Epiphyton in Agricultural Streams: Structural Control and Comparison to Epilithon
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Environmental Variables
2.3. Epiphyton and Epilithon Sampling
2.4. Epiphyton and Epilithon Structure Characterization
2.5. Statistical Analyses
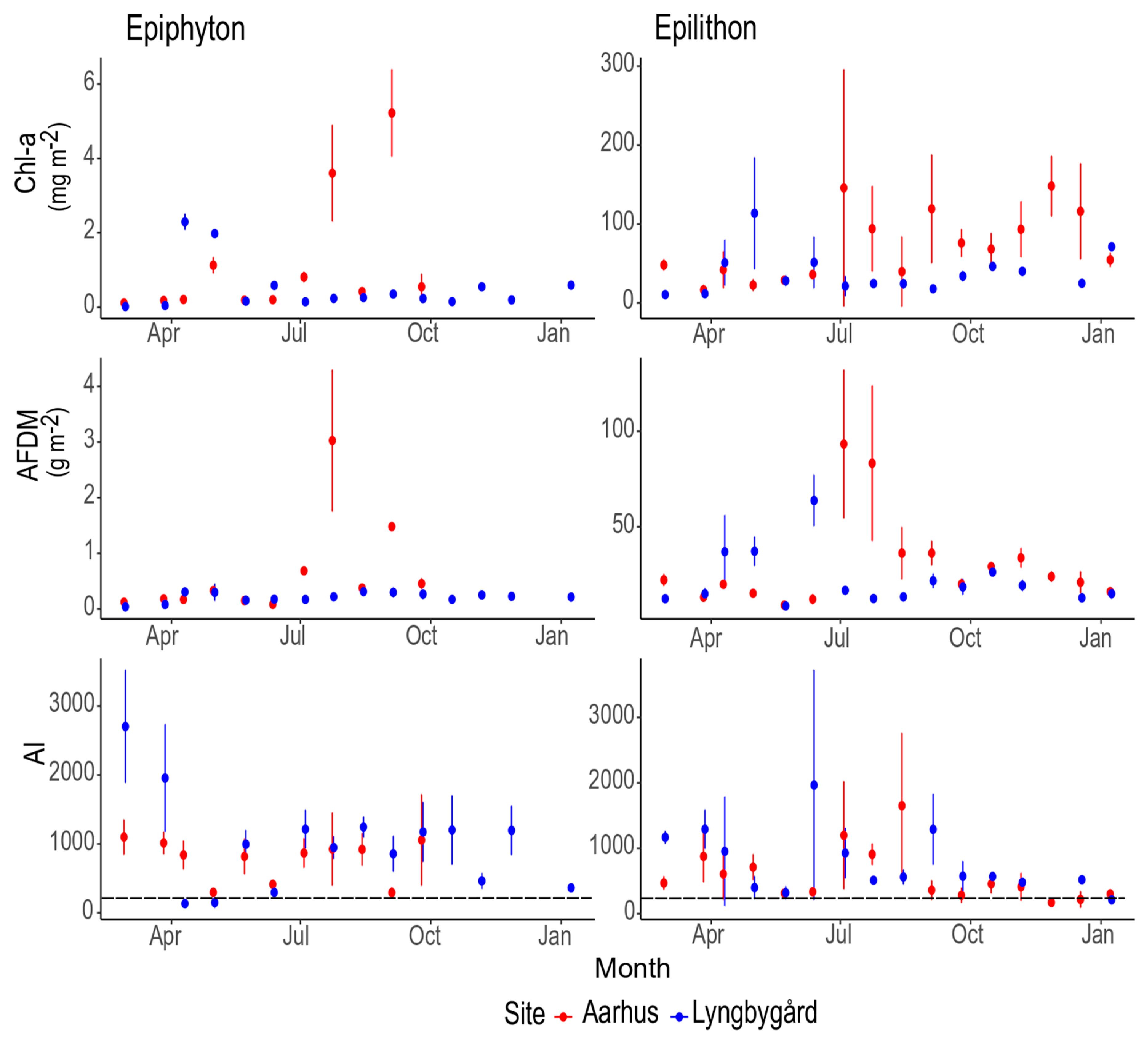
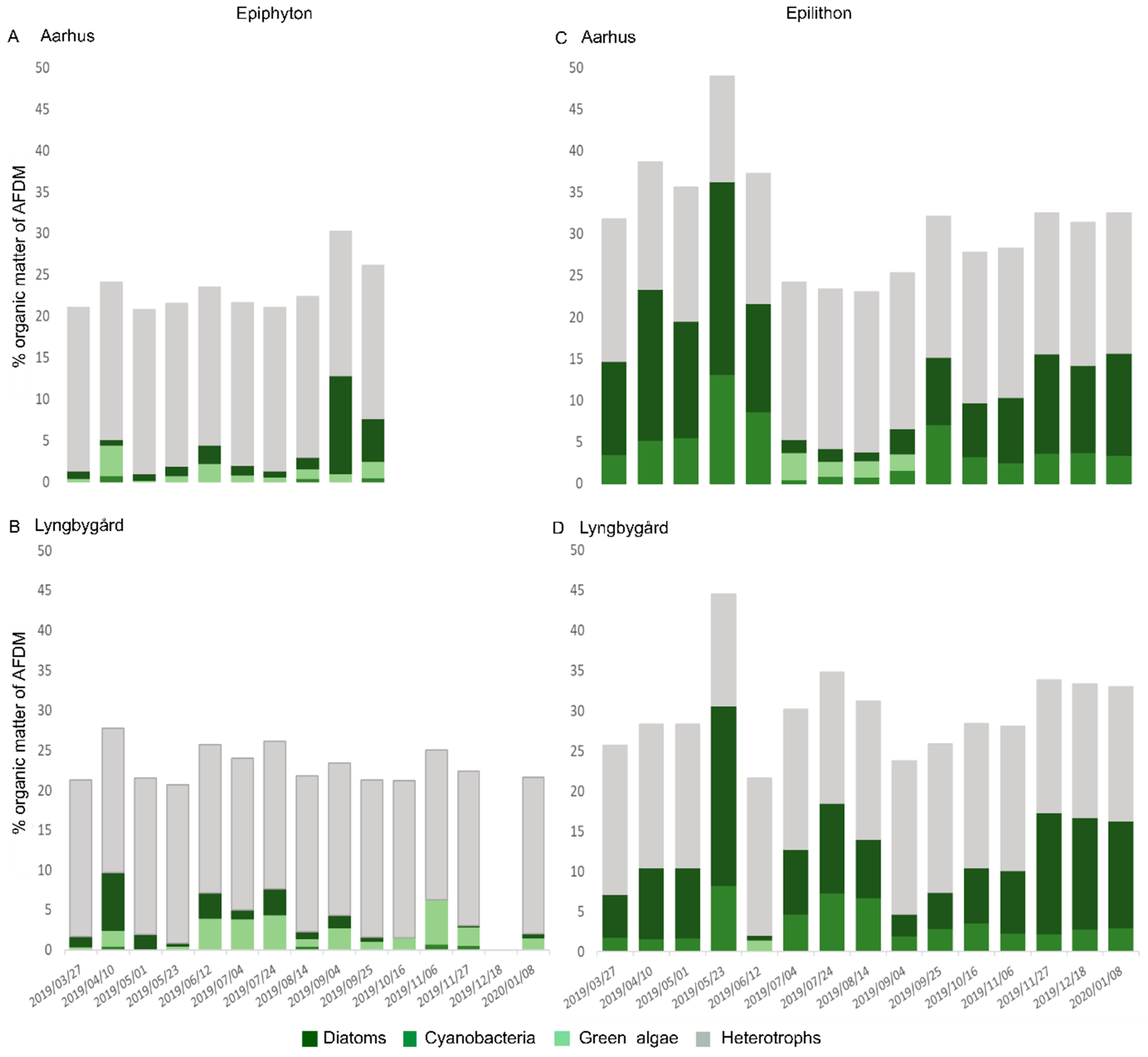
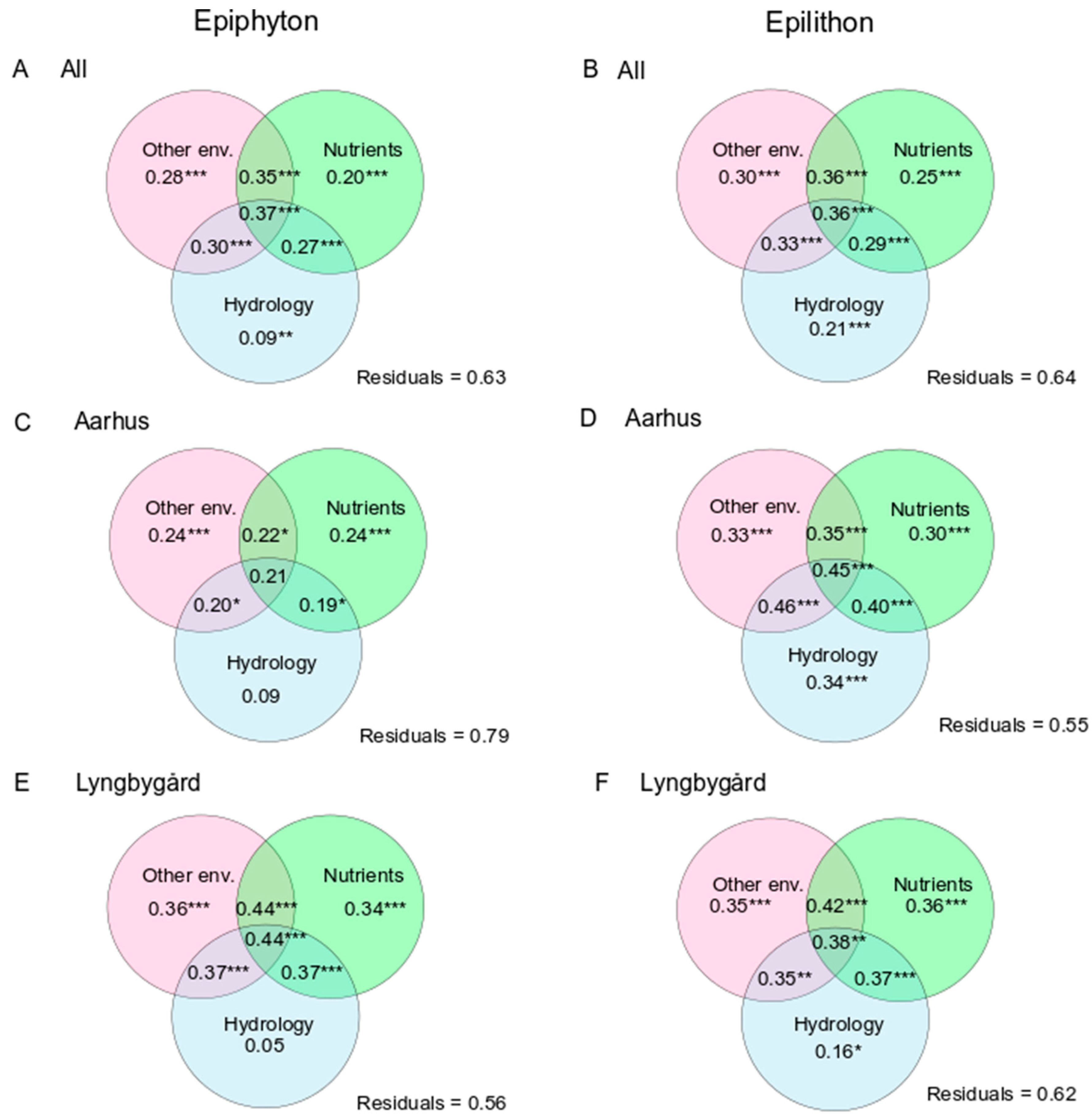
3. Results
3.1. Changes in Environmental Variables
3.2. Biomass, AI and Main Drivers
3.3. Algal Composition and Main Drivers
3.4. Diatom Species Composition and Main Drivers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Battin, T.J.; Kaplan, L.A.; Newbold, J.D.; Hansen, C.M. Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature 2003, 426, 439–442. [Google Scholar] [CrossRef]
- Besemer, K.; Peter, H.; Logue, J.B.; Langenheder, S.; Lindström, E.S.; Tranvik, L.J.; Battin, T.J. Unraveling assembly of stream biofilm communities. ISME J. 2012, 6, 1459–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romaní, A.M. Freshwater Biofilms. In Biofouling; Wiley-Blackwell: Oxford, UK, 2009; pp. 137–153. [Google Scholar]
- Biggs, B.J.; Thomsen, H.A. Disturbance of stream periphyton by perturbations in shear stress: Time to structural failure and differences in community resistance. J. Phycol. 1995, 31, 233–241. [Google Scholar] [CrossRef]
- Biggs, B.J. Hydraulic habitat of plants in streams. Regul. Rivers Res. Manag. 1996, 12, 131–144. [Google Scholar] [CrossRef]
- Guo, K.; Wu, N.; Manolaki, P.; Baattrup-Pedersen, A.; Riis, T. Short-period hydrological regimes outperform physicochemical variables in shaping stream diatom traits and biofilm community functions. Sci. Total Environ. 2020, 743, 140720. [Google Scholar] [CrossRef]
- Wu, N.; Thodsen, H.; Andersen, H.E.; Tornbjerg, H.; Baattrup-Pedersen, A.; Riis, T. Flow regimes filter species traits of benthic diatom communities and modify the functional features of lowland streams-a nationwide scale study. Sci. Total Environ. 2019, 651, 357–366. [Google Scholar] [CrossRef]
- Alnoee, A.B.; Riis, T.; Baattrup-Pedersen, A. Comparison of metabolic rates among macrophyte and nonmacrophyte habitats in streams. Freshw. Sci. 2016, 35, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Levi, P.S.; Riis, T.; Alnøe, A.B.; Peipoch, M.; Maetzke, K.; Bruus, C.; Baattrup-Pedersen, A. Macrophyte complexity controls nutrient uptake in lowland streams. Ecosystems 2015, 18, 914–931. [Google Scholar] [CrossRef]
- Demarty, M.; Prairie, Y.T. In situ dissolved organic carbon (DOC) release by submerged macrophyte–epiphyte communities in southern Quebec lakes. Can. J. Fish. Aquat. Sci. 2009, 66, 1522–1531. [Google Scholar] [CrossRef]
- Zhai, X.; Piwpuan, N.; Arias, C.A.; Headley, T.; Brix, H. Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems? Ecol. Eng. 2013, 61, 555–563. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Wetzel, R.G. Epiphytic alkaline phosphatase on natural and artificial plants in an oligotrophic lake: Re-evaluation of the role of macrophytes as a phosphorus source for epiphytes. Limnol. Oceanogr. 1990, 35, 736–747. [Google Scholar] [CrossRef]
- Bojorge-García, M.; Carmona, J.; Ramírez, R. Species richness and diversity of benthic diatom communities in tropical mountain streams of Mexico. Inland Waters 2014, 4, 279–292. [Google Scholar] [CrossRef] [Green Version]
- Wijewardene, L.; Wu, N.; Fohrer, N.; Riis, T. Epiphytic biofilms in freshwater and interactions with macrophytes: Current understanding and future directions. Aquat. Bot. 2022, 176, 103467. [Google Scholar] [CrossRef]
- Brodersen, K.E.; Koren, K.; Revsbech, N.P.; Kühl, M. Strong leaf surface basification and CO2 limitation of seagrass induced by epiphytic biofilm microenvironments. Plant Cell Environ. 2020, 43, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.L. Primary productivity, chemo-organotrophy, and nutritional interactions of epiphytic algae and bacteria on macrophytes in the littoral of a lake. Ecol. Monogr. 1971, 41, 97–127. [Google Scholar] [CrossRef]
- Cantonati, M.; Spitale, D. The role of environmental variables in structuring epiphytic and epilithic diatom assemblages in springs and streams of the Dolomiti Bellunesi National Park (south-eastern Alps). Fundam. Appl. Limnol. Arch. Für Hydrobiol. 2009, 174, 117–133. [Google Scholar] [CrossRef]
- Roberts, B.J.; Mulholland, P.J.; Hill, W.R. Multiple Scales of Temporal Variability in Ecosystem Metabolism Rates: Results from 2 Years of Continuous Monitoring in a Forested Headwater Stream. Ecosystems 2007, 10, 588–606. [Google Scholar] [CrossRef]
- Lowe, R.L.; LaLiberte, G.D. Benthic stream algae: Distribution and structure. In Methods in Stream Ecology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 193–221. [Google Scholar]
- Tang, T.; Cai, Q.; Liu, R.; Li, D.; Xie, Z. Distribution of epilithic algae in the Xiangxi River system and their relationships with environmental factors. J. Freshw. Ecol. 2002, 17, 345–352. [Google Scholar] [CrossRef]
- Zlatanović, S.; Fabian, J.; Premke, K.; Mutz, M. Shading and sediment structure effects on stream metabolism resistance and resilience to infrequent droughts. Sci. Total Environ. 2018, 621, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Costică, M.; Costică, N.; Stratu, A. Contributions to the Knowledge of Aquatic and Paludous Macrophytes and of Some Epiphytic Algae with Role in Processes of Self-Cleaning in the Urban Sector of Nicolina River-Iasi. Present Environ. Sustain. Dev. 2018, 12, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Shamsudin, L.; Sleigh, M.A. Seasonal changes in composition and biomass of epiphytic algae on the macrophyte Ranunculus penicillatus in a chalk stream, with estimates of production, and observations on the epiphytes of Cladophora glomerata. Hydrobiologia 1995, 306, 85–95. [Google Scholar] [CrossRef]
- Xia, P.; Yan, D.; Sun, R.; Song, X.; Lin, T.; Yi, Y. Community composition and correlations between bacteria and algae within epiphytic biofilms on submerged macrophytes in a plateau lake, southwest China. Sci. Total Environ. 2020, 727, 138398. [Google Scholar] [CrossRef]
- Winter, J.G.; Duthie, H.C. Stream epilithic, epipelic and epiphytic diatoms: Habitat fidelity and use in biomonitoring. Aquat. Ecol. 2000, 34, 345–353. [Google Scholar] [CrossRef]
- Soininen, J. Environmental and spatial control of freshwater diatoms—A review. Diatom Res. 2007, 22, 473–490. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, J.-X.; Pei, G.-F.; Liu, G.-X. Using benthic diatom assemblages to assess human impacts on streams across a rural to urban gradient. Environ. Sci. Pollut. Res. 2015, 22, 18093–18106. [Google Scholar] [CrossRef] [PubMed]
- Munn, M.D.; Waite, I.; Konrad, C.P. Assessing the influence of multiple stressors on stream diatom metrics in the upper Midwest, USA. Ecol. Indic. 2018, 85, 1239–1248. [Google Scholar] [CrossRef]
- Biggs, B.J.F.; Stevenson, R.J.; Lowe, R.L. A habitat matrix conceptual model for stream periphyton. Arch. Fur Hydrobiol. 1998, 143, 21–56. [Google Scholar] [CrossRef]
- Biggs, B.J.F.; Nikora, V.I.; Snelder, T.H. Linking scales of flow variability to lotic ecosystem structure and function. River Res. Appl. 2005, 21, 283–298. [Google Scholar] [CrossRef]
- Riis, T. Dispersal and colonisation of plants in lowland streams: Success rates and bottlenecks. Hydrobiologia 2008, 596, 341–351. [Google Scholar] [CrossRef]
- Thimijan, R.W.; Heins, R.D. Photometric, radiometric, and quantum light units of measure: A review of procedures for interconversion. HortScience 1983, 18, 818–822. [Google Scholar]
- Steinman, A.D.; Lamberti, G.A.; Leavitt, P.R.; Uzarski, D.G. Biomass and pigments of benthic algae. In Methods in Stream Ecology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 223–241. [Google Scholar]
- Lakatos, G. Composition of reed periphyton (biotecton) in the Hungarian part of lake Fertö. BFB-Bericht 1989, 71, 125–134. [Google Scholar]
- Li, H.-P.; Gong, G.-C.; Hsiung, T.-M. Phytoplankton pigment analysis by HPLC and its application in algal community investigations. Bot. Bull. Acad. Sin. 2002, 43, 283–290. [Google Scholar]
- Kahlert, M.; McKie, B.G. Comparing new and conventional methods to estimate benthic algal biomass and composition in freshwaters. Environ. Sci. Process. Impacts 2014, 16, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Cecchi, P.; Malet, N.; Bec, B.; Torre, F.; Pasqualini, V. Evaluation of FluoroProbe® performance for the phytoplankton-based assessment of the ecological status of Mediterranean coastal lagoons. Environ. Monit. Assess. 2019, 191, 204. [Google Scholar] [CrossRef]
- Rosero-López, D.; Walter, M.T.; Flecker, A.S.; Ontaneda, D.F.; Dangles, O. Standardization of instantaneous fluoroprobe measurements of benthic algal biomass and composition in streams. Ecol. Indic. 2021, 121, 107185. [Google Scholar] [CrossRef]
- Sanzone, D.M.; Tank, J.L.; Meyer, J.L.; Mulholland, P.J.; Findlay, S.E. Microbial incorporation of nitrogen in stream detritus. Hydrobiologia 2001, 464, 27–35. [Google Scholar] [CrossRef]
- Bey, M.; Ector, L. Atlas des Diatrs d’eau de la Région Rhône-Alpes. 2013, pp. 1–1182. Available online: http://www.auvergne-rhone-alpes.developpement-durable.gouv.fr/atlas-des-diatomees-a3480.html (accessed on 10 October 2021).
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen im Süßwasser-Benthos von Mitteleuropa: Bestimmungsflora Kieselalgen für die ökologische Praxis; über 700 der häufigsten Arten und ihrer Ökologie; Gantner: Ruggell, Liechtenstein, 2011. [Google Scholar]
- Cantonati, M.; Kelly, M.G.; Lange-Bertalot, H. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; Koeltz Botanical Books: Oberreifenberg, Germany, 2017; pp. 1–942. [Google Scholar]
- Bąk, M.; Witkowski, A.; Żelazna-Wieczorek, J.; Wojtal, A.; Szczepocka, E.; Szulc, K.; Szulc, B. Klucz do Oznaczania Okrzemek w Fitobentosie na Potrzeby Oceny Stanu Ekologicznego wód Powierzchniowych w Polsce; Biblioteka Monitoringu Srodowiska: Warszawa, Poland, 2012. [Google Scholar]
- R Core Team. R Software: Version 4.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for DATA Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Wei, T.; Simko, V. R Package “corrplot”: Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 10 October 2021).
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Stevens, M. Package—Vegan: Community Ecology Package. R Package Version 2.5-6. 2020. Available online: https://github.com/vegandevs/vegan (accessed on 10 October 2021).
- De Caceres, M. Package ‘Vegclust’. 2010. Available online: https://emf-creaf.github.io/vegclust (accessed on 10 October 2021).
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Cornejo, A.; Tonin, A.M.; Checa, B.; Tuñon, A.R.; Pérez, D.; Coronado, E.; González, S.; Ríos, T.; Macchi, P.; Correa-Araneda, F.; et al. Effects of multiple stressors associated with agriculture on stream macroinvertebrate communities in a tropical catchment. PLoS ONE 2019, 14, e0220528. [Google Scholar] [CrossRef] [Green Version]
- Inkscape Project. Inkscape; Prentice hall press: Hoboken, NJ, USA, 2020. [Google Scholar]
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D.; Ripley, M.B. Package ‘mass’. Cran R 2013, 538, 113–120. [Google Scholar]
- Belyaeva, P. Photosynthetic pigments of phytoperiphyton in the Sylva River (Middle Ural). Inland Water Biol. 2017, 10, 52–58. [Google Scholar] [CrossRef]
- Kahlert, M.; Pettersson, K. The impact of substrate and lake trophy on the biomass and nutrient status of benthic algae. Hydrobiologia 2002, 489, 161–169. [Google Scholar] [CrossRef]
- Soininen, J.; Eloranta, P. Seasonal persistence and stability of diatom communities in rivers: Are there habitat specific differences? Eur. J. Phycol. 2004, 39, 153–160. [Google Scholar] [CrossRef]
- Zelnik, I.; Sušin, T. Epilithic Diatom Community Shows a Higher Vulnerability of the River Sava to Pollution during the Winter. Diversity 2020, 12, 465. [Google Scholar] [CrossRef]
- Fernandes, V.; Esteves, F. The use of indices for evaluating the periphytic community in two kinds of substrate in Imboassica Lagoon, Rio de Janeiro, Brazil. Braz. J. Biol. 2003, 63, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Lock, M.A.; Wallace, R.R.; Costerton, J.W.; Ventullo, R.M.; Charlton, S.E. River Epilithon: Toward a Structural-Functional Model. Oikos 1984, 42, 10–22. [Google Scholar] [CrossRef]
- Wolters, J.-W.; Reitsema, R.E.; Verdonschot, R.C.M.; Schoelynck, J.; Verdonschot, P.F.M.; Meire, P. Macrophyte-specific effects on epiphyton quality and quantity and resulting effects on grazing macroinvertebrates. Freshw. Biol. 2019, 64, 1131–1142. [Google Scholar] [CrossRef]
- Piirsoo, K.; Vilbaste, S.; Truu, J.; Pall, P.; Trei, T.; Tuvikene, A.; Viik, M. Origin of phytoplankton and the environmental factors governing the structure of microalgal communities in lowland streams. Aquat. Ecol. 2007, 41, 183–194. [Google Scholar] [CrossRef]
- Shen, R.; Ren, H.; Yu, P.; You, Q.; Pang, W.; Wang, Q. Benthic Diatoms of the Ying River (Huaihe River Basin, China) and Their Application in Water Trophic Status Assessment. Water 2018, 10, 1013. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Liu, Y.; Fan, Y. Diatom Taxonomic Composition as a Biological Indicator of the Ecological Health and Status of a River Basin under Agricultural Influence. Water 2020, 12, 2067. [Google Scholar] [CrossRef]
- Ferreiro, N.; Giorgi, A.; Feijoó, C. Effects of macrophyte architecture and leaf shape complexity on structural parameters of the epiphytic algal community in a Pampean stream. Aquat. Ecol. 2013, 47, 389–401. [Google Scholar] [CrossRef]
- Pettit, N.E.; Ward, D.P.; Adame, M.F.; Valdez, D.; Bunn, S.E. Influence of aquatic plant architecture on epiphyte biomass on a tropical river floodplain. Aquat. Bot. 2016, 129, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Bowden, W.B.; Peterson, B.J.; Finlay, J.C.; Tucker, J. Epilithic chlorophyll a, photosynthesis, and respiration in control and fertilized reaches of a tundra stream. Hydrobiologia 1992, 240, 121–131. [Google Scholar] [CrossRef]
- Hill, W.R.; Fanta, S.E.; Roberts, B.J. Quantifying phosphorus and light effects in stream algae. Limnol. Oceanogr. 2009, 54, 368–380. [Google Scholar] [CrossRef] [Green Version]
- Ács, É.; Kiss, K.T. Effects of the water discharge on periphyton abundance and diversity in a large river (River Danube, Hungary). Hydrobiologia 1993, 249, 125–133. [Google Scholar] [CrossRef]
- Matthaei, C.D.; Piggott, J.J.; Townsend, C.R. Multiple stressors in agricultural streams: Interactions among sediment addition, nutrient enrichment and water abstraction. J. Appl. Ecol. 2010, 47, 639–649. [Google Scholar] [CrossRef]
- Moulton, T.P.; Souza, M.L.; Walter, T.L.; Krsulovic, F.A.M. Patterns of periphyton chlorophyll and dry mass in a neotropical stream: A cheap and rapid analysis using a hand-held fluorometer. Mar. Freshw. Res. 2009, 60, 224–233. [Google Scholar] [CrossRef]
- Gosselain, V.; Hudon, C.; Cattaneo, A.; Gagnon, P.; Planas, D.; Rochefort, D. Physical variables driving epiphytic algal biomass in a dense macrophyte bed of the St. Lawrence River (Quebec, Canada). Hydrobiologia 2005, 534, 11–22. [Google Scholar] [CrossRef]
- Tank, J.L.; Martí, E.; Riis, T.; von Schiller, D.; Reisinger, A.J.; Dodds, W.K.; Whiles, M.R.; Ashkenas, L.R.; Bowden, W.B.; Collins, S.M.; et al. Partitioning assimilatory nitrogen uptake in streams: An analysis of stable isotope tracer additions across continents. Ecol. Monogr. 2018, 88, 120–138. [Google Scholar] [CrossRef] [Green Version]
- Biggs, B.J.F. Eutrophication of streams and rivers: Dissolved nutrient-chlorophyll relationships for benthic algae. J. N. Am. Benthol. Soc. 2000, 19, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Lévesque, D.; Hudon, C.; James, P.M.A.; Legendre, P. Environmental factors structuring benthic primary producers at different spatial scales in the St. Lawrence River (Canada). Aquat. Sci. 2017, 79, 345–356. [Google Scholar] [CrossRef]
- Morin, J.O.n.; Kimball, K.D. Relationship of macrophyte-mediated changes in the water column to periphyton composition and abundance. Freshw. Biol. 1983, 13, 403–414. [Google Scholar] [CrossRef]
- Sobczak, W.V.; Findlay, S. Variation in Bioavailability of Dissolved Organic Carbon among Stream Hyporheic Flowpaths. Ecology 2002, 83, 3194–3209. [Google Scholar] [CrossRef]
- Lange, H. Time-Series Analysis in Ecology. In eLS; Wiley-VCH GmbH: Weinheim, Germany, 2006. [Google Scholar]


| Model | Response Variables | Environmental Variables | Estimate | p Value | Model adj.R2 | Model Significance | AIC |
|---|---|---|---|---|---|---|---|
| Epiphyton | Chl-a | No significant model | |||||
| AFDM | Temperature | 0.306 | 0.112 | 0.218 | 0.023 | 72.215 | |
| DOC | −0.352 | 0.069 | |||||
| AI | No significant model | ||||||
| Epilithon | Chl-a | CV of Q | −0.259 | 0.128 | 0.224 | 0.01 | 87.531 |
| PO43− | 0.386 | 0.027 | |||||
| AFDM | No significant model | ||||||
| AI | CV of Q | 0.298 | 0.093 | 0.164 | 0.029 | 89.946 | |
| PO43− | −0.282 | 0.111 | |||||
| Model | Response Variables | Environmental Variables | Estimate | p Value | Model adj.R2 | Model Significance | AIC |
|---|---|---|---|---|---|---|---|
| Epiphyton | Diatom | DOC | −0.502 | 0.012 | 0.218 | 0.012 | 66.106 |
| Cyanobacteria | Qmed | 0.349 | 0.087 | 0.217 | 0.03 | 67.048 | |
| CV of Q | 0.309 | 0.127 | |||||
| Green algae | No significant model | ||||||
| Epilithon | Diatom | Temperature | −0.562 | 0.001 | 0.291 | 0.001 | 78.752 |
| Cyanobacteria | No significant model | ||||||
| Green algae | Temperature | 0.624 | 0.028 | 0.365 | 0.002 | 77.231 | |
| DOC | −0.596 | 0.006 | |||||
| Qmed | 0.460 | 0.161 | |||||
| Epiphyton—Lyngbygård | Diatom | Light | 0.390 | 0.147 | 0.388 | 0.027 | 37.480 |
| DOC | −0.413 | 0.127 | |||||
| Cyanobacteria | DOC | 0.570 | 0.033 | 0.269 | 0.033 | 39.183 | |
| Green algae | No significant model | ||||||
| Epilithon—Aarhus | Diatom | Temperature | −0.679 | 0.005 | 0.419 | 0.005 | 38.270 |
| Cyanobacteria | No significant model | ||||||
| Green algae | Temperature | 0.353 | 0.179 | 0.543 | 0.004 | 35.466 | |
| DOC | −0.495 | 0.069 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijewardene, L.; Wu, N.; Giménez-Grau, P.; Holmboe, C.; Fohrer, N.; Baattrup-Pedersen, A.; Riis, T. Epiphyton in Agricultural Streams: Structural Control and Comparison to Epilithon. Water 2021, 13, 3443. https://doi.org/10.3390/w13233443
Wijewardene L, Wu N, Giménez-Grau P, Holmboe C, Fohrer N, Baattrup-Pedersen A, Riis T. Epiphyton in Agricultural Streams: Structural Control and Comparison to Epilithon. Water. 2021; 13(23):3443. https://doi.org/10.3390/w13233443
Chicago/Turabian StyleWijewardene, Lishani, Naicheng Wu, Pau Giménez-Grau, Cecilie Holmboe, Nicola Fohrer, Annette Baattrup-Pedersen, and Tenna Riis. 2021. "Epiphyton in Agricultural Streams: Structural Control and Comparison to Epilithon" Water 13, no. 23: 3443. https://doi.org/10.3390/w13233443
APA StyleWijewardene, L., Wu, N., Giménez-Grau, P., Holmboe, C., Fohrer, N., Baattrup-Pedersen, A., & Riis, T. (2021). Epiphyton in Agricultural Streams: Structural Control and Comparison to Epilithon. Water, 13(23), 3443. https://doi.org/10.3390/w13233443







