Benthic Metabolism in Fluvial Sediments with Larvae of Lampetra sp.
Abstract
1. Introduction
2. Materials and Methods
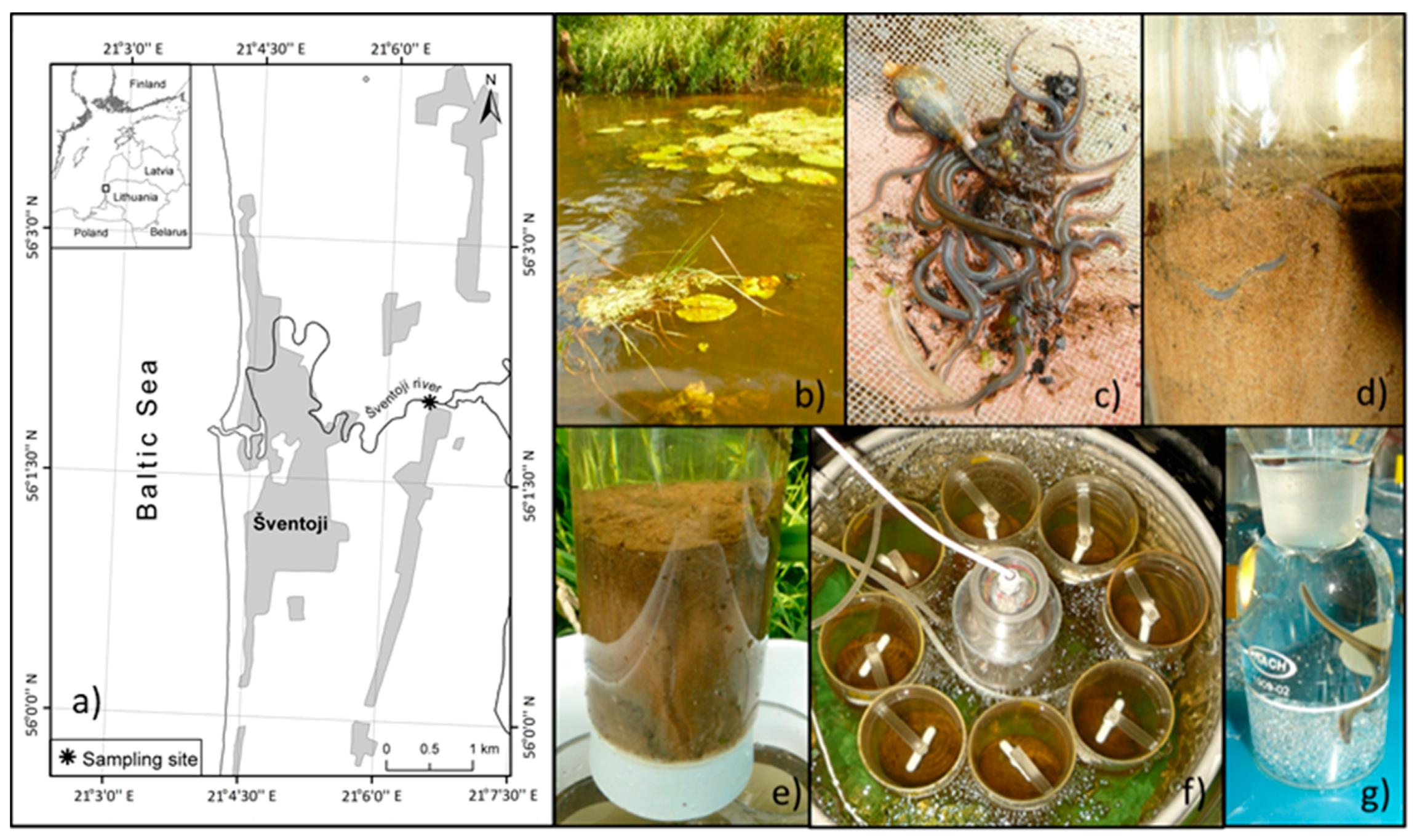
2.1. Sampling Procedure and Microcosms Set-Up
2.2. Measurement of Ammocoetes Respiration and Excretion Rates
2.3. Measurements of Benthic Fluxes in Lamprey Larvae Bioturbated Sediments
2.4. Sediment Characterization and Analytical Methods for Water Samples
2.5. Statistical Analysis
3. Results and Discussion
3.1. Sampling Site Features
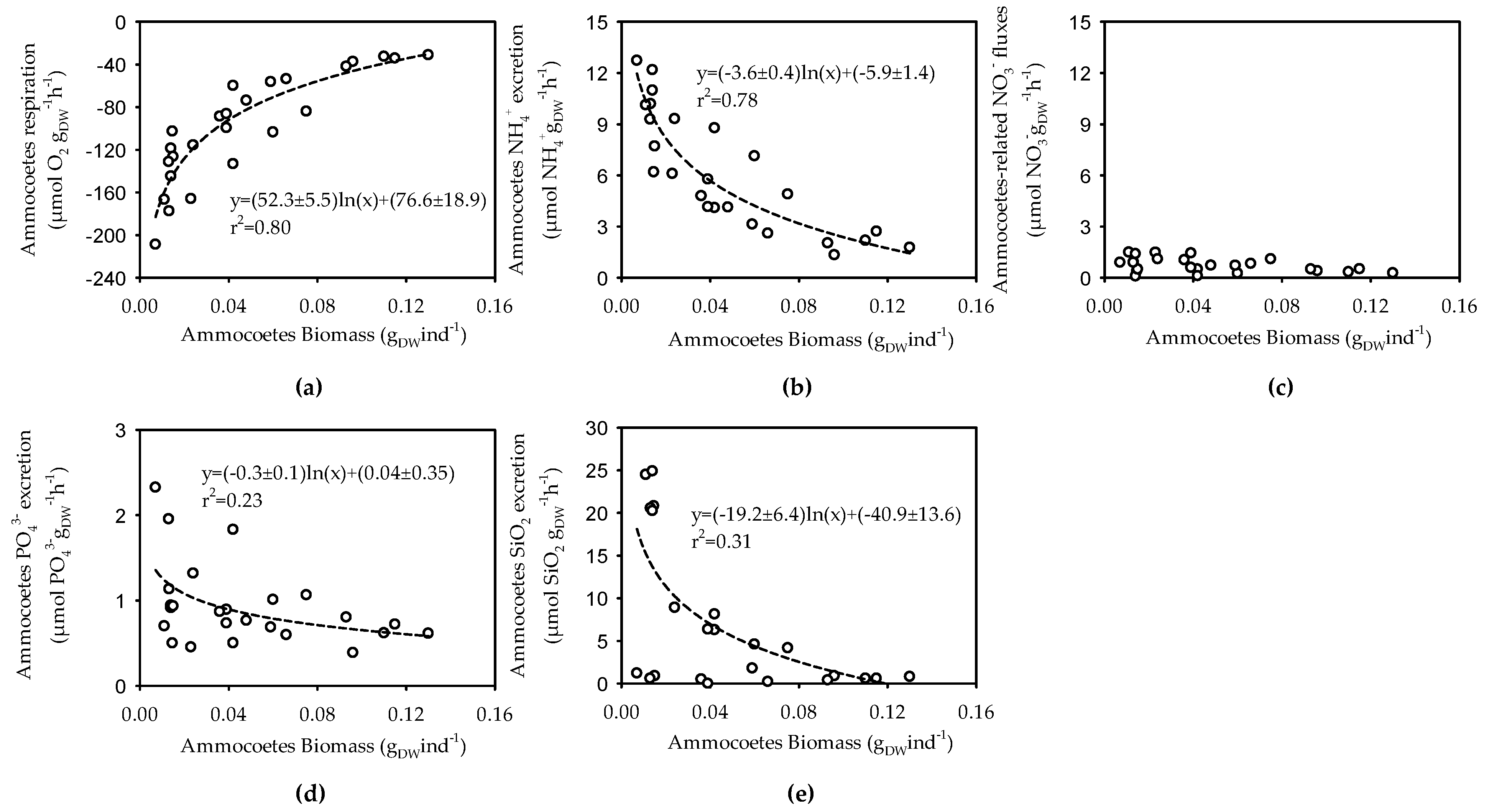
3.2. Size, Weight and Metabolic Activity of the Ammocoetes Retrieved in the Šventoji River
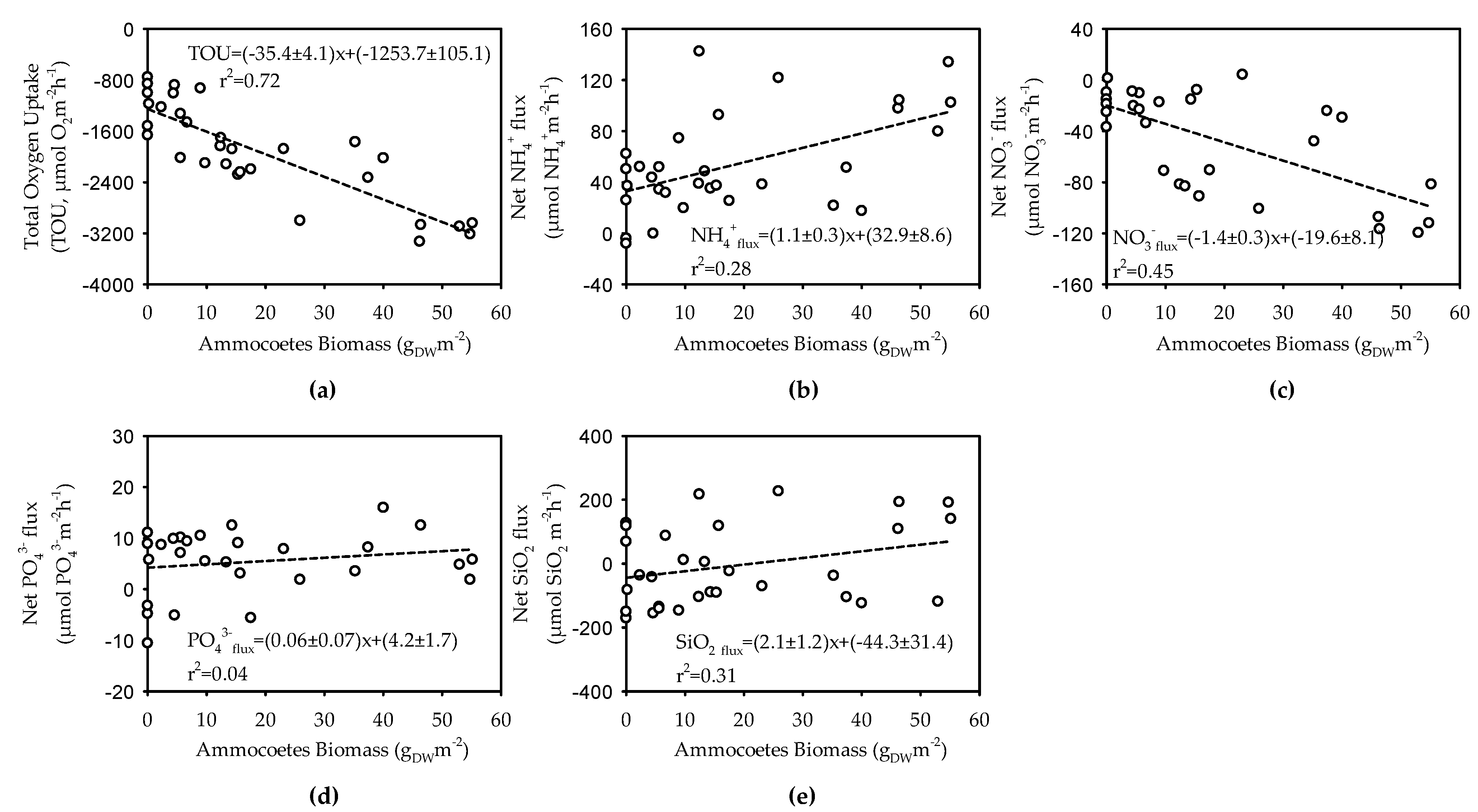
3.3. Benthic Respiration and Nutrient Fluxes in Sediments Bioturbated by Ammocoetes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kristensen, E. Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. In Life at Interfaces and under Extreme Conditions; Liebezeit, G., Dittmann, S., Kröncke, I., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 1–24. ISBN 978-94-010-5808-7. [Google Scholar]
- Kristensen, E.; Penha-Lopes, G.; Delefosse, M.; Valdemarsen, T.; Quintana, C.; Banta, G. What Is Bioturbation? The Need for a Precise Definition for Fauna in Aquatic Sciences. Mar. Ecol. Prog. Ser. 2012, 446, 285–302. [Google Scholar] [CrossRef]
- Adámek, Z.; Maršáalek, B. Bioturbation of Sediments by Benthic Macroinvertebrates and Fish and Its Implication for Pond Ecosystems: A Review. Aquacult. Int. 2013, 1–17. [Google Scholar] [CrossRef]
- Moore, J.W. Animal Ecosystem Engineers in Streams. BioScience 2006, 56, 237–246. [Google Scholar] [CrossRef]
- Boeker, C.; Lueders, T.; Mueller, M.; Pander, J.; Geist, J. Alteration of Physico-Chemical and Microbial Properties in Freshwater Substrates by Burrowing Invertebrates. Limnologica 2016, 59, 131–139. [Google Scholar] [CrossRef]
- Pelegrí, S.P.; Blackburn, T.H. Effect of Bioturbation by Nereis Sp., Mya Arenaria and Cerastoderma Sp. on Nitrification and Denitrification in Estuarine Sediments. Ophelia 1995, 42, 289–299. [Google Scholar] [CrossRef]
- Nielsen, O.; Gribsholt, B.; Kristensen, E.; Revsbech, N. Microscale Distribution of Oxygen and Nitrate in Sediment Inhabited by Nereis Diversicolor: Spatial Patterns and Estimated Reaction Rates. Aquat. Microb. Ecol. 2004, 34, 23–32. [Google Scholar] [CrossRef]
- Benelli, S.; Bartoli, M.; Ribaudo, C.; Fano, E. Contrasting Effects of an Alien Worm on Benthic N Cycling in Muddy and Sandy Sediments. Water 2019, 11, 465. [Google Scholar] [CrossRef]
- Bartoli, M.; Benelli, S.; Magri, M.; Ribaudo, C.; Moraes, P.C.; Castaldelli, G. Contrasting Effects of Bioturbation Studied in Intact and Reconstructed Estuarine Sediments. Water 2020, 12, 3125. [Google Scholar] [CrossRef]
- Nascimento, F.J.A.; Näslund, J.; Elmgren, R. Meiofauna Enhances Organic Matter Mineralization in Soft Sediment Ecosystems. Limnol. Oceanogr. 2012, 57, 338–346. [Google Scholar] [CrossRef]
- Bonaglia, S.; Nascimento, F.J.A.; Bartoli, M.; Klawonn, I.; Brüchert, V. Meiofauna Increases Bacterial Denitrification in Marine Sediments. Nat. Commun. 2014, 5, 5133. [Google Scholar] [CrossRef]
- Rosenberg, R. Marine Benthic Faunal Successional Stages and Related Sedimentary Activity. Sci. Mar. 2001, 65, 107–119. [Google Scholar] [CrossRef]
- Kristensen, E.; Delefosse, M.; Quintana, C.O.; Flindt, M.R.; Valdemarsen, T. Influence of Benthic Macrofauna Community Shifts on Ecosystem Functioning in Shallow Estuaries. Front. Mar. Sci. 2014, 1, 41. [Google Scholar] [CrossRef]
- Benelli, S.; Bartoli, M.; Zilius, M.; Vybernaite-Lubiene, I.; Ruginis, T.; Vaiciute, D.; Petkuviene, J.; Fano, E.A. Stoichiometry of Regenerated Nutrients Differs between Native and Invasive Freshwater Mussels with Implications for Algal Growth. Freshw. Biol. 2019, 64, 619–631. [Google Scholar] [CrossRef]
- Breukelaar, A.W.; Lammens, E.H.R.R.; Breteler, J.G.P.K.; Tatrai, I. Effects of Benthivorous Bream (Abramis Brama) and Carp (Cyprinus Carpio) on Sediment Resuspension and Concentrations of Nutrients and Chlorophyll a. Freshw. Biol. 1994, 32, 113–121. [Google Scholar] [CrossRef]
- Hogg, R.S.; Coghlan, S.M.; Zydlewski, J.; Simon, K.S. Anadromous Sea Lampreys (Petromyzon Marinus) Are Ecosystem Engineers in a Spawning Tributary. Freshw. Biol. 2014, 59, 1294–1307. [Google Scholar] [CrossRef]
- Gottesfeld, A.S.; Hassan, M.A.; Tunnicliffe, J.F. Salmon Bioturbation and Stream Process. Am. Fish. Soc. Symp. 2008, 65, 175–193. [Google Scholar]
- Nika, N.; Virbickas, T. Brown Trout Salmo Trutta Redd Superimposition by Spawning Lampetra Species in a Lowland Stream. J. Fish Biol. 2010, 77, 2358–2372. [Google Scholar] [CrossRef]
- Boeker, C.; Geist, J. Lampreys as Ecosystem Engineers: Burrows of Eudontomyzon sp. and Their Impact on Physical, Chemical, and Microbial Properties in Freshwater Substrates. Hydrobiologia 2016, 777, 171–181. [Google Scholar] [CrossRef]
- Shirakawa, H.; Yanai, S.; Goto, A. Lamprey Larvae as Ecosystem Engineers: Physical and Geochemical Impact on the Streambed by Their Burrowing Behavior. Hydrobiologia 2013, 701, 313–322. [Google Scholar] [CrossRef]
- Potter, I.C. Ecology of Larval and Metamorphosing Lampreys. Can. J. Fish. Aquat. Sci. 1980, 37, 1641–1657. [Google Scholar] [CrossRef]
- Hardisty, M.W.; Potter, I.C. The Behaviour, Ecology and Growth of Larval Lampreys. In The Biology of Lampreys; Hardisty, M.W., Potter, I.C., Eds.; Academic Press: London, UK, 1971; pp. 85–125. [Google Scholar]
- Smith, D.M.; Welsh, S.A.; Turk, P.J. Available Benthic Habitat Type May Influence Predation Risk in Larval Lampreys: Predation Risk in Larval Lampreys. Ecol. Freshw. Fish 2012, 21, 160–163. [Google Scholar] [CrossRef]
- Aronsuu, K.; Virkkala, P. Substrate Selection by Subyearling European River Lampreys (Lampetra Fluviatilis) and Older Larvae (Lampetra spp). Ecol. Freshw. Fish 2014, 23, 644–655. [Google Scholar] [CrossRef]
- Ojutkangas, E.; Aronen, K.; Laukkanen, E. Distribution and Abundance of River Lamprey (Lampetra fluviatilis) Ammocoetes in the Regulated River Perhonjoki. Regul. Rivers Res. Mgmt. 1995, 10, 239–245. [Google Scholar] [CrossRef]
- Enequist, P. Das Bachneunauge Als Ökologische Modification Des Flussneunauges. Über Die Fluss- Und Bachneunaugen Schwedens. Ark. Für Zool. 1937, 29, 1–22. [Google Scholar]
- Hardisty, M.W. The Life History and Growth of the Brook Lamprey (Lampetra planeri). J. Anim. Ecol. 1944, 13, 110–122. [Google Scholar] [CrossRef]
- Hardisty, M.W. Biology of the Cyclostomes; Chapman & Hall: London, UK, 1979. [Google Scholar]
- Vaughn, C.C. Biodiversity Losses and Ecosystem Function in Freshwaters: Emerging Conclusions and Research Directions. BioScience 2010, 60, 25–35. [Google Scholar] [CrossRef]
- Renaud, C.B. Conservation Status of Northern Hemisphere Lampreys (Petromyzontidae). J. Appl. Ichthyol. 1997, 13, 143–148. [Google Scholar] [CrossRef]
- Maitland, P.S. Ecology of the River, Brook and Sea Lamprey. In Conserving Natura 2000 Rivers Monitoring; English Nature: Peterborough, UK, 2003; p. 52. [Google Scholar]
- Ruginis, T.; Bartoli, M.; Petkuviene, J.; Zilius, M.; Lubiene, I.; Laini, A.; Razinkovas-Baziukas, A. Benthic Respiration and Stoichiometry of Regenerated Nutrients in Lake Sediments with Dreissena polymorpha. Aquat. Sci. 2014, 76, 405–417. [Google Scholar] [CrossRef]
- Lorenzen, C.J. Determination of Chlorophyll and Pheo-pigments: Spectrophotometric Equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Grasshoff, K.; Ehrhardt, M.; Kremling, K. Methods of Seawater Analysis, 2nd ed.; Verlag Chemie: Berlin, Germany, 1983. [Google Scholar]
- Bower, C.E.; Holm-Hansen, T. A Salicylate–Hypochlorite Method for Determining Ammonia in Seawater. Can. J. Fish. Aquat. Sci. 1980, 37, 794–798. [Google Scholar] [CrossRef]
- Smith, D.M.; Welsh, S.A.; Turk, P.J. Selection and Preference of Benthic Habitat by Small and Large Ammocoetes of the Least Brook Lamprey (Lampetra aepyptera). Environ. Biol. Fish 2011, 91, 421–428. [Google Scholar] [CrossRef]
- Moser, M.; Bayer, J.; MacKinlay, D. The Biology of Lampreys—Symposium Proceedings. In Proceedings of the International Congress on the Biology of Fish University of British Columbia, Vancouver, BC, Canada, 22–25 July 2002. [Google Scholar]
- Wotton, R.S. The Ubiquity and Many Roles of Exopolymers (EPS) in Aquatic Systems. Sci. Mar. 2004, 68, 13–21. [Google Scholar] [CrossRef]
- Manion, P.J. Diatoms as Food of Larval Sea Lampreys in a Small Tributary of Northern Lake Michigan. Trans. Am. Fish. Soc. 1967, 96, 224–226. [Google Scholar] [CrossRef]
- Benelli, S.; Bartoli, M.; Racchetti, E.; Moraes, P.C.; Zilius, M.; Lubiene, I.; Fano, E.A. Rare but Large Bivalves Alter Benthic Respiration and Nutrient Recycling in Riverine Sediments. Aquat. Ecol. 2017, 51, 1–16. [Google Scholar] [CrossRef]
- Clarke, A.; Johnston, N.M. Scaling of Metabolic Rate with Body Mass and Temperature in Teleost Fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- Samuiloviene, A.; Bartoli, M.; Bonaglia, S.; Cardini, U.; Vybernaite-Lubiene, I.; Marzocchi, U.; Petkuviene, J.; Politi, T.; Zaiko, A.; Zilius, M. The Effect of Chironomid Larvae on Nitrogen Cycling and Microbial Communities in Soft Sediments. Water 2019, 11, 1931. [Google Scholar] [CrossRef]
- Wilkie, M.P.; Wang, Y.; Walsh, P.J.; Youson, J.H. Nitrogenous Waste Excretion by the Larvae of a Phylogenetically Ancient Vertebrate: The Sea Lamprey (Petromyzon marinus). Can. J. Zool. 1999, 77, 707–715. [Google Scholar] [CrossRef]
- Welsh, D.T.; Castadelli, G. Bacterial Nitrification Activity Directly Associated with Isolated Benthic Marine Animals. Mar. Biol. 2004, 144, 1029–1037. [Google Scholar] [CrossRef]
- Staponkus, R.; Kesminas, V. Status Assessment of Lampreys in Natura 2000 Network in Lithuania. Biologija 2014, 60, 1–7. [Google Scholar] [CrossRef][Green Version]
- Geist, J.; Auerswald, K. Physicochemical Stream Bed Characteristics and Recruitment of the Freshwater Pearl Mussel (Margaritifera margaritifera). Freshw. Biol. 2007, 52, 2299–2316. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nika, N.; Zilius, M.; Ruginis, T.; Giordani, G.; Bagdonas, K.; Benelli, S.; Bartoli, M. Benthic Metabolism in Fluvial Sediments with Larvae of Lampetra sp. Water 2021, 13, 1002. https://doi.org/10.3390/w13071002
Nika N, Zilius M, Ruginis T, Giordani G, Bagdonas K, Benelli S, Bartoli M. Benthic Metabolism in Fluvial Sediments with Larvae of Lampetra sp. Water. 2021; 13(7):1002. https://doi.org/10.3390/w13071002
Chicago/Turabian StyleNika, Nerijus, Mindaugas Zilius, Tomas Ruginis, Gianmarco Giordani, Kasparas Bagdonas, Sara Benelli, and Marco Bartoli. 2021. "Benthic Metabolism in Fluvial Sediments with Larvae of Lampetra sp." Water 13, no. 7: 1002. https://doi.org/10.3390/w13071002
APA StyleNika, N., Zilius, M., Ruginis, T., Giordani, G., Bagdonas, K., Benelli, S., & Bartoli, M. (2021). Benthic Metabolism in Fluvial Sediments with Larvae of Lampetra sp. Water, 13(7), 1002. https://doi.org/10.3390/w13071002






