water-Borne Disease: A Century of the Activated Sludge Sewage Treatment Process; University of Bath: Bath, UK, 2014. [Google Scholar]
- Bordalo, A.A.; Teixeira, R.; Wiebe, W.J. A water quality index applied to an international shared river basin: The case of the Douro River. Environ. Manag. 2006, 38, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.P.; Marques, C. Faecal coliform bacteria in Febros River (Northwest Portugal): Temporal variation, correlation with water parameters, and species identification. Environ. Monit. Assess. 2006, 118, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.S.A. Modelação do Transporte e Dispersão de um Marcador Corado no Rio Febros. Ph.D. Thesis, Instituto Superior de Engenharia do Porto, Porto, Portugal, 2009. [Google Scholar]
- Bio, A.; Vieira, N.; Costa, M.J.; Valente, A. Assessment of habitat and water quality of the Portuguese Febros River and one of its tributaries. Limnetica 2011, 30, 103–116. [Google Scholar] [CrossRef]
- Boix, C.; Ibáñez, M.; Bijlsma, L.; Sancho, J.V.; Hernández, F. Investigation of cannabis biomarkers and transformation products in waters by liquid chromatography coupled to time of flight and triple quadrupole mass spectrometry. Chemosphere 2014, 99, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Ujváry, I.; Grotenhermen, F. 11-Nor-9-carboxy-∆ 9-tetrahydrocannabinol–a ubiquitous yet underresearched cannabinoid. A review of the literature. Cannabinoids 2014, 9, 1–8. [Google Scholar]
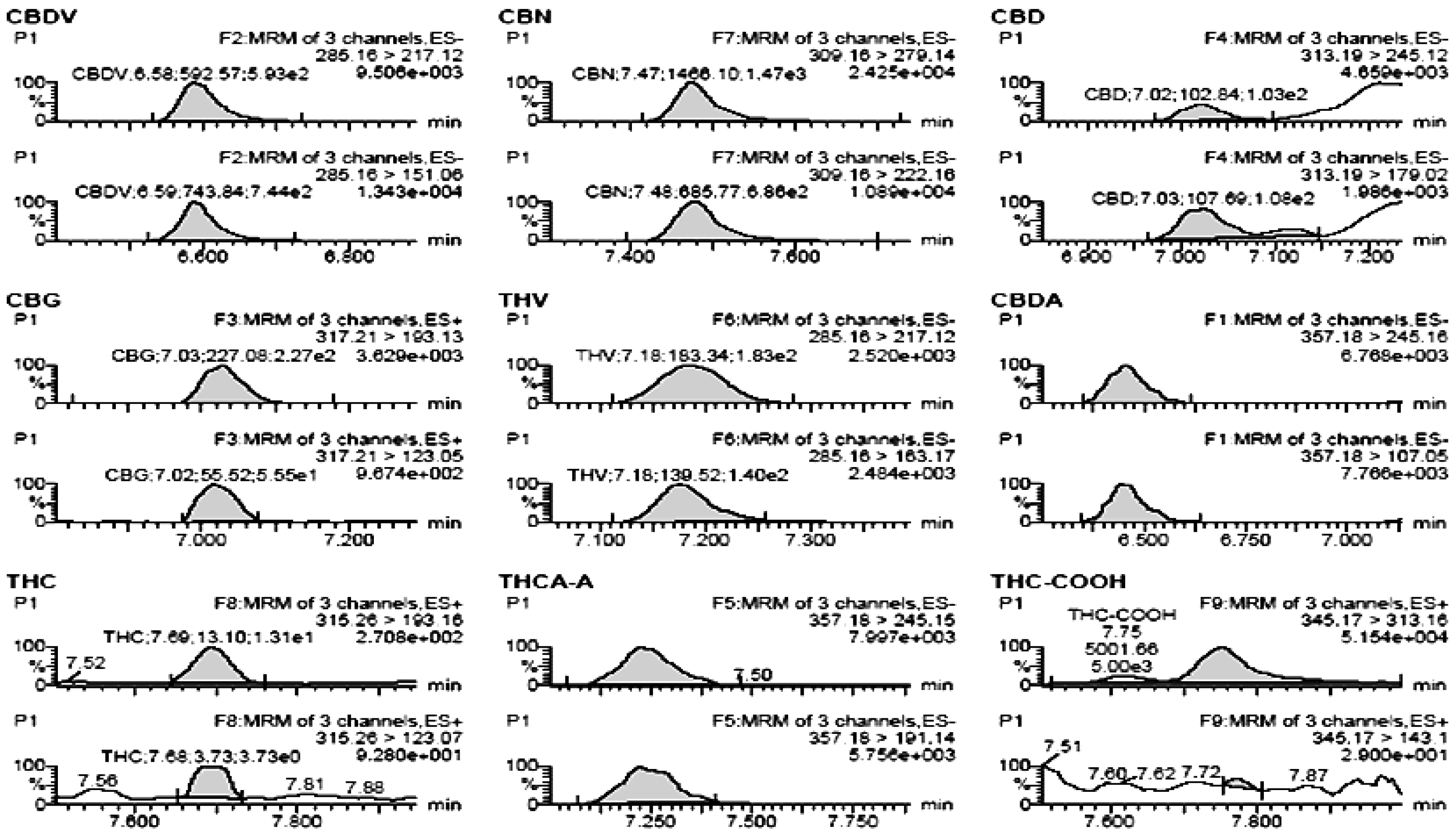
| Compound | Rt min | RSD min | 1st Standard S/N | LOQ (S/N) 10 μg L−1 | LOD (S/N) 3 μg L−1 |
|---|---|---|---|---|---|
| CBDV | 6.58 | 0.001 | 383.5 | 0.13 | 0.039 |
| CBN | 7.48 | 0.077 | 734.4 | 0.068 | 0.020 |
| CBD | 7.03 | 0.082 | 8.122 | 6.2 | 1.86 |
| CBG | 7.02 | 0.000 | 23.35 | 2.1 | 0.64 |
| THV | 7.19 | 0.080 | 16.78 | 3.0 | 0.89 |
| CBDA | 6.61 | 0.262 | 68.27 | 0.73 | 0.22 |
| THC | 7.69 | 0.075 | 20.25 | 0.25 | 0.74 |
| THCA-A | 7.40 | 0.135 | 40.97 | 1.2 | 0.37 |
| THC-COOH | 7.75 | 0.000 | 84.26 | 0.59 | 0.18 |
| Compound | Febros River | C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C (μg L−1) | RSD (%) | QC LOQ (%) | QC LOQ RSD (%) | QC 10LOQ (%) | Rec RSD (%) | C (μg L−1) | RSD (%) | QC LOQ (%) | QC LOQ RSD (%) | QC 10LOQ (%) | Rec RSD (%) | |
| CBDV | <LOQ | - | 99 | 3.5 | 101 | 2.5 | <LOQ | - | 98 | 7.2 | 96 | 9.4 |
| CBN | 0.079 | 3.0 | 91 | 1.9 | 91 | 1.1 | 0.076 | 4.8 | 94 | 3.1 | 92 | 2.2 |
| CBD | <LOQ | - | 102 | 11.4 | 100 | 13.7 | 0.096 | 4.3 | 129 | 4.5 | 133 | 1.8 |
| CBG | <LOQ | - | 76 | 7.6 | 73 | 8.5 | <LOQ | - | 67 | 10.8 | 64 | 15.0 |
| THV | <LOQ | - | 118 | 2.3 | 123 | 1.3 | <LOQ | - | 89 | 2.9 | 84 | 0.9 |
| CBDA | 0.070 | 7.9 | 114 | 1.7 | 110 | 1.2 | 0.065 | 0.0 | 124 | 5.2 | 125 | 3.2 |
| THC | <LOQ | - | 116 | 2.2 | 112 | 3.2 | <LOQ | - | 128 | 1.5 | 133 | 1.3 |
| THCA-A | 0.073 | 8.7 | 104 | 2.4 | 110 | 1.7 | 0.071 | 5.8 | 108 | 3.6 | 116 | 4.3 |
| THC-COOH | <LOQ | - | 98 | 7.5 | 102 | 6.3 | <LOQ | - | 74 | 8.5 | 71 | 9.8 |
| Compound | Febros River Water | WWPT Effluent Water | ||||
|---|---|---|---|---|---|---|
| Area | RSD (%) | C (μg L−1) | Area | RSD (%) | C (μg L−1) | |
| CBDV | 22.6 | 0.6 | <LOQ | 24.6 | 0.6 | <LOQ |
| CBN | 2503 | 4 | 0.079 | 2274 | 5 | 0.076 |
| CBD | 72.4 | 0.7 | <LOQ | 1205 | 4 | 0.096 |
| CBG | 20.5 | 0.3 | <LOQ | 8.0 | 0.4 | <LOQ |
| THV | 9.2 | 0.5 | <LOQ | 7.8 | 0.4 | <LOQ |
| CBDA | 2026 | 8 | 0.070 | 1350 | 5 | 0.065 |
| THC | 326.6 | - | <LOQ | 133.7 | 2.7 | <LOQ |
| THCA-A | 2098 | 9 | 0.073 | 1768 | 6 | 0.071 |
| THC-COOH | 87,000 | 33 | <LOQ | 5507 | 8 | <LOQ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milan, S.; Lelario, F.; Scrano, L.; Ottati, C.; Bufo, S.A.; Alpendurada, M.d.F. Detection of Eight Cannabinoids and One Tracer in Wastewater and River Water by SPE-UPLC–ESI-MS/MS. Water 2022, 14, 588. https://doi.org/10.3390/w14040588
Milan S, Lelario F, Scrano L, Ottati C, Bufo SA, Alpendurada MdF. Detection of Eight Cannabinoids and One Tracer in Wastewater and River Water by SPE-UPLC–ESI-MS/MS. Water. 2022; 14(4):588. https://doi.org/10.3390/w14040588
Chicago/Turabian StyleMilan, Simone, Filomena Lelario, Laura Scrano, Chiara Ottati, Sabino Aurelio Bufo, and Maria de Fátima Alpendurada. 2022. "Detection of Eight Cannabinoids and One Tracer in Wastewater and River Water by SPE-UPLC–ESI-MS/MS" Water 14, no. 4: 588. https://doi.org/10.3390/w14040588
APA StyleMilan, S., Lelario, F., Scrano, L., Ottati, C., Bufo, S. A., & Alpendurada, M. d. F. (2022). Detection of Eight Cannabinoids and One Tracer in Wastewater and River Water by SPE-UPLC–ESI-MS/MS. Water, 14(4), 588. https://doi.org/10.3390/w14040588









