A Review of Recently Discovered Remains of the Pleistocene Branchiopods (Anostraca, Notostraca) from NE Siberia and Arctic Canada
Abstract
:1. Introduction
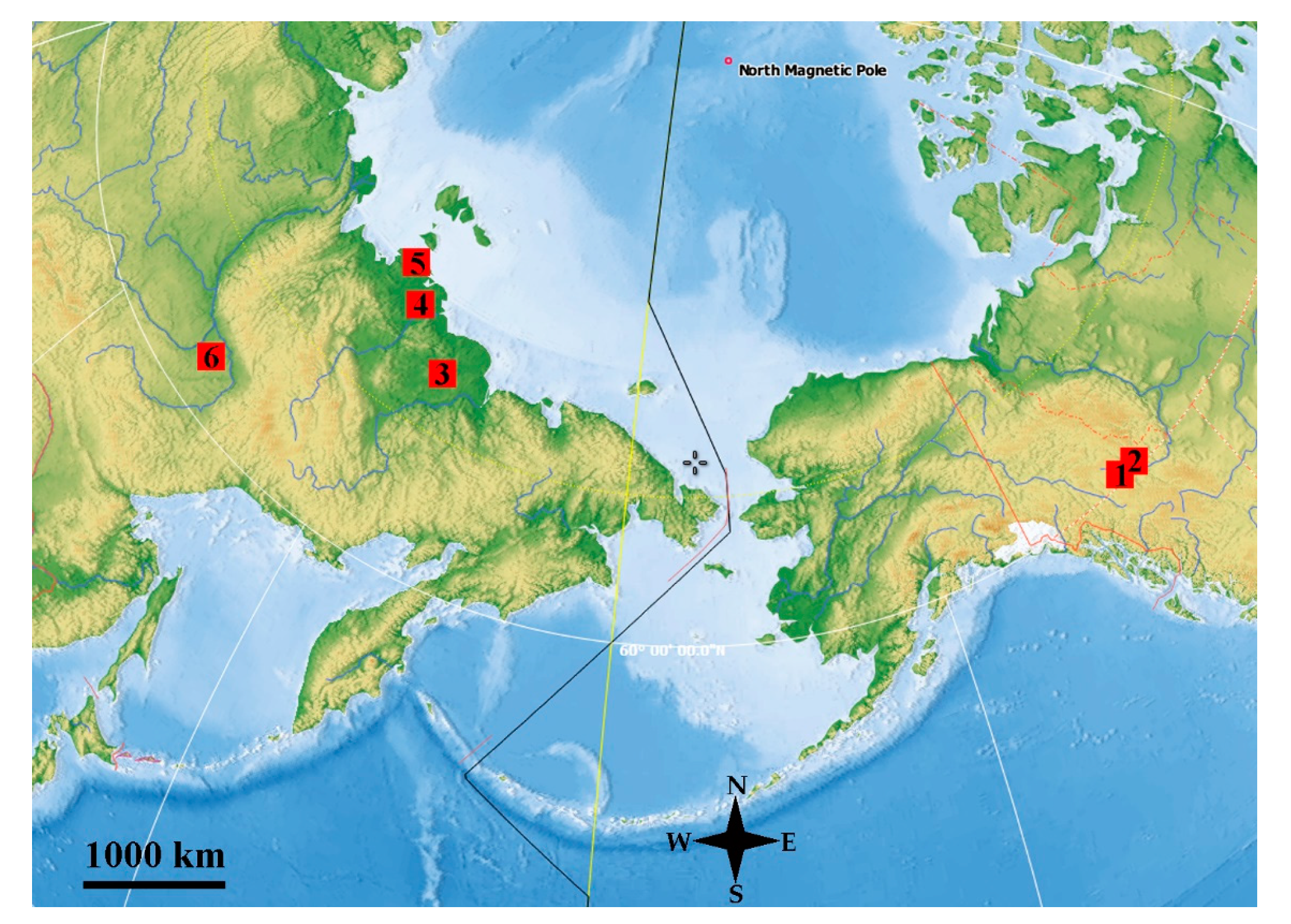
2. Materials and Methods
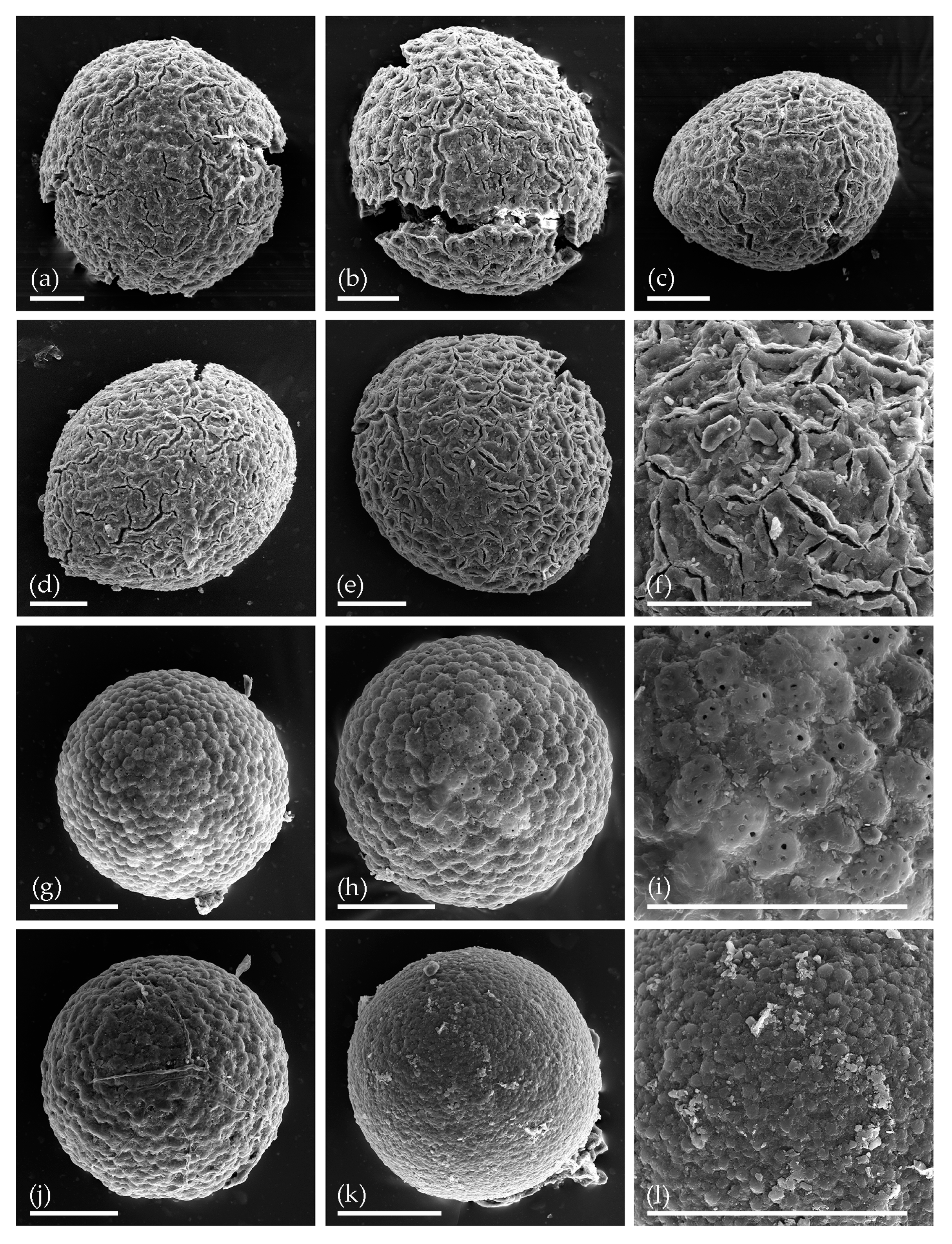
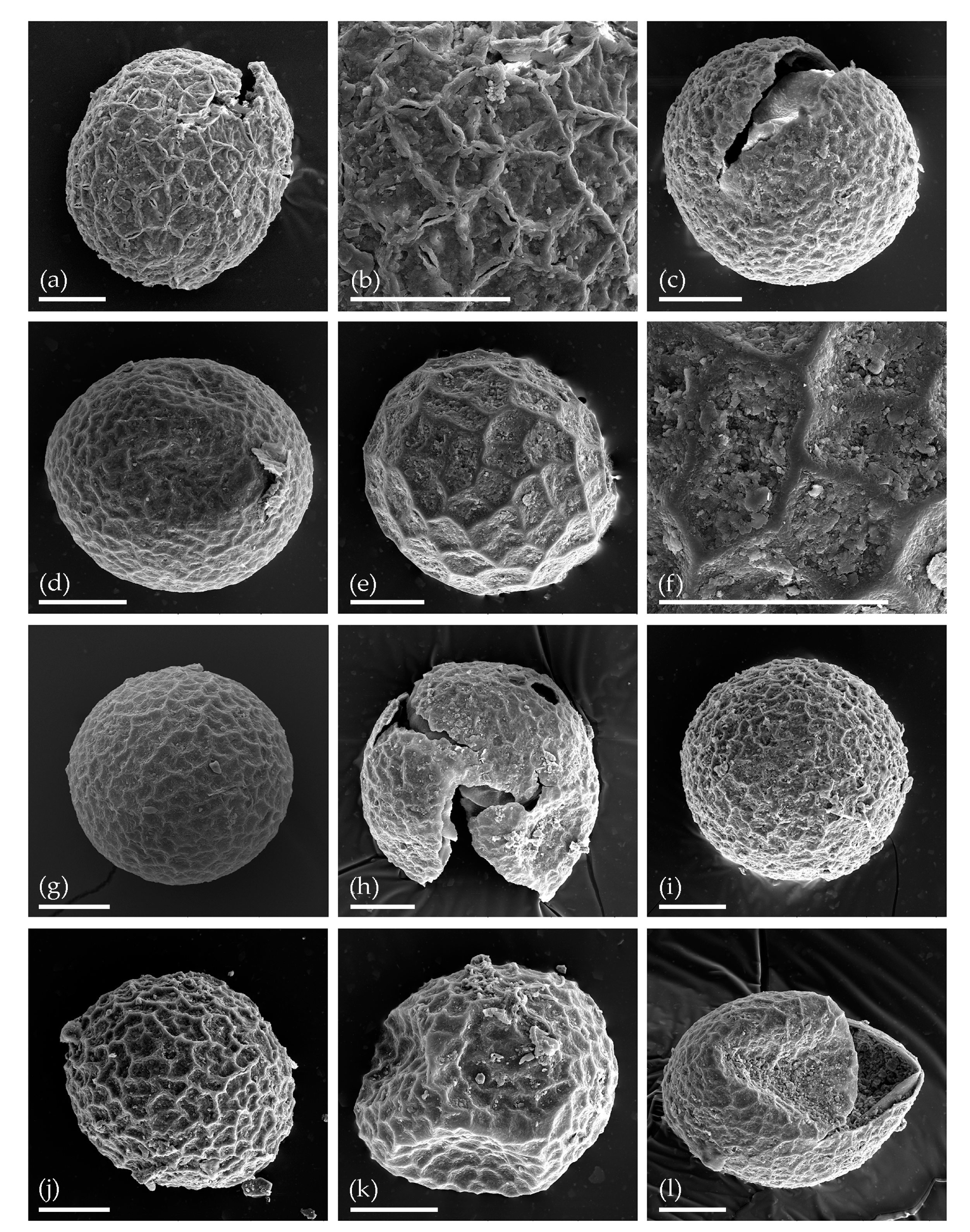
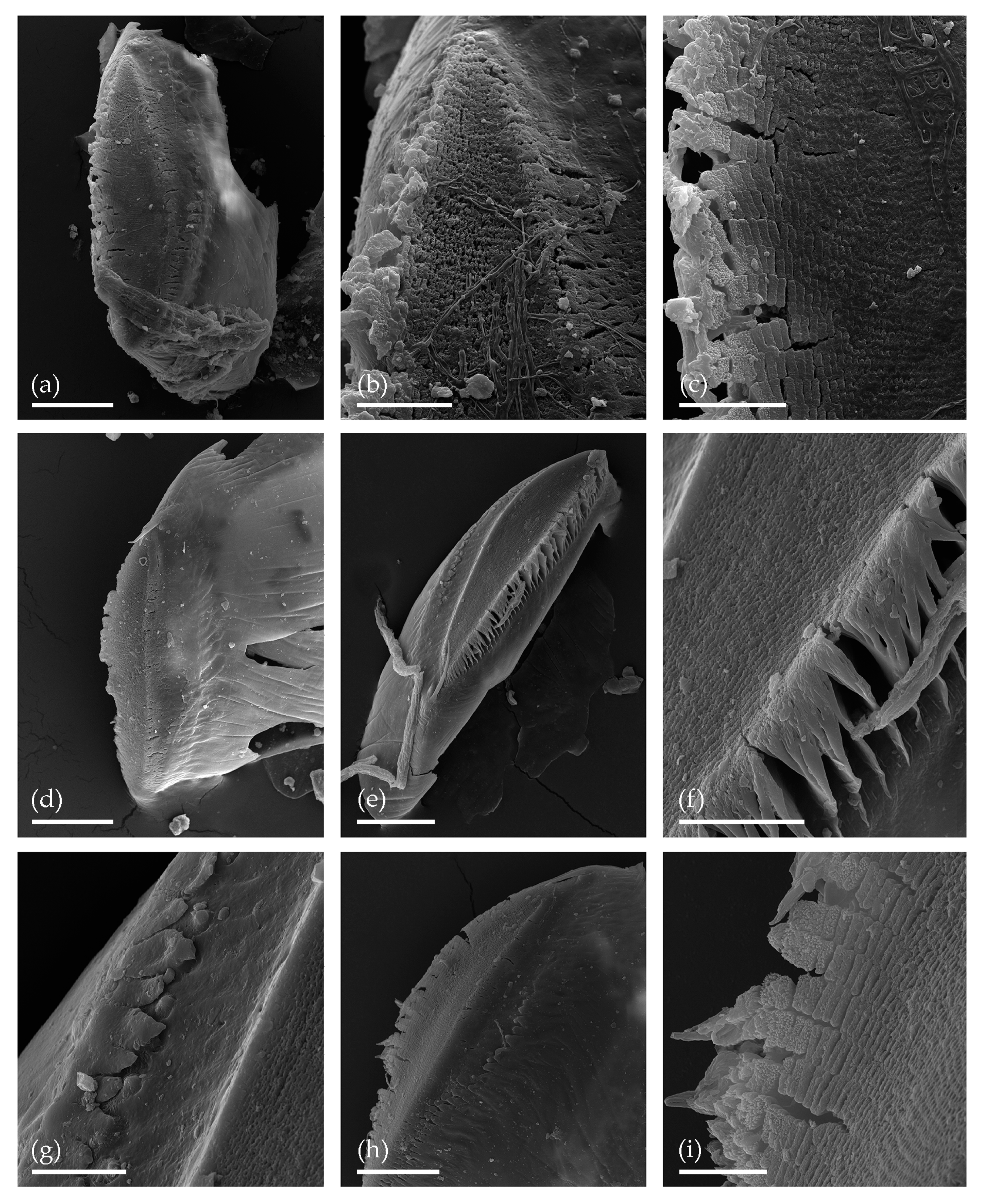
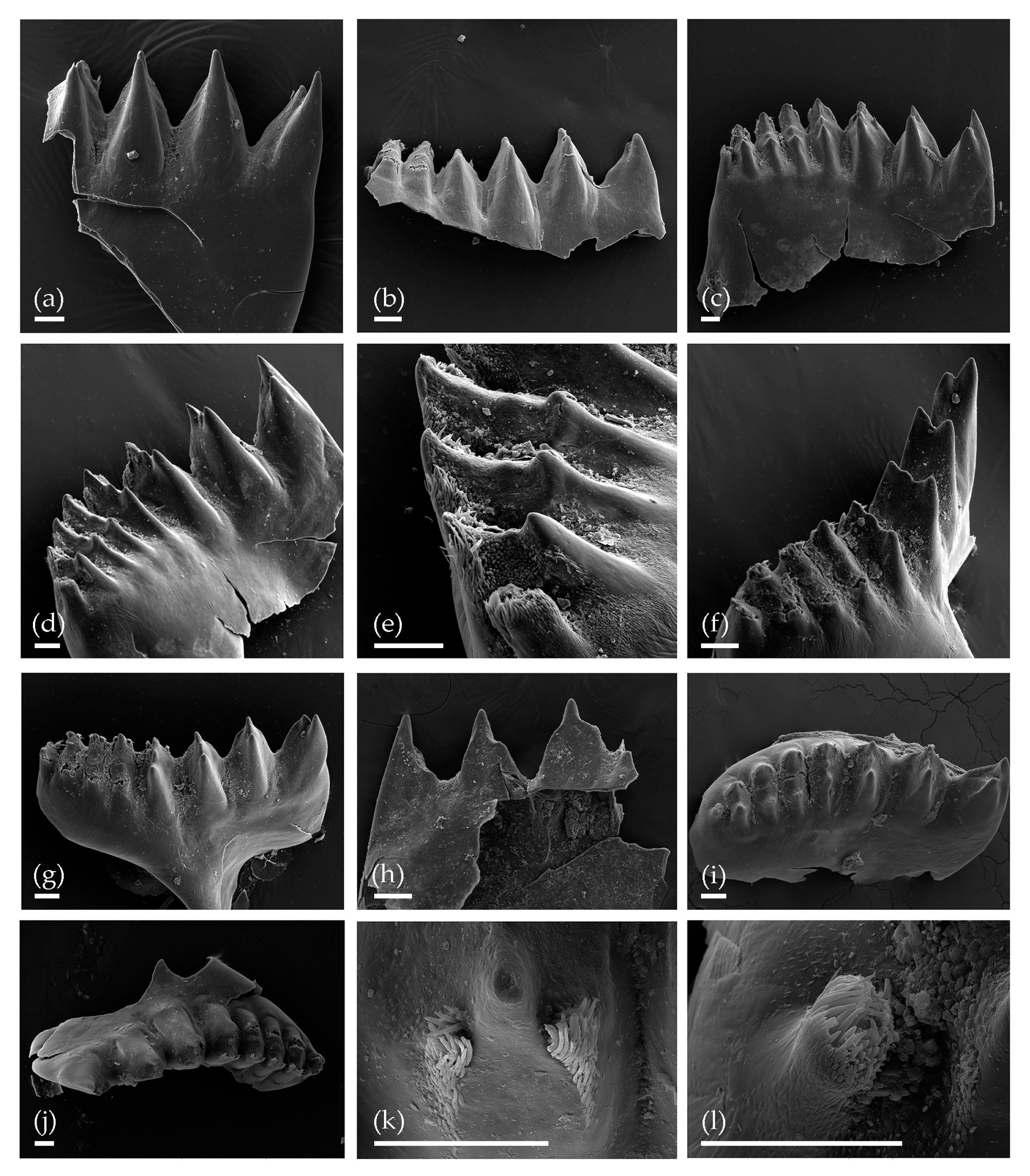
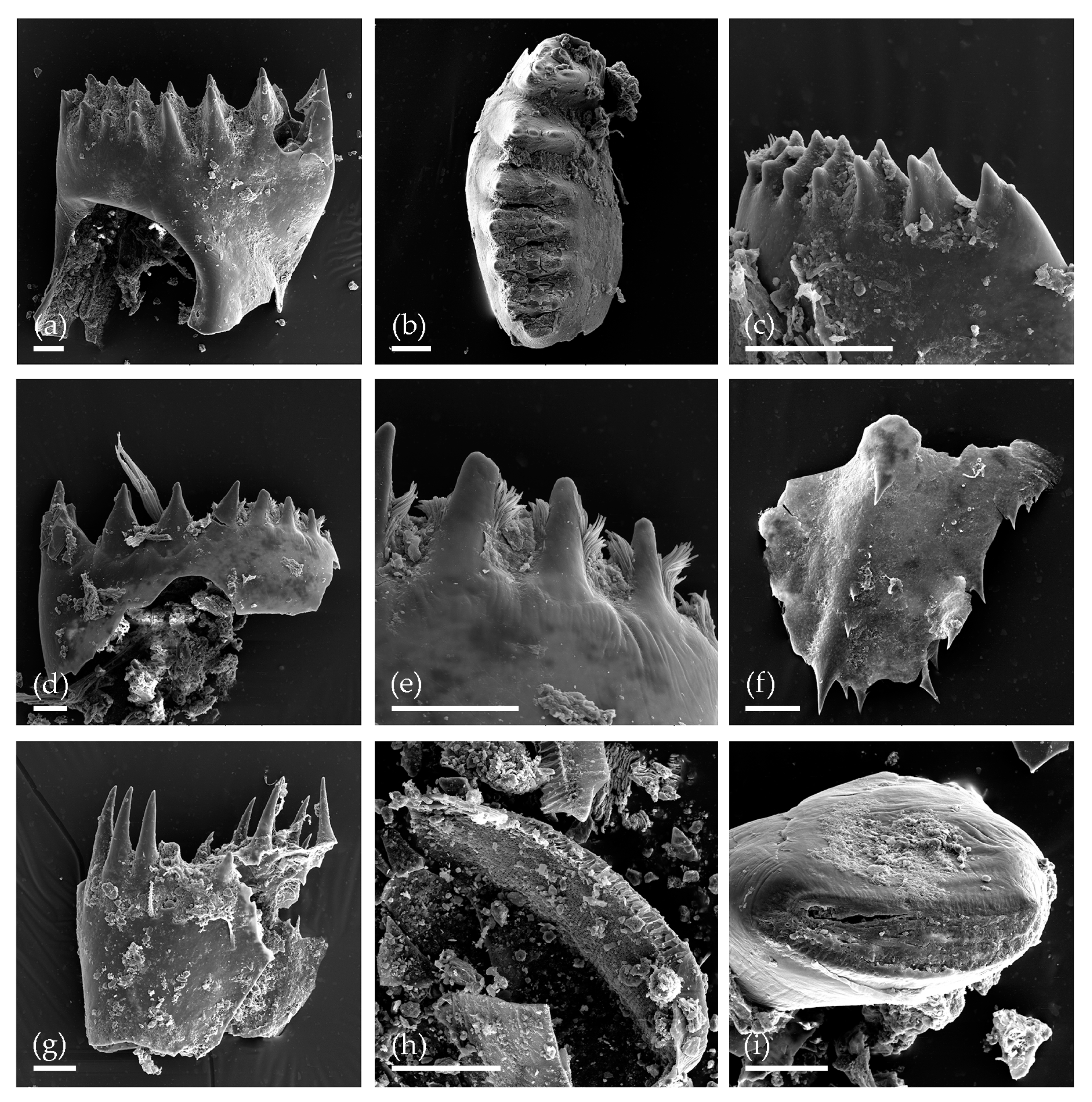
3. Results
3.1. Tom Creek
3.2. Alan Creek
3.3. Bol’shaya Chukochya Mammoth Hair
3.4. Staraya Allaikha Mammoth Hair
3.5. Yuka Baby Mammoth Skull
3.6. Churapcha Rhino
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brendonck, L.; Rogers, D.C.; Olesen, J.; Weeks, S.; Hoeh, W.R. Global diversity of large branchiopods (Crustacea: Branchiopoda) in freshwater. Hydrobiologia 2008, 198, 167–176. [Google Scholar] [CrossRef]
- Harvey, T.H.P.; Vélez, M.I.; Butterfield, N.J. Exceptionally preserved crustaceans from western Canada reveal a cryptic Cambrian radiation. Proc. Natl. Acad. Sci. USA 2012, 109, 1589–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagebro, L.; Gueriau, P.; Hegna, T.A.; Rabet, N.; Butler, A.D.; Budd, G.E. The oldest notostracan (Upper Devonian Strud locality, Belgium). Palaeontology 2015, 58, 497–509. [Google Scholar] [CrossRef]
- Gueriau, P.; Rabet, N.; Clément, G.; Lagebro, L.; Vannier, J.; Briggs, D.E.; Charbonnier, S.; Olive, S.; Béthoux, O. A 365-million-year-old freshwater community reveals morphological and ecological stasis in branchiopod crustaceans. Curr. Biol. 2016, 26, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueriau, P.; Rabet, N.; Du Tien Hat, E. The Strud crustacean fauna (Late Devonian, Belgium): Updated review and palaeoecology of an early continental ecosystem. Earth Environ. Sci. Trans. R. Soc. Edinb. 2016, 107, 79–90. [Google Scholar] [CrossRef]
- Hegna, T.A.; Astrop, T.I. The fossil record of the clam shrimp (Crustacea; Branchiopoda). Zool. Stud. 2020, 59, 43. [Google Scholar] [CrossRef]
- Van Damme, K.; Kotov, A.A. The fossil record of the Cladocera (Crustacea: Branchiopoda): Evidence and hypotheses. Earth-Sci. Rev. 2016, 163, 162–189. [Google Scholar] [CrossRef]
- Smirnov, N.N. Mesozoic Anomopoda (Crustacea) from Mongolia. Zool. J. Linn. Soc. 1992, 104, 97–116. [Google Scholar] [CrossRef]
- Kotov, A.A. Jurassic Cladocera (Crustacea, Branchiopoda) with a description of an extinct Mesozoic order. J. Nat. Hist. 2007, 41, 13–37. [Google Scholar] [CrossRef]
- Kotov, A.A.; Korovchinsky, N.M. First record of fossil Mesozoic Ctenopoda (Crustacea, Cladocera). Zool. J. Linn. Soc. 2006, 146, 269–274. [Google Scholar] [CrossRef]
- Rogers, D.C. Branchiopoda (Anostraca, Notostraca, Laevicaudata, Spinicaudata, Cyclestherida); Encyclopedia of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780123706263. [Google Scholar]
- Martin, J.W.; Rogers, D.C.; Olesen, J. Collecting and processing branchiopods. J. Crust. Biol. 2016, 36, 396–401. [Google Scholar] [CrossRef]
- Rogers, D.C. Anostracan (Crustacea: Branchiopoda) zoogeography II. Relating distribution to geochemical substrate properties in the USA. Zootaxa 2014, 3856, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.C. Larger hatching fractions in avian dispersed Anostracan eggs (Branchiopoda). J. Crust. Biol. 2014, 34, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Frey, D.G. The ecological significance of Cladocera remains in lake sediments. Ecology 1960, 41, 684–699. [Google Scholar] [CrossRef]
- Frey, D.G. Cladocera analysis. In Handbook of Holocene Paleoecology and Paleohydrology; Berglund, B.E., Ed.; J. Wiley & Sons Ltd.: London, UK, 1986; pp. 667–692. [Google Scholar]
- Korhola, A.; Rautio, M. Cladocera and other branchiopod crustaceans. In Tracking Environmental Change Using Lake Sediments; Volume 4: Zoological Indicators; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 5–41. [Google Scholar]
- Kotov, A.A.; Ibragimova, A.G.; Neretina, A.N. Identification of Ceriodaphnia Dana, 1853 (Crustacea: Cladocera) taxa from European Russia based on ephippial morphology. Zootaxa 2018, 4527, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Wojewódka, M.; Sinev, A.Y.; Zawisza, E.; Stańczak, J. A guide to the identification of subfossil chydorid Cladocera (Crustacea: Branchiopoda) from lake sediments of Central America and the Yucatan Peninsula, Mexico: Part II. J. Paleolimnol. 2020, 63, 37–64. [Google Scholar] [CrossRef]
- Kotov, A.A.; Kuzmina, S.A.; Frolova, L.A.; Zharov, A.A.; Neretina, A.N.; Smirnov, N.N. Ephippia of the Daphniidae (Branchiopoda: Cladocera) in Late Caenozoic deposits: Untapped source of information for palaeoenvironment reconstructions in the Northern Holarctic. Invert. Zool. 2019, 16, 183–199. [Google Scholar] [CrossRef]
- Zharov, A.A.; Neretina, A.N.; Rogers, D.C.; Reshetova, S.A.; Sinitsa, S.M.; Kotov, A.A. Pleistocene Branchiopods (Cladocera, Anostraca) from Transbaikalian Siberia demonstrate morphological and ecological stasis. Water 2020, 12, 3063. [Google Scholar] [CrossRef]
- Kirillova, I.V.; Van der Plicht, J.; Gubin, S.V.; Zanina, O.G.; Chernova, O.F.; Lapteva, E.G.; Trofimova, S.S.; Zinoviev, E.V.; Zharov, A.A.; Fadeeva, E.O.; et al. Taphonomic phenomenon of ancient mammal fur from Glacial Beringia. Boreas 2016, 45, 455–469. [Google Scholar] [CrossRef]
- Kotov, A.A.; Neretina, A.N.; Zharov, A.A.; Izyumova, E.I.; Boeskorov, G.G.; Kosintsev, P.A.; Shidlovskiy, F.K. A new glance at old samples: Remains of freshwater invertebrates associated with mummified carcasses of large quaternary mammals. Zool. Zhurn. 2019, 98, 1247–1255. [Google Scholar]
- Kotov, A.A.; Zharov, A.A.; Chernova, O.F.; Neretina, A.N.; Gololobova, M.A.; Trofimova, S.S.; Zinovyev, E.V.; Izymova, E.I.; Zanina, O.G.; Kirillova, I.V.; et al. Crustacea (Branchiopoda) among organic remains from mammoth hair. Biol. Bull. 2020, 46, 850–863. [Google Scholar] [CrossRef]
- Neretina, A.N.; Gololobova, M.A.; Neplyukhina, A.A.; Zharov, A.A.; Rogers, C.D.; Horne, D.J.; Protopopov, A.V.; Kotov, A.A. Crustacean remains from the Yuka mammoth raise questions about non-analogue freshwater communities in the Beringian region during the Pleistocene. Sci. Rep. 2020, 10, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennike, O. Palaeoecology of two lake basins from Disko, West Greenland. J. Quat. Sci. 1995, 10, 149–155. [Google Scholar] [CrossRef]
- Bos, D.G.; Cumming, B.F.; Smol, J.P. Cladocera and Anostraca from the Interior Plateau of British Columbia, Canada, as paleolimnological indicators of salinity and lake level. Hydrobiologia 1999, 392, 129–141. [Google Scholar] [CrossRef]
- Szeroczyñska, K.; Sarmaja-Korjonen, K. Atlas of Subfossil Cladocera from Central and Northern Europe; Friends of the Lower Vistula Society: Świecie, Poland, 2007; ISBN 9788392491965. [Google Scholar]
- Thiery, A.; Gasc, C. Resting eggs of Anostraca, Notostraca and Spinicaudata (Crustacea, Branchiopoda) occurring in France: Identification and taxonomical value. Hydrobiologia 1991, 212, 245–259. [Google Scholar] [CrossRef]
- Thiery, A.; Brtek, J.; Gasc, C. Cyst morphology of European branchiopods (Crustacea: Anostraca, Notostraca, Spinicaudata, Laevicaudata). Bull. Mus. Nat. Hist. Paris Ser. 4 1995, 17, 107–140. [Google Scholar]
- Vekhov, N.V. Branchiopod crustaceans (Anostraca and Notostraca) of ephemeral water bodies of the steppe zone. Zool. Zhurnal 1989, 68, 241–250. [Google Scholar]
- Belk, D.; Mura, G.; Weeks, S. Untangling confusion between Eubranchipus vernalis and Eubranchipus neglectus (Branchiopoda: Anostraca). J. Crust. Biol. 1998, 18, 147–152. [Google Scholar] [CrossRef]
- Takahashi, N.; Kitano, T.; Hatanaka, Y.; Nagahata, Y.; Tshistjakov, Y.A.; Hamasaki, M.; Moriya, H.; Igarashi, K.; Umetsu, K. Three new species of the fairy shrimp Eubranchipus Verill, 1870 (Branchiopoda: Anostraca) from northern Japan and far Eastern Russia. BMC Zool. 2018, 3, 5. [Google Scholar] [CrossRef]
- Mura, G. Scanning electron microscopic study of the molar surfaces of the mandibles of Chirocephalus diaphanus Prévost (Anostraca). Crustaceana 1991, 60, 178–185. [Google Scholar] [CrossRef]
- Mura, G. Morphological features of the mandible related to feeding habits of some Anostraca species. Crustaceana 1995, 68, 83–102. [Google Scholar] [CrossRef]
- Rogers, D.C. A conceptual model for anostracan biogeography. J. Crust. Biol. 2015, 35, 686–699. [Google Scholar] [CrossRef] [Green Version]
- Belk, D.; Schram, F.R. A new species of anostracan from the Miocene of California. J. Crust. Biol. 2001, 21, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Schram, F.R. Crustacea; Oxford Univ. Press: New York, NY, USA, 1986. [Google Scholar]
- Knecht, R.J.; Benner, J.S.; Rogers, D.C.; Ridge, J.C. Surculichnus bifurcauda n. igen., n. isp., a trace fossil from Late Pleistocene glaciolacustrine varves of the Connecticut River Valley, USA, attributed to notostracan crustaceans based on neoichnological experimentation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 272, 232–239. [Google Scholar] [CrossRef]
- Hegna, T.A.; Rogers, D.C. The World’s First Clam Shrimp Symposium: Drawing paleontology and biology together. Zool. Stud. 2020, 59, 46. [Google Scholar] [CrossRef]
- Mura, G. Pattern of egg shell morphology in Thamnocephalids and Streptocephalids of the New World (Anostraca). Crustaceana 1992, 62, 300–311. [Google Scholar] [CrossRef]
- Mura, G.; Thierry, A. Taxonomical significance of scanning electron microscopic morphology of the Euphyllopod’s resting eggs from Morocco, Part I. Anostraca. Vie et Milieu 1986, 36, 125–131. [Google Scholar]
- Vanschoenwinkel, B.A.; Vandecaetsbeek, T.; Pineau, O.; Grillas, P.; Brendonck, L. Dispersal of freshwater invertebrates by large terrestrial mammals: A case study with wild boar (Sus scrofa) in Mediterranean wetlands. Freshwat. Biol. 2008, 53, 2264–2273. [Google Scholar] [CrossRef]
- Vanschoenwinkel, B.A.; Waterkeyn, A.; Nhiwatiwa, T.; Pinceel, T.; Spooren, E.; Geerts, A.; Clegg, B.; Brendonck, L. Passive external transport of freshwater invertebrates by elephant and other mud-wallowing mammals in an African savannah habitat. Freshwat. Biol. 2011, 56, 1365–2427. [Google Scholar] [CrossRef]
- Frey, D.G. Questions concerning cosmopolitanism in Cladocera. Arch. Hydrobiol. 1982, 93, 484–502. [Google Scholar]
- Frey, D.G. The taxonomy and biogeography of the Cladocera. Hydrobiologia 1987, 145, 5–17. [Google Scholar] [CrossRef]
- Fryer, G. Structure and habits of living branchiopod crustaceans and their bearing on the interpretation of fossil forms. Earth Environ. Sci. Trans. R. Soc. Edinb. 1985, 76, 103–113. [Google Scholar] [CrossRef]
- Fryer, G. Studies on the functional morphology and biology of the Notostraca (Crustacea: Branchiopoda). Phil. Trans. R. Soc. Lond. B 1988, 321, 27–124. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, D.C.; Zharov, A.A.; Neretina, A.N.; Kuzmina, S.A.; Kotov, A.A. A Review of Recently Discovered Remains of the Pleistocene Branchiopods (Anostraca, Notostraca) from NE Siberia and Arctic Canada. Water 2021, 13, 280. https://doi.org/10.3390/w13030280
Rogers DC, Zharov AA, Neretina AN, Kuzmina SA, Kotov AA. A Review of Recently Discovered Remains of the Pleistocene Branchiopods (Anostraca, Notostraca) from NE Siberia and Arctic Canada. Water. 2021; 13(3):280. https://doi.org/10.3390/w13030280
Chicago/Turabian StyleRogers, D. Christopher, Anton A. Zharov, Anna N. Neretina, Svetlana A. Kuzmina, and Alexey A. Kotov. 2021. "A Review of Recently Discovered Remains of the Pleistocene Branchiopods (Anostraca, Notostraca) from NE Siberia and Arctic Canada" Water 13, no. 3: 280. https://doi.org/10.3390/w13030280
APA StyleRogers, D. C., Zharov, A. A., Neretina, A. N., Kuzmina, S. A., & Kotov, A. A. (2021). A Review of Recently Discovered Remains of the Pleistocene Branchiopods (Anostraca, Notostraca) from NE Siberia and Arctic Canada. Water, 13(3), 280. https://doi.org/10.3390/w13030280







