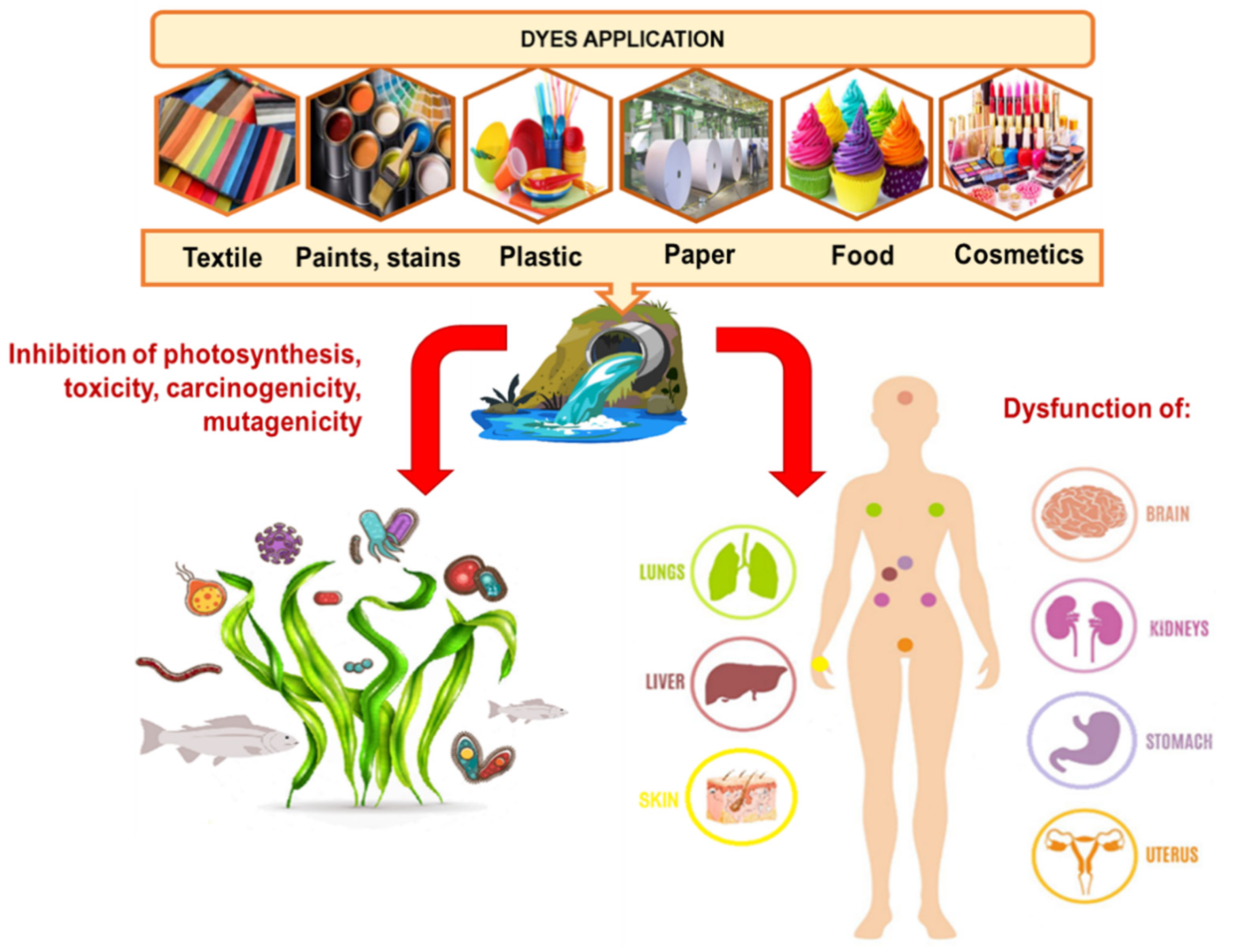
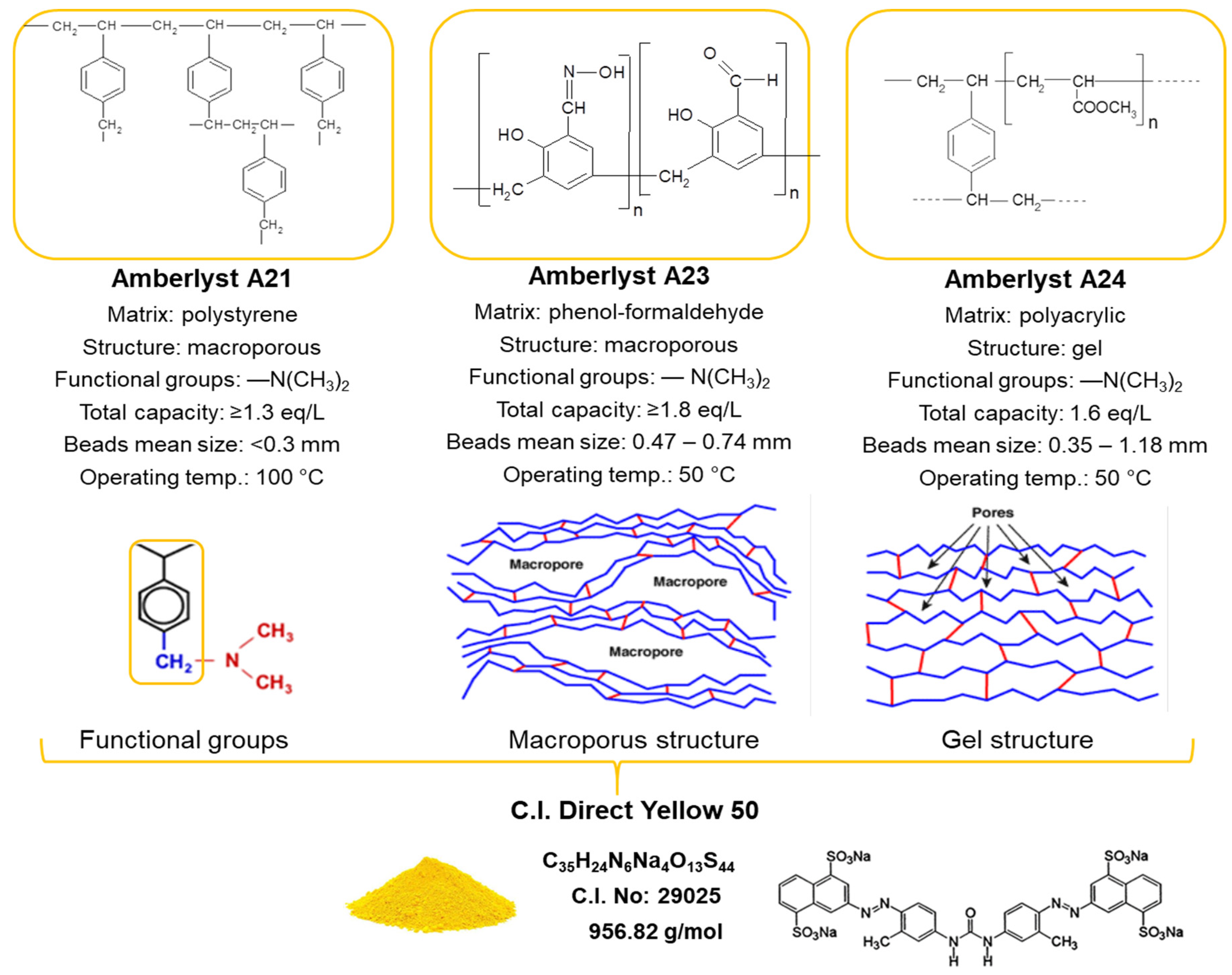
Physicochemical Interactions in Systems C.I. Direct Yellow 50—Weakly Basic Resins: Kinetic, Equilibrium, and Auxiliaries Addition Aspects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbents and Adsorbate
2.2. Other Chemicals
2.3. Batch Adsorption Method
2.4. Kinetic Experiments
2.4.1. Pseudo First-Order Model (PFO)
2.4.2. Pseudo Second-Order Model (PSO)
2.4.3. Weber and Morris Intraparticle Diffusion Model (ID)
2.5. Equilibrium Experiments
2.5.1. Langmuir Model
2.5.2. Freundlich Model
2.5.3. The Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
2.6. Desorption Experiments
3. Results and Discussion
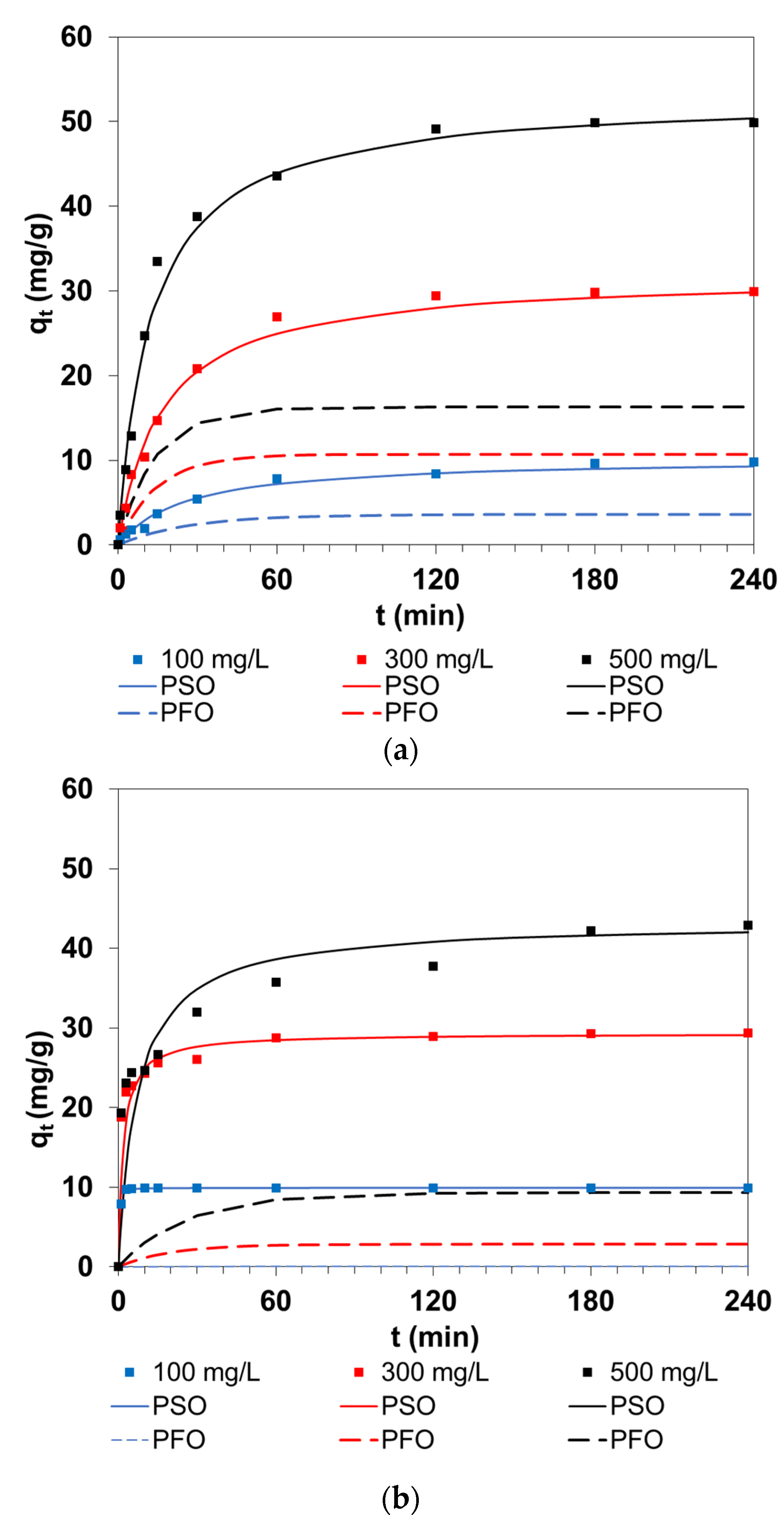
3.1. Effects of Initial Dye Concentration and Phase Contact Time
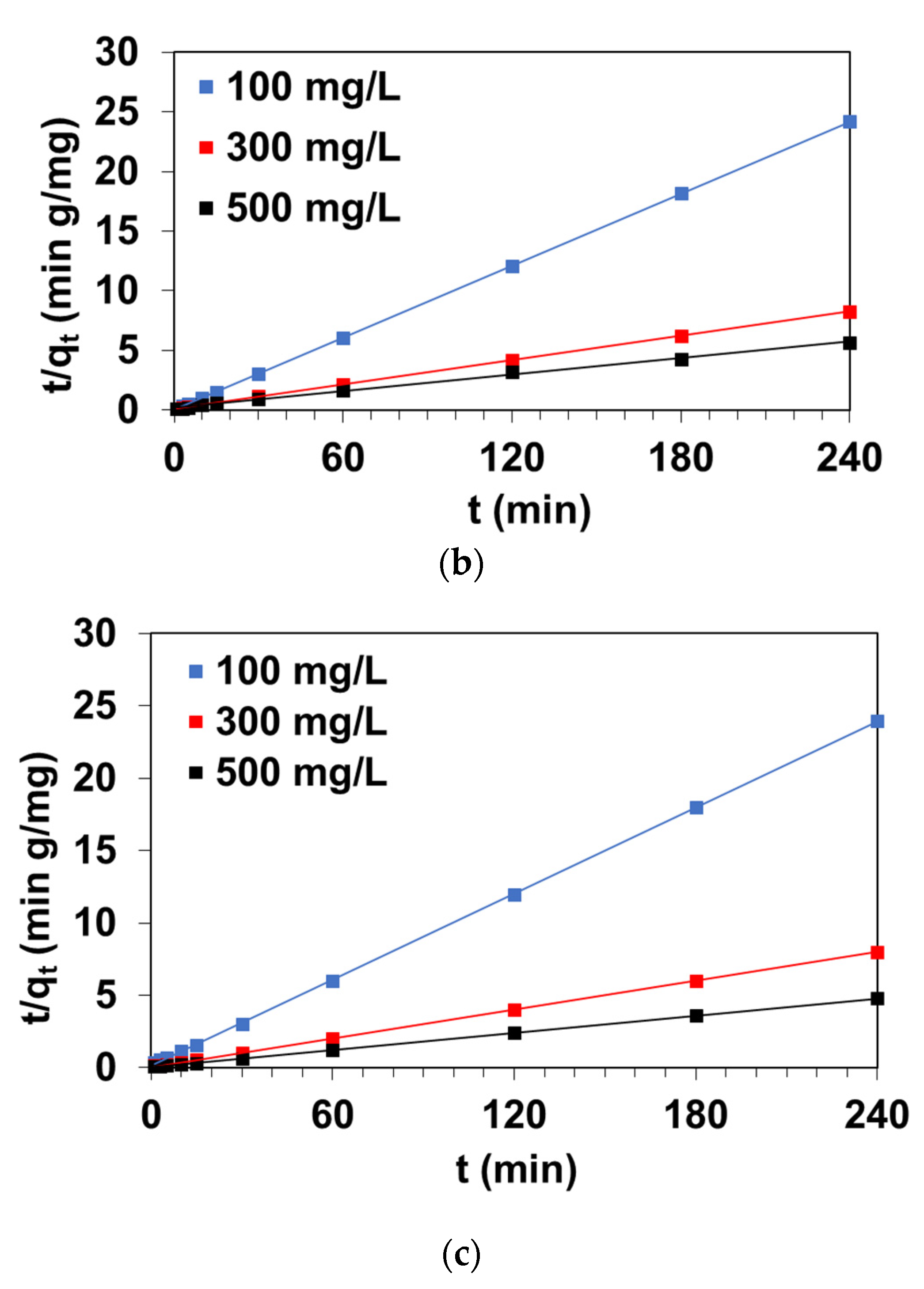
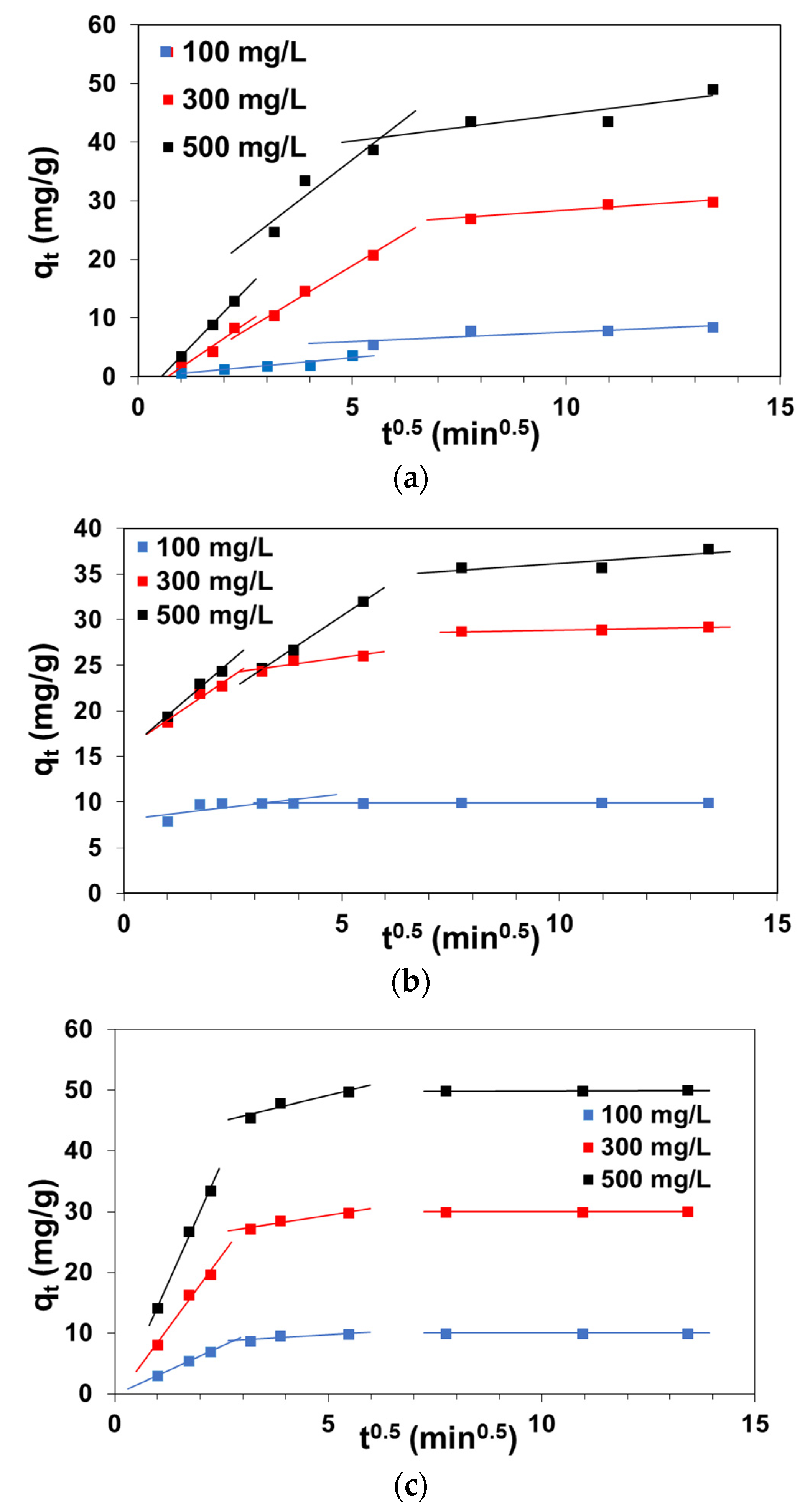
3.2. Kinetic Parameters of Sorption
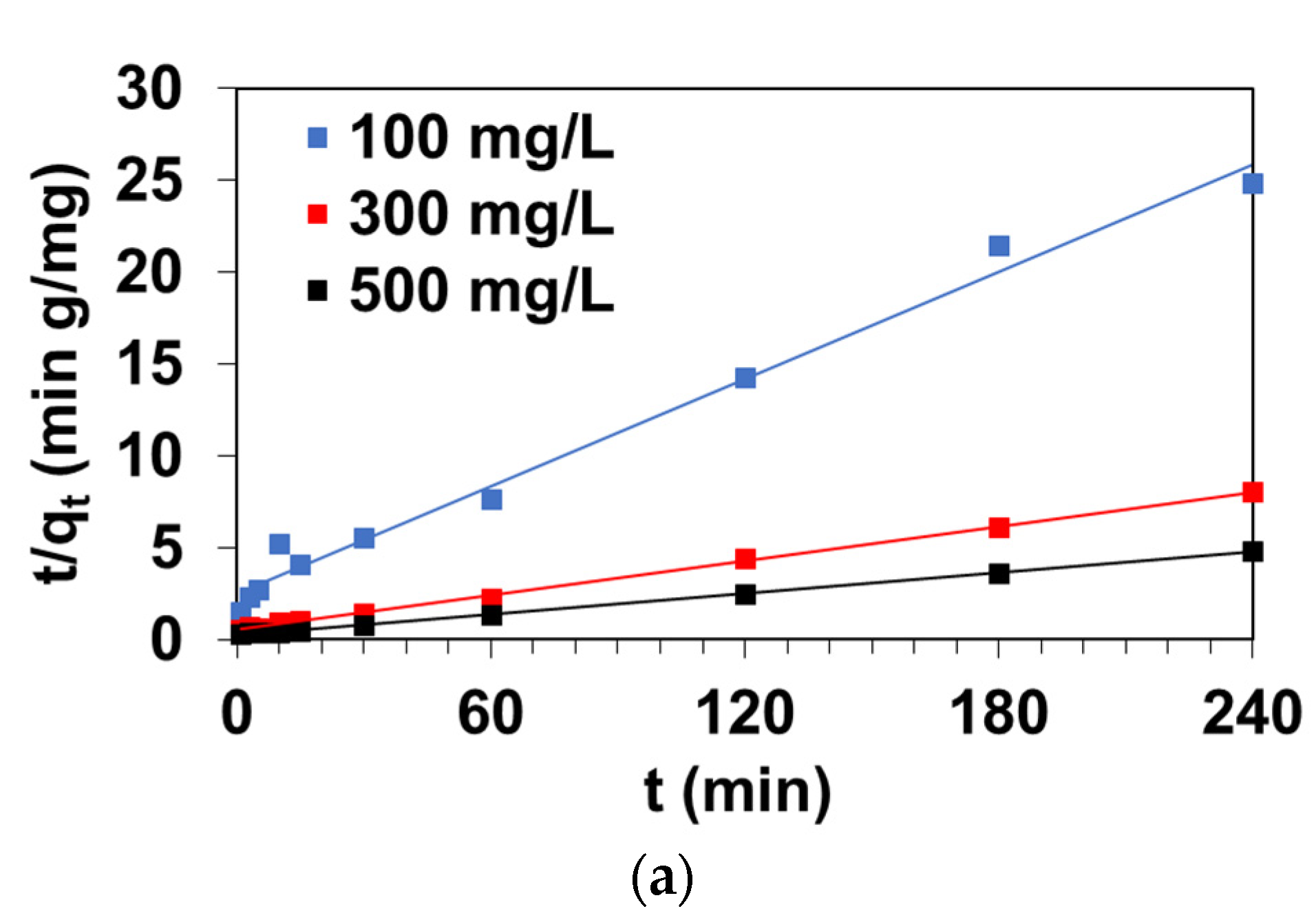
3.2.1. Pseudo First-Order Model
3.2.2. Pseudo Second-Order Model
| Sorbent | Kinetic Studies | Equilibrium Studies | Ref. |
|---|---|---|---|
| Cotton fibre | PSO, k2 = 11.4 × 102 − 12.95 × 102 g/mg min, qe = 0.684–0.965 mg/g, C0 = 113.4 mg/L, T = 40–90 °C, pH = 7.03, a.d. = 1 g/30 mL | Langmuir model, qe = 4.4–5.8 mg/g, T = 40–90 °C, pH = 7.03, a.d. = 1 g/30 mL | [60] |
| Organoclay adsorbent | PSO, k2 = 0.087 g/mg min, qe = 1.66 mg/g, C0 = 50 mg/L, T = 25 °C, pH = 3, a.d. = 0.2 g/50 mL | Freundlich model, k = 0.617 mg1–1/n L1/n/g, n = 1.93, pH = 3, a.d. = 0.2 g/50 mL | [61] |
| Sugarcane bagasse biomas | PSO, k2 = 0.0051 g/mg min, qe = 18.8 mg/g, C0 = 50 mg/L, T = 30 °C, pH = 2, a.d. = 0.05 g/50 mL | Langmuir model, qe = 51.8 mg/g, T = 30 °C, pH = 2, a.d. = 0.05 g/50 mL | [62] |
| Sugarcane bagasse biomas treated with polyethyleneimine | PSO, k2 = 0.012 g/mg min, qe = 20.7 mg/g, C0 = 50 mg/L, T = 30 °C, pH = 2, a.d. = 0.05 g/50 mL | Langmuir model, qe = 68.4 mg/g, T = 30 °C, pH = 2, a.d. = 0.05 g/50 mL | |
| Rice straw | No model, qe = 2.8–1.0 mg/g, pH = 4–10, C0 = 100 mg/L, t = 8 days, a.d. = 0.5 mg/50 mL | Freundlich model, k = 0.28–0.77 mg1−1/n L1/n/g, n = 1.34–1.86, pH = 4–10, a.d. = 0.5 mg/50 mL | [63] |
| Radiation-modified maize starch/acrylonitrile | No model, qe = 2.8–1.2 mg/g, pH = 4–10, C0 = 100 mg/L, t = 8 days, a.d. = 0.5 mg/50 mL | Freundlich model, k = 0.33–0.66 mg1−1/n L1/n/g, n = 1.74–2.1, pH = 4–10, a.d. = 0.5 mg/50 mL | |
| Marble powder | PSO, k2 = 0.81–35.31 g/mg min, qe = 1.39–26.3 mg/g, C0 = 10–60 mg/L, T = 25 °C, a.d. = 2 g/L | Langmuir model, qe = 50 mg/g, T = 25 °C, a.d. = 2 g/L | [64] |
| Amberlite XAD7 (PA non functionalyzed resin) | PSO, k2 = 0.015–2.34 g/mg min, qe = 0.9–9.4 mg/g, C0 = 10–100 mg/L, T = 25 °C a.d. = 0.5 g/50 mL | Langmuir model, qe = 27.9 mg/g, T = 25 °C, a.d. = 0.5 g/50 mL | [48] |
| Amberlite IRA958 (PA with quaternary ammonium groups) | PSO, k2 = 0.020–0.036 g/mg min, qe = 10–50 mg/g, C0 = 100–500 mg/L, T = 25 °C a.d. = 0.5 g/50 mL | Langmuir model, qe = 534.8 mg/g, T = 25 °C, a.d. = 0.5 g/50 mL | |
| Lewatit VPOC1064 (PS-DVB non functionalyzed resin) | PSO, k2 = 0.016–0.41 g/mg min, qe = 0.9–6.8 mg/g, C0 = 10–100 mg/L, T = 25 °C a.d. = 0.5 g/50 mL | Langmuir model, qe = 19.4 mg/g, T = 25 °C, a.d. = 0.5 g/50 mL | [48] |
| Amberlite IRA900 (PS-DVB with quaternary ammonium groups) | PSO, k2 = 0.01–0.06 g/mg min, qe = 7.7–39.1 mg/g, C0 = 10–500 mg/L, T = 25 °C a.d. = 0.5 g/50 mL | Langmuir model, qe = 487.2 mg/g, T = 25 °C, a.d. = 0.5 g/50 mL | |
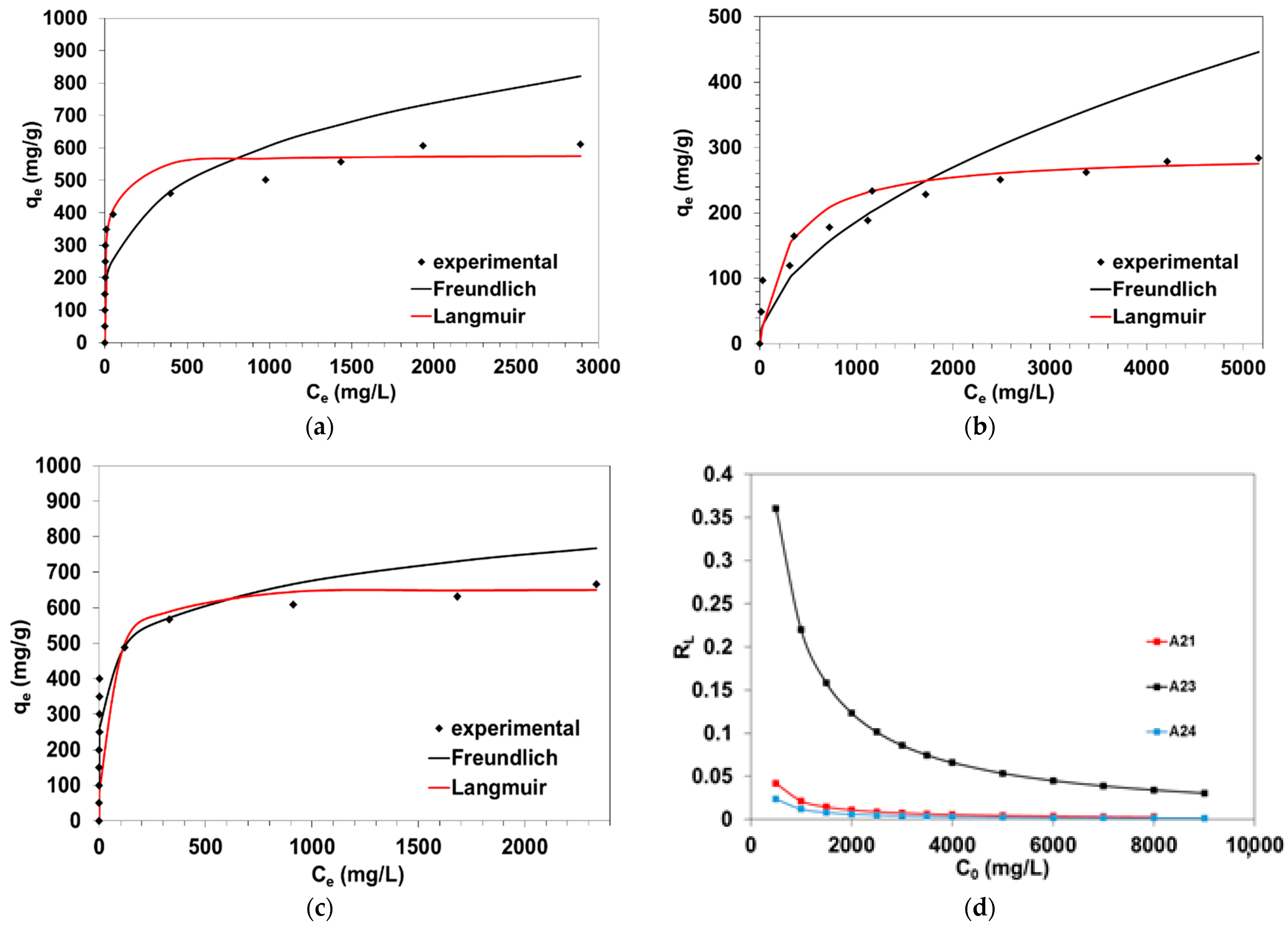
| Amberlyst A23 Amberlyst A21 Amberlyst A24 | PSO, see Table 2 | qe = 284.3 mg/g qe = 610.9 mg/g qe = 666.5 mg/g Langmuir model, See Table 4 | This study |
| Anion Exchanger | qe,exp (mg/g) | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | Q0 (mg/g) | b (L/mg) | R2 | n | 1/n | kF (mg1−1/n L1/n/g) | ||
| A24 | 666.5 | 0.998 | 652.9 | 0.0834 | 0.726 | 6.661 | 0.150 | 239.5 |
| A21 | 610.9 | 0.991 | 577.5 | 0.0460 | 0.308 | 3.526 | 0.284 | 84.3 |
| A23 | 284.3 | 0.990 | 290.3 | 0.0035 | 0.799 | 1.889 | 0.529 | 4.8 |
3.2.3. Weber and Morris Intraparticle Diffusion Model
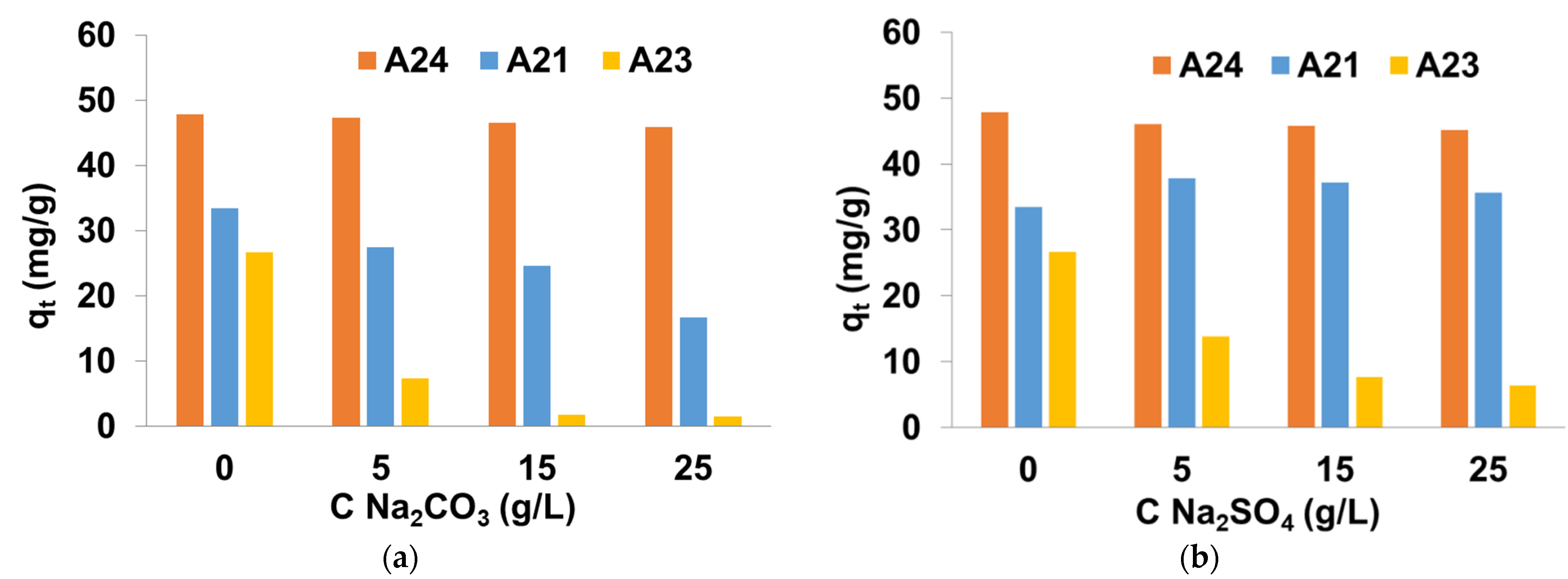
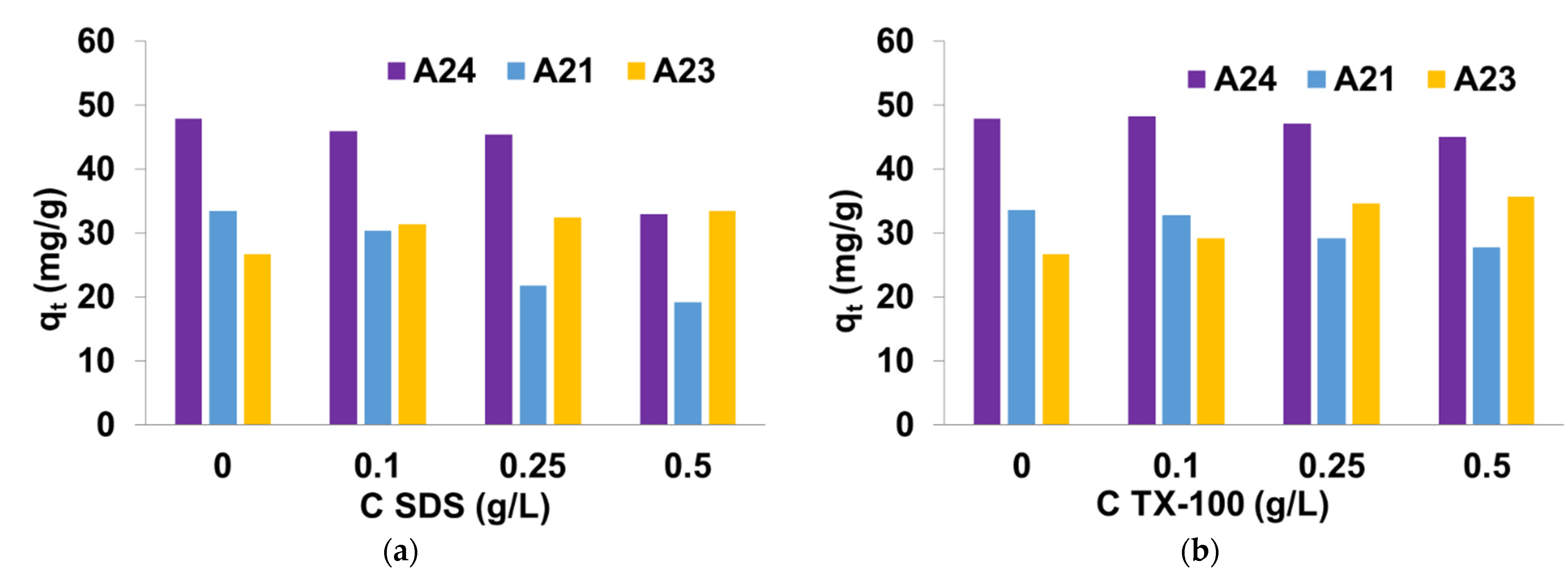
3.3. Effect of Salts and Surfactants Presence on DY50 Adsorption
3.4. Adsorption Isotherms
3.4.1. Langmuir Isotherm
3.4.2. Freundlich Isotherm
3.4.3. Adsorption Mechanism
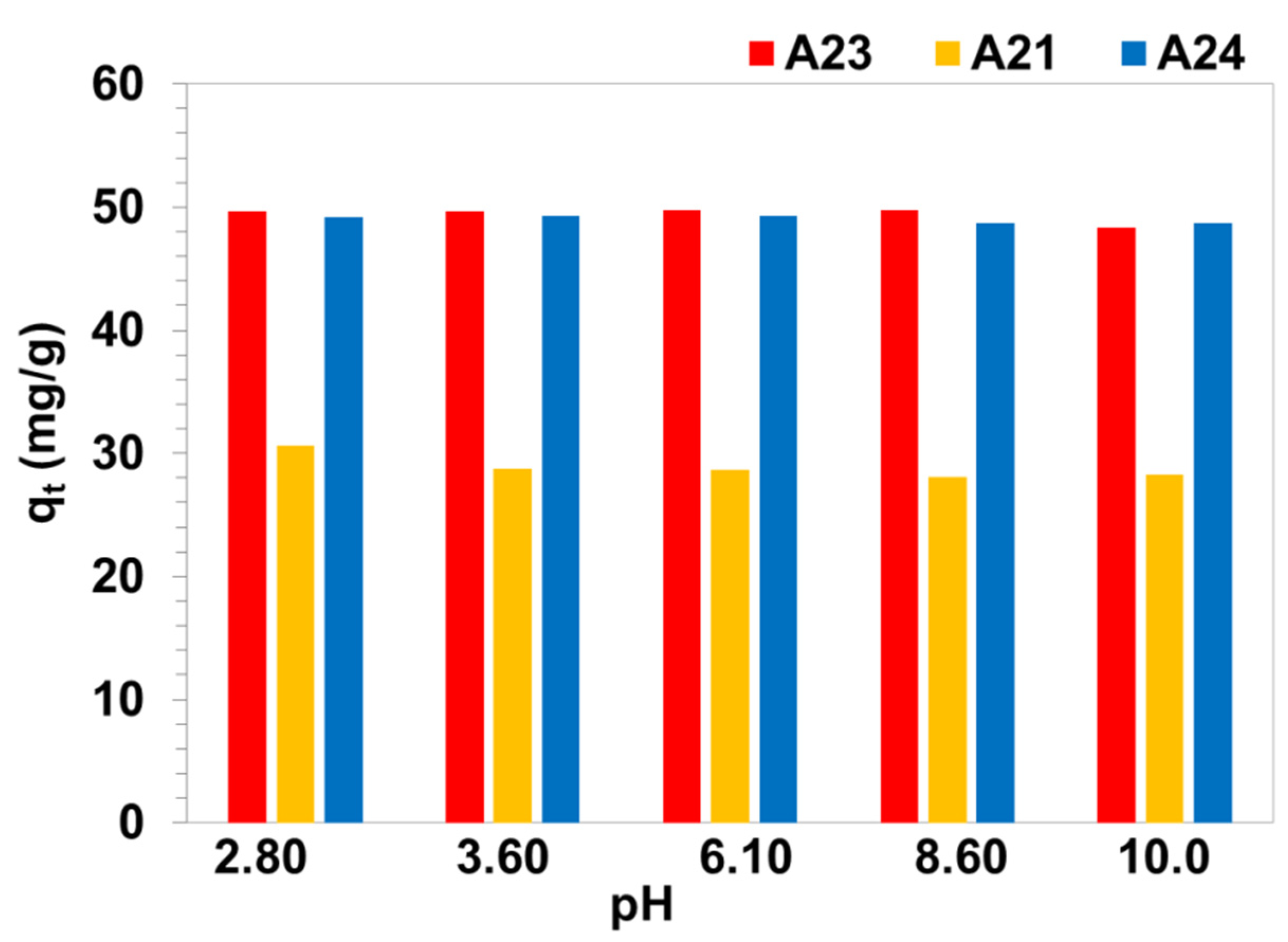
3.5. Impact of Solution pH
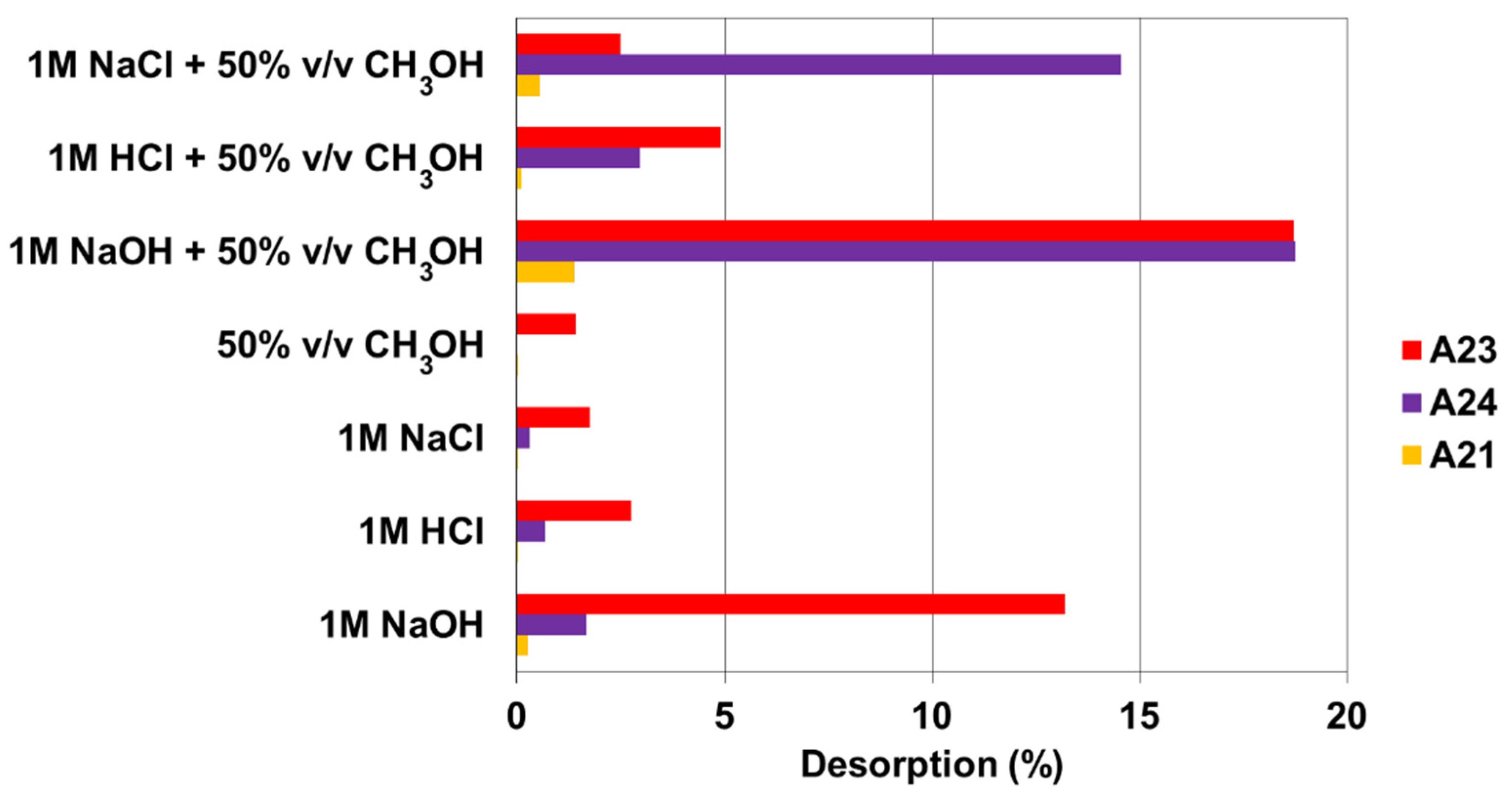
3.6. Desorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rijsberman, F.R. Water Scarcity: Fact or Fiction. Agric. Water Manag. 2006, 80, 5–22. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, S. Handbook of Water Purity and Quality; IWA Publishing: London, UK, 2009. [Google Scholar]
- Anielak, A.M. Chemiczne i Fizykochemiczne Oczyszczanie Ścieków; PWN: Warszawa, Poland, 2002. [Google Scholar]
- Maciejewska-Nowak, K. Usuwanie barwników ze ścieków przemysłowych. Ochr. Sr. 1986, 488/4, 17–23. [Google Scholar]
- Al-Asheh, S.; Banat, F.; Abu-Aitah, L. Adsorption of phenol using different types of activated bentonites. Sep. Purif. Technol. 2003, 33, 1–10. [Google Scholar] [CrossRef]
- Boni, M.R.; Chiavola, A.; Marzeddu, S. Application of biochar to the remediation of Pb-contaminated solutions. Sustainability 2018, 10, 4440. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodi, N.M.; Arami, M.J. Degradation and toxicity reduction of textile wastewater using immobilized titania nanophotocatalysis. Photochem. Photobiol. B 2009, 94, 20–24. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.J. Modeling and sensitivity analysis of dyes adsorption onto natural adsorbent from colored textile wastewater. Appl. Polym. Sci. 2008, 109, 4043–4048. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.J. Bulk phase degradation of Acid Red 14 by nanophotocatalysis using immobilized titanium(IV) oxide nanoparticles. Photochem. Photobiol. A 2006, 182, 60–66. [Google Scholar] [CrossRef]
- Bemska, J.; Bilińska, L.; Ledakowicz, S. Analiza ścieków włókienniczych pod kątem wyboru najlepszej metody ich oczyszczania. In Nauka i Przemysł–Metody Spektroskopowe, Nowe Wyzwania i Możliwości; Hubicki, Z., Ed.; Uniwersytet Marii Curie-Skłodowskiej w Lublinie Wydział Chemii: Lublin, Poland, 2012. [Google Scholar]
- Zhou, Y.; Lu, J.; Liu, Y. Recent advences for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Arica, M.Y.; Bayramŏglu, G. Biosorption of Reactive Red-120 dye from aqueous solution by native and modified fungus biomass preparations of Lentinus sajorcaju. J. Hazard. Mater. 2007, 149, 499–507. [Google Scholar] [CrossRef]
- Aksu, Z.; That, A.I.; Tunç, O. A comperative adsorption/biosorption study of Acid Blue 161: Effect of temperature on equilibrium and kinetic parameters. Chem. Eng. J. 2008, 142, 23–39. [Google Scholar] [CrossRef]
- Cho, I.H.; Zoch, K.D. Photocatalytic degradation of azo dye (Reactive Red 120) in TiO2/UV system: Optimization and modeling using response surface methodology (RSM) based on the central composite design. Dyes Pigments 2007, 75, 533–543. [Google Scholar] [CrossRef]
- Tsui, S.M.; Chu, W. Photocatalytic degradation of dye pollutants in the presence of acetone. Water Sci. Technol. 2001, 44, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.P.; Thiruvenkatachari, R.; Shim, W.G.; Moon, H. Evaluation of the performance of adsorption and coagulation process for the maximum removal of reactive dyes. Dyes Pigments 2006, 69, 196–203. [Google Scholar] [CrossRef]
- Golob, V.; Vinder, A.; Simoni, M. Efficiency of the coagulation/flocculation method for the treatment of dyebath effluents. Dyes Pigments 2005, 67, 93–97. [Google Scholar] [CrossRef]
- Shuang, C.; Wang, J.; Li, H.; Li, A.; Zhou, O. Effect of the chemical structure of anion exchange resin on the adsorption of humic acid: Behavior and mechanism. J. Colloid Interface Sci. 2015, 437, 163–169. [Google Scholar] [CrossRef]
- Mohd Salleh, M.A.; Mahmoud, D.K.; Wan Abdul Karim, W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Altintas, B.; Yakup Arica, M. Adsorption kinetics and thermodynamic parameters of cationic dyes from aqueous solutions by using a new strong cation-exchange resin. Chem. Eng. J. 2009, 152, 339–346. [Google Scholar] [CrossRef]
- Valix, M.; Cheung, W.H.; McKay, G. Preparation of activated carbon using low temperaturę carbonisation and physical activation of high ash raw bagasse for acid dye adsorption. Chemosphere 2004, 56, 493–501. [Google Scholar] [CrossRef]
- Thinakaran, N.; Panneerselvam, P.; Baskaralingam, P.; Elango, D.; Sivanesan, S. Equilibrium and kinetic studies on the removal of acid red 114 from aqueous solutions using activated carbons prepared from seed shells. J. Hazard. Mater. 2008, 158, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Akmil-Basar, C.; Onal, Y.; Kilicer, T.; Eren, D. Adsorptions of high concentration malachite green by two activated carbons having different porous structures. J. Hazard. Mater. 2005, 127, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Konicki, W.; Cendrowski, K.; Chen, X.; Mijowska, E. Application of hollow mesoporous carbon nanospheres as an high effective adsorbent for the fast removal of acid dyes from aqueous solutions. Chem. Eng. J. 2013, 228, 824–833. [Google Scholar] [CrossRef]
- Seifikar, F.; Azizian, S.; Sillanpää, M. Microwave-assisted synthesis of carbon powder for rapid dye removal. Mater. Chem. Phys. 2020, 250, 123057. [Google Scholar] [CrossRef]
- Thamer, B.M.; Aldalbahi, A.; Moydeen, M.; El-Hamshary, H.; Al-Enizi, A.M.; El-Newehy, M.H. Effective adsorption of coomassie brilliant blue dye using poly(phenylene diamine)grafted electrospun carbon nanofibers as a novel adsorbent. Mater. Chem. Phys. 2019, 234, 133–145. [Google Scholar] [CrossRef]
- Boni, M.R.; Chiavola, A.; Marzeddu, S. Remediation of lead-contaminated water by Virgin Coniferous wood biochar adsorbent: Batch and column application. Water Air Soil Pollut. 2020, 231, 171. [Google Scholar] [CrossRef]
- Cukierman, A.L.; Nunell, G.V.; Bonelli, P.R. Removal of emerging pollutants from water through adsorption onto carbon-based materials. In Emerging and Nanomaterial Contaminants in Wastewater: Advanced Treatment Technologies; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Hameed, B.H.; Aziz, N. Adsorption of direct dye on palm ash: Kinetic and equilibrium modeling. J. Hazard. Mater. 2007, 141, 70–76. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Hong, G.B.; Wang, Y.K. Synthesis of low-cost adsorbent from rice bran for the removal of reactive dye based on the response surface methodology. Appl. Surf. Sci. 2017, 423, 800–809. [Google Scholar]
- Kallel, F.; Chaari, F.; Bouaziz, F.; Bettaieb, F.; Ghorbel, R.; Chaabouni, S.E. Sorption and desorption characteristics for the removal of a toxic dye, methylene blue from aqueous solution by a low cost agricultural by-product. J. Mol. Liq. 2016, 219, 279–288. [Google Scholar] [CrossRef]
- Zou, W.; Zhao, L.; Zhu, L. Efficient uranium(VI) biosorption on grapefruit peel: Kinetic study and thermodynamic parameters. J. Radioanal. Nucl. Chem. 2012, 292, 1303–1315. [Google Scholar] [CrossRef]
- Asfaram, A.; Fathi, M.R.; Khodadoust, S.; Naraki, M. Removal of Direct Red 12B by garlic peel as a cheap adsorbent: Kinetics, thermodynamic and equilibrium isotherms study of removal. Spectrochim. Acta A 2014, 127, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Wawrzkiewicz, M. Comparison of gel anion exchangers of various basicity in direct dye removal from aqueous solutions and wastewaters. Chem. Eng. J. 2011, 173, 773–781. [Google Scholar]
- Srivatsav, P.; Sriharsha Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Panchamoorthy Gopinath, K.; Bhatnagar, A. Biochar as an eco-friendly and economical adsorbent for the removal of colorants (dyes) from aqueous environment: A review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Afroze, A.; Kanti Sen, T. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Aisyah Ishak, S.; Fared Murshed, M.; Md Akil, H.; Ismail, N.; Zalifah Md Rasib, S.; Ali Saeed Al-Gheethi, A. The application of modified natural polymers in toxicant dye compounds wastewater: A review. Water 2020, 12, 2032. [Google Scholar] [CrossRef]
- Argumedo-Delira, R.; Gómez-Martínez, M.J.; Uribe-Kaffure, R. Trichoderma biomass as an alternative for removal of congo red and malachite green industrial dyes. Appl. Sci. 2021, 11, 448. [Google Scholar] [CrossRef]
- Duhan, M.; Kaur, R. Nano-structured polyaniline as a potential adsorbent for methylene blue dye removal from effluent. J. Compos. Sci. 2021, 5, 7. [Google Scholar] [CrossRef]
- Ma, C.M.; Hong, G.B.; Wang, Y.K. Performance evaluation and optimization of dyes removal using rice bran-based magnetic composite adsorbent. Materials 2020, 13, 2764. [Google Scholar] [CrossRef]
- Available online: Samcotech.com/how-much-does-it-cost-to-buy-maintain-and-dispose-of-ion-exchange-resins/ (accessed on 15 January 2021).
- Zong, L.; Liu, F.; Chen, D.; Zhang, X.; Ling, C.; Li, A. A novel pyridine based polymer for highly efficient separation of nickel from high-acidity and high-concentration cobalt solutions. Chem. Eng. J. 2018, 334, 995–1005. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Amberlite and Amberlyst Resins-Technical Information Bulletin; Sigma-Aldrich: St. Louis, MO, USA, 2013. [Google Scholar]
- Dąbrowski, A.; Hubicki, Z.; Podkościelny, P.; Barczak, M. Selektywne usuwanie jonów metali ciężkich z wód oraz ścieków przemysłowych poprzez wymianę jonową. Przem. Chem. 2006, 85, 232–241. [Google Scholar]
- Greluk, M.; Hubicki, Z. Effect of basicity of anion exchangers and number and positions of sulfonic groups of acid dyes on dyes adsorption on macroporous anion exchangers with styrenic polymer matrix. Chem. Eng. J. 2013, 215–216, 731–739. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Polska-Adach, E.; Hubicki, Z. Polacrylic and polystyrene functionalized resins for direct dye removal from textile effluents. Sep. Sci. 2019, 53, 1065–1075. [Google Scholar] [CrossRef]
- Greluk, M.; Hubicki, Z. Kinetics, isotherm and thermodynamic studies of Reactive Black 5 removal by acid acrylic resins. Chem. Eng. J. 2010, 162, 919–926. [Google Scholar] [CrossRef]
- Ekici, S.; Gamze Guntekin, G. Utilization of polyampholyte hydrogels for simultaneous removal of textile dyes from aqueous solutions. Sep. Sci. 2018, 53, 1777–1790. [Google Scholar] [CrossRef]
- Available online: http://dardel.info/IX/resin_structure.html#other (accessed on 15 January 2021).
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Kinetics models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Plaziński, W.; Dziuba, J.; Rudziński, W. Modeling of sorption kinetics: The pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorption 2013, 19, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Weber, W.; Morris, J. Kinetics of adsorption on carbon from solutions. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surfacearea activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Equilibrium, kinetics and mechanism of Malachite Green adsorption on activated carbon prepared from bamboo by K2CO3 activation and subsequent gasification with CO2. J. Hazard. Mater. 2008, 157, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H.M.F. Ober dies adsorption in Losungen. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Ismail, L.F.M.; Sallam, H.B.; Abo Farha, S.A.; Gamal, A.M.; Mahmoud, G.E.A. Adsorption behaviour of direct yellow 50 onto cotton fiber: Equilibrium, kinetic and thermodynamic profile. Spectrochim. Acta A 2014, 131, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Khodaeer, E.A. Removal of direct dyes from aqueous solution using natural clay and organoclay adsorbents. Baghdad Sci. J. 2015, 12, 158–166. [Google Scholar]
- Sadaf, S.; Bhatti, H.N.; Nausheen, S.; Amin, M. Application of a novel lignocellulosic biomaterial for the removal of Direct Yellow 50 dye from aqueous solution: Batch and column study. J. Taiwan Inst. Chem. Eng. 2015, 47, 160–170. [Google Scholar] [CrossRef]
- Abdel-Aal, S.E.; Gad, Y.H.; Dessouki, A.M. Use of rice straw and radiation-modified maize starch/acrylonitrile in the treatment of wastewater. J. Hazard. Mater. 2006, B129, 204–215. [Google Scholar] [CrossRef]
- Diouri, K.; Chaqroune, A.; Kherbeche, A.; Miyah, Y.; Lahrichi, A. Kinetics of Direct Yellow 50 dye adsorption onto marble powder sorbents. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 16626–16637. [Google Scholar]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of C. I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Kowalska, I. Usuwanie anionowych substancji powierzchniowo czynnych w procesie wymiany jonowej. Ochr. Sr. 2009, 31, 25–29. [Google Scholar]
- Majewska-Nowak, K. Usuwanie barwników organicznych z roztworów wodnych w procesie ultrafiltracji w obecności anionowej substancji powierzchniowo czynnej. Ochr. Sr. 2006, 3, 15–24. [Google Scholar]
- Ghoreishi, S.M.; Behpour, M.; Shabani-Nooshabadi, M. Interaction of anionic azo dye and TTAB–cationic surfactant. J. Braz. Chem. Soc. 2009, 20, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.N.; Sarwar, A. Study of dye-surfactant interaction: Aggregation and dissolution of yellowish in N-dodecyl pyridinum choride. Fluid Phase Equilib. 2006, 239, 166–171. [Google Scholar] [CrossRef]
- Arami, M.; Limaee, N.Y.; Mahmoodi, N.M.; Tabrizi, N.S. Equilibrium and kinetics studies for the adsorption of direct and acid dyes from aqueous solution by soy meal hull. J. Hazard. Mater. 2006, 135, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Mohseni, M. The role of hydrophobic properties in ion exchange removal of organic compounds from water. Can. J. Chem. Eng. 2017, 95, 1449–1455. [Google Scholar] [CrossRef]
- Li, P.; SenGupta, A.K. Sorption of hydrophobic ionizable organic compounds (HIOCs) onto polymeric ion exchangers. React. Funct. Polym. 2004, 60, 27–39. [Google Scholar] [CrossRef]
- Li, P.; SenGupta, A.K. Entropy-driven selective ion exchange for aromtic ions and the role of cosolvents. Colloid Surf. A 2001, 191, 123–132. [Google Scholar] [CrossRef]
- Stevenson, D.M.; Duff, D.G.; Kirkwood, D.J. The behaviour of dyes in aqueous solutions part II–anionic dye-nonionic surfactant interactions. J. Soc. Dyers Colour. 1981, 97, 13–17. [Google Scholar] [CrossRef]
- Harland, C.E. Ion Exchange: Theory and Practice; The Royal Society of Chemistry: Cambridge, UK, 1994. [Google Scholar]
- Zagorodni, A.A.; Kotova, D.L.; Selemenev, V.F. Infrared spectroscopy of ion exchange resins: Chemical deterioration of the resins. React. Funct. Polym. 2002, 53, 157–171. [Google Scholar] [CrossRef]
- Clavijo, C.; Osma, J.F. Functionalized Leather: A novel and effective hazardous solid waste adsorbent for the removal of the diazo dye congo red from aqueous solution. Water 2019, 11, 1906. [Google Scholar] [CrossRef] [Green Version]
- Karcher, S.; Kornmüller, A.; Jekel, M. Screening of commercial sorbents for the removal of reactive dyes. Dyes Pigments 2011, 51, 111–125. [Google Scholar] [CrossRef]



| Conditions | System | |
|---|---|---|
| Na2SO4/Na2CO3 | SDS/TX-100 | |
| Salts/surfactants concentration (g/L) | 5, 15, 25 | 0.1, 0.25, 0.5 |
| Dye concentration (mg/L) | 500 | 500 |
| Time (min) | 15 | 15 |
| Volume (mL) | 50 | 50 |
| Anion exchanger mass (g) | 0.5 | 0.5 |
| Temp. (°C) | 25 | 25 |
| Vibration amplitude, rotary (cycle/min) Maximum absorbance wavelength (nm) | 8, 180 399/403 | 8, 180 394/397 |
| Resin | C0 (mg/L) | qe,exp (mg/g) | PFO | PSO | ID | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (1/min) | R2 | qe (mg/g) | k2 (g/mg∙min) | R2 | ki (mg/g min0.5) | R2 | |||
| A24 | 100 | 10.0 | 2.38 | 0.0435 | 0.779 | 10.12 | 0.0609 | 0.999 | 0.441 | 0.809 |
| 300 | 30.0 | 4.65 | 0.0334 | 0.715 | 30.28 | 0.0201 | 0.999 | 1.106 | 0.946 | |
| 500 | 50.0 | 9.19 | 0.0608 | 0.907 | 50.44 | 0.0128 | 0.999 | 1.732 | 0.924 | |
| A21 | 100 | 9.8 | 8.3 | 0.0163 | 0.915 | 10.3 | 0.0038 | 0.999 | 0.923 | 0.874 |
| 300 | 29.9 | 24.6 | 0.0297 | 0.991 | 31.9 | 0.0019 | 0.999 | 4.397 | 0.987 | |
| 500 | 49.9 | 37.5 | 0.0311 | 0.989 | 52.9 | 0.0015 | 0.999 | 5.612 | 0.874 | |
| A23 | 100 | 9.9 | 0.2 | 0.0137 | 0.257 | 9.9 | 1.1945 | 0.999 | 0.560 | 0.529 |
| 300 | 29.3 | 6.5 | 0.0219 | 0.917 | 29.3 | 0.0188 | 0.999 | 0.668 | 0.789 | |
| 500 | 42.9 | 21.5 | 0.0169 | 0.944 | 43.2 | 0.0032 | 0.999 | 3.184 | 0.998 | |
| Bath Components | Light Intensity | Deep Intensity |
|---|---|---|
| Dye | up 0.5% | 0.5–3% |
| Na2CO3 | 05–1% | 1–2% |
| Na2SO4 | 4–10% | 10–30% |
| Surfactants | up 0.5% | 0.5–1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzkiewicz, M.; Polska-Adach, E. Physicochemical Interactions in Systems C.I. Direct Yellow 50—Weakly Basic Resins: Kinetic, Equilibrium, and Auxiliaries Addition Aspects. Water 2021, 13, 385. https://doi.org/10.3390/w13030385
Wawrzkiewicz M, Polska-Adach E. Physicochemical Interactions in Systems C.I. Direct Yellow 50—Weakly Basic Resins: Kinetic, Equilibrium, and Auxiliaries Addition Aspects. Water. 2021; 13(3):385. https://doi.org/10.3390/w13030385
Chicago/Turabian StyleWawrzkiewicz, Monika, and Ewelina Polska-Adach. 2021. "Physicochemical Interactions in Systems C.I. Direct Yellow 50—Weakly Basic Resins: Kinetic, Equilibrium, and Auxiliaries Addition Aspects" Water 13, no. 3: 385. https://doi.org/10.3390/w13030385
APA StyleWawrzkiewicz, M., & Polska-Adach, E. (2021). Physicochemical Interactions in Systems C.I. Direct Yellow 50—Weakly Basic Resins: Kinetic, Equilibrium, and Auxiliaries Addition Aspects. Water, 13(3), 385. https://doi.org/10.3390/w13030385