Habitat-Diversity Relations between Sessile Macrobenthos and Benthic Copepods in the Rocky Shores of a Marine Protected Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Field Sampling
2.2. Meiofaunal and Harpacticoid Assemblages
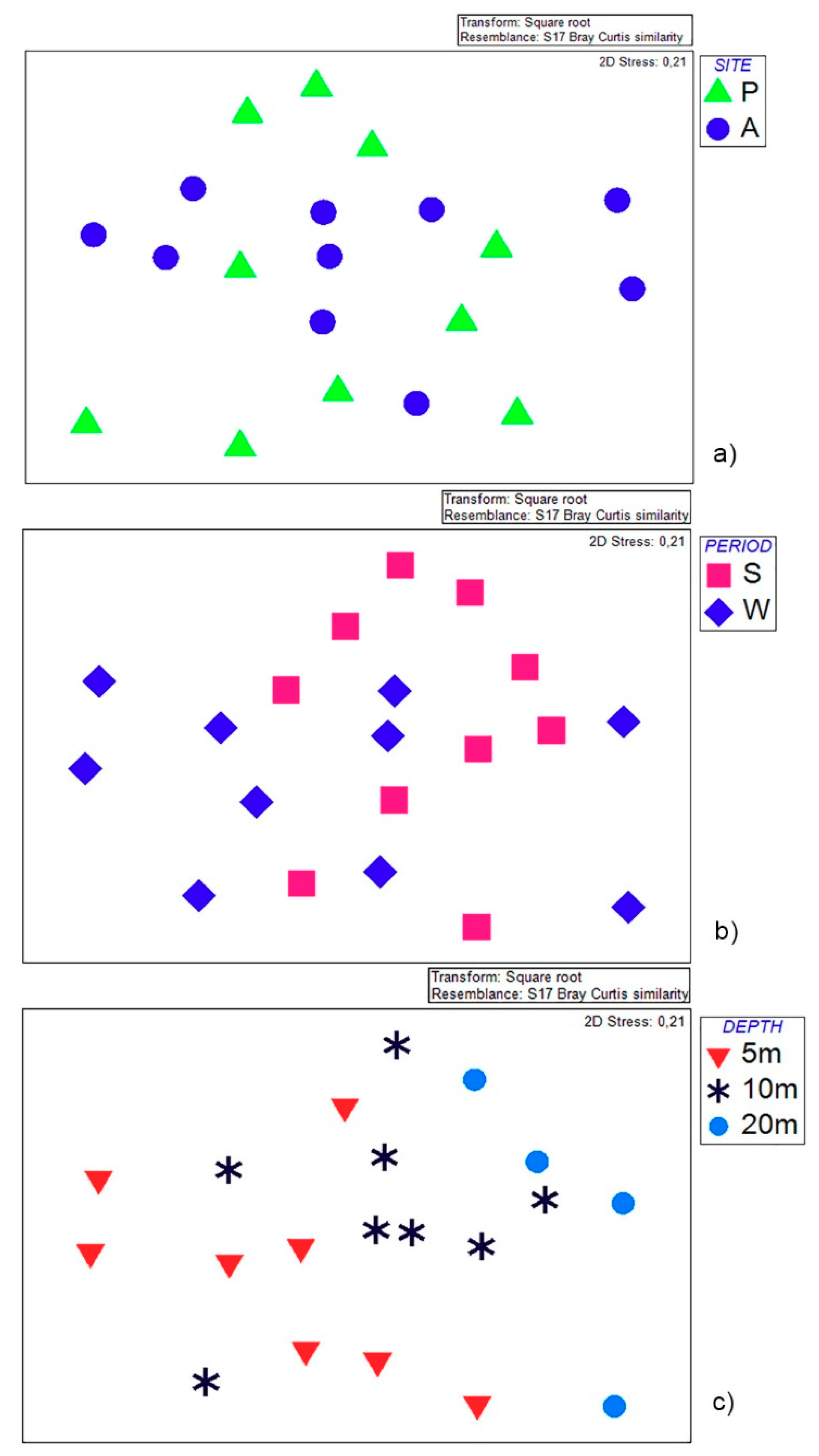
2.3. Statistical Analyses
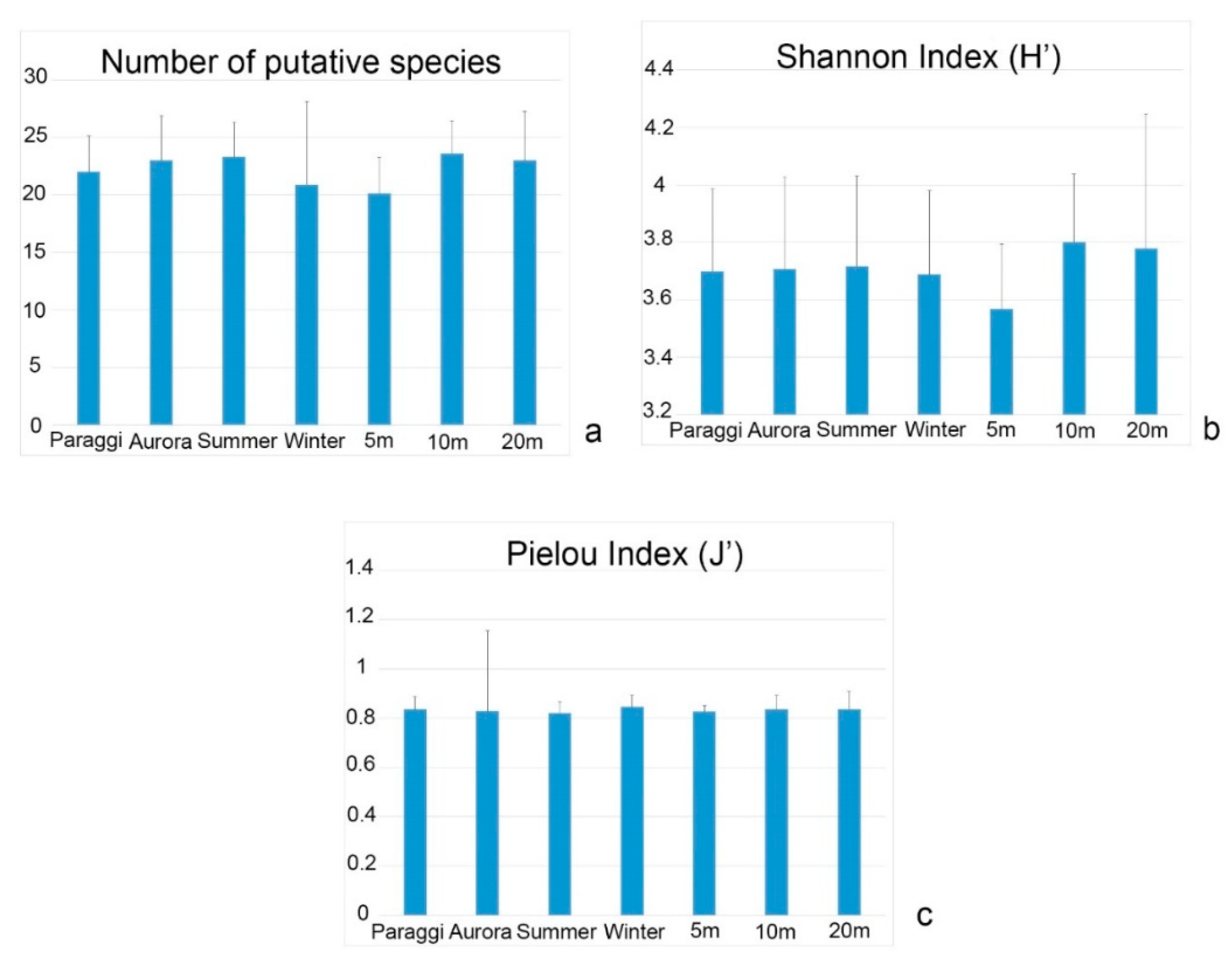
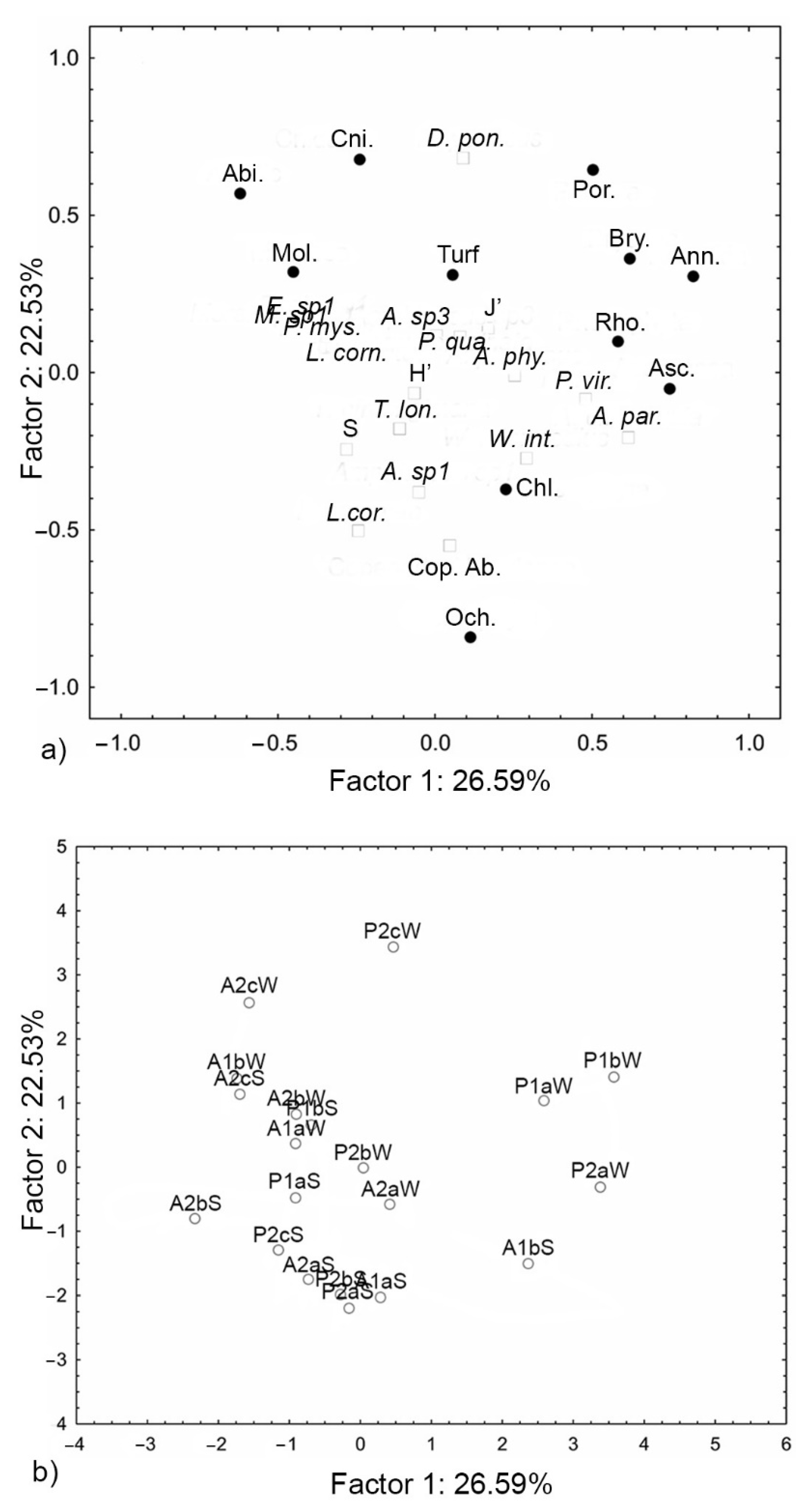
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, P.D.; Wilson, M.A. Palaeoecology and evolution of marine hard substrate communities. Earth Sci. Rev. 2003, 62, 1–103. [Google Scholar] [CrossRef]
- Thrush, S.F.; Chiantore, M.; Asnaghi, V.; Hewitt, J.; Fiorentino, D.; Cattaneo-Vietti, R. Habitat- diversity relationships in rocky shore algal turf infaunal communities. Mar. Ecol. Prog. Ser. 2011, 424, 119–132. [Google Scholar] [CrossRef]
- Largaespada, C.; Guichard, F.; Archambault, P. Meta-ecosystem engineering: Nutrient fluxes reveal intraspecific and interspecific feedbacks in fragmented mussel beds. Ecology 2012, 93, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Danovaro, R.; Fraschetti, S. Meiofaunal vertical zonation on hard bottoms: Comparison with soft-bottom meiofauna. Mar. Ecol. Prog. Ser. 2002, 230, 159–169. [Google Scholar] [CrossRef]
- Bianchelli, S.; Pusceddu, A.; Canese, S.; Greco, S.; Danovaro, R. High meiofaunal and nematodes diversity around mesophotic coral oases in the Mediterranean Sea. PLoS ONE 2013, 8, e66553. [Google Scholar] [CrossRef][Green Version]
- Ape, F.; Sarà, G.; Airoldi, L.; Mancuso, F.P.; Mirto, S. Influence of environmental factors and biogenic habitats on intertidal meiofauna. Hydrobiologia 2018, 807, 349–366. [Google Scholar] [CrossRef]
- Losi, V.; Sbrocca, C.; Gatti, G.; Semprucci, F.; Rocchi, M.; Bianchi, C.N.; Balsamo, M. Sessile macrobenthos (Ochrophyta) drives seasonal change of meiofaunal community structure on temperate rocky reefs. Mar. Environ. Res. 2018, 142, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Frame, K.; Hunt, G.; Roy, K. Intertidal meiofaunal biodiversity with respect to different algal habitats: A test using phytal ostracodes from Southern California. Hydrobiologia 2007, 586, 331–342. [Google Scholar] [CrossRef]
- Passarelli, C.; Olivier, F.; Paterson, D.M.; Hubas, C. Impacts of biogenic structures on benthic assemblages: Microbes, meiofauna, macrofauna and related ecosystem functions. Mar. Ecol. Prog. Ser. 2012, 465, 85–97. [Google Scholar] [CrossRef]
- Gheerardyn, H.; De Troch, M.; Vincx, M.; Vanreusel, A. Diversity and community structure of harpacticoid copepods associated with cold-water coral substrates in the Porcupine Seabight (North-East Atlantic). Helgol. Mar. Res. 2010, 64, 53–62. [Google Scholar] [CrossRef]
- Jayabarathi, R.; Padmavati, G.; Anandavelu, I. Spatial heterogeneity of benthic copepods: A comparative aspect on composition, abundance, and correlation. Zool. Stud. 2015, 54, 51. [Google Scholar] [CrossRef]
- Bell, S.S.; Walters, K.; Hall, M.O. Habitat utilization by harpacticoid copepods: A morphometric approach. Mar. Ecol. Prog. Ser. 1987, 35, 59–64. [Google Scholar] [CrossRef]
- Hicks, G.R.F.; Coull, B.C. The ecology of marine meiobenthic harpacticoid copepods. Oceanogr. Mar. Biol. Annu. Rev. 1983, 21, 67–175. [Google Scholar]
- Gheerardyn, H.; De Troch, M.; Ndaro, S.G.M.; Raes, M.; Vincx, M.; Vanreusel, A. Community structure and microhabitat preferences of harpacticoid copepods in a tropical reef lagoon (Zanzibar Island, Tanzania). J. Mar. Biol. Assoc. UK 2008, 88, 747–758. [Google Scholar] [CrossRef]
- Sarmento, V.C.; Santos, P.J.P. Species of Harpacticoida (Crustacea, Copepoda) from the phytal of Porto de Galinhas coral reefs, north-eastern. Check List 2012, 8, 936–939. [Google Scholar] [CrossRef][Green Version]
- Mascart, T.; Lepoint, G.; De Troch, M. Meiofauna and harpacticoid copepods in different habitats of a Mediterranean seagrass meadow. J. Mar. Biol. Assoc. UK 2013, 93, 1557–1566. [Google Scholar] [CrossRef]
- Mazzocchi, M.G.; Di Capua, I. Copepodi planctonici/planktonic copepods: Calanoida, Cyclopoida, Harpacticoida, Mormonilloida, Siphonostomatoida. Biol. Mar. Mediterr. 2010, 17, 420–431. [Google Scholar]
- Giere, O. Meiobenthology: The Microscopic Motile Fauna of Aquatic Sediments, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2009; p. 527. [Google Scholar]
- Balsamo, M.; Albertelli, G.; Ceccherelli, V.U.; Coccioni, R.; Colangelo, M.A.; Curini-Galletti, M.; Danovaro, R.; D’Addabbo, R.; De Leonardis, C.; Fabiano, M.; et al. Meiofauna of the Adriatic Sea: Present knowledge and future perspectives. Chem. Ecol. 2010, 26, 45–63. [Google Scholar] [CrossRef]
- Guerrini, A.; Colangelo, M.A.; Ceccherelli, V.U. Recolonization patterns of meiobenthic communities in brackish vegetated and unvegetated habitats after induced hypoxia/anoxia. Hydrobiologia 1998, 375–376, 73–87. [Google Scholar] [CrossRef]
- Lee, M.R.; Correa, J.A.; Castilla, J.C. An assessment of the potential use of the nematode to copepod ratio in the monitoring of metals pollution. The Chãnaral Case. Mar. Pollut. Bull. 2001, 42, 606–701. [Google Scholar] [CrossRef]
- Grego, M.; Riedel, B.; Stachowitsch, M.; De Troch, M. Meiofauna winners and losers of coastal hypoxia: Case study harpacticoid copepods. Biogeosciences 2014, 11, 281–292. [Google Scholar] [CrossRef]
- Semprucci, F.; Balsamo, M.; Sandulli, R. Assessment of the ecological quality (EcoQ) of the Venice lagoon using the structure and biodiversity of the meiofaunal assemblages. Ecol. Indic. 2016, 67, 451–457. [Google Scholar] [CrossRef]
- Fraschetti, S.; Gambi, C.; Giangrande, A.; Musco, L.; Terlizzi, A.; Danovaro, R. Structural and functional response of meiofauna rocky assemblages to sewage pollution. Mar. Pollut. Bull. 2006, 52, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Scopa, M.; Gambi, C.; Fraschetti, S. Trophic importance of subtidal metazoan meiofauna: Evidence from in situ exclusion experiments on soft and rocky substrates. Mar. Biol. 2007, 152, 339–350. [Google Scholar] [CrossRef]
- Appeltans, W.; Ahyong, S.T.; Anderson, G.; Angel, M.V.; Artois, T.; Bailly, N.; Costello, M.J. The magnitude of global marine species diversity. Curr. Biol. 2012, 22, 2189–2202. [Google Scholar] [CrossRef]
- Rossel, S.; Martínez Arbizu, P. Revealing higher than expected diversity of Harpacticoida (Crustacea: Copepoda) in the North Sea using MALDI-TOF MS and molecular barcoding. Sci. Rep. 2019, 9, 9182. [Google Scholar] [CrossRef]
- Venturini, S.; Campodonico, P.; Cappanera, V.; Fanciulli, G. Recreational fisheries in Portofino Marine Protected Area, Italy: Some implications for the management. Fish. Manag. Ecol. 2017, 24. [Google Scholar] [CrossRef]
- Hiscock, K. Subtidal rock and shallow sediments using diving. In Biological Surveys of Estuaries and Coasts; Baker, J.M., Walff, W.J., Eds.; Cambridge University Press: Cambridge, UK, 1987; pp. 198–237. [Google Scholar]
- Dethier, M.N.; Graham, E.S.; Cohen, S.; Tear, L.M. Visual versus random-point percent cover estimations: “Obiective” is not always better. Mar. Ecol. Prog. Ser. 1993, 96, 93–100. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Pronzato, R.; Cattaneo-Vietti, R.; Benedetti-Cecchi, L.; Morri, C.; Pansini, M.; Chemello, R.; Milazzo, M.; Fraschetti, S.; Terlizzi, A.; et al. Mediterranean marine benthos: A manual of methods for its sampling and study. Hard bottoms. Biol. Mar. Mediterr. 2004, 11, 185–215. [Google Scholar]
- Danovaro, R.; Gambi, C.; Mirto, S.; Sandulli, R.; Ceccherelli, V.U. Meiofauna. In Mediterranean Marine Benthos: A Manual of Methods for Its Sampling and Study; Gambi, M.C., Dappiano, M., Eds.; SIBM: Genoa, Italy, 2004; Volume 11, pp. 55–97. [Google Scholar]
- Heip, C.; Vincx, M.; Vranken, G. The ecology of marine nematodes. Oceanogr. Mar. Biol. Annu. Rev. 1985, 23, 399–489. [Google Scholar]
- De Troch, M.; Gurdebeke, S.; Fiers, F.; Vincx, M. Zonation and structuring factors of meiofauna communities in a tropical seagrass bed (Gazi Bay, Kenya). J. Sea Res. 2001, 45, 45–61. [Google Scholar] [CrossRef]
- Humes, A.G.; Gooding, R.U. A method for studying the external anatomy of copepods. Crustaceana 1964, 6, 238–240. [Google Scholar] [CrossRef]
- Reid, J.W. Workshop on Taxonomic Techniques for Copepods. 2006. Available online: http://www.nmnh.si.edu/iz/copepod/techniques.htm (accessed on 2 December 2015).
- Huys, R.; Boxshall, G. Copepod Evolution; The Ray Society: London, UK, 1991; p. 468. [Google Scholar]
- Lang, K. Monographie der Harpacticiden I & II; Håkan Ohlssons Boktryckeri: Lund, Sweden, 1948; p. 1682. [Google Scholar]
- Lang, K. Copepoda: Harpacticoidea from the Californian Pacific coast. K. Sven. Vetensk. Akad. Handl. 1965, 10, 1–566. [Google Scholar]
- Huys, R.; Gee, J.M.; Moore, C.G.; Hamond, R. Marine and brackish water harpacticoid copepods. In Part I. Synopsys of the British Fauna (New Series), 5th ed.; Cambridge University Press: Cambridge, UK, 1996; pp. 1–352. [Google Scholar]
- Boxshall, G.A.; Halsey, S.H. An Introduction to Copepod Diversity; The Ray Society: London, UK, 2004; p. 996. [Google Scholar]
- WoRMS Editorial Board 2019. World Register of Marine Species. Available online: http://www.marinespecies.org (accessed on 12 November 2019).
- Todaro, A.M.; Ceccherelli, V.U. Harpacticoida. Biol. Mar. Mediterr. 2010, 17, 452–464. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Primer V6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; p. 192. [Google Scholar]
- Semprucci, F.; Boi, P.; Manti, A.; Covazzi Harriague, A.; Rocchi, M.; Colantoni, P.; Papa, S.; Balsamo, M. Benthic communities along a littoral of the Central Adriatic Sea (Italy). Helgol. Mar. Res. 2010, 64, 101–115. [Google Scholar] [CrossRef]
- Mac Arthur, R.H.; Mac Arthur, J.W. On bird species diversity. Ecology 1961, 42, 594–598. [Google Scholar] [CrossRef]
- Ferreiro, N.; Giorgi, A.; Feijoo, C. Effects of macrophyte architecture and leaf shape complexity on structural parameters of the epiphytic algal community in a Pampean stream. Aquat. Ecol. 2013, 47, 389–401. [Google Scholar] [CrossRef]
- Olafsson, E. Marine Macrophytes as Foundation Species; Taylor & Francis Inc.: Abingdon, UK, 2016; p. 285. [Google Scholar] [CrossRef]
- Gee, J.M.; Warwick, R.M. Metazoan community structure in relation to the fractal dimensions of marine macroalgae. Mar. Ecol. Prog. Ser. 1994, 103, 141–150. [Google Scholar] [CrossRef]
- Hicks, G.R.F. Meiofauna associated with rocky shore algae. In The Ecology of Rocky Coasts; Moore, P.G., Seed, R., Eds.; Columbia University Press: New York, NY, USA, 1985; pp. 36–56. [Google Scholar]
- Arroyo, N.L.; Maldonado, M.; Perez-Portela, R.; Benito, J. Distribution patterns of meiofauna associated with a sublittoral Laminaria bed in the Cantabrian Sea (north-eastern Atlantic). Mar. Biol. 2004, 144, 231–242. [Google Scholar] [CrossRef]
- Arroyo, L.N.; Maldonado, M.; Walters, K. Within- and between plant distribution of harpacticoid copepods in a North Atlantic bed of Laminaria ochroleuca. J. Mar. Biol. Assoc. UK 2006, 86, 309–316. [Google Scholar] [CrossRef]
- Aviz, D.; Silva, R.F.D.; Rosa Filho, J.S. Sabellaria wilsoni (Polychaeta: Sabellariidae): An ecosystem engineer and promoter of zoobenthos diversity in the Brazilian Amazon coast. J. Mar. Biol. Assoc. UK 2019, 99, 1099–1109. [Google Scholar] [CrossRef]
- Ataide, M.B.; Venekey, V.; Rosa-Filho, J.S.; Dos Santos, P.J.P. Sandy reefs of Sabellaria wilsoni (Polychaeta—Sabellariidae) as ecosystem engineers for meiofauna in the Amazon coastal region, Brazil. Mar. Biodivers. 2014, 44, 403–413. [Google Scholar] [CrossRef]
- Jones, C.G.; Gutierrez, J.L.; Byers, J.E.; Crooks, J.A.; Lambrinos, J.G.; Talley, T.S. A framework for understanding physical ecosystem engineering by organisms. Oikos 2010, 119, 1862–1869. [Google Scholar] [CrossRef]
- Russo, R.; Valente, S.; Colangelo, G.; Belmonte, G. Meiofauna distribution on hard substrata in a submarine cave. J. Mar. Biol. Assoc. UK 2015, 95, 1555–1564. [Google Scholar] [CrossRef]
- Hicks, G.R.F. Species associations and seasonal population densities of marine phytal harpacticoid copepods from Cook Strait. N. Z. J. Mar. Freshwater Res. 1977, 11, 621–643. [Google Scholar] [CrossRef]
- Hopper, G.J.; Davenport, J. Epifaunal composition and fractal dimensions of intertidal marine macroalgae in relation to emersion. J. Mar. Biol. Assoc. UK 2006, 86, 1297–1304. [Google Scholar] [CrossRef]
- Noodt, W. Ecology of the Copepoda. In Proceedings of the First International Conference on Meiofauna, Tunis, Tunisia, 1–11 July 1969; Hulings, N.C., Ed.; Smithsonian Contributions to Zoology. Smithsonian Institution: Washington, DC, USA, 1971; Volume 76, pp. 97–102. [Google Scholar]
- Callens, M.; Gheerardyn, H.; Ndaro, S.G.M.; De Troch, M.; Vanreusel, A. Harpacticoid copepod colonization of coral fragments in a tropical reef lagoon (Zanzibar, Tanzania). J. Mar. Biol. Assoc. UK 2012, 92, 1535–1545. [Google Scholar] [CrossRef][Green Version]
- Semprucci, F.; Frontalini, F.; Covazzi-Harriague, A.; Coccioni, R.; Balsamo, M. Meio- and macrofauna in the marine area of the Monte St. Bartolo Natural Park (Central Adriatic Sea, Italy). Sci. Mar. 2013, 77, 189–199. [Google Scholar] [CrossRef]
- Gibbons, M.J.; Griffiths, C.L. A comparison of macrofaunal and meiofaunal distribution and standing stock across a rocky shore, with an estimate of their productivities. Mar. Biol. 1986, 93, 181–188. [Google Scholar] [CrossRef]
- Johnson, S.C.; Scheibling, R.E. Structure and dynamics of epifaunal assemblages of intertidal rock weeds (Ascophyllum nodosum and Fucus vesiculosus) in Nova Scotia, Canada. Mar. Ecol. Prog. Ser. 1987, 37, 209–227. [Google Scholar] [CrossRef]
- Prathep, A.; Marrs, R.H.; Norton, T.A. Spatial and temporal variations in sediment accumulation in an algal turf and their impact on associated fauna. Mar. Biol. 2003, 142, 381–390. [Google Scholar] [CrossRef]
- De Troch, M.; Raes, M.; Muthumbi, A.; Gheerardyn, H.; Vanreusel, A. Spatial diversity of nematode and copepod genera of the coral degradation zone along the Kenyan coast, including a test for the use of higher-taxon surrogacy. Afr. J. Mar. Sci. 2008, 30, 25–33. [Google Scholar] [CrossRef]
- Buschmann, A.H. Amphipod food preference and Iridaea spp. (Rhodophyta) spore release and dispersal. J. Mar. Biol. Assoc. UK 1991, 71, 891–897. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Kihara, T.C.; Huys, R. Collecting and Processing Non-Planktonic Copepods. J. Crust. Biol. 2016, 36, 576–583. [Google Scholar] [CrossRef][Green Version]
- Noli, N.; Sbrocca, C.; Sandulli, R.; Balsamo, M.; Semprucci, F. Contribution to the knowledge of the Harpacticoida (Crustacea, Copepoda) from the Sardinian coast, Italy. Arx. Misc. Zool. 2018, 16, 121–133. [Google Scholar] [CrossRef]
- Bakir, A.K.; Katağan, T.; Aker, H.V.; Özcan, T.; Sezgin, M.; Ateş, A.S.; Koçak, C.; Kirkim, F. The marine arthropods of Turkey. Turk. Zool. Derg. 2014, 38, 1–67. [Google Scholar] [CrossRef]
- Hicks, G.R.F.; Grahame, J. Mucus production and its role in the feeding behaviour of Diarthrodes nobilis (Copepoda: Harpacticoida). J. Mar. Biol. Assoc. UK 1979, 59, 321–330. [Google Scholar] [CrossRef]
- De Troch, M.; Steinarsdóttir, M.B.; Chepurnov, V.; Ólafsson, E. Grazing on diatoms by harpacticoid copepods: Species-specific density-dependent uptake and microbial gardening. Aquat. Microb. Ecol. 2005, 3, 135–144. [Google Scholar] [CrossRef]
- Sarmento, V.C.; Lage, L.M.; Santos, P.J.P. Copepoda Harpacticoida community of a rocky shore under the influence of upwelling (Arraial do Cabo, southeastern Brazil). J. Mar. Biol. Assoc. UK 2012, 92, 1117–1126. [Google Scholar] [CrossRef]
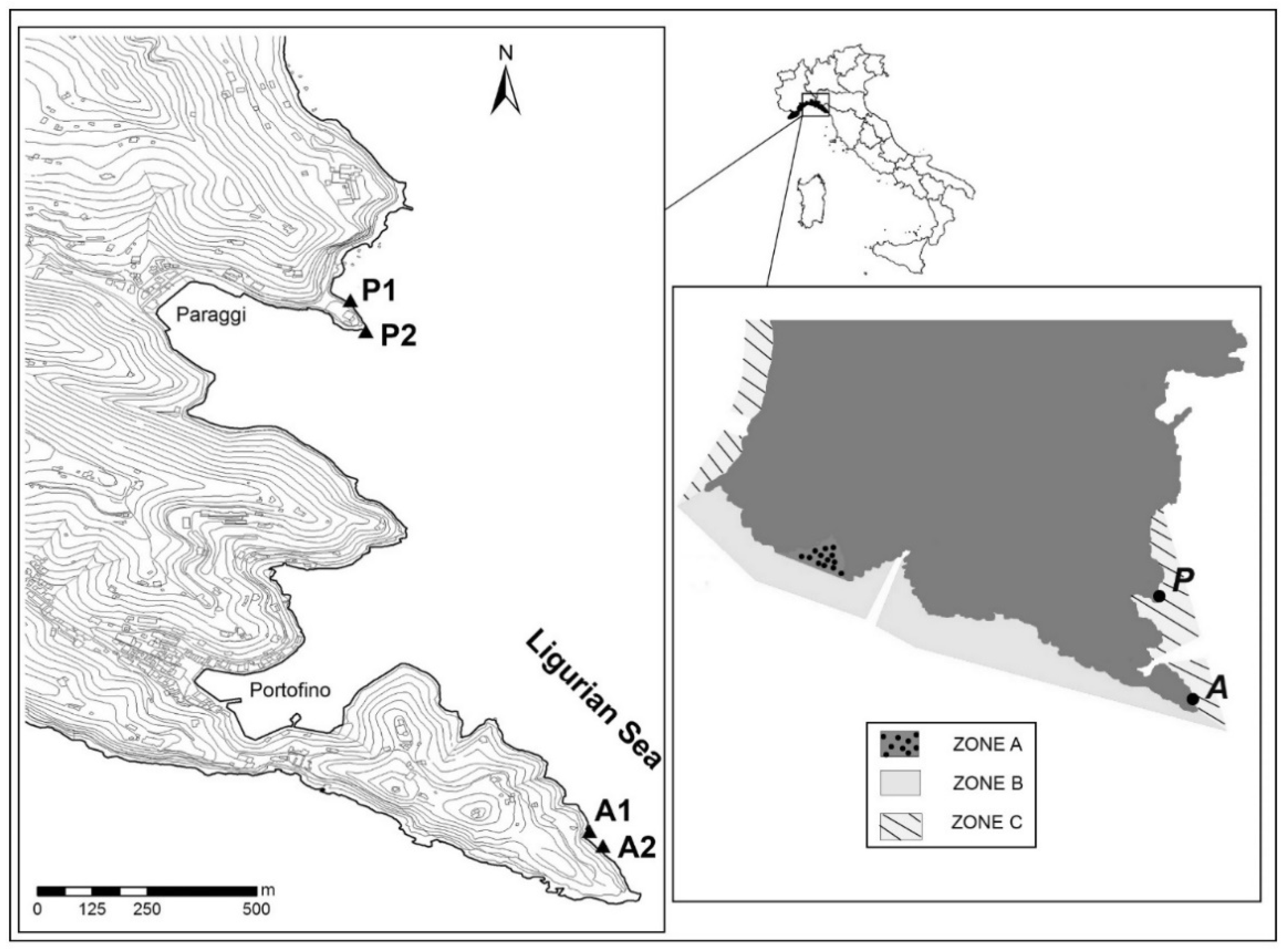
| Code | Site | Slope | Depth (m) | Season |
|---|---|---|---|---|
| P1a S | Paraggi | Sub-vertical | 5 | Summer |
| P2a S | Inclined | 5 | ||
| P1b S | Sub-vertical | 10 | ||
| P2b S | Inclined | 10 | ||
| P2c S | Inclined | 20 | ||
| A1a S | Aurora | Sub-vertical | 5 | |
| A2a S | Inclined | 5 | ||
| A1b S | Sub-vertical | 10 | ||
| A2b S | Inclined | 10 | ||
| A2c S | Inclined | 20 | ||
| P1a W | Paraggi | Sub-vertical | 5 | Winter |
| P2a W | Inclined | 5 | ||
| P1b W | Sub-vertical | 10 | ||
| P2b W | Inclined | 10 | ||
| P2c W | Inclined | 20 | ||
| A1a W | Aurora | Sub-vertical | 5 | |
| A2a W | Inclined | 5 | ||
| A1b W | Sub-vertical | 10 | ||
| A2b W | Inclined | 10 | ||
| A2c W | Inclined | 20 |
| Variables | PC1 | PC2 |
|---|---|---|
| Active variables | ||
| Abiotic | −0.62 | 0.57 |
| Annelida | 0.82 | 0.31 |
| Ascidiacea | 0.75 | −0.05 |
| Bryozoa | 0.62 | 0.36 |
| Chlorophyta | 0.23 | −0.37 |
| Cnidaria | −0.24 | 0.68 |
| Mollusca | −0.45 | 0.32 |
| Ochrophyta | 0.11 | −0.84 |
| Porifera | 0.50 | 0.64 |
| Rhodophyta | 0.58 | 0.10 |
| Turf | 0.05 | 0.31 |
| Supplememtary variables | ||
| Longipedia coronata | −0.24 | −0.50 |
| Porcellidium viride | 0.48 | −0.09 |
| Thalestris longimana | −0.11 | −0.18 |
| Phyllothalestris mysis | −0.34 | 0.08 |
| Diarthrodes ponticus | 0.09 | 0.68 |
| Amphiascus sp.1 | −0.05 | −0.38 |
| Amphiascus sp.3 | 0.01 | 0.12 |
| Amonardia phyllopus | 0.25 | −0.01 |
| Ameira parvula | 0.62 | −0.21 |
| Wellsopsyllus intermedius | 0.29 | −0.27 |
| Morariopsis sp.1 | −0.38 | 0.08 |
| Laophonte cornuta | −0.20 | 0.08 |
| Paralaophonte quaterspinata | 0.08 | 0.11 |
| Esola sp.1 | −0.33 | 0.08 |
| Species number | −0.28 | −0.25 |
| J’ | 0.17 | 0.14 |
| H’ | −0.07 | −0.06 |
| Copepod Abundance | 0.05 | −0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbrocca, C.; De Troch, M.; Losi, V.; Grassi, E.; Balsamo, M.; Semprucci, F. Habitat-Diversity Relations between Sessile Macrobenthos and Benthic Copepods in the Rocky Shores of a Marine Protected Area. Water 2021, 13, 1020. https://doi.org/10.3390/w13081020
Sbrocca C, De Troch M, Losi V, Grassi E, Balsamo M, Semprucci F. Habitat-Diversity Relations between Sessile Macrobenthos and Benthic Copepods in the Rocky Shores of a Marine Protected Area. Water. 2021; 13(8):1020. https://doi.org/10.3390/w13081020
Chicago/Turabian StyleSbrocca, Claudia, Marleen De Troch, Valentina Losi, Eleonora Grassi, Maria Balsamo, and Federica Semprucci. 2021. "Habitat-Diversity Relations between Sessile Macrobenthos and Benthic Copepods in the Rocky Shores of a Marine Protected Area" Water 13, no. 8: 1020. https://doi.org/10.3390/w13081020
APA StyleSbrocca, C., De Troch, M., Losi, V., Grassi, E., Balsamo, M., & Semprucci, F. (2021). Habitat-Diversity Relations between Sessile Macrobenthos and Benthic Copepods in the Rocky Shores of a Marine Protected Area. Water, 13(8), 1020. https://doi.org/10.3390/w13081020







