Oxidation to Control Cyanobacteria and Cyanotoxins in Drinking Water Treatment Plants: Challenges at the Laboratory and Full-Scale Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Drinking Water Treatment Plant and Sampling Description
2.2. Oxidation and Stagnation Procedures
2.2.1. Oxidation
2.2.2. Stagnation
2.3. Sample Preparation and Analysis
2.3.1. Taxonomic Cell Counts
2.3.2. Dissolved Organic Carbon (DOC)
2.3.3. Microcystins (MCs)
2.3.4. DNA Extraction, Shotgun Metagenomic Sequencing Protocols, Bioinformatics Description, and Statistical Analysis
2.3.5. Droplet Digital PCR (ddPCR)
2.3.6. Solid Analysis
3. Results
3.1. Characteristics of Untreated Sludge
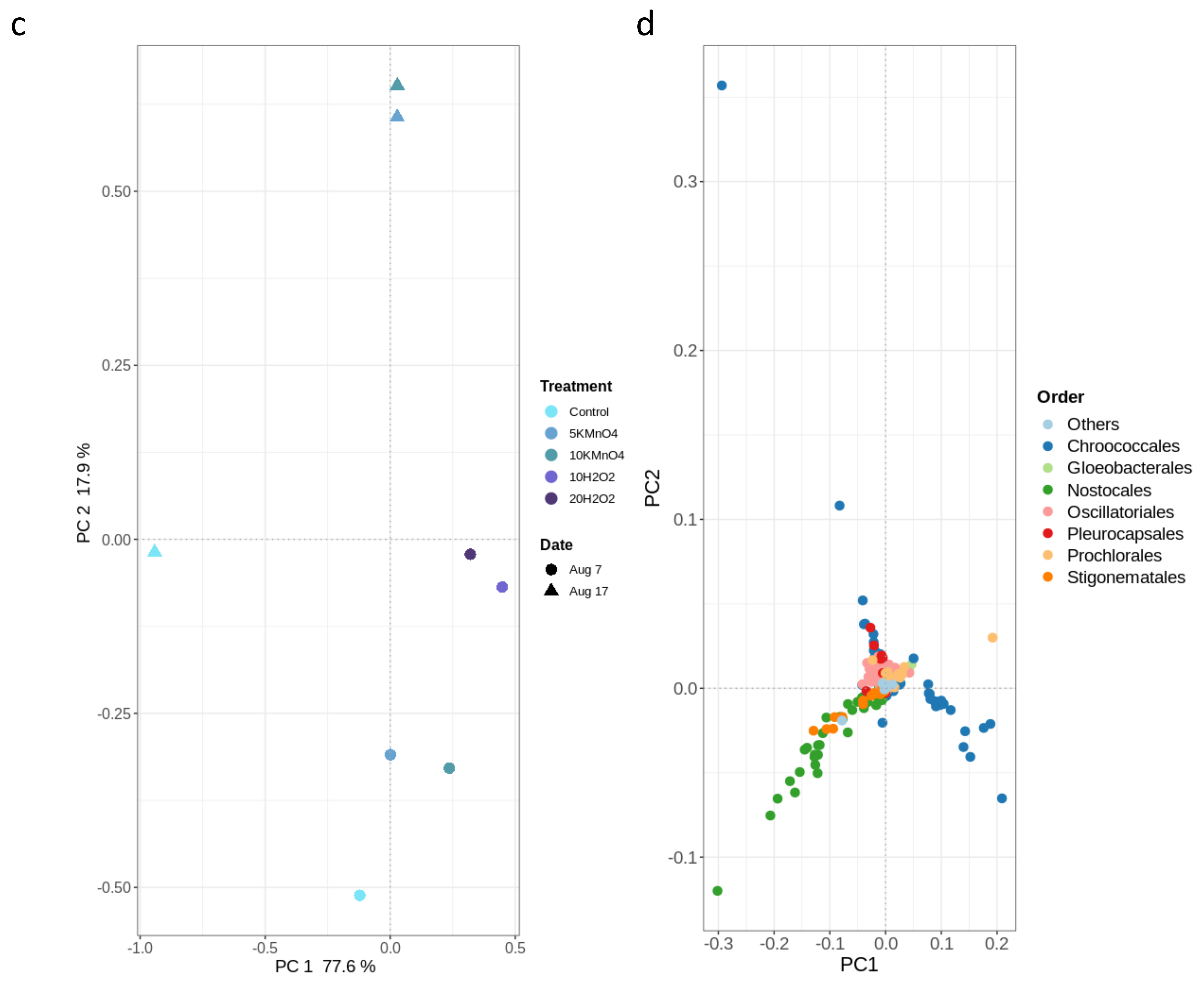
3.2. Oxidation of Cyanobacteria-Laden Sludge in Controlled Conditions
3.3. Oxidation of Cyanobacteria-Laden Sludge in a Full-Scale Plant
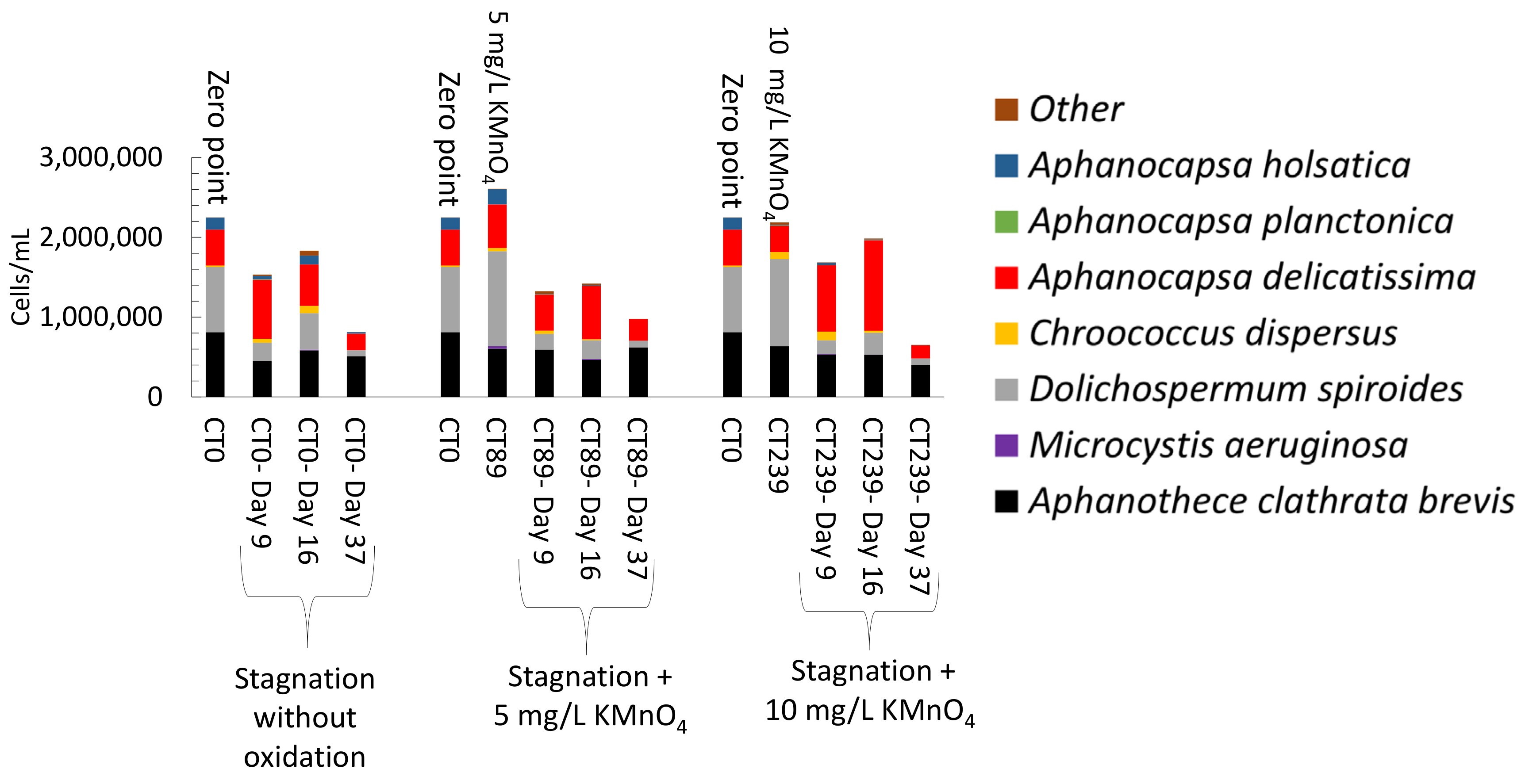
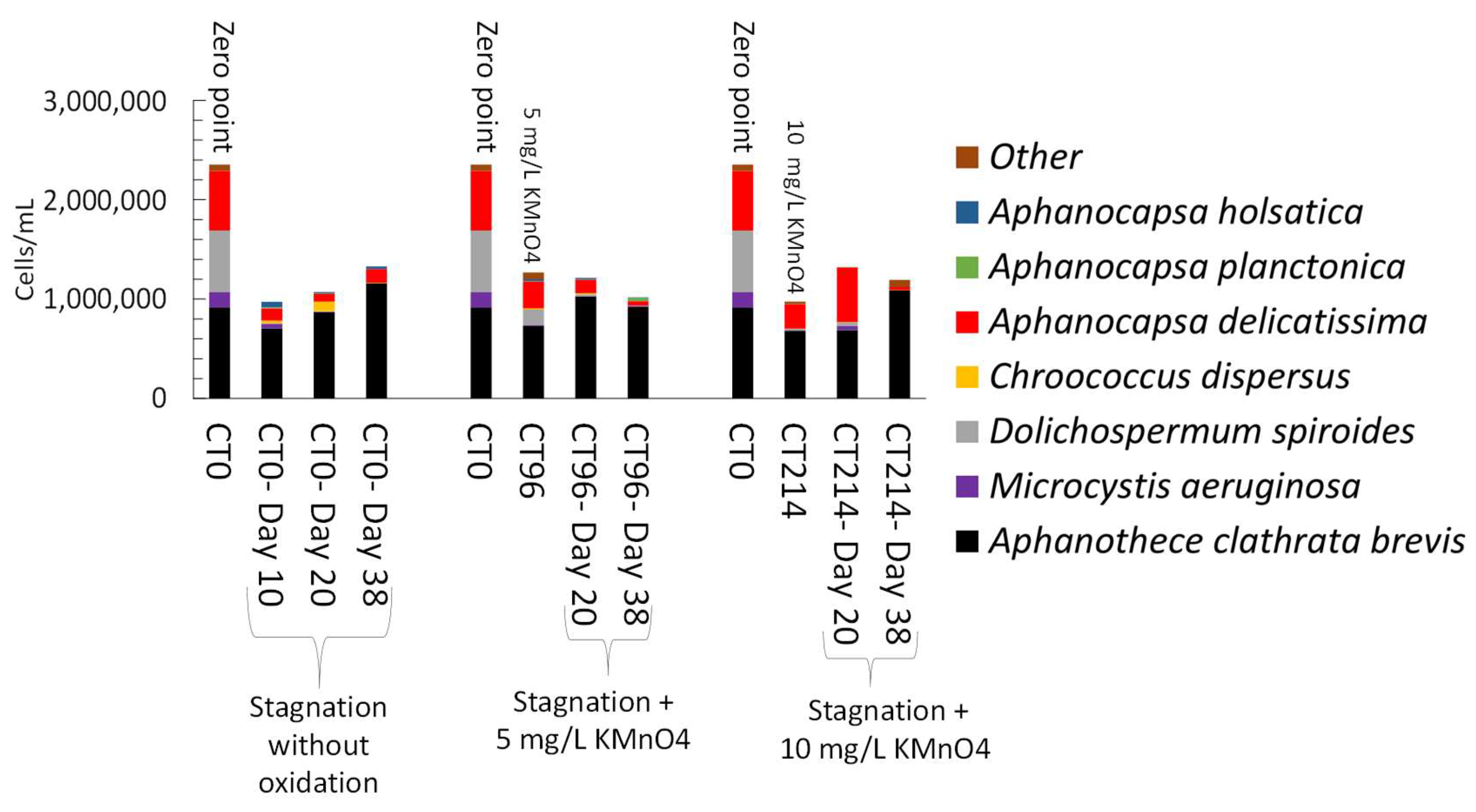
3.4. Impact of Stagnation on Oxidized Cyanobacteria-Laden Sludge
3.4.1. Stagnation after Laboratory Oxidation
3.4.2. Stagnation after On-Site Oxidation
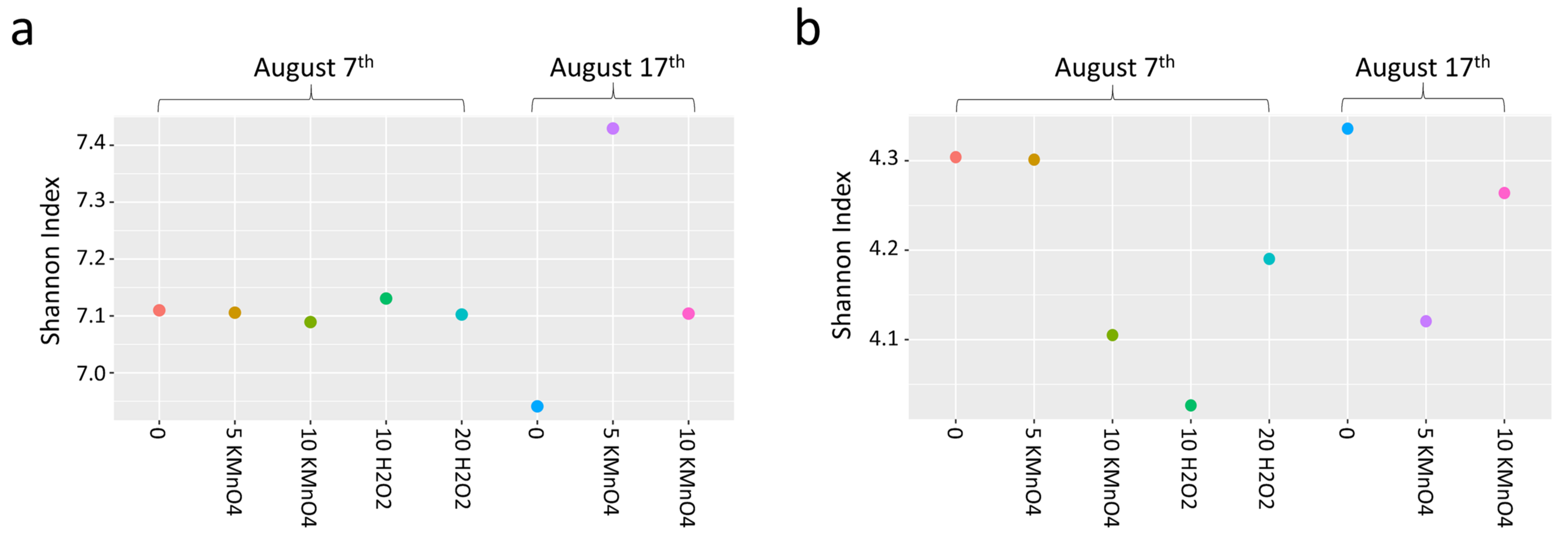
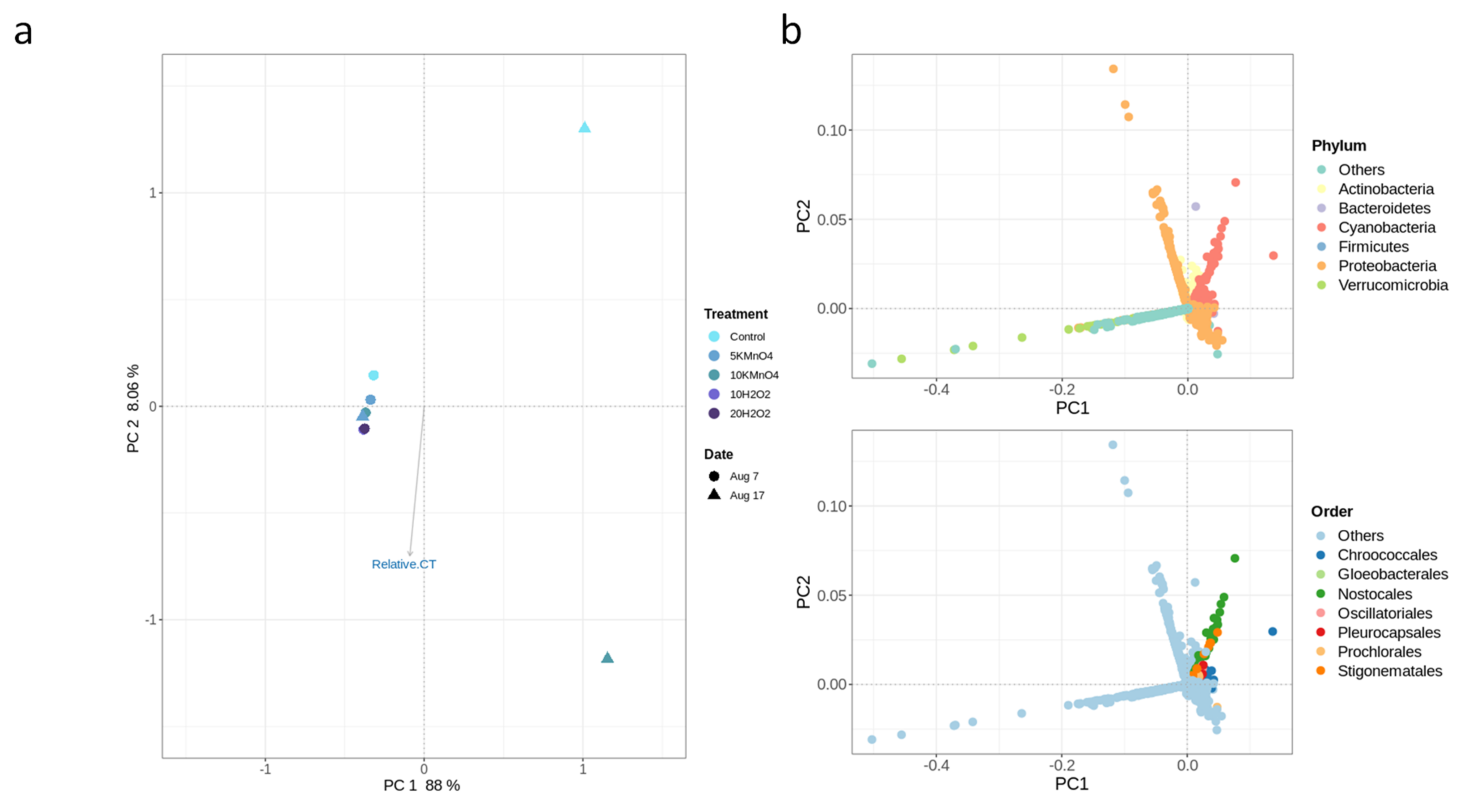
3.5. Factors Affecting the Fate of Cyanobacteria and MCs in Sludge
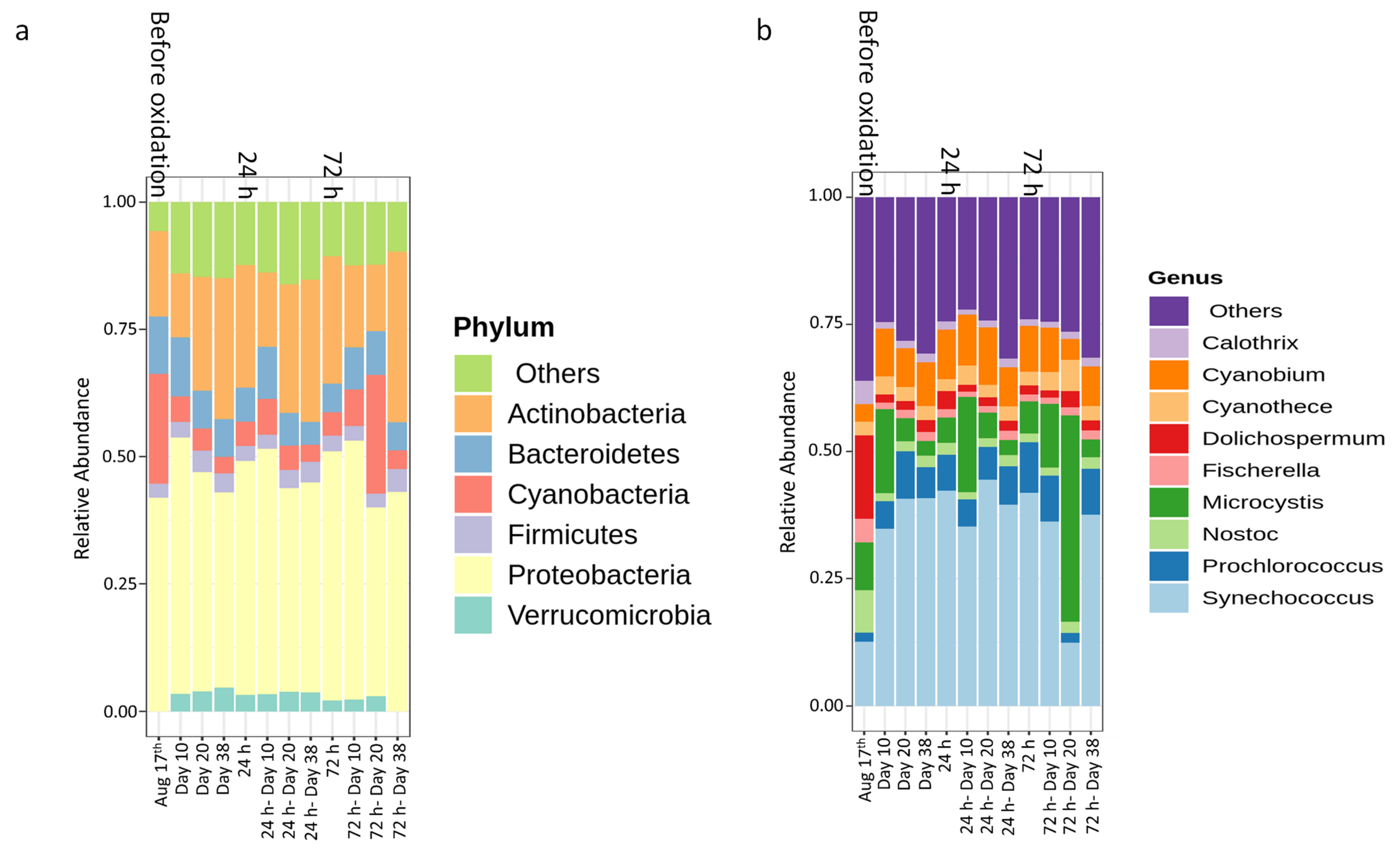
- MCs detected in the sludge are present in dissolved and cell-bound forms and this partitioning is affected by storage and oxidation. Cyanobacterial cell integrity losses can lead to the release of cell-bound cyanotoxins after oxidation [27,28] and as revealed by this study, after stagnation. Released dissolved MCs can be oxidized [31], adsorbed onto PAC or flocs [11,53] or can be biodegraded by species belonging to Proteobacteria, Actinobacteria, and Firmicutes [54,55].
- Oxidation causes significant changes in microbial diversity leading to the selective persistence of some cyanobacterial species over other communities [29,30] and nutrient availability [11] as obtained here by the increase of DOC (data not shown). These factors could explain the observed growth of some cyanobacterial species proceeded by oxidation and stagnation.
- Oxidative stress (ex. H2O2 and nutrient depletion) may cause gene expression regulation of mcy genes, leading to MCs production [56,57]. There are no data about gene expression regulation in the presence of KMnO4 in water or sludge samples. In our investigation, the combination of oxidation and stagnation represents an increase in oxidative stress.
4. Conclusions
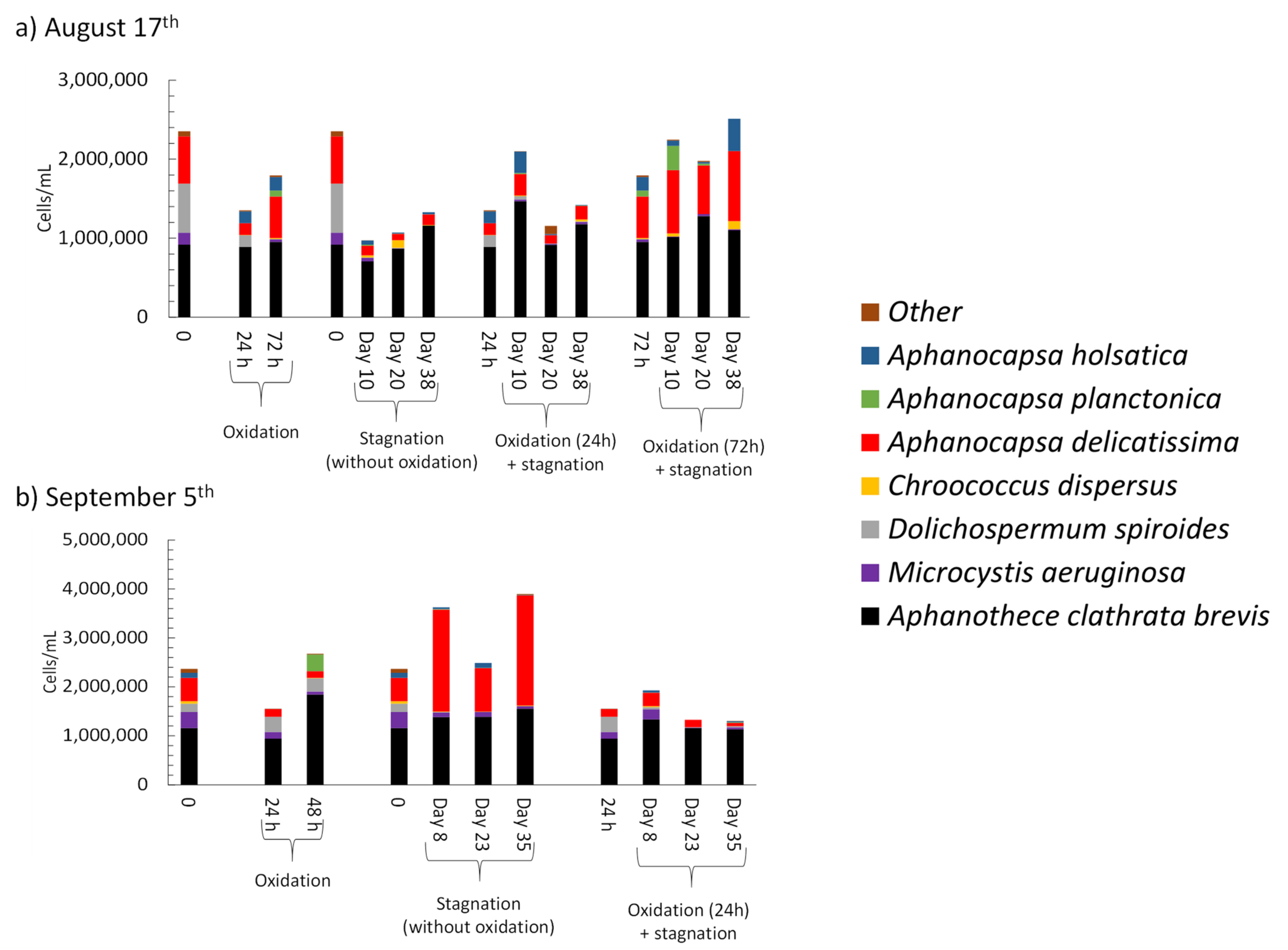
- Controlled laboratory oxidation with KMnO4 and H2O2 decreased total taxonomic counts, as well as potential MC producers. The highest observed cell count decrease was from 2.7 × 106 cells/mL to 6.2 × 105 cells/mL (77%) when applying 20 mg/L H2O2. However, stagnation after controlled oxidation (laboratory scale) led to an increase of cyanobacterial cells in 8 out of 22 samples as compared to oxidation alone. The highest cell count increase after stagnation was from 6.2 × 105 cells/mL to 1.6 × 106 cells/mL (2.6-fold increase) observed in the sludge sample oxidized by 20 mg/L H2O2 and stagnated for 17 days. KMnO4 (10 mg/L) and H2O2 (20 mg/L) could decrease MC concentration up to 98% (from 63 ng/L to below detection limit) and 41% (from 139 ng/L to 77 ng/L), respectively. Laboratory oxidation did not lead to higher production of MCs during stagnation. When comparing H2O2 and KMnO4 oxidation results in the same day, H2O2, with greater half-lives than KMnO4, was more effective than KMnO4 for a decrease of cyanobacterial cells and cyanotoxin concentrations. Similarly, oxidation/stagnation caused cell growth in 14 out of 22 samples by up to 145% as compared to stagnation alone.
- Laboratory oxidation shifted cyanobacterial diversity from Nostocales (Dolichospermum) towards Chroococcales (Microcystis) and Synechococcales (Synechococcus) as the persistent orders (genus). Opposite to KMnO4 oxidation, in which no selective removal within the cyanobacterial community was observed, H2O2 selectively reduced Chroococcales.
- Short-term (24 h) reduction on total taxonomic cell counts was observed during shock on-site oxidation. Indeed, on-site shock oxidation could decrease total taxonomic cell counts up to 43% (after 24 h). In contrast, it did not deplete cyanobacteria in the sludge supernatant. However, stagnation led to exceeding total cell counts by up to 55% in six out of nine samples as compared to on-site oxidation alone. Partial cell growth was also observed in MC producer genera such as Microcystis, Chroococcus, and Aphanocapsa.
- Total MCs after stagnation preceded by shock on-site oxidation (10 mg/L KMnO4) remained below initial MCs concentrations. However, mcyD gene copy numbers increased during stagnation, suggesting the growth of potential MC producer species.
- As compared to storage (stagnation) only, sludge oxidation with KMnO4 and H2O2 at the dosages studied did not bring remarkable additional benefits for the mitigation of cyanobacterial cells and cyanotoxins during subsequent storage. In some cases, oxidation prior to storage led to enhanced growth of potential MC producers in the sludge. Continuous application of oxidants or higher concentrations may prove to be more effective.
- These results demonstrate the interest in developing strategies that minimize cyanobacteria and cyanotoxin accumulation in the stored sludge, such as pre-oxidation, PAC application, and source treatment.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, J.C.; Michalak, A.M. Exploring temperature and precipitation impacts on harmful algal blooms across continental U.S. lakes. Limnol. Oceanogr. 2019, 65, 992–1009. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Pick, F.R. Blooming algae: A Canadian perspective on the rise of toxic cyanobacteria. Can. J. Fish. Aquat. Sci. 2016, 73, 1149–1158. [Google Scholar] [CrossRef] [Green Version]
- Kimambo, O.N.; Gumbo, J.R.; Chikoore, H. The occurrence of cyanobacteria blooms in freshwater ecosystems and their link with hydro-meteorological and environmental variations in Tanzania. Heliyon 2019, 5, e01312. [Google Scholar] [CrossRef] [Green Version]
- Winter, J.G.; DeSellas, A.M.; Fletcher, R.; Heintsch, L.; Morley, A.; Nakamoto, L.; Utsumi, K. Algal blooms in Ontario, Canada: Increases in reports since 1994. Lake Reserv. Manag. 2011, 27, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Chorus, I.; Fastner, J.; Welker, M. Cyanobacteria and Cyanotoxins in a Changing Environment: Concepts, Controversies, Challenges. Water 2021, 13, 2463. [Google Scholar] [CrossRef]
- Drikas, M.; Chow, C.W.K.; House, J.; Burch, M.D. Using coagulation, flocculation, and settling to remove toxic cyanobacteria. J. Am. Water Works Assoc. 2001, 93, 100–111. [Google Scholar] [CrossRef]
- Zamyadi, A.; Dorner, S.; Ellis, D.; Bolduc, A.; Bastien, C.; Prévost, M. Species-dependence of cyanobacteria removal efficiency by different drinking water treatment processes. Water Res. 2013, 47, 2689–2700. [Google Scholar] [CrossRef]
- Newcombe, G.; House, J.; Ho, L.; Baker, P.; Burch, M. Management Strategies For Cyanobacteria (Blue-Green Algae): A Guide for Water Utilities; The Cooperative Research Centre for Water Quality and Treatment: Adelaïde, Australia, 2010; p. 112. [Google Scholar]
- Chorus, I.; Welker, M. Toxic Cyanobacteria In Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Jalili, F.; Trigui, H.; Guerra Maldonado, J.F.; Dorner, S.; Zamyadi, A.; Shapiro, B.J.; Terrat, Y.; Fortin, N.; Sauvé, S.; Prévost, M. Can cyanobacterial diversity in the source predict the diversity in sludge and the risk of toxin release in a drinking water treatment plant? Toxins 2021, 13, 25. [Google Scholar] [CrossRef]
- Shang, L.; Feng, M.; Xu, X.; Liu, F.; Ke, F.; Li, W. Co-occurrence of microcystins and taste-and-odor compounds in drinking water source and their removal in a full-scale drinking water treatment plant. Toxins 2018, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Zamyadi, A.; MacLeod, S.; Fan, Y.; McQuaid, N.; Dorner, S.; Sauvé, S.; Prévost, M. Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: A monitoring and treatment challenge. Water Res. 2012, 46, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Pei, H.-Y.; Hu, W.-R.; Ma, C.-X. The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes. Chem. Eng. J. 2012, 193–194, 196–202. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Rosa, M.J. Comparing dissolved air flotation and conventional sedimentation to remove cyanobacterial cells of Microcystis aeruginosa. Part II. The effect of water background organics. Sep. Purif. Technol. 2007, 53, 126–134. [Google Scholar] [CrossRef]
- Almuhtaram, H.; Cui, Y.; Zamyadi, A.; Hofmann, R. Cyanotoxins and cyanobacteria cell accumulations in drinking water treatment plants with a low risk of bloom formation at the source. Toxins 2018, 10, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamyadi, A.; Dorner, S.; Ndong, M.; Ellis, D.; Bolduc, A.; Bastien, C.; Prévost, M. Low-risk cyanobacterial bloom sources: Cell accumulation within full-scale treatment plants. J. Am. Water Work. Assoc. 2013, 102, E651–E663. [Google Scholar] [CrossRef] [Green Version]
- Zamyadi, A.; Henderson, R.K.; Stuetz, R.; Newcombe, G.; Newtown, K.; Gladman, B. Cyanobacterial management in full-scale water treatment and recycling processes: Reactive dosing following intensive monitoring. Environ. Sci. Water Res. Technol. 2016, 2, 362–375. [Google Scholar] [CrossRef]
- Zamyadi, A.; Romanis, C.; Mills, T.; Neilan, B.; Choo, F.; Coral, L.A.; Gale, D.; Newcombe, G.; Crosbie, N.; Stuetz, R.; et al. Diagnosing water treatment critical control points for cyanobacterial removal: Exploring benefits of combined microscopy, next-generation sequencing, and cell integrity methods. Water Res. 2019, 152, 96–105. [Google Scholar] [CrossRef]
- Ho, L.; Dreyfus, J.; Boyer, J.E.; Lowe, T.; Bustamante, H.; Duker, P.; Meli, T.; Newcombe, G. Fate of cyanobacteria and their metabolites during water treatment sludge management processes. Sci. Total Environ. 2012, 424, 232–238. [Google Scholar] [CrossRef]
- Sun, F.; Pei, H.-Y.; Hu, W.-R.; Li, X.-Q.; Ma, C.-X.; Pei, R.-T. The cell damage of Microcystis aeruginosa in PACl coagulation and floc storage processes. Sep. Purif. Technol. 2013, 115, 123–128. [Google Scholar] [CrossRef]
- Xu, H.; Pei, H.; Xiao, H.; Jin, Y.; Li, X.; Hu, W.; Ma, C.; Sun, J.; Li, H. Behaviors of Microcystis aeruginosa cells during floc storage in drinking water treatment process. Sci. Rep. 2016, 6, 34943. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Xu, H.; Pei, H.; Jin, Y.; Li, H.; Ma, C. Worse than cell lysis: The resilience of Oscillatoria sp. during sludge storage in drinking water treatment. Water Res. 2018, 142, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pei, H.; Xu, H.; Jin, Y.; Sun, J. Behavior of Cylindrospermopsis raciborskii during coagulation and sludge storage-higher potential risk of toxin release than Microcystis aeruginosa? J. Hazard. Mater. 2018, 347, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, G.; Dreyfus, J.; Monrolin, Y.; Pestana, C.; Reeve, P.; Sawade, E.; Ho, L.; Chow, C. Optimizing Conventional Treatment for the Removal of Cyanobacteria and Toxins; Water Research Foundation (WRF): Denver, CO, USA, 2015; ISBN 978-1-60573-216-9. [Google Scholar]
- Jalili, F. Optimal Treatment Strategies to Prevent and Manage Cyanobacteria and Cyanotoxins in Drinking Water Sludge. Ph.D. Thesis, Polytechnique Montréal, Montréal, QC, Canada, 2022. [Google Scholar]
- Fan, J.; Ho, L.; Hobson, P.; Brookes, J. Evaluating the effectiveness of copper sulphate, chlorine, potassium permanganate, hydrogen peroxide and ozone on cyanobacterial cell integrity. Water Res. 2013, 47, 5153–5164. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Daly, R.; Hobson, P.; Ho, L.; Brookes, J. Impact of potassium permanganate on cyanobacterial cell integrity and toxin release and degradation. Chemosphere 2013, 92, 529–534. [Google Scholar] [CrossRef]
- Moradinejad, S.; Trigui, H.; Guerra Maldonado, J.F.; Shapiro, J.; Terrat, Y.; Zamyadi, A.; Dorner, S.; Prévost, M. Diversity assessment of toxic cyanobacterial blooms during oxidation. J. Toxins 2020, 12, 728. [Google Scholar] [CrossRef] [PubMed]
- Lusty, M.W.; Gobler, C.J. The efficacy of hydrogen peroxide in mitigating cyanobacterial blooms and altering microbial communities across four lakes in NY, USA. Toxins 2020, 12, 428. [Google Scholar] [CrossRef]
- Piezer, K.; Li, L.; Jeon, Y.; Kadudula, A.; Seo, Y. Review: The application of potassium permanganate to treat cyanobacteria-laden water. Proc. Saf. Environ. Prot. 2021, 148, 400–414. [Google Scholar] [CrossRef]
- Matthijs, H.C.P.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef]
- Pestana, C.J.; Reeve, P.J.; Sawade, E.; Voldoire, C.F.; Newton, K.; Praptiwi, R.; Collingnon, L.; Dreyfus, J.; Hobson, P.; Gaget, V.; et al. Fate of cyanobacteria in drinking water treatment plant lagoon supernatant and sludge. Sci. Total Environ. 2016, 565, 1192–1200. [Google Scholar] [CrossRef]
- Fan, J.J.; Hobson, P.; Ho, L.; Daly, R.; Brookes, J. The effects of various control and water treatment processes on the membrane integrity and toxin fate of cyanobacteria. J. Hazard. Mater. 2014, 264, 313–322. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 5, p. 1360. [Google Scholar]
- Fan, J.J.; Ho, L.; Hobson, P.; Daly, R.; Brookes, J. Application of various oxidants for cyanobacteria control and cyanotoxin removal in wastewater treatment. J. Environ. Eng. 2014, 140, 04014022. [Google Scholar] [CrossRef]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal number and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Lund, J.W.G. A simple counting chamber for Nannoplankton. Limnol. Oceanogr. 1959, 4, 57–65. [Google Scholar] [CrossRef]
- Planas, D.; Desrosiers, M.; Groulx, S.R.; Paquet, S.; Carignan, R. Pelagic and benthic algal responses in eastern Canadian Boreal Shield lakes following harvesting and wildfires. Can. J. Fish. Aquat. Sci. 2000, 57, 136–145. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Method 415.1. Organic Carbon, Total (Combustion or Oxidation); United States Environmental Protection Agency: Washington, DC, USA, 1974; p. 3. [Google Scholar]
- Munoz, G.; Vo Duy, S.; Roy-Lachapelle, A.; Husk, B.; Sauve, S. Analysis of individual and total microcystins in surface water by on-line preconcentration and desalting coupled to liquid chromatography tandem mass spectrometry. J. Chromatogr. 2017, 1516, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Roy-Lachapelle, A.; Vo Duy, S.; Munoz, G.; Dinh, Q.T.; Bahl, E.; Simon, D.F.; Sauvé, S. Analysis of multiclass cyanotoxins (microcystins, anabaenopeptins, cylindrospermopsin and anatoxins) in lake waters using on-line SPE liquid chromatography high-resolution Orbitrap mass spectrometry. Anal. Methods 2019, 11, 5289–5300. [Google Scholar] [CrossRef] [Green Version]
- Moradinejad, S. Characterization of Phenomenon Governing Oxidation of Cyanobacteria through Metagenomics. Ph.D. Thesis, Polytechnique Montréal, Montréal, QC, Canada, 2021. [Google Scholar]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Greenacre, M. Compositional Data Analysis in Practice; Chapman and Hall/CRC Press: London, UK, 2018; 136p, ISBN 978-1-138-31661-4. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward selection of explanatory variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef]
- Fortin, N.; Aranda-Rodriguez, R.; Jing, H.; Pick, F.; Bird, D.; Greer, C.W. Detection of microcystin-producing cyanobacteria in Missisquoi Bay, Quebec, Canada, using quantitative PCR. Appl. Environ. Microbiol. 2010, 76, 5105–5112. [Google Scholar] [CrossRef] [Green Version]
- Moradinejad, S.; Trigui, H.; Maldonado, J.F.G.; Shapiro, B.J.; Terrat, Y.; Sauvé, S.; Fortin, N.; Zamyadi, A.; Dorner, S.; Prévost, M. Metagenomic study to evaluate functional capacity of a cyanobacterial bloom during oxidation. Chem. Eng. J. Adv. 2021, 8, 100151. [Google Scholar] [CrossRef]
- Szlag, D.C.; Spies, B.; Szlag, R.G.; Westrick, J.A. Permanganate Oxidation of Microcystin-LA: Kinetics, Quantification, and Implications for Effective Drinking Water Treatment. J. Toxicol. 2019, 2019, 3231473. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, H.J.; Lee, K.M.; Seo, J.; Lee, C. Oxidation of Microcystins by Permanganate: pH and Temperature-Dependent Kinetics, Effect of DOM Characteristics, and Oxidation Mechanism Revisited. Environ. Sci. Technol. 2018, 52, 7054–7063. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shao, C.; Lin, T.-F.; Shen, J.; Yu, S.; Shang, R.; Yin, D.; Zhang, K.; Gao, N. Kinetics of cell inactivation, toxin release, and degradation during permanganation of Microcystis aeruginosa. Environ. Sci. Technol. 2014, 48, 2885–2892. [Google Scholar] [CrossRef]
- Dreyfus, J.; Monrolin, Y.; Pestana, C.J.; Reeve, P.J.; Sawade, E.; Newton, K.; Ho, L.; Chow, C.W.K.; Newcombe, G. Identification and assessment of water quality risks associated with sludge supernatant recycling in the presence of cyanobacteria. J. Water Suppl. Res. Technol. Aqua 2016, 65, 441–452. [Google Scholar] [CrossRef]
- Ma, C.; Pei, H.; Hu, W.; Xu, H.; Jin, Y. The lysis and regrowth of toxic cyanobacteria during storage of achitosan–aluminium chloride composite coagulated sludge: Implications for drinking water sludge treatment. RSC Adv. 2016, 6, 112756–112764. [Google Scholar] [CrossRef]
- Kormas, K.A.; Lymperopoulou, D.S. Cyanobacterial toxin degrading bacteria: Who are they? Biomed. Res. Int. 2013, 2013, 463894. [Google Scholar] [CrossRef] [Green Version]
- Maghsoudi, E.; Fortin, N.; Greer, C.; Maynard, C.; Page, A.; Duy, S.V.; Sauve, S.; Prevost, M.; Dorner, S. Cyanotoxin degradation activity and mlr gene expression profiles of a Sphingopyxis sp. isolated from Lake Champlain, Canada. Environ. Sci. Proc. Impacts 2016, 18, 1417–1426. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, J.S.; Giani, A. Microcystin production and regulation under nutrient stress conditions in toxic microcystis strains. Appl. Environ. Microbiol. 2014, 80, 5836–5843. [Google Scholar] [CrossRef] [Green Version]
- Schatz, D.; Keren, Y.; Vardi, A.; Sukenik, A.; Carmeli, S.; Börner, T.; Dittmann, E.; Kaplan, A. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol. 2007, 9, 965–970. [Google Scholar] [CrossRef]



| Date | Oxidant | Initial Applied Dose (mg/L) | Contact Time (h) | Oxidant/DOC Ratio |
|---|---|---|---|---|
| 31 July | KMnO4 | 5 | 1: Laboratory assay | 1.4 |
| 10 | 2.8 | |||
| 7 August | 5 | 1.6 | ||
| 10 | 3.1 | |||
| H2O2 | 10 | 24: Laboratory assay | 3.1 | |
| 20 | 6.3 | |||
| 17 August | KMnO4 | 5 | 1: Laboratory assay 24, 72: On-site assay (10 mg/L) | 1.5 |
| 10 | 3.1 | |||
| 5 September | KMnO4 | 10 | 24, 48: On-site assay (10 mg/L) | 1.0 |
| Date | 31 July | 7 August | 17 August | 17 August | ||||
|---|---|---|---|---|---|---|---|---|
| Oxidant | KMnO4 | KMnO4 | KMnO4 | H2O2 | ||||
| Dose (mg/L) | 5 | 10 | 5 | 10 | 5 | 10 | 10 | 20 |
| k (min−1) | 0.054 | 0.037 | 0.044 | 0.042 | 0.049 | 0.043 | 0.0024 | 0.0022 |
| Half-life (min) | 12.8 | 18.7 | 15.8 | 16.5 | 14.1 | 16.1 | 288.8 | 315.0 |
| R2 | 0.92 | 0.90 | 0.90 | 0.92 | 0.91 | 0.93 | 0.95 | 0.99 |
| CT (mg·min/L) | 89 | 239 | 105 | 218 | 96 | 214 | 4090 | 7781 |
| Relative CT | 0.37 | 1.0 | 0.44 | 0.91 | 0.40 | 0.90 | 0.53 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalili, F.; Trigui, H.; Maldonado, J.F.G.; Dorner, S.; Zamyadi, A.; Shapiro, B.J.; Terrat, Y.; Fortin, N.; Sauvé, S.; Prévost, M. Oxidation to Control Cyanobacteria and Cyanotoxins in Drinking Water Treatment Plants: Challenges at the Laboratory and Full-Scale Plants. Water 2022, 14, 537. https://doi.org/10.3390/w14040537
Jalili F, Trigui H, Maldonado JFG, Dorner S, Zamyadi A, Shapiro BJ, Terrat Y, Fortin N, Sauvé S, Prévost M. Oxidation to Control Cyanobacteria and Cyanotoxins in Drinking Water Treatment Plants: Challenges at the Laboratory and Full-Scale Plants. Water. 2022; 14(4):537. https://doi.org/10.3390/w14040537
Chicago/Turabian StyleJalili, Farhad, Hana Trigui, Juan Francisco Guerra Maldonado, Sarah Dorner, Arash Zamyadi, B. Jesse Shapiro, Yves Terrat, Nathalie Fortin, Sébastien Sauvé, and Michèle Prévost. 2022. "Oxidation to Control Cyanobacteria and Cyanotoxins in Drinking Water Treatment Plants: Challenges at the Laboratory and Full-Scale Plants" Water 14, no. 4: 537. https://doi.org/10.3390/w14040537
APA StyleJalili, F., Trigui, H., Maldonado, J. F. G., Dorner, S., Zamyadi, A., Shapiro, B. J., Terrat, Y., Fortin, N., Sauvé, S., & Prévost, M. (2022). Oxidation to Control Cyanobacteria and Cyanotoxins in Drinking Water Treatment Plants: Challenges at the Laboratory and Full-Scale Plants. Water, 14(4), 537. https://doi.org/10.3390/w14040537








