Intestinal Microbial Ecology and Fillet Metal Chemistry of Wild Grey Mullets Reflect the Variability of the Aquatic Environment in a Western Mediterranean Coastal Lagoon (Santa Giusta, Sardinia, Italy)
Abstract
1. Introduction
2. Materials and Methods
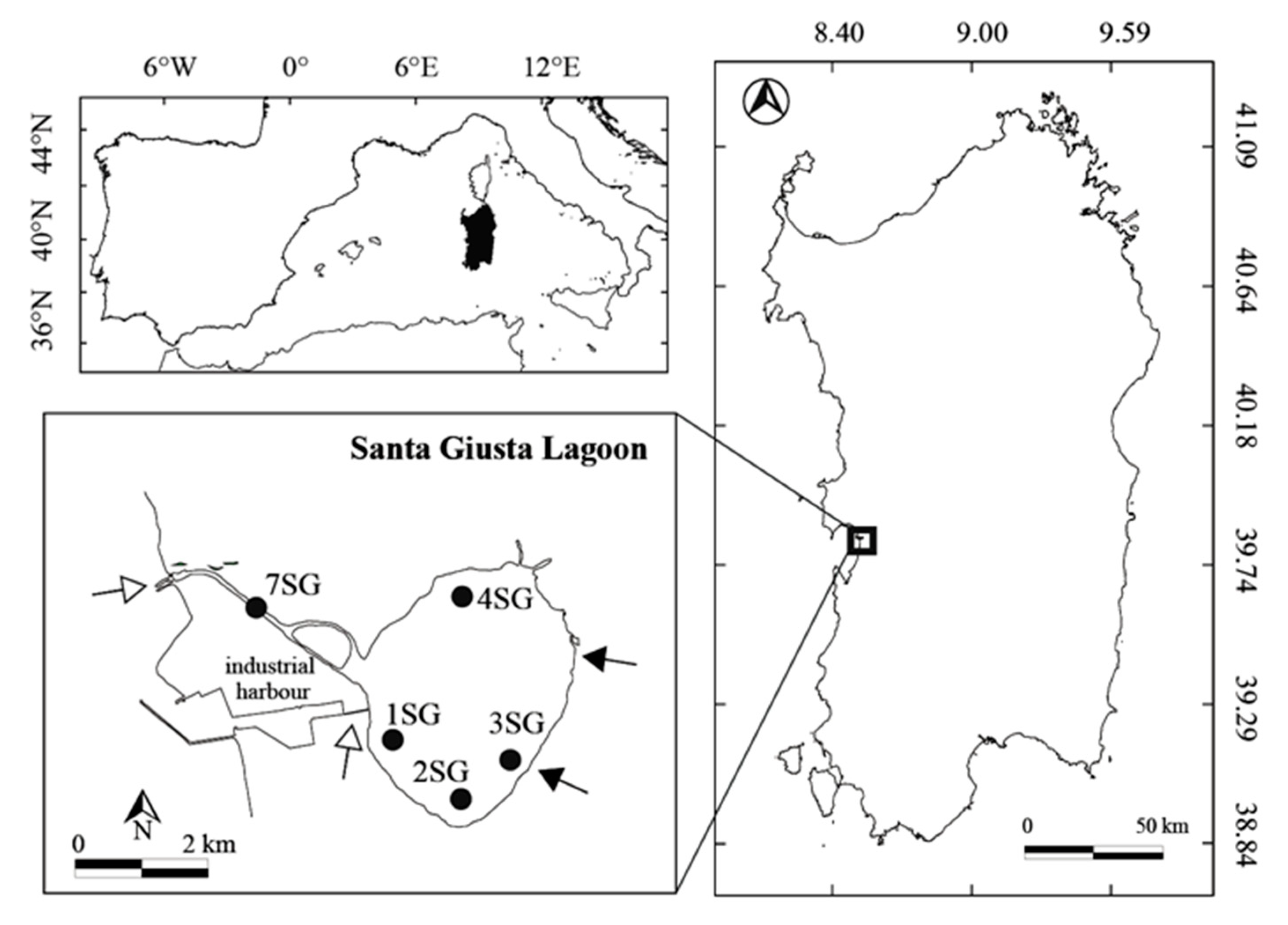
2.1. Study Area
2.2. Environmental Characterization
2.3. Microbiological Analyses of Fish Gut
2.3.1. Samplings, Biometry and Conventional Microbiological Analyses
2.3.2. Estimation of Bacterial Abundance
- membrane conversion factor = filtration area/area of micrometer field;
- N = total number of bacteria counted/number of micrometer fields counted;
- D = dilution factor, i.e., volume of sample stained/total volume of sample.
2.3.3. Taxonomical Identification of Bacteria
2.4. Heavy Metal Analyses
2.5. Statistical Analyses
3. Results
3.1. Environmental Context
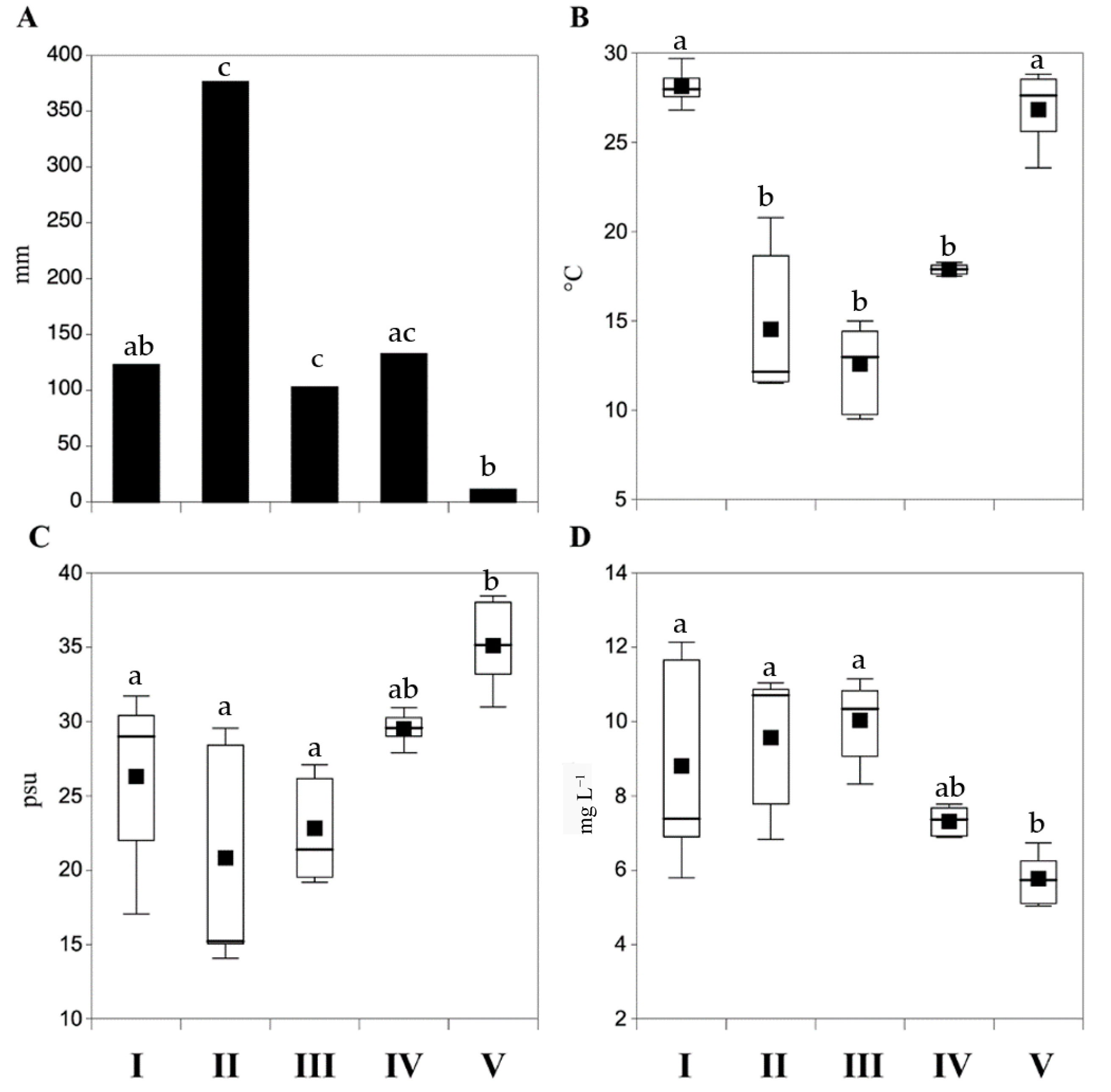
3.1.1. Rainfalls, Water Temperature, Salinity and Dissolved Oxygen
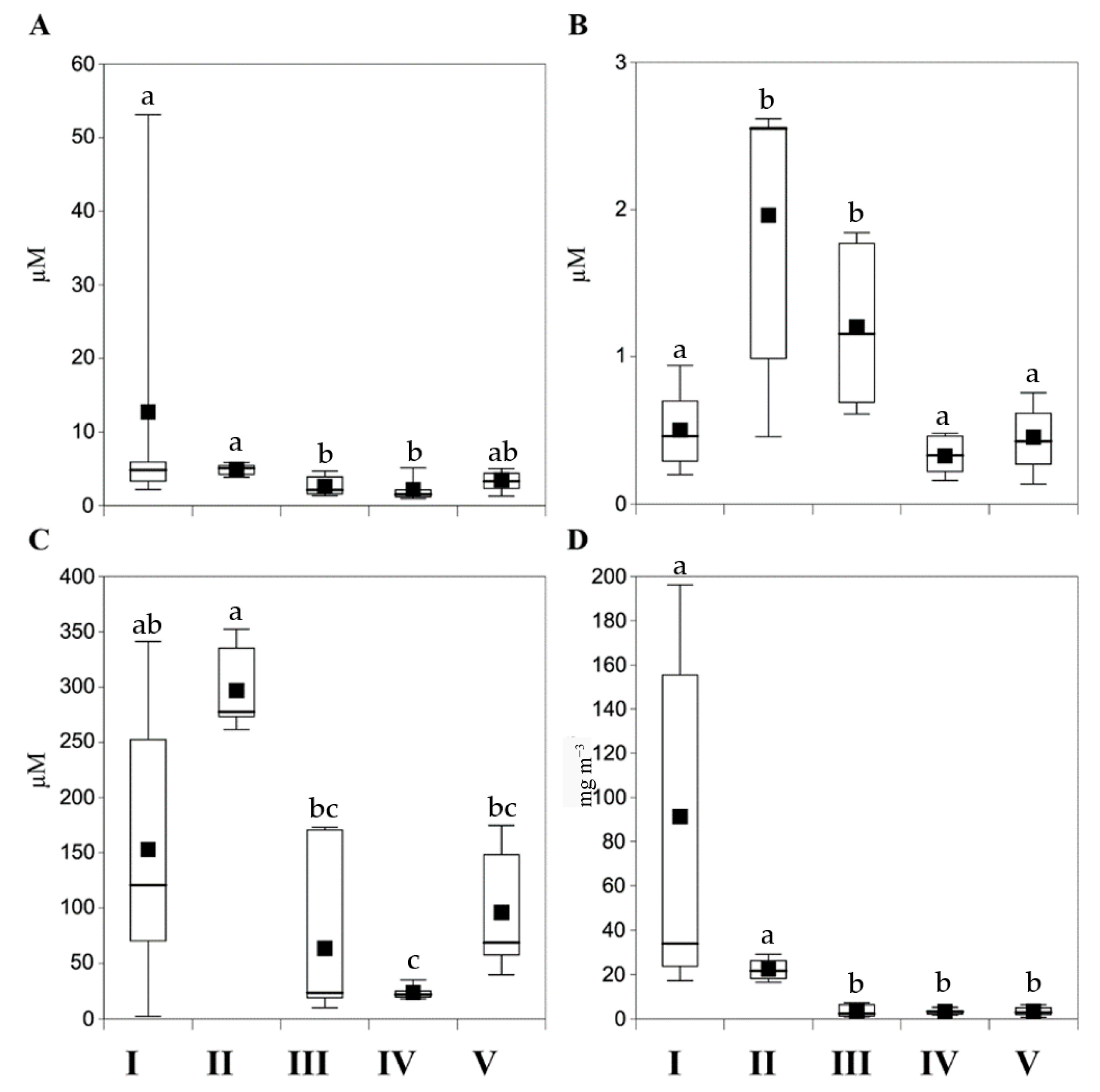
3.1.2. Nutrients and Chlorophyll a
3.2. Fish Gut Microbiological Analysis
3.2.1. Cultivable Bacteria Enumeration
3.2.2. Bacterial Abundance (Cultivable and Not Cultivable Bacteria)
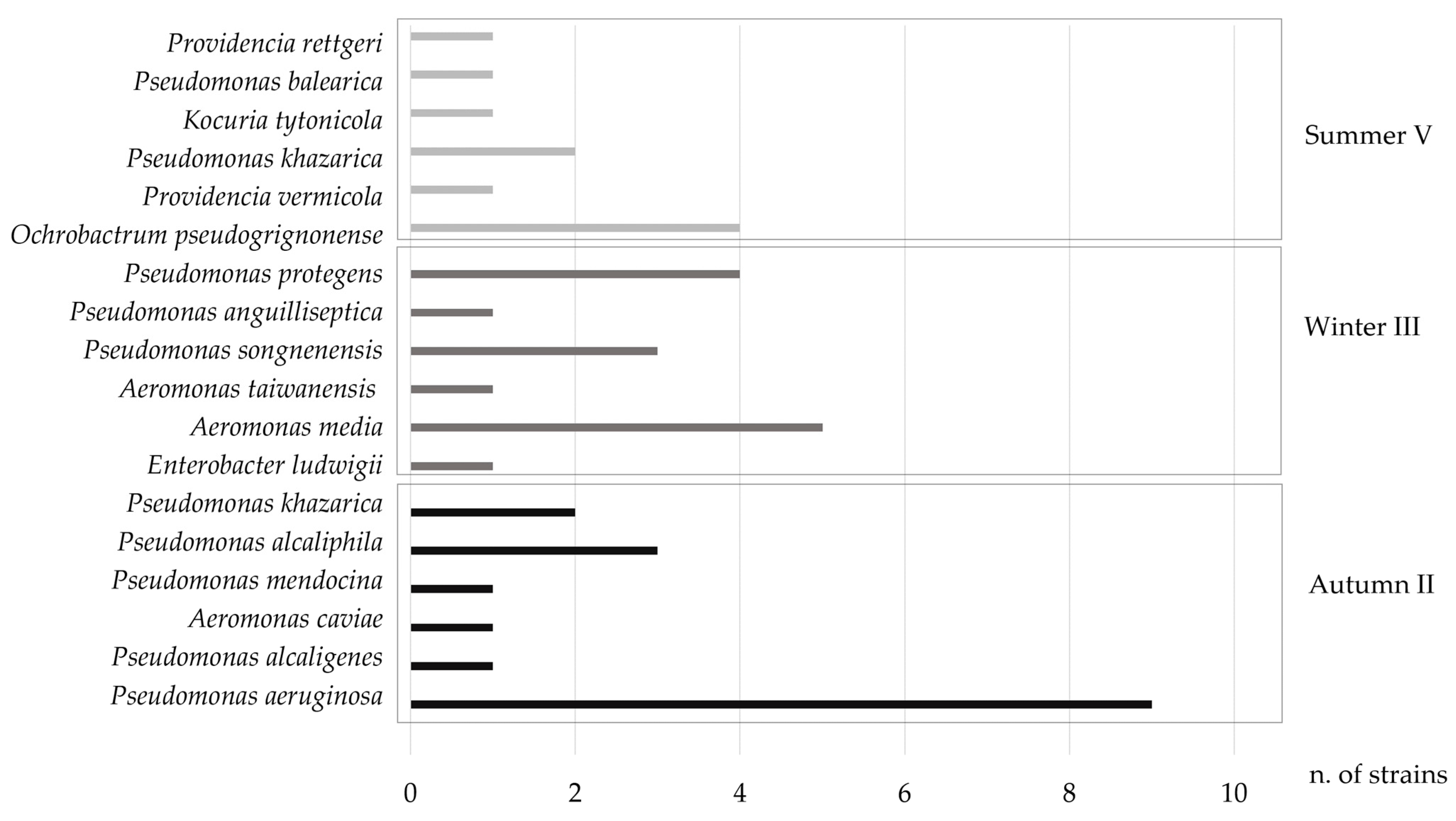
3.2.3. Identification of Intestinal Bacteria by 16S rDNA Sequence
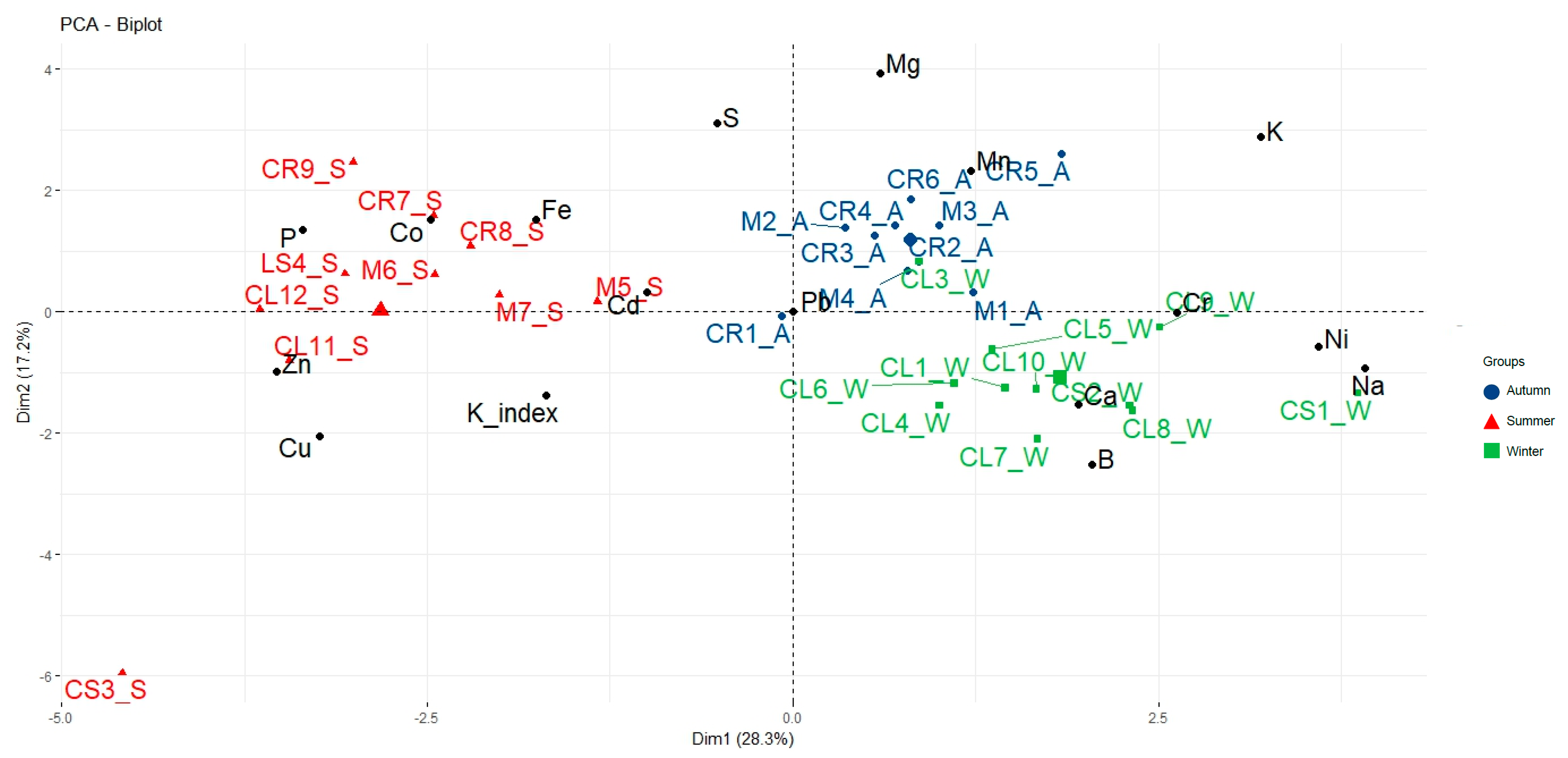
3.3. Fish Metal Analysis and Biometrics
4. Discussion
5. Conclusions
- The present work gives a novel knowledge contribution on the cultivable gut microflora and fillet metal composition of wild mullets in a transitional ecosystem of the Mediterranean area.
- The quali-quantitative microbiological composition of mullets’ gut and the fillet metal quantities appeared to be influenced by several environmental variables, characterized by clear seasonal dynamics (e.g., meteorological conditions, temperature, salinity and nutrients).
- Metagenomic studies are in progress to clarify the ecological role of mullet gut microbiota, both for fish and the ecosystem.
- The multi-disciplinarity of this work proved to be a good approach for studying complex ecosystems, such as Mediterranean lagoons, characterized by a notable variability of environmental factors due to anthropogenetic and natural stressors.
- Our findings can be valuable for management practices, especially in critical and instable aquatic environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newton, A.; Brito, A.C.; Icely, J.D.; Derolez, V.; Clara, I.; Angus, S.; Schernewski, G.; Inácio, M.; Lillebø, A.I.; Sousa, A.I.; et al. Assessing, quantifying and valuing the ecosystem services of coastal lagoons. J. Nat. Conserv. 2018, 44, 50–65. [Google Scholar] [CrossRef]
- Beraldi, G.Q.; de Rezende, C.E.; de Almeida, G.M.; Carvalho, C.; de Lacerda, L.D.; de Farias, R.N.; Vidal, M.S.; Mouillot, D.; Souza, M.D.P.; Molisani, M.M. Assessment of a coastal lagoon metal distribution through natural and anthropogenic processes (SE, Brazil). Mar. Poll. Bull. 2019, 146, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Holmlund, C.M.; Hammer, M. Ecosystem services generated by fish populations. Ecol. Econom. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Yilmaz, F. The comparison of heavy metal concentrations (Cd, Cu, Mn, Pb, and Zn) in tissues of three economically important fish (Anguilla anguilla, Mugil cephalus and Oreochromis niloticus) inhabiting Koycegiz Lake-Mugia (Turkey). Turk. J. Sci. Technol. 2009, 4, 7–15. [Google Scholar]
- Wilson, R.W.; Millero, F.J.; Taylor, J.R.; Walsh, P.J.; Christensen, V.; Jennings, S.; Grosell, M. Contribution of fish to the marine inorganic carbon cycle. Science 2009, 323, 359–362. [Google Scholar] [CrossRef]
- Balk, L.; Larsson, A.; Frolin, L. Baseline studies of biomarkers in the feral female perch (Perca fluviatilis) as tools in biological monitoring of anthropogenic substances. Mar. Environ. Res. 1996, 42, 203–208. [Google Scholar] [CrossRef]
- Curson, A.R.J.; Matthew, J.S.; Todd, J.D.; Carpenter, S.R.; Cottingham, K.L. Resilience and restoration of lakes. Conserv. Ecol. 1997, 1, 2. Available online: www.consecol.org (accessed on 1 March 2021).
- Diop, M.; Howsam, M.; Diop, C.; Goossens, J.F.; Diouf, A.; Amara, R. Assessment of trace element contamination and bioaccumulation in algae (Ulva lactuca), mussels (Perna perna), shrimp (Penaeus kerathurus), and fish (Mugil cephalus, Saratherondon melanotheron) along the Senegalese coast. Mar. Poll. Bull. 2016, 103, 339–343. [Google Scholar] [CrossRef]
- Ihunwo, O.C.; Dibofori-Orji, A.N.; Olowu, C.; Ibezim-Ezeani, M.U. Distribution and risk assessment of some heavy metals in surface water, sediment and grey mullet (Mugil cephalus) from contaminated creek in Woji, southern Nigeria. Mar. Poll. Bull. 2020, 154, 111042. [Google Scholar] [CrossRef]
- Jones, J.; DiBattista, J.D.; Stat, M.; Bunce, M.; Boyce, M.C.; Fairclough, D.V.; Travers, M.J.; Huggett, M.J. The microbiome of the gastrointestinal tract of a range-shifting marine herbivorous fish. Front. Microbiol. 2018, 9, 2000. [Google Scholar] [CrossRef]
- Talwar, C.; Nagar, S.; Lal, R.; Negi, R.K. Fish gut microbiome: Current Approaches and Future Perspectives. Indian J. Microbiol. 2018, 58, 397–414. [Google Scholar] [CrossRef]
- Floris, R.; Manca, S.; Fois, N. Microbial ecology of intestinal tract of gilthead sea bream (Sparus aurata Linnaeus, 1758) from two coastal lagoons of Sardinia (Italy). Trans. Water Bullet. 2013, 7, 4–12. [Google Scholar] [CrossRef]
- Nikouli, E.; Meziti, A.; Antonopoulou, E.; Mente, E.; Kormas, K.A. Gut Bacterial Communities in Geographically Distant Populations of Farmed Sea Bream (Sparus aurata) and Sea Bass (Dicentrarchus labrax). Microorganisms 2018, 6, 92. [Google Scholar] [CrossRef]
- Hovda, M.B.; Fontanillas, R.; McGurk, C.; Obach, A.; Rosnes, J.T. Seasonal variations in the intestinal microbiota of farmed Atlantic salmon (Salmo salar L.). Aquac. Res. 2012, 43, 154–159. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Lee Merrifield, D.; Spring, P.; Sweetman, J.; Moate, R.; Davies, S.J. Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata). Aquaculture 2010, 300, 182–188. [Google Scholar] [CrossRef]
- Carnevali, O.; Zamponi, M.C.; Sulpizio, R.; Rollo, A.; Nardi, M.; Orpianesi, C.; Silvi, S.; Caggiano, M.; Polzonetti, A.M.; Cresci, A. Administration of probiotic strain to improve sea bream wellness during development. Aquac. Int. 2004, 12, 377–386. [Google Scholar] [CrossRef]
- Floris, R.; Scanu, G.; Fois, N.; Rizzo, C.; Malavenda, R.; Spanò, N.; Lo Giudice, A. Intestinal bacterial flora of Mediterranean gilthead sea bream (Sparus aurata Linnaeus) as a novel source of natural surface active compounds. Aquac. Res. 2018, 49, 1262–1273. [Google Scholar] [CrossRef]
- Crosetti, D. Biology, Ecology and Culture of Grey Mullet (Mugilidae), 1st ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 42–127. [Google Scholar]
- Thomson, J.M. The Mugilidae of the world. Mem. Queens. Mus. 1997, 41, 457–562. [Google Scholar]
- Cataudella, S.; Bronzi, P. Aquacoltura Responsabile Verso le Produzioni Acquatiche del Terzo Millennio; Libreria Internazionale Hoepli: Milan, Italy, 2001; pp. 559–560. ISBN 978880000463. [Google Scholar]
- Bledsoe, G.E.; Bledsoe, C.D.; Rasco, B. Caviars and Fish Roe Products. Crit. Rev. Food Sci. Nutr. 2003, 43, 317–356. [Google Scholar] [CrossRef] [PubMed]
- Caredda, M.; Addis, M.; Pes, M.; Fois, N.; Sanna, G.; Piredda, G.; Sanna, G. Physico-chemical, colorimetric, rheological parameters and chemometric discrimination of the origin of Mugil cephalus’ roes during the manufacturing process of Bottarga. Food Res. Int. 2018, 108, 128–135. [Google Scholar] [CrossRef]
- Lugliè, A.; Sechi, N.; Oggiano, G.; Sanna, G.; Tapparo, A. Ecological assessment of Santa Giusta lagoon (Sardinia, Italy). Annali di Chimica 2002, 92, 239–247. [Google Scholar]
- Sechi, N.; Fiocca, F.; Sannio, A.; Lugliè, A. Santa Giusta Lagoon (Sardinia): Phytoplancton and nutrients before and after waste diversion. J. Limnol. 2001, 60, 194–200. [Google Scholar] [CrossRef]
- Satta, C.T.; Anglès, S.; Garcés, E.; Sechi, N.; Pulina, S.; Padedda, B.M.; Stacca, D.; Lugliè, A. Dinoflagellate cyst assemblages in surface sediments from three shallow Mediterranean Lagoons (Sardinia, North Western Mediterranean Sea). Estuar. Coast. 2014, 37, 646–663. [Google Scholar] [CrossRef]
- Cannas, A.; Cataudella, S.; Rossi, R. Gli Stagni Della Sardegna; CIRSPE: Cagliari, Italy, 1998. [Google Scholar]
- Satta, C.T.; Padedda, B.M.; Sechi, N.; Pulina, S.; Loria, A.; Lugliè, A. Multiannual Chattonella subsalsa Biecheler (Raphidophyceae) blooms in a Mediterranean lagoon (Santa Giusta Lagoon, Sardinia Island, Italy). Harmful Algae 2017, 67, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972; p. 167. [Google Scholar]
- SCOR-UNESCO. Determination of Photosynthetic Pigments in Sea Water. In Monographs on Oceanographic Methodology; UNESCO: Paris, France, 1997. [Google Scholar]
- Porter, K.G.; Feig, Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980, 25, 943–948. [Google Scholar] [CrossRef]
- Wetzel, R.G.; Likens, G.E. Limnological Analyses, 2nd ed.; Springer-Verlag: New York, NY, USA, 1991; p. 391. [Google Scholar]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations, Bulletin 191; Bulletin of the Fisheries Research Board of Canada: Ottawa, ON, Canada, 1975; p. 209. [Google Scholar]
- Larsen, A.; Tao, Z.; Bullard, S.A.; Arias, C.R. Diversity of the skin microbiota of fishes: Evidence for host species specificity. FEMS Microbiol. Ecol. 2013, 85, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Khemis, I.B.; Aridh, N.B.; Hamza, N.; M’hetli, M.; Sadok, S. Heavy metals and minerals contents in pikeperch (Sander lucioperca), carp (Cyprinus carpio) and flathead grey mullets (Mugil cephalus) from Sidi Salem reservoir (Tunisia): Health risk assessment related to fish consumption. Environ. Sci. Pollut. Res. 2017, 24, 19494–19507. [Google Scholar] [CrossRef]
- Pulina, S.; Suikkanen, S.; Satta, C.T.; Mariani, M.A.; Padedda, B.M.; Virdis, T.; Caddeo, T.; Sechi, N.; Lugliè, A. Multiannual phytoplankton trends in relation to environmental changes across aquatic domains: A case study from Sardinia (Mediterranean Sea). Plant. Biosyst. 2016, 150, 660–670. [Google Scholar] [CrossRef]
- Pulina, S.; Satta, C.T.; Padedda, B.M.; Sechi, N.; Lugliè, A. Seasonal variations of phytoplankton size structure in relation to environmental variables in three Mediterranean shallow coastal lagoons. Estuar. Coast. Shelf Sci. 2018, 212, 95–104. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Bouvier, C.; Bouvier, T.; Troussellier, M.; Escalas, A.; Mouillot, D. Genetic difference but functional similarity among fish gut bacterial communities through molecular and biochemical fingerprints. FEMS Microbiol. Ecol. 2012, 79, 568–680. [Google Scholar] [CrossRef]
- Sala, M.M.; Estrada, M.; Gasol, J.M. Seasonal changes in the functional diversity of bacteriolplankton in contrasting coastal environments of the NW Mediterranean. Aquat. Microb. Ecol. 2006, 44, 1–9. [Google Scholar] [CrossRef]
- Rizzo, C.; Michaud, L.; Hörmann, B.; Gerçe, B.; Syldatk, C.; Hausmann, R.; De Domenico, E.; Lo Giudice, A. Bacteria associated with sabellids (Polychaeta: Annelida) as a novel source of surface active compounds. Mar. Poll. Bull. 2013, 70, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Skrodenyte-Arbaciauskiene, V.; Sruoga, A.; Butkauskas, D.; Skrupskelis, K. Phylogenetic analysis of intestinal bacteria of freshwater salmon Salmo salar and sea trout Salmo trutta trutta and diet. Fish. Sci. 2008, 74, 1307–1314. [Google Scholar] [CrossRef]
- Curson, A.R.J.; Sullivan, M.J.; Todd, J.D.; Johnston, A.W.B. Identification of genes for dimethyl sulfide production in bacteria in the gut of Atlantic Herring (Clupea harengus). Int. Soc. Microb. Ecol. J. Short Commun. 2010, 4, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, X.; Zhang, R. Draft genome sequence of Ochrobactrum pseudogrignonense strain CDB2, a highly efficient arsenate-resistant soil bacterium from arsenic-contaminated cattle dip sites. Genome Announc. 2013, 1, e00173-13. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.S.; Wang, E.; Zimmermann, S.; Boutin, S.; Wagner, H.; Wink, M. Kocuria tytonicola, new bacteria from the preen glands of Americanbarn owls (Tyto furcata). Syst. Appl. Microbiol. 2018, 42, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ashforth, E.; Ren, B.; Song, F.; Dai, H.; Liu, M.; Wang, J.; Xie, Q.; Zhang, L. Bioprospecting microbial natural product libraries from the marine environment for drug discovery. J. Antibiot. 2010, 63, 415–422. [Google Scholar] [CrossRef]
- Oali, N.; Belabed, B.E.; Chenchouni, H. Modelling environment contamination with heavy metals in flathead grey mullet Mugil cephalus and upper sediments from North African coasts of the Mediterranean Sea. Sci. Total Environ. 2018, 39, 156–174. [Google Scholar] [CrossRef]
- Prado, P.; Vergara, C.; Caiola, N.; Ibáñez, C. Influence of salinity regime on the food-web structure and feeding ecology of fish species from Mediterranean coastal lagoons. Estuar. Coast. Shelf Sci. 2014, 139, 1–10. [Google Scholar] [CrossRef]
- International Standard Guidelines (UE 1881/2006). Available online: http://www.fao.org/faolex/results/details/en/c/LEX-FAOC068134/ (accessed on 1 March 2021).
- Zhang, Y.; Zhang, R.; Sun, H.; Chen, Q.; Yu, X.; Zhang, T.; Yi, M.; Liu, J.X. Copper inhibits hatching of fish embryos via inducing reactive oxygen species and down-regulating Wnt signalling. Aquat. Toxicol. 2018, 205, 156–164. [Google Scholar] [CrossRef]
- Esposito, M.; Maglio, P.; Hauber, T.; Miedico, O.; Serpe, P.; Chiaravalle, E.A. Studio sulla contaminazione da metalli in prodotti ittici provenienti dall’area marina di Crotone. La Rivista di Scienza dell’Alimentazione 2012, 1, 7–15. Available online: https://www.researchgate.net/publication/256766114 (accessed on 10 January 2020).
- Annabi, A.; Said, K.; Messaoudi, I. Heavy metal levels in gonad and liver tissues—effects on the reproductive parameters of natural populations of Aphanius facsiatus. Environ. Sci. Pollut. Res. 2013, 20, 7309–7319. [Google Scholar] [CrossRef] [PubMed]
- Katselis, G.; Koukou, K.; Dimitriou, E.; Koutsikopoulos, C. Short-term seaward fish migration in the Messolonghie-Etoliko lagoons (Western Greek coast) in relation to climatic variables and the lunar cycle. Estuar. Coast. Shelf Sci. 2007, 73, 571e582. [Google Scholar] [CrossRef]
- Marks, P.J.; Plaskett, D.; Potter, I.C.; Bradley, J.S. Relationship between concentration of heavy metals in muscle tissue and body weight of fish from the swan-Avon estuary, Western Australia. Mar. Freshw. Res. 1980, 31, 783–793. [Google Scholar] [CrossRef]

| Autumn II | Winter III | Summer V | ||
|---|---|---|---|---|
| Total heterotrophic bacteria | min-max | 1.1 × 104–3.1 × 105 | 5.5 × 103–8.2 × 104 | 7.0 × 103–7.0 × 104 |
| mean | 1.3 × 105 | 3.7 × 104 | 3.8 × 104 | |
| Marine bacteria | min-max | 1.0 × 104–1.0 × 105 | 1.2 × 105–3.8 × 105 | 1.2 × 105–1.1 × 106 |
| mean | 6.3 × 104 a | 2.5 × 105 a | 7.4 × 105 b | |
| Enterobacteriaceae | min-max | 3.1 × 102–5.0 × 104 | 5.0 × 102–2.4 × 103 | 1.7 × 102–1.3 × 104 |
| mean | 2.4 × 104 | 1.5 × 103 | 5.4 × 103 | |
| Coliforms | min-max | 7.0 × 102–2.0 × 104 | 3.5 × 102–2.5 × 103 | 60.0 × 104 |
| mean | 1.1 × 104 | 1.1 × 103 | 4.3 × 103 | |
| Dapi | min-max | ND | 9.0 × 105–1.9 × 106 | 8.9 × 107–1.1 × 108 |
| mean | 1.5 × 106 a | 9.8 × 107 b | ||
| Phylum or Class | Strain | Bacterial Affiliation | Accession Number | Fish Species |
|---|---|---|---|---|
| Gammaproteobacteria | 1 | Pseudomonas aeruginosa | MW369461 | Chelon ramada |
| Gammaproteobacteria | 6 | Pseudomonas aeruginosa | MW369462 | Chelon ramada |
| Gammaproteobacteria | 10 | Pseudomonas alcaligenes | MW369463 | Chelon ramada |
| Gammaproteobacteria | 11 | Aeromonas caviae | MW369464 | Chelon ramada |
| Gammaproteobacteria | 13 | Pseudomonas aeruginosa | MW369465 | Chelon ramada |
| Gammaproteobacteria | 15 | Pseudomonas aeruginosa | MW369466 | Chelon ramada |
| Gammaproteobacteria | 17 | Pseudomonas mendocina | MW369467 | Chelon ramada |
| Gammaproteobacteria | 20 | Pseudomonas alcaliphila | MW369468 | Mugil cephalus |
| Gammaproteobacteria | 24 | Pseudomonas khazarica | MW369469 | Mugil cephalus |
| Gammaproteobacteria | 26 | Pseudomonas khazarica | MW369470 | Mugil cephalus |
| Gammaproteobacteria | 28 | Enterobacter ludwigii | MW369471 | Chelon saliens |
| Gammaproteobacteria | 30 | Aeromonas media | MW369472 | Chelon saliens |
| Gammaproteobacteria | 35 | Aeromonas taiwanensis | MW369473 | Chelon saliens |
| Gammaproteobacteria | 37 | Aeromonas media | MW369474 | Chelon labrosus |
| Gammaproteobacteria | 38 | Pseudomonas songnenensis | MW369475 | Chelon labrosus |
| Gammaproteobacteria | 40 | Aeromonas media | MW369476 | Chelon labrosus |
| Gammaproteobacteria | 41 | Pseudomonas anguilliseptica | MW369477 | Chelon labrosus |
| Gammaproteobacteria | 47 | Pseudomonas protegens | MW369478 | Chelon labrosus |
| Gammaproteobacteria | 54 | Aeromonas media | MW369479 | Chelon labrosus |
| Gammaproteobacteria | 55 | Pseudomonas protegens | MW369480 | Chelon labrosus |
| Alphaproteobacteria | 58 | Ochrobactrum pseudogrignonense | MW369481 | Mugil cephalus |
| Gammaproteobacteria | 66 | Providencia vermicola | MW369482 | Mugil cephalus |
| Gammaproteobacteria | 67 | Pseudomonas khazarica | MW369483 | Chelon ramada |
| Actinobacteria | 69 | Kocuria tytonicola | MW369484 | Chelon ramada |
| Gammaproteobacteria | 71 | Pseudomonas balearica | MW369485 | Chelon ramada |
| Alphaproteobacteria | 77 | Ochrobactrum pseudogrignonense | MW369486 | Chelon labrosus |
| Gammaproteobacteria | 82 | Providencia rettgeri | MW369487 | Chelon labrosus |
| Autumn II | Winter III | Summer V | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| Ca | 134.42 b | 73.52 | 86.45 | 338.97 | 283.79 a | 108.54 | 128.54 | 472.81 | 141.50 b | 53.78 | 90.58 | 255.59 |
| Mg | 364.5 | 15.1 | 329.99 | 380.45 | 344.32 | 22.55 | 307.42 | 392.58 | 346.94 | 41.11 | 239.93 | 392.85 |
| Na | 323.40 b | 42.99 | 271.04 | 422.07 | 405.13 a | 47.09 | 325.21 | 488.01 | 238.84 c | 30.57 | 188.35 | 285.05 |
| K * | 5.14 a | 0.2 | 4.9 | 5.56 | 4.91 a | 0.18 | 4.68 | 5.21 | 4.37 b | 0.5 | 3 | 4.76 |
| P * | 2.60 b | 10 | 2.45 | 2.79 | 2.79 b | 0.14 | 2.6 | 3.02 | 3.60 a | 0.4 | 2.55 | 3.96 |
| S * | 2.97 | 0.24 | 2.6 | 3.37 | 2.95 | 0.14 | 2.76 | 3.18 | 3.05 | 0.32 | 2.28 | 3.41 |
| B | 0.00 c | 0 | 0 | 0 | 1.92 a | 0.52 | 1.26 | 2.65 | 0.63 b | 0.37 | 0.35 | 1.43 |
| Zn | 5.05 b | 1.35 | 3.21 | 7.52 | 3.83 c | 0.27 | 3.36 | 4.31 | 9.02 a | 4.69 | 3.72 | 16.15 |
| Cu | 0.25 b | 0.08 | 0.15 | 0.4 | 0.22 b | 0.11 | 0.07 | 0.4 | 6.70 a | 8.3 | 0.4 | 19.59 |
| Fe | 7.78 b | 2.28 | 4.01 | 11.21 | 4.98 b | 1.61 | 2.52 | 7.35 | 9.06 a | 3.83 | 4.17 | 16.03 |
| Cr | 0.23 | 0.1 | 0.1 | 0.4 | 0.3 | 0.21 | 0.14 | 0.8 | 0.11 | 0.06 | 0.05 | 0.21 |
| Ni | 0.10 a | 0.03 | 0.07 | 0.17 | 0.15 a | 0.1 | 0.07 | 0.36 | 0.00 b | 0 | 0 | 0 |
| Mn | 0.2 a | 0.15 | 0.05 | 0.56 | 0.00 b | 0 | 0 | 0 | 0.00 b | 0 | 0 | 0 |
| Co | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.65 | 0.75 | 0 | 2.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floris, R.; Sanna, G.; Satta, C.T.; Piga, C.; Sanna, F.; Lugliè, A.; Fois, N. Intestinal Microbial Ecology and Fillet Metal Chemistry of Wild Grey Mullets Reflect the Variability of the Aquatic Environment in a Western Mediterranean Coastal Lagoon (Santa Giusta, Sardinia, Italy). Water 2021, 13, 879. https://doi.org/10.3390/w13060879
Floris R, Sanna G, Satta CT, Piga C, Sanna F, Lugliè A, Fois N. Intestinal Microbial Ecology and Fillet Metal Chemistry of Wild Grey Mullets Reflect the Variability of the Aquatic Environment in a Western Mediterranean Coastal Lagoon (Santa Giusta, Sardinia, Italy). Water. 2021; 13(6):879. https://doi.org/10.3390/w13060879
Chicago/Turabian StyleFloris, Rosanna, Gabriele Sanna, Cecilia Teodora Satta, Carlo Piga, Francesco Sanna, Antonella Lugliè, and Nicola Fois. 2021. "Intestinal Microbial Ecology and Fillet Metal Chemistry of Wild Grey Mullets Reflect the Variability of the Aquatic Environment in a Western Mediterranean Coastal Lagoon (Santa Giusta, Sardinia, Italy)" Water 13, no. 6: 879. https://doi.org/10.3390/w13060879
APA StyleFloris, R., Sanna, G., Satta, C. T., Piga, C., Sanna, F., Lugliè, A., & Fois, N. (2021). Intestinal Microbial Ecology and Fillet Metal Chemistry of Wild Grey Mullets Reflect the Variability of the Aquatic Environment in a Western Mediterranean Coastal Lagoon (Santa Giusta, Sardinia, Italy). Water, 13(6), 879. https://doi.org/10.3390/w13060879







