Fish Habitat Reclamation Based on Geographical Morphology Heterogeneity in the Yangtze River and the Short-Term Effects on Fish Community Structure
Abstract
:1. Introduction
2. Materials and Methods
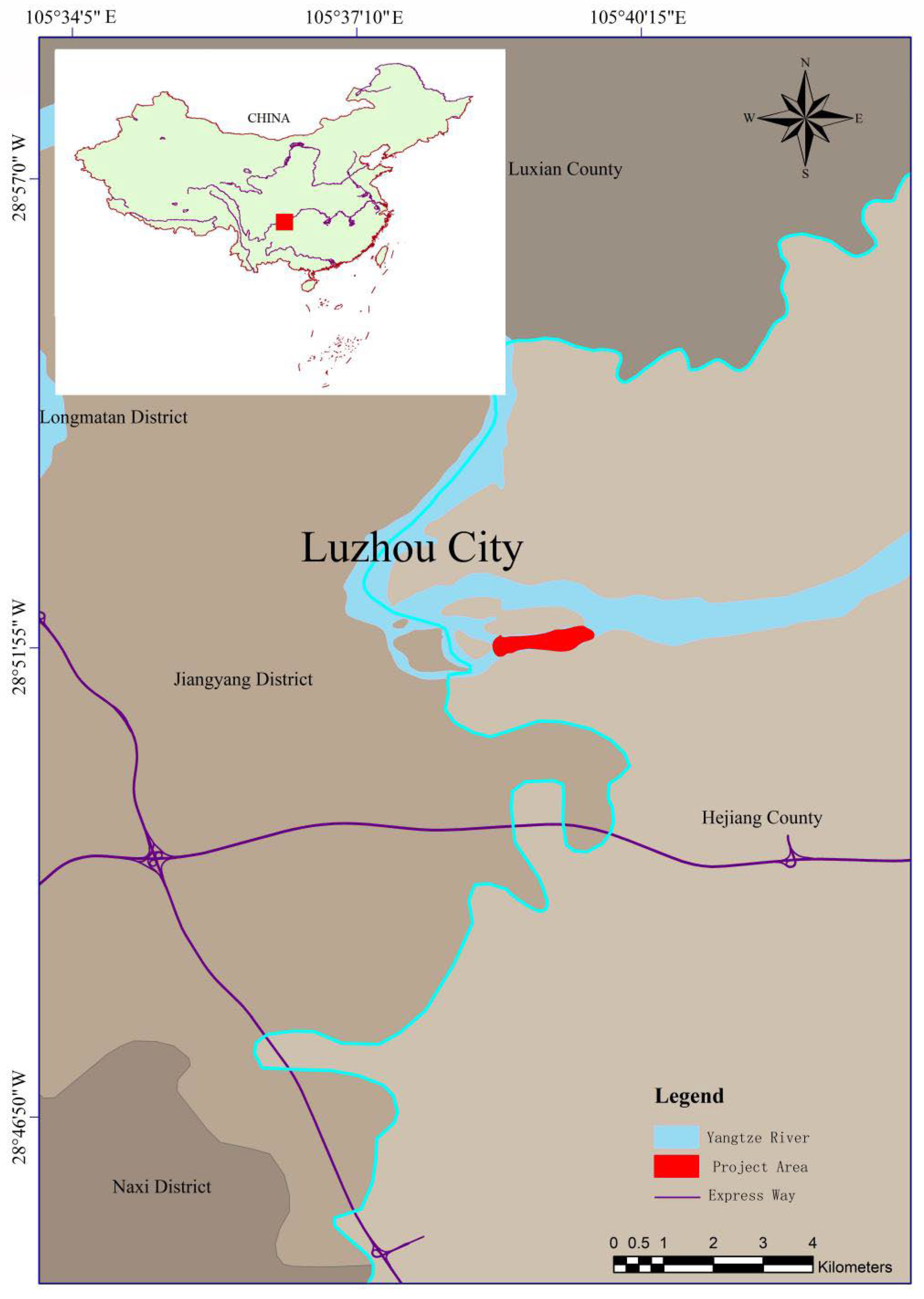
2.1. Study Area
2.2. Restoration Project
2.3. Simulation of the Influence of Projects on Hydrological Conditions
2.3.1. Numerical Computational Model
2.3.2. Study Design for Water Flow Pattern Change
2.3.3. Governing Equations and Turbulence Models
2.4. Numerical Grid Parameters
2.5. Hydromorphological Survey and Model Validation
2.6. Fish Survey
2.7. Statistical Analysis
2.7.1. Fish Composition Analysis
2.7.2. Fish Ecological Behavior Trait Analysis
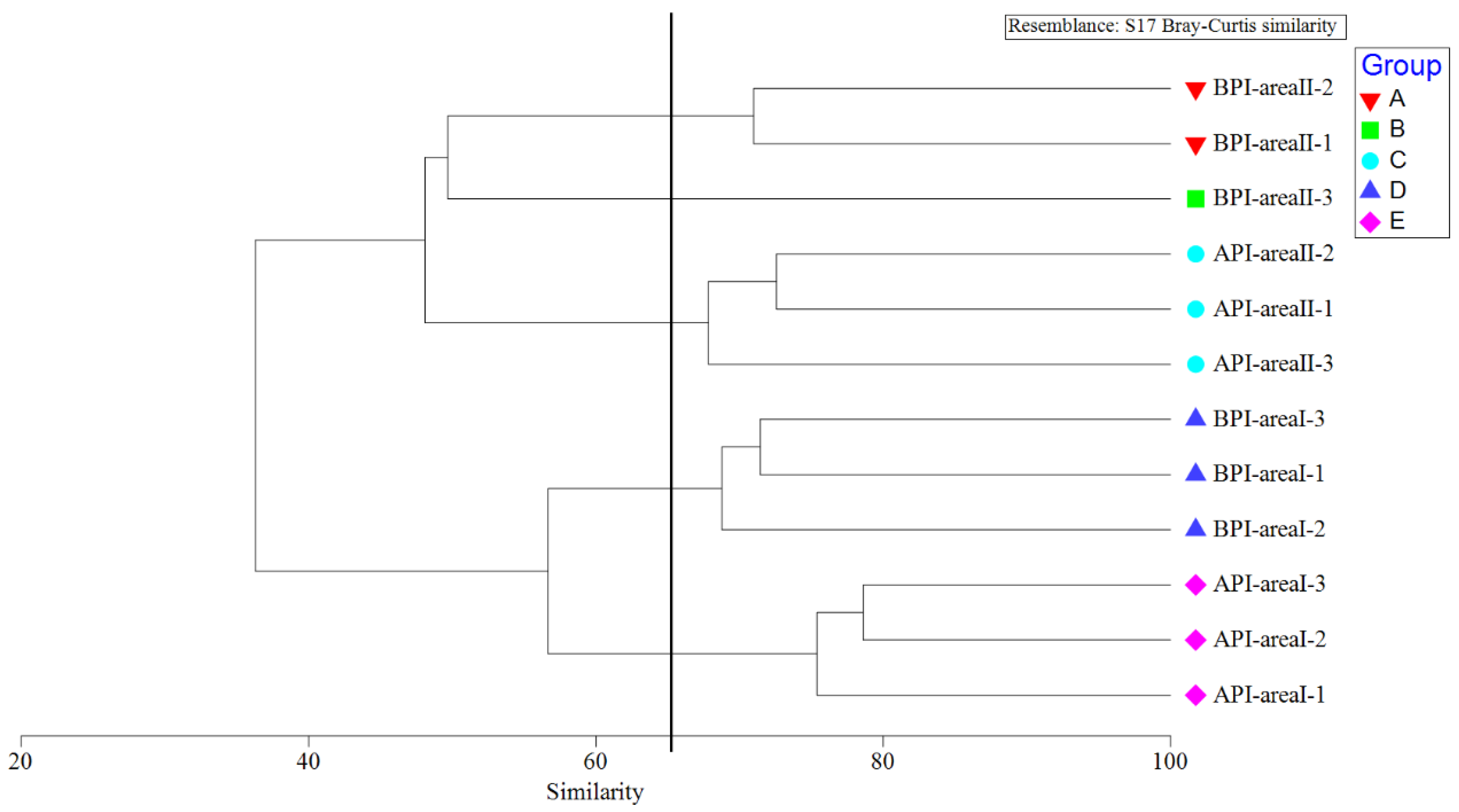
2.7.3. Cluster Analysis of Fish Community Structure
3. Results
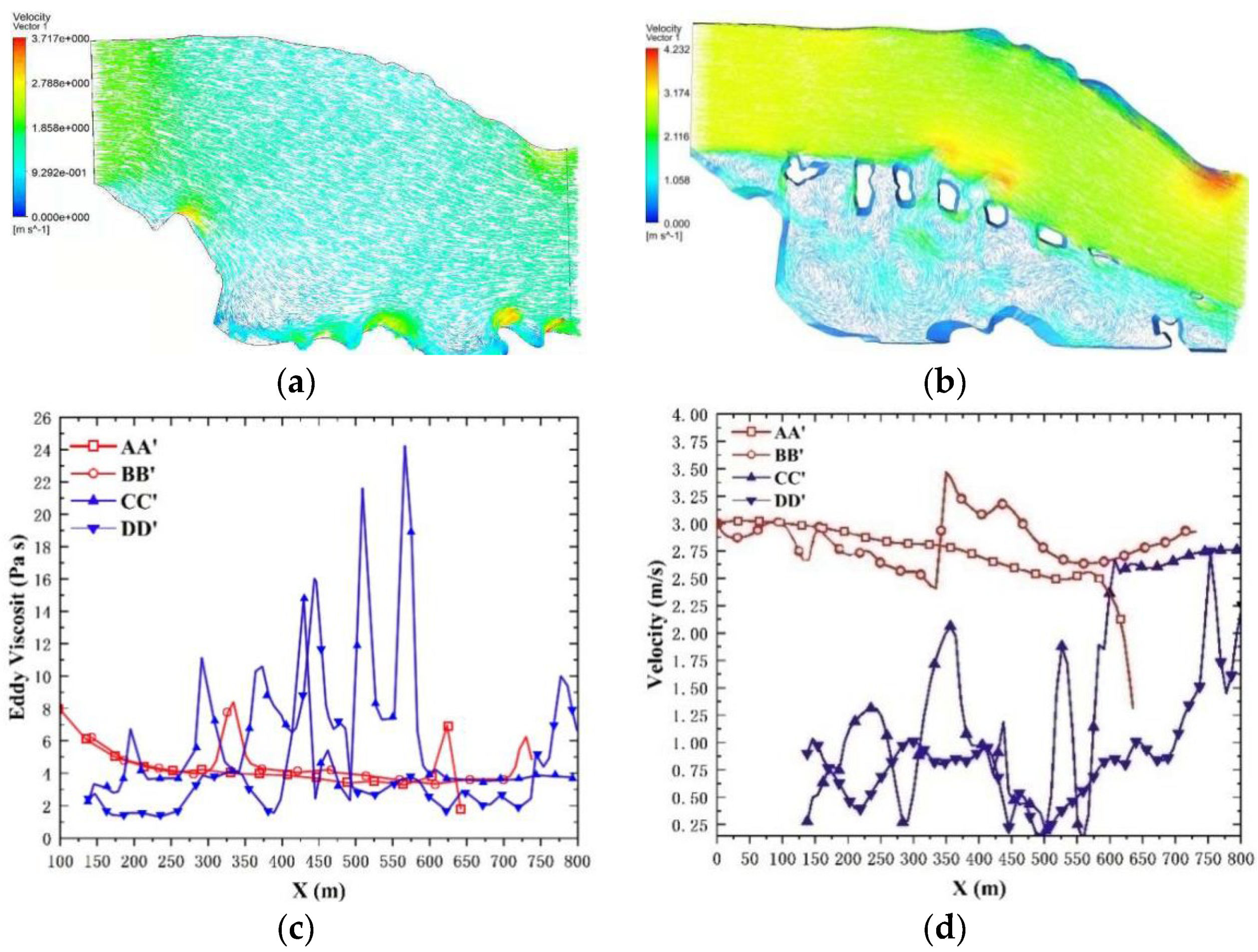
3.1. Analysis of Flow Characteristics
3.1.1. Hydromorphological Condition Verification
3.1.2. Flow Field Simulation
3.1.3. Turbulence Characteristics Simulation
3.1.4. Velocity Distribution Simulation on Longitudinal Cross-Sections
3.2. Fish Resources
3.2.1. Fish Composition
3.2.2. Fish Ecological Behavior Trait
3.2.3. Analysis of Differences among Fish Communities
4. Discussion
4.1. Projected Effect on Hydromorphology
4.2. Projected Effect on Fish Species
4.3. Summary and Future Research Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFD | Computational fluid dynamics |
| ADCP | Acoustic Doppler current profiler |
| SIMPLEC | Semi-Implicit Method for Pressure Linked Equations |
| SIMPER | Similarity percentage |
References
- Mccluney, K.E.; Poff, L.R.; Palmer, M.A.; Thorp, J.H.; Poole, G.C.; Williams, B.S.; Williams, M.R.; Baron, J.S. Riverine macrosystems ecology: Sensitivity, resistance, and resilience of whole river basins with human alterations. Front. Ecol. Environ. 2014, 12, 48–58. [Google Scholar] [CrossRef]
- Palmer, M.A.; Richardson, D.C. Provisioning Services: Afocus on Fresh Water; Princeton University Press: Princeton, NJ, USA, 2009. [Google Scholar]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef] [Green Version]
- Winemiller, K.O.; McIntyre, P.B.; Castello, L.; Fluet-Chouinard, E.; Saenz, L. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 2016, 351, 128–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlisle, D.M.; Falcone, J.; Wolock, D.M.; Meador, M.R.; Norrjs, R.H. Predicting the natural flow regime: Models for assessing hydrological alteration in streams. River Res. Appl. 2010, 26, 118–136. [Google Scholar] [CrossRef]
- Poff, L.R.; Olden, J.D.; Merritt, D.M.; Pepin, D.M. Homogenization of regional river dynamics by dams and global biodiversity implications. Proc. Natl. Acad. Sci. USA 2007, 104, 5732–5737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunn, S.E.; Arthington, A.H. Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity. Environ. Manag. 2002, 30, 492–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehner, B.; Liermann, C.R.; Revenga, C.; Vorosmarty, C.; Fekete, B.; Crouzet, P.; Döll, P.; Endejan, M.; Frenken, K.; Magome, J.; et al. High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front. Ecol. Environ. 2011, 9, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Power, M.E.; Dietrich, W.E.; Finlay, J.C. Dams and downstream aquatic biodiversity: Potential food web consequences of hydrologic and geomorphic change. Environ. Manag. 1996, 20, 887–895. [Google Scholar] [CrossRef]
- Cao, W.X. Several problems on the protection of fish resources in the Yangtze River Basin. Resour. Environ. Yangtze Basin 2008, 17, 163–164. [Google Scholar]
- Duan, X.B.; Tian, H.W.; Gao, T.H.; Liu, S.P.; Wang, K.; Chen, D.Q. Resources status of ichthyoplankton in the Upperyangtze River before the storage of JinSha River first stage project. Resour. Environ. Yangtze Basin 2015, 24, 1358–1366. [Google Scholar]
- Fu, C. Freshwater fish biodiversity in the Yangtze River basin of China patterns, threats and conservation. Biodivers Conserv. 2003, 12, 1649–1685. [Google Scholar] [CrossRef]
- Gao, T.H.; Tian, H.W.; Ye, C.; Duan, X.B. Diversity and composition of fish in the mainstream of national nature reserve of rare and endemic fish in the upper Yangtze River. Freshw. Fish 2013, 43, 36–42. [Google Scholar]
- Zhao, W.H.; Cao, H.Q.; Huang, Z.; Wang, Z.H. Assessment of physical integrity of National Nature Reserve for rare and endemic fishes in upstream Yangtze River before and after xiangjiaba dam impoundment. J. Yangtze River Sci. Res. Inst. 2015, 32, 76–80. [Google Scholar]
- Palmer, M.A.; Hondula, K.L.; Koch, B.J. Ecological Restoration of Streams and Rivers: Shifting Strategies and Shifting Goals. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 247–269. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, C.; Aradottir, A.L.; Hagen, D.; Halldórsson, G.; Høegh, K.; Mitchell, R.J.; Raulund-Rasmussen, K.; Svavarsdóttir, K.; Tolvanen, A.; Wilson, S.D. Evaluating the process of ecological restoration. Ecol. Soc. 2016, 21, 41. [Google Scholar] [CrossRef] [Green Version]
- Tockner, K.; Uehlinger, U.; Robinson, C.T. Rivers of Europe; Academic Press: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Boedeltje, G.; Smolders, A.J.P.; Roelofs, J.G.M.; Van Groenendael, J.M. Constructed shallow zones along navigation canals: Vegetation establishment and change in relation to environmental characteristics. Aquat. Conserv. Mar. Freshw. Ecosyst. 2001, 11, 453–471. [Google Scholar] [CrossRef]
- Brookes, A. The distribution and management of channelized streams in Denmark. Regul. Rivers Res. Manag. 1987, 1, 3–16. [Google Scholar] [CrossRef]
- Jähnig, S.C.; Lorenz, A.W. Substrate-specific macroinvertebrate diversity patterns following stream restoration. Aquat. Sci. 2008, 70, 292–303. [Google Scholar] [CrossRef]
- Jähnig, S.C.; Brabec, K.; Buffagni, A.; Erba, S.; Lorenz, A.W.; Ofenböck, T.; Verdonschot, P.F.M.; Hering, D. A comparative analysis of restoration measures andtheir effects on hydromorphology and benthic invertebrates in 26 central andsouthern European rivers. J. Appl. Ecol. 2010, 47, 671–680. [Google Scholar] [CrossRef]
- Sundermann, A.; Antons, C.; Cron, N.; Lorenz, A.W.; Hering, D.; Haase, P. Hydromorphological restoration of running waters: Effects on benthic invertebrate assemblages. Freshw. Biol. 2011, 56, 1689–1702. [Google Scholar] [CrossRef]
- Vogt, T.; Hoehn, E.; Schneider, P.; Freund, A.; Schirmer, M.; Cirpka, O.A. Fluctuations of electrical conductivity as a natural tracer for bank filtration in a losing stream. Adv. Water Resour. 2010, 33, 1296–1308. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. The ecological value of stream restoration measures: An evaluation on ecosystem and target species scales. Ecol. Eng. 2014, 62, 129–139. [Google Scholar] [CrossRef]
- Pander, J.; Mueller, M.; Geist, J. A comparison of four stream substratum restoration techniques concerning interstitial conditions and downstream effects. River Res. Appl. 2015, 31, 239–255. [Google Scholar] [CrossRef]
- Lu, W.; Font, R.A.; Cheng, S.; Wang, J.; Kollmann, J. Assessing the context and ecological effects of river restoration—A meta-analysis. Ecol. Eng. 2019, 136, 30–37. [Google Scholar] [CrossRef]
- Ebel, G. Untersuchungen zur Stabilisierung von Barbenpopulationen-Dargestellt am Beispiel Eines Mitteldeutschen Fließgewässers; Eigenverlag: Halle, Germany, 2002. [Google Scholar]
- Hauer, C.; Unfer, G.; Schmutz, S.; Habersack, H. The importance of morphody-namic processes at riffles used as spawning grounds during the incubation timeof nase (Chondrostoma nasus). Hydrobiologia 2007, 579, 15–27. [Google Scholar] [CrossRef]
- Hauer, C.; Unfer, G.; Schmutz, S.; Habersack, H. Morphodynamic effects onthe habitat of juvenile cyprinids (Chondrostoma nasus) in a restored Austrianlowland river. Environ. Manag. 2008, 42, 279–296. [Google Scholar] [CrossRef]
- Kondolf, G.M. Assessing Salmonid Spawning Gravel Quality. Trans. Am. Fish. Soc. 2000, 129, 262–281. [Google Scholar] [CrossRef]
- Pulg, U.; Barlaup, B.T.; Sternecker, K.; Trepl, L.; Unfer, G. Restoration of spawning habitats of brown trout (Salmo trutta) in a regulated chalk stream. River Res. Appl. 2013, 29, 172–182. [Google Scholar] [CrossRef]
- Schmutz, S.; Kremser, H.; Melcher, A.; Jungwirth, M.; Muhar, S.; Waidbacher, H.; Zauner, G. Ecological effects of rehabilitation measures at the Austrian Danube: A meta-analysis of fish assemblages. Hydrobiologia 2014, 729, 49–60. [Google Scholar] [CrossRef]
- Lorenz, A.W.; Stoll, S.; Sundermann, A.; Haase, P. Do adult and YOY fish benefit from river restoration measures? Ecol. Eng. 2013, 61, 174–181. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Kottelat, Cornol & Freyhof: Berlin, Germany, 2007. [Google Scholar]
- Zhou, L.; Wang, Z. Numerical simulation of cavitation around a hydrofoil and evaluation of a RNG κ-ε model. J. Fluids Eng. Trans. ASME 2008, 130, 011302. [Google Scholar] [CrossRef]
- Fischer, J.L.; Filip, G.P.; Alford, L.K.; Roseman, E.F.; Vaccaro, L. Supporting aquatic habitat remediation in the Detroit River through numerical simulation. Geomorphology 2020, 353, 107001. [Google Scholar] [CrossRef]
- Underwood, A.J. On beyond baci: Sampling designs that might reliably detect environmental disturbances-sciencedirect. In Detecting Ecological Impacts; Academic Press: New York, NY, USA, 1996. [Google Scholar]
- Zhang, M.; Zhu, M. Vertebrate Paleontology of China, Volume I, Fish; China Science Press: Beijing, China, 2015. [Google Scholar]
- Ni, Y.; Wu, H.L. Ichthyology of Jiangsu; China Agricultural Press: Beijing, China, 2006. [Google Scholar]
- Wang, M.; Zhu, F.Y.; Liu, S.P.; Duan, X.; Chen, D. Seasonal variations of fish community structure of Lake Hanfeng in three gorges reservoir region. J. Lake Sci. 2017, 29, 439–447. [Google Scholar]
- Wo, J.; Xu, B.; Xue, Y.; Ren, Y.; Zhang, C. Temporo-spatial heterogeneity of dominant fish species in the Jiaozhou Bay community. J. Fish. Sci. China 2017, 24, 1091–1098. [Google Scholar] [CrossRef]
- Wootton, R.J. Fish Ecology; Springer: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Lv, B.; Li, F.; Wang, B.; Xu, B.Q.; Wei, Z.H.; Zhang, H.J.; Zhang, P.C. Community structure of fish resources in spring and autumn in the Yellow Sea of Shandong. J. Fish Sci. China 2011, 35, 692–699. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Li, J.; Li, X.H.; Jia, X.P.; Tan, X.C.; Wang, C.; Li, Y.F.; Shao, X.F. Fish community diversity and its relationship with environmental factors in Lianjiang. Acta Ecol. Sin. 2012, 32, 5795–5805. [Google Scholar]
- Guo, J.Z.; Chen, Z.Z.; Tian, J.; Tian, Y.J.; Zhang, K.; Li, C.H. Species composition and diversity of fish communities in Jiaozhou Bay. Acta Ecol. Sin. 2019, 39, 7002–7013. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Feld, C.K.; Birk, S.; Bradley, D.C.; Hering, D.; Kail, J.; Marzin, A.; Melcher, A.; Nemitz, D.; Pedersen, M.L.; Pletterbauer, F.; et al. From natural to degraded rivers and back again: A test of restoration ecology theory and practice. In Advances in Ecological Research; Woodward, G., Ed.; Academic Press: London, UK, 2001. [Google Scholar]
- Palmer, M.A.; Menninger, H.L.; Bernhardt, E. River restoration, habitat heterogeneity and biodiversity: A failure of theory or practice? Freshw. Biol. 2010, 55, 205–222. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Sudduth, E.B.; Palmer, M.A.; Allan, J.D.; Meyer, J.L.; Alexander, G.; Follastad-Shah, J.; Hassett, B.; Jenkinson, R.; Lave, R.; et al. Restoring Rivers One Reach at a Time: Results from a Survey of U.S. River Restoration Practitioners. Restor. Ecol. 2007, 15, 482–493. [Google Scholar] [CrossRef] [Green Version]
- Kondolf, G.M.; Anderson, S.; Lave, R.; Pagano, L.; Merenlender, A.; Bernhardt, E.S. Two decades of river restoration in California: What can we learn? Restor. Ecol. 2007, 15, 516–523. [Google Scholar] [CrossRef]
- European Commission. Directive of the European Parliament and of the Council 2000/60/EC establishing a framework for community action in the field of water policy. J. Int. Wildl. Law Policy 2000, 3, 93–97. [Google Scholar] [CrossRef]
- Pander, J.; Geist, J. Ecological indicators for stream restoration success. Ecol. Indic. 2013, 30, 106–118. [Google Scholar] [CrossRef]
- Geist, J. Trends and Directions in Water Quality and Habitat Management in the Context of the European Water Framework Directive. Fisheries 2014, 39, 219–220. [Google Scholar] [CrossRef]
- Geist, J. Seven steps towards improving freshwater conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 447–453. [Google Scholar] [CrossRef]
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Palmer, M.; Allan, J.D.; Meyer, J.; Bernhardt, E.S. River Restoration in the Twenty-First Century: Data and Experiential Knowledge to Inform Future Efforts. Restor. Ecol. 2007, 15, 472–481. [Google Scholar] [CrossRef] [Green Version]
- Pander, J.; Geist, J. Can fish habitat restoration for rheophilic species in highly modified rivers be sustainable in the long run? Ecol. Eng. 2016, 88, 28–38. [Google Scholar] [CrossRef]
- Roni, P.; Hanson, K.; Beechie, T. Global Review of the Physical and Biological Effectiveness of Stream Habitat Rehabilitation Techniques. N. Am. J. Fish. Manag. 2008, 28, 856–890. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Tactics, Strategies and Templets. Oikos 1988, 52, 3–18. [Google Scholar] [CrossRef]
- Manfrin, A.; Teurlincx, S.; Lorenz, A.W.; Haase, P.; Marttila, M.; Syrjänen, J.T.; Thomas, G.; Stoll, S. Effect of river restoration on life-history strategies in fish communities. Sci. Total Environ. 2019, 663, 486–495. [Google Scholar] [CrossRef] [Green Version]
- King, A.J.; Tonkin, Z.; Mahoney, J. Environmental flow enhances native fish spawning and recruitment in the Murray River, Australia. River Res. Appl. 2009, 25, 1205–1218. [Google Scholar] [CrossRef]
- Koebel, J.W. An Historical Perspective on the Kissimmee River Restoration Project. Restor. Ecol. 1995, 3, 149–159. [Google Scholar] [CrossRef]
- Petruck, A.; Beckereit, M.; Hurck, R. Restoration of the River Emscher; ASCE Library: Philadelphia, PA, USA, 2003. [Google Scholar]
- Stammel, B.; Cyffka, B.; Geist, J.; Müller, M.; Pander, J.; Blasch, G.; Fischer, P.; Gruppe, A.; Haas, F.; Kilg, M.; et al. Floodplain restoration on the Upper Danube (Germany) by re-establishing water and sediment dynamics: A scientific monitoring as part of the implementation. River Syst. 2012, 20, 55–70. [Google Scholar] [CrossRef]
- Xue, S.; Sun, T.; Zhang, H.; Shao, D. Suitable habitat mapping in the Yangtze River Estuary influenced by land reclamations. Ecol. Eng. 2016, 97, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Duerregger, A.; Pander, J.; Palt, M.; Mueller, M.; Nagel, C.; Geist, J. The importance of stream interstitial conditions for the early-life-stage development of the European nase (Chondrostoma nasus L.). Ecol. Freshw. Fish 2018, 27, 920–932. [Google Scholar] [CrossRef]
- Baras, E.; Nindaba, J. Diel dynamics of habitat use by riverine young-of-the-year Barbus barbus and Chondrostoma nasus (Cyprinidae). Fundam. Appl. Limnol. 1999, 146, 431–448. [Google Scholar] [CrossRef]
- Ramler, D.; Keckeis, H. Effects of large-river restoration measures on ecological fish guilds and focal species of conservation in a large European river (Danube, Austria). Sci. Total Environ. 2019, 686, 1076–1089. [Google Scholar] [CrossRef]
- Britton, J.R.; Pegg, J. Ecology of european barbel Barbus Barbus: Implications for river, fishery, and conservation management. Rev. Fish Sci. 2011, 19, 321–330. [Google Scholar] [CrossRef]
- Keckeis, H.; Winkler, G.; Flore, L.; Reckendorfer, W.; Schiemer, F. Spatial and seasonal characteristics of 0+ fish nursery habitats of nase, Chondrostoma nasus in the river Danube, Austria. Folia Zool. 1997, 46, 133–150. [Google Scholar]
- Ovidio, M.; Philippart, J.C. Movement patterns and spawning activity of individual nase (Chondrostoma nasus L.) in flow-regulated and weir-fragmented rivers. J. Appl. Ichthyol. 2008, 24, 256–262. [Google Scholar] [CrossRef]
- Zbinden, S.; Maier, K.J. Contribution to the knowledge of the distribution and spawning grounds of Chondrostoma nasus and Chondrostoma toxostoma (Pisces, Cyprinidae) in Switzerland. In Conservation of Endangered Freshwater Fish in Europe; Kirchhofer, A., Hefti, D., Eds.; Birkhäuser: Basel, Switzerland, 1996. [Google Scholar]
- Fischer, J.L.; Roseman, E.; Mayer, C.M.; Qian, S. Effectiveness of shallow water habitat remediation for improving fish habitat in a large temperate river. Ecol. Eng. 2018, 123, 54–64. [Google Scholar] [CrossRef]
- Grenouillet, G.; Pont, D.; Olivier, J.M. Habitat occupancy patterns of juvenile fishes in a large lowland river: Interactions with macrophytes. Fundam. Appl. Limnol. 2000, 149, 307–326. [Google Scholar] [CrossRef]
- Lapointe, N.W.R.; Corkum, L.D.; Mandrak, N.E. Seasonal and Ontogenic Shifts in Microhabitat Selection by Fishes in the Shallow Waters of the Detroit River, a Large Connecting Channel. Trans. Am. Fish. Soc. 2007, 136, 155–166. [Google Scholar] [CrossRef]
- Lapointe, N.W.R.; Corkum, L.D.; Mandrak, N.E. Macrohabitat associations of fishes in shallow waters of the Detroit River. J. Fish Biol. 2010, 76, 446–466. [Google Scholar] [CrossRef]
- Pander, J.; Mueller, M.; Geist, J. Succession of fish diversity after reconnecting a large floodplain to the upper Danube River. Ecol. Eng. 2015, 75, 41–50. [Google Scholar] [CrossRef]
- Stoll, S.; Kail, J.; Lorenz, A.W.; Sundermann, A.; Haase, P. The Importance of the Regional Species Pool, Ecological Species Traits and Local Habitat Conditions for the Colonization of Restored River Reaches by Fish. PLoS ONE 2014, 9, e84741. [Google Scholar] [CrossRef]




| Species | Rare and Endemic Fish | Capture Period | Abundance/Ind. | Biomass/g |
|---|---|---|---|---|
| Monopterus albus (Zuiew) | BPI | 1 | 30.20 | |
| Acrossocheilus monticola (Günther) | ☐ | BPI | 2 | 35.80 |
| Hemiculterella sauvagei | ☐ | API | 48 | 412.80 |
| Ancherythroculter kurematsui (Kimura) | ☐ | API | 1 | 33.00 |
| Xenocypris davidi (Bleeker) | API | 1 | 76.60 | |
| Culter mongolicus (Basilewsky) | API | 9 | 2095.20 | |
| Rhodeus sinensis (Kaer) | API | 7 | 130.10 | |
| Pseudobrama simoni (Bleeker) | API | 3 | 106.80 | |
| Procypris rabaudi (Tchang) | ☐ | API | 1 | 82.70 |
| Squalidus argentatus | API | 17 | 207.70 | |
| Xenocypris yunnanensis (Nichols) | ☐ | API | 1 | 243.90 |
| Acipenser dabryarus (Dumeril) | ▲ | API | 1 | 54.80 |
| Rhinogobio ventralis (Savage et Dabry) | ☐ | API | 1 | 112.20 |
| Species | Abundance/ Ind. | Biomass/g | IRI | |||
|---|---|---|---|---|---|---|
| BPI | API | BPI | API | BPI | API | |
| Carassius auratus (Linnaeus) | 135 | 224 | 3145 | 5105 | 2393 | 2353 |
| Saurogobio dabryi (Bleeker) | 69 | 230 | 1332 | 3329 | 848 | 1991 |
| Hemiculter leucisculus (Basilewsky) | 93 | 166 | 1742 | 2673 | 1632 | 1613 |
| Cyprinus carpio (Linnaeus) | 31 | 34 | 4029 | 4217 | 1102 | 691 |
| Trilophysa bleekeri (Sauvage et Dabry) | 77 | 107 | 346 | 369 | 808 | 613 |
| Pseudolaubuca engraulis (Nichols) | 34 | 59 | 825 | 1334 | 307 | 380 |
| Culter ilishaeformis (Bleeker) | 3 | 74 | 176 | 1770 | \ | 367 |
| Culter mongolicus (Basilewsky) | \ | 9 | \ | 2095 | \ | 237 |
| Abbottina rivularis (Basilewsky) | 17 | 41 | 129 | 208 | 124 | 187 |
| Rhodeus sinensis (Kaer) | 8 | 29 | 54 | 161 | \ | 179 |
| Ctenopharyngodon idellus (Cuvier et Valenciennes) | 2 | 9 | 1573 | 3082 | \ | 169 |
| Hemiculterella sauvagei | \ | 48 | \ | 413 | \ | 163 |
| Hemibarbus maculatus (Bleeker) | 13 | 43 | 262 | 297 | \ | 139 |
| Zacco platypus (Temminck et Schlegel) | 12 | 15 | 898 | 1144 | 175 | 129 |
| PelteobagrusVachelli (Richardson) | 6 | 26 | 125 | 379 | \ | 122 |
| Rhinogobius giurinus (Rutter) | 39 | 21 | 196 | 78 | 302 | \ |
| Sinibrama taeniatus (Nichols) | 19 | 13 | 248 | 263 | 136 | \ |
| Leiocassis crassilabris (Günther) | 19 | 8 | 507 | 241 | 119 | \ |
| Species | A Group and B Group | A Group and C Group | D Group and E Group | |||
|---|---|---|---|---|---|---|
| Av.Diss | Contrib% | Av.Diss | Contrib% | Av.Diss | Contrib% | |
| Trilophysa bleekeri (Sauvage et Dabry) | 7.5 | 14.9 | 3.7 | 7.0 | 2.1 | 4.7 |
| Hemiculter leucisculus (Basilewsky) | 7.1 | 14.2 | \ | \ | 6.1 | 14.1 |
| Pseudolaubuca engraulis (Nichols) | 4.7 | 9.4 | \ | \ | 1.7 | 4.0 |
| Carassius auratus (Linnaeus) | 4.7 | 9.4 | 8.0 | 15.2 | 4.0 | 9.2 |
| Liobagrus marginatus (Günther) | 3.7 | 7.4 | 2.5 | 4.7 | \ | \ |
| Abbottina rivularis (Basilewsky) | 3.4 | 6.8 | \ | \ | 1.7 | 3.9 |
| Rhinogobius giurinus (Rutter) | 2.7 | 5.4 | \ | \ | 1.5 | 3.5 |
| Sinibrama taeniatus (Nichols) | 2.6 | 5.2 | 2.5 | 4.7 | \ | \ |
| Saurogobio dabryi (Bleeker) | \ | \ | 10.1 | 19.3 | 7.3 | 16.9 |
| Hemiculterella sauvagei | \ | \ | 8.5 | 16.2 | \ | \ |
| Leiocassis crassilabris (Günther) | \ | \ | 2.5 | 4.7 | \ | \ |
| Culter ilishaeformis (Bleeker) | \ | \ | \ | \ | 4.5 | 10.3 |
| Hemibarbus maculates (Bleeker) | \ | \ | \ | \ | 2.1 | 4.9 |
| Cumulative contribution rate | 72.8 | 70.2 | 71.5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, X.; Liu, X.; Zhang, J.; He, B.; Tian, C.; Zhou, Y.; Chen, X.; Zhu, L. Fish Habitat Reclamation Based on Geographical Morphology Heterogeneity in the Yangtze River and the Short-Term Effects on Fish Community Structure. Water 2022, 14, 1554. https://doi.org/10.3390/w14101554
Che X, Liu X, Zhang J, He B, Tian C, Zhou Y, Chen X, Zhu L. Fish Habitat Reclamation Based on Geographical Morphology Heterogeneity in the Yangtze River and the Short-Term Effects on Fish Community Structure. Water. 2022; 14(10):1554. https://doi.org/10.3390/w14101554
Chicago/Turabian StyleChe, Xuan, Xingguo Liu, Jun Zhang, Bin He, Changfeng Tian, Yin Zhou, Xiaolong Chen, and Lin Zhu. 2022. "Fish Habitat Reclamation Based on Geographical Morphology Heterogeneity in the Yangtze River and the Short-Term Effects on Fish Community Structure" Water 14, no. 10: 1554. https://doi.org/10.3390/w14101554
APA StyleChe, X., Liu, X., Zhang, J., He, B., Tian, C., Zhou, Y., Chen, X., & Zhu, L. (2022). Fish Habitat Reclamation Based on Geographical Morphology Heterogeneity in the Yangtze River and the Short-Term Effects on Fish Community Structure. Water, 14(10), 1554. https://doi.org/10.3390/w14101554






