Phytoplankton Growth Rate and Microzooplankton Grazing under Conditions of Climatic Changes and Anthropogenic Pollution in the Coastal Waters of the Black Sea (Sevastopol Region)
Abstract
:1. Introduction
2. Materials and Methods
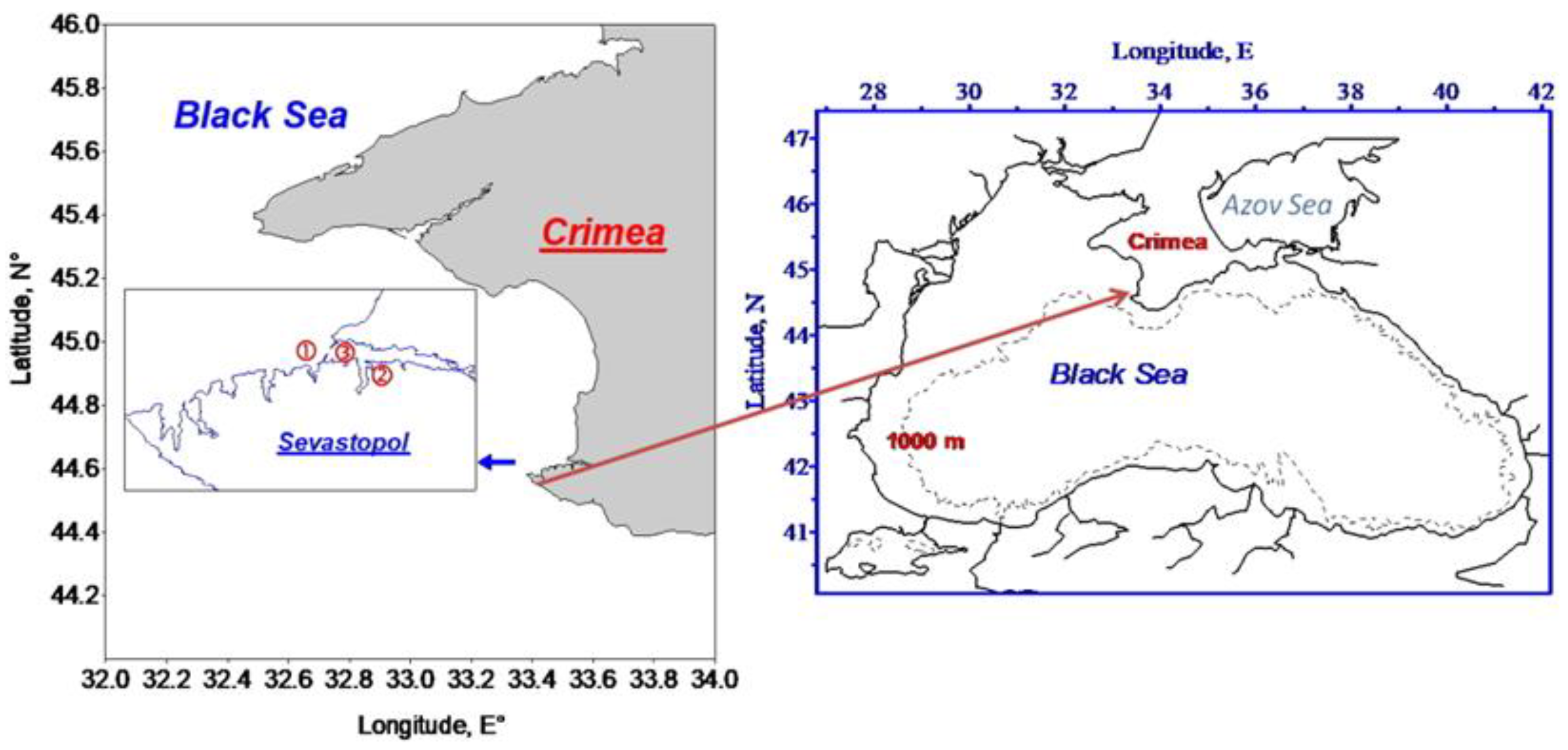
2.1. Study Area and Sampling
2.2. Processing
2.3. Data Analysis
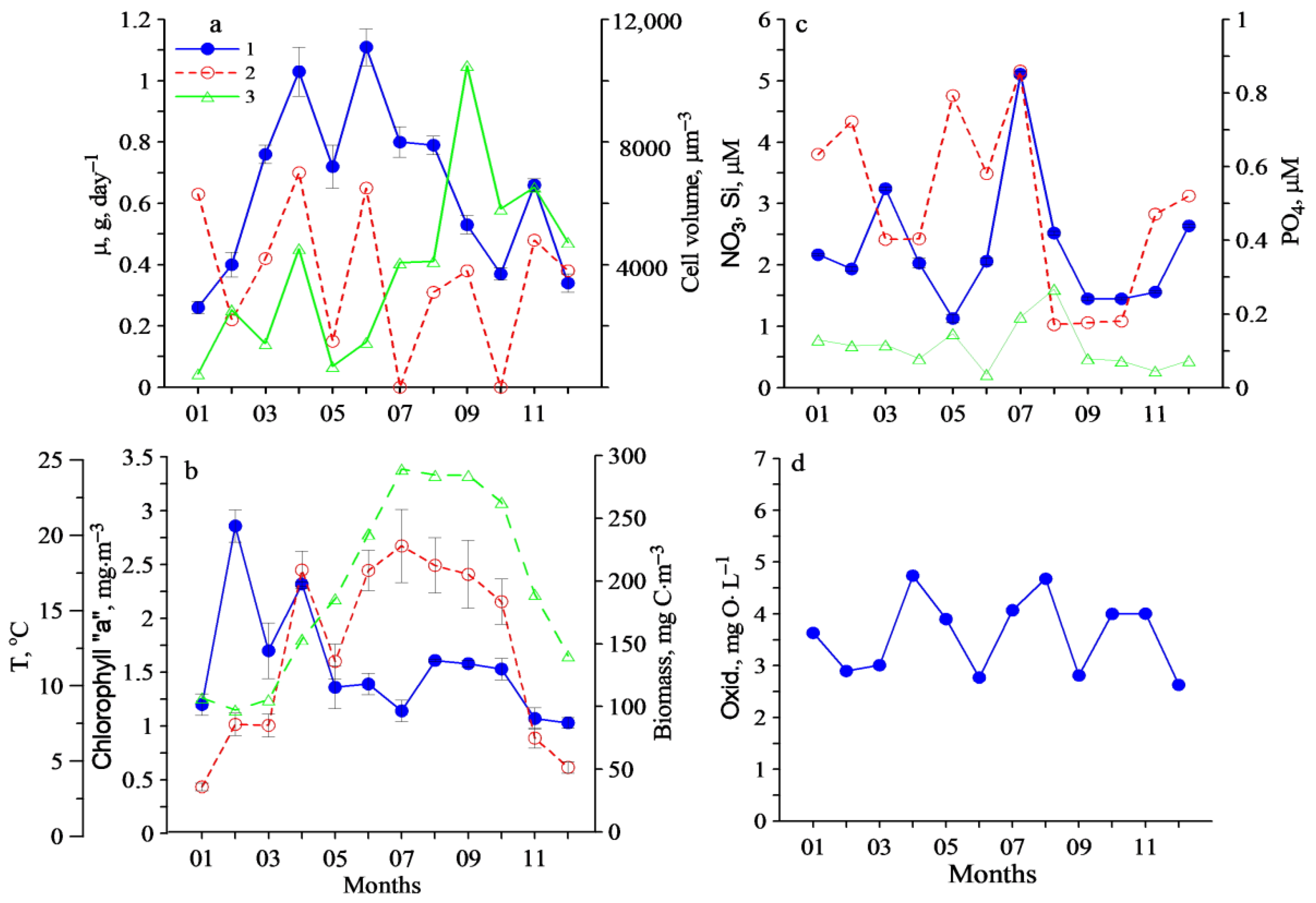
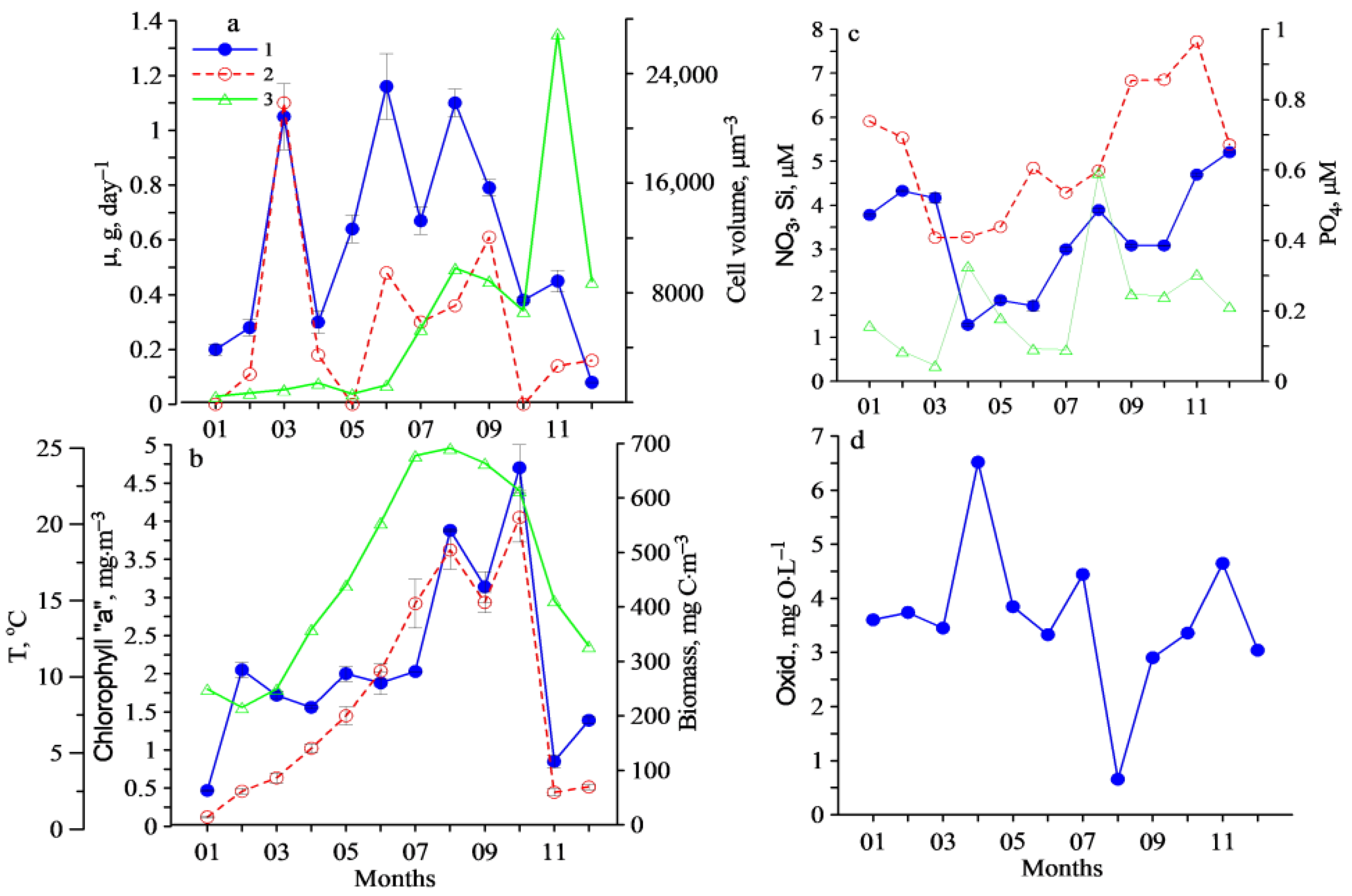
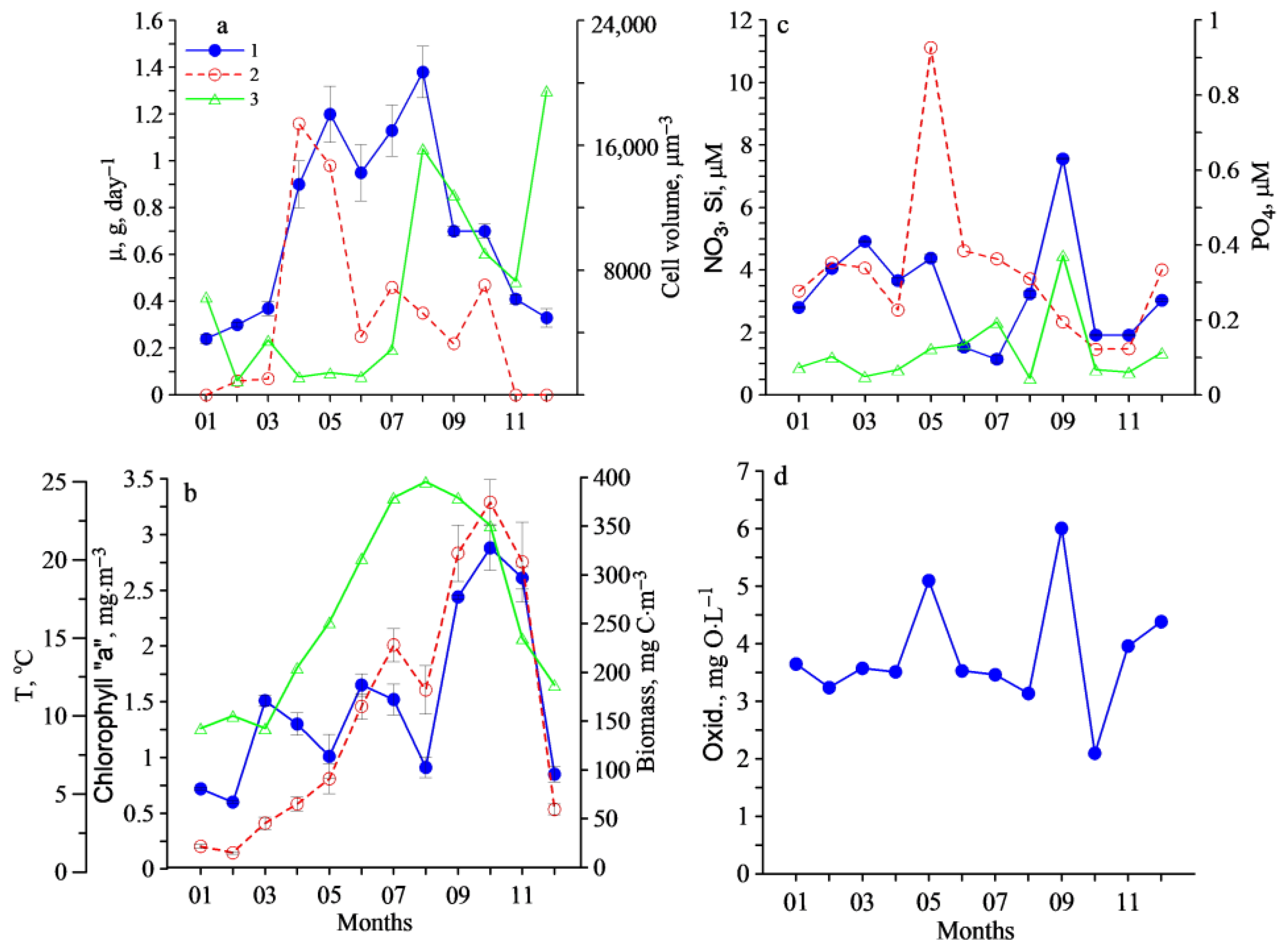
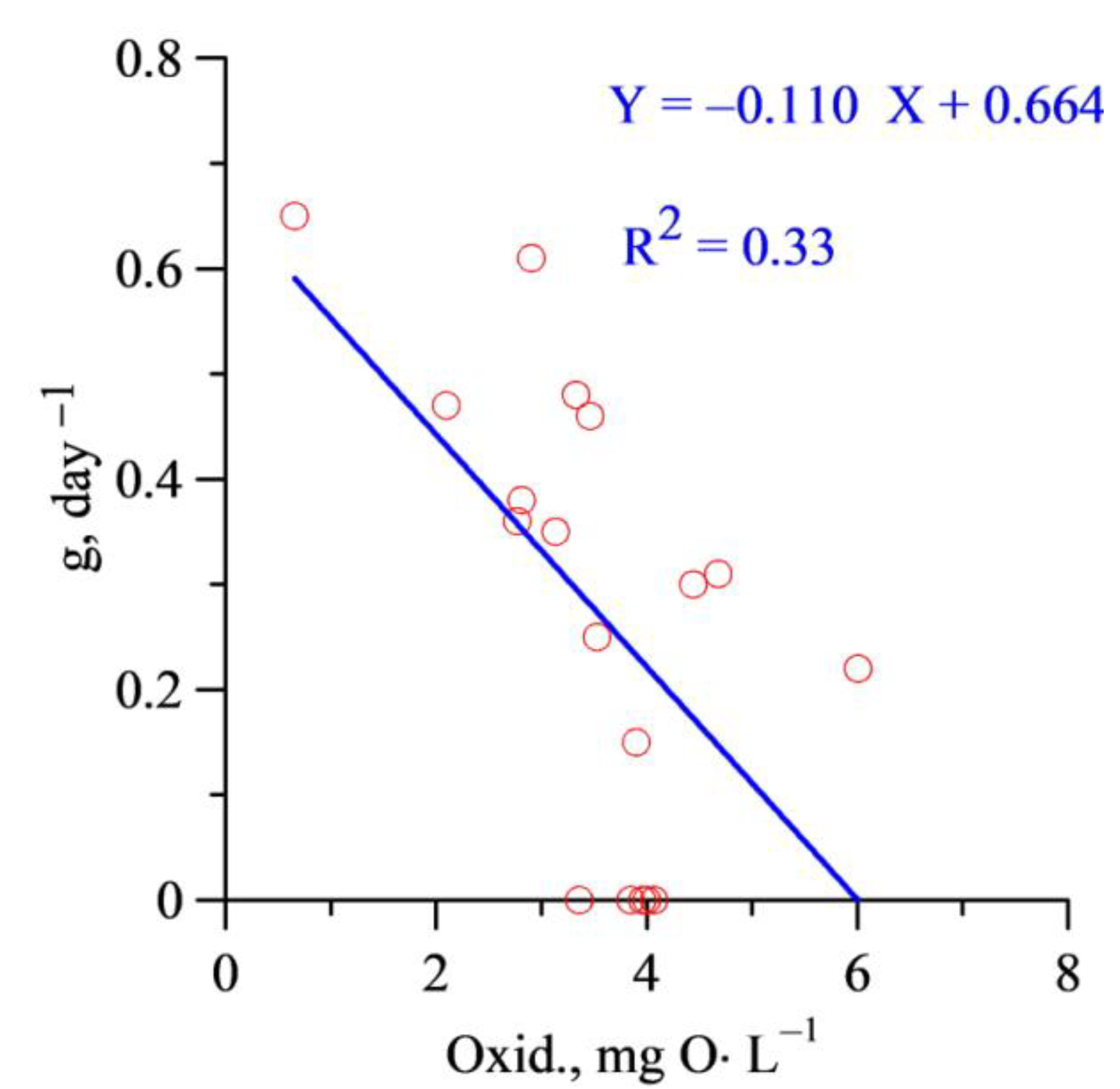
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Häder, D.-P.; Gao, K. Interactions of anthropogenic stress factors on marine phytoplankton. Front. Environ. Sci. 2015, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Oguz, T.; Gilbert, D. Abrupt transitions of the top-down controlled Black Sea pelagic ecosystem during 1960–2000: Evidence for regime-shifts under strong fishery exploitation and nutrient enrichment modulated by climate-induced variations. Deep Sea Res. I 2007, 54, 220–242. [Google Scholar] [CrossRef]
- Mikaelyan, A.S.; Kubryakov, A.A.; Silkin, V.A.; Pautova, L.A.; Chasovnikov, V.K. Regional climate and patterns of phytoplankton annual succession in the open waters of the Black Sea. Deep Sea Res. I 2018, 142, 44–57. [Google Scholar] [CrossRef]
- Repetin, L.N. Spatial and temporal variability of the temperature regime of the Black Sea coastal zone. Environ. Saf. Coast. Shelf Zones Integr. Use Shelf Resour. 2012, 26, 99–116. (In Russian) [Google Scholar]
- Stelmakh, L.; Kovrigina, N.; Gorbunova, T. Phytoplankton adaptation strategies under the influence of climatic changes and anthropogenic pressure on the Black Sea coastal ecosystems on the example Sevastopol Bay. Ecol. Monten. 2020, 37, 34–42. [Google Scholar] [CrossRef]
- Orekhova, N.A.; Varenik, A.V. Current Hydrochemical Regime of the Sevastopol Bay. Phys. Oceanogr. 2018, 25, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Schmoker, C.; Hernandes-Leon, S.; Calbet, A. Microzooplankton grazing in the oceans: Impacts, data variability, knowledge gaps and future directions. J. Plankton Res. 2013, 35, 691–706. [Google Scholar] [CrossRef]
- Stelmakh, L.V.; Georgieva, E.Y. Microzooplankton: The trophic role and involvement in the phytoplankton loss and bloom-formation in the Black Sea. Turk. J. Fish. Aquat. Sci. 2014, 14, 955–964. [Google Scholar] [CrossRef]
- Sorokin, Y.I.; Sukhanova, I.N.; Konovalova, G.V. The primary production and the phytoplankton of the equatorial divergence area in the eastern Pacific Ocean. Proc. Inst. Oceanol. Ac. Sci. USSR 1975, 102, 108–122. (In Russian) [Google Scholar]
- Bryantzeva, Y.; Liah, A.M.; Sergeeva, A.V. Estimation of Biovolume and Surface Area of Black Sea UnicelularAlagae; IBSS: Sevastopol, Ukraine, 2005; 25p. (In Russian) [Google Scholar]
- Tomas, C.R. Identifying Marine Diatoms and Dinoflagellates; Academic Press: New York, NY, USA, 1997; 858p. [Google Scholar]
- Landry, M.R.; Hassett, R.P. Estimating the Grazing Impact of Marine Micro-Zooplankton. Mar. Biol. 1982, 67, 283–288. [Google Scholar] [CrossRef]
- JGOFS Protocols. Protocols for the Joint Global Ocean Flux Study (JGOFS) Core Measurements. Manual and Guides; UNESCO: Paris, France, 1994; Volume 29. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1999; 600p. [Google Scholar] [CrossRef]
- Skopintsev, B.A. Formation of the Modern Chemical Composition of the Black Sea Waters; Gidrometeoizdat: Leningrad, Russia, 1975; 336p. (In Russian) [Google Scholar]
- Vollenweider, R.A.; Giovanardi, F.; Montanari, G.; Rinaldi, A. Characterization of the trophic conditions of marine coastal waters with special reference to the NW Adriatic Sea: Proposal for a trophic scale, turbidity and generalized water quality index. Environmetrics 1998, 9, 329–357. [Google Scholar] [CrossRef]
- Sovga, E.E.; Mezenceva, I.V.; Hmara, T.V.; Slepchuk, K.A. On the prospects and possibilities of assessing the self-cleaning ability of the water area of Sevastopol Bay. Environ. Saf. Coast. Shelf Zones Integr. Use Shelf Resour. 2014, 28, 153–164. (In Russian) [Google Scholar]
- Gubanov, V.I.; Stelmakh, L.V.; Klimenko, N.P. Complex assessments of the water quality of the Sevastopol seaside (Black Sea). Ecol. Sea 2002, 62, 76–80. (In Russian) [Google Scholar]
- Kuftarkova, E.A.; Kovrigina, N.P.; Rodionova, N.Y.; Bobko, N.I. Hydrochemical characteristics of the coastal waters of the Crimean peninsula. In Mariculture of Mussels in the Black Sea; Ivanov, V.A., Ed.; ECOSI-Hydrophysica: Sevastopol, Ukraine, 2007; pp. 74–93. (In Russian) [Google Scholar]
- Gruzinov, V.M.; Dyakov, N.N.; Mezenceva, I.V.; Malchenko, Y.A.; Zhonova, N.V.; Korshenko, A.N. Sources of coastal waterpollution near Sevastopol. Oceanology 2019, 59, 523–532. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Interactions between NH+4 and NO−3 uptake and assimilation: Comparison of diatoms and dinoflagellates at several growth temperatures. Mar. Biol. 1999, 133, 541–551. [Google Scholar] [CrossRef]
- Akimov, A.I.; Solomonova, E.S. Characteristics of growth and fluorescence of certain types of algae during acclimation to different temperatures under culture conditions. Oceanology 2019, 59, 316–326. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F. Heterotrophic dinoflagellates: A significant component of microzooplankton biomass and major grazers of diatoms in the sea. Mar. Ecol. Prog. Ser. 2007, 352, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Strom, S.L.; Brainard, M.A.; Holmes, J.L.; Olson, L.B. Phytoplankton blooms are strongly impacted by microzooplankton grazing in coastal North Pacific waters. Mar. Biol. 2001, 138, 355–368. [Google Scholar] [CrossRef]
| Month | Bacillariophyceae | Dinophyceae | Other | ||
|---|---|---|---|---|---|
| B, % | Dominant Species | B, % | Dominant Species | B, % | |
| January | 8.4 | Skeletonema sp. Dactyliosolen fragilissimus (Bergon) Hasle | 63.8 | Gymnodinium agile Kofoid & Swezy, Prorocentrum micans Ehrenberg, Protoceratium reticulatum(Claparède andLachmann) Bütschli | 27.8 |
| February | 70.0 | Pseudo-nitzschia sp., Chaetoceros curvisetus Cleve | 21.5 | Prorocentrum cordatum (Ostenfeld) J.D.Dodge, P. micans, Prorocentrum scutellum Schröder | 8.5 |
| March | 78.5 | Skeletonema sp., Pseudo-nitzchia sp., C. curvisetus | 20.7 | Gonyaulax spinifera (Claparède andLachmann) Diesing, P. cordatum | 0.8 |
| April | 97.0 | Striatella unipunctata(Lyngbye) C.Agardh, Chaetoceros compressus Lauder, Licmophora abbreviate C.Agardh | 2.6 | Prorocentrum compressum (Bailey) T.H.Abé ex J.D.Dodge | 0.4 |
| May | 45.0 | Proboscia alata(Brightwell) Sundström, Pseudo-nitzchia sp. | 15.8 | Gymnodinium variabile E.C.Herdman, G. spinifera | 39.2 |
| June | 61.1 | Thalassiosira sp., Chaetoceros affinis Lauder, C. curvisetus | 37.6 | Kryptoperidinium foliaceum (F.Stein) Lindemann, G. variabile | 1.3 |
| July | 65.4 | Pseudosolenia calcar-avis(Schultze) B.G.Sundström, P. alata | 31.0 | Scrippsiella trochoidea(F.Stein) A.R. Loeblich III, Gymnodinium sp. | 3.6 |
| August | 52.0 | P. calcar-avis, P. alata | 44.0 | P. cordatum, Ceratium furca (Ehrenberg) Claparède andLachmann | 4.0 |
| September | 87.0 | P. calcar-avis, P. alata | 11.0 | Gymnodinium wulffii J.Schiller Gymnodinium simplex | 2.0 |
| October | 96.5 | P. calcar-avis, P. alata | 3.5 | G. simplex, Gymnodinium sp. | 0.0 |
| November | 46.0 | P. calcar-avis, C. curvisetus | 53.0 | C. furca, Lingulodinium polyedrum (Stein) Dodge | 1.0 |
| December | 24.8 | C. affinis, Nitzschia sigma (Kützing) W.Smith | 74.6 | P. micans, P. cordatum | 0.6 |
| Month | Bacillariophyceae | Dinophyceae | Other | ||
|---|---|---|---|---|---|
| B, % | Dominant Species | B, % | Dominant Species | B, % | |
| January | 70.2 | Skeletonema sp., Licmophora sp. | 29.6 | S. trochoidea, P. micans | 0.2 |
| February | 82.0 | Skeletonema sp., Pseudo-nitzschia sp., C. curvisetus | 0 | - | 18.0 |
| March | 71.9 | Pseudonitzchia sp., Chaetoceros insignis Müller Melchers, Skeletonema sp. | 27.9 | S. trochoidea, Gymnodinium sp. | 0.3 |
| April | 35.9 | P. alata, C insignis, Chaetoceros socialis H.S.Lauder | 63.2 | G. spinifera, G. agile | 0.9 |
| May | 78.9 | Pseudo-nitzchia sp., D. fragilissimus, C. affinis | 15.6 | P. micans, P. cordatum, S. trochoidea | 5.5 |
| June | 41.5 | Thalassiosira sp, C. affinis, C. curvisetus | 56.4 | P. cordatum, G. variabile, G. simplex | 2.1 |
| July | 73.9 | P. calcar-avis, P. alata | 25.2 | P. micans, Glenodinium pilula (Ostenfeld) J.Schiller | 0.9 |
| August | 94.0 | P. calcar-avis, P. alata | 5.7 | P. micans, P. compressum | 0.2 |
| September | 67.1 | P. calcar-avis, P. alata | 32.6 | P. micans, P. compressum | 0.3 |
| October | 93.2 | P. calcar-avis, P. alata | 6.6 | P. micans, P. cordatum | 0.1 |
| November | 19.0 | C. affinis, Entomoneis paludosa (W.Smith) Reimer | 80.9 | Goniaulax poligramma Stein, C. furca, C. fusus | 0.1 |
| December | 51.0 | P. calcar-avis, P. alata | 37.2 | P. micans, P. cordatum | 11.8 |
| Month | Bacillariophyceae | Dinophyceae | Other | ||
|---|---|---|---|---|---|
| B, % | Dominant Species | B, % | Dominant Species | B, % | |
| January | 41.5 | Thalassionema nitzschoides (Grunow) Mereschkowsky, P. calcar-avis, Skeletonema sp., | 45.6 | G. variabile, Gymnodinium sp. | 12.9 |
| February | 62.4 | Skeletonema sp., Pseudo-nitzchia sp., Striatella unipunctata (Lyngbye) C.Agardh | 36.6 | P. micans | 1.0 |
| March | 92.2 | C. insignis, Amphora hyalina Kützing, Licmophora flabellata(Greville) C.Agardh, | 7.7 | P. cordatum | 0.1 |
| April | 99.1 | Licmophora abbreviata C. Agardh, Navicula pennata A.W.F.Schmidt, C. affinis | 0.9 | G. poligramma | 0.1 |
| May | 75.7 | Pseudo-nitzchia sp., D. fragilissimus, C. affinis | 23.7 | G. variabile, G. spinifera, C. furca | 0.6 |
| June | 10.7 | Cyclotella caspia Grunow, C. affinis | 89.3 | G. variabile, Gymnodinium sp., P. cordatum, C. furca | 0.8 |
| July | 97.8 | P. calcar-avis, P. alata | 2.1 | S. trochoidea, Gyrodinium sp. | 0.0 |
| August | 92.0 | P. calcar-avis, P. alata | 7.9 | P. micans, S. trochoidea | 0.1 |
| September | 88.2 | P. calcar-avis, P. alata, Pseudo-nitzchia sp. | 11.8 | P. micans, Gymnodinium sp. | 0.1 |
| October | 98.0 | P. calcar-avis, P. alata | 2.0 | P. micans, P. cordatum | 0.0 |
| November | 47.6 | Melosira moniliformis (O.F.Müller) C.A. Gardh, Cylindroteca closterium (Ehrenberg) Reimann and J.C.Lewin | 52.4 | C. furca, P. micans | 0.0 |
| December | 10.9 | Nitzschia sigma (Kützing) W.Smith, Thalassiosira parva Pr.-Lavr. | 88.9 | C. furca, P. micans | 0.0 |
| Parameters | Warm Period (May–October) | Cold Period (November–April) | ||
|---|---|---|---|---|
| Range | Mean (±SE) | Range | Mean (±SE) | |
| Chla, mg·m−3 | 0.91–4.70 | 2.13 (±0.20) | 0.47–2.86 | 1.37 (±0.20) |
| B, mg C·m−3 | 136–564 | 285 (±29) | 36–209 | 72 (±31) |
| µ, day−1 | 0.37–1.38 | 0.80 (±0.07) | 0.08–1.20 | 0.52 (±0.08) |
| g, day−1 | 0–0.61 | 0.28 (±0.05) | 0–1.16 | 0.48 (±0.07) |
| µ/ g, % | 0–77 | 32 (±6) | 0–200 | 74 (±11) |
| T, °C | 16.0–25.0 | 21.9 (±0.7) | 8.0–15.1 | 11.1 (±0.6) |
| NO3, µM | 1.13–7.59 | 2.65 (0.37) | 1.29–5.21 | 3.33 (0.33) |
| NH4, µM | 0.11–2.69 | 0.78 (0.19) | 0.12–0.83 | 0.36 (0.14) |
| PO4, µM | 0.04–0.59 | 0.17 (0.03) | 0.05–0.21 | 0.12 (0.02) |
| Si, µM | 1.02–6.83 | 3.65 (0.43) | 2.41–7.73 | 4.36 (0.46) |
| Oxidizability, mg O·L−1 | 0.66–6.01 | 3.50(±0.26) | 2.63–6.52 | 3.84 (±0.27) |
| TRIX | 1.13–4.73 | 2.87 (±0.36) | 1.93–3.85 | 3.06 (±0.12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelmakh, L.; Kovrigina, N. Phytoplankton Growth Rate and Microzooplankton Grazing under Conditions of Climatic Changes and Anthropogenic Pollution in the Coastal Waters of the Black Sea (Sevastopol Region). Water 2021, 13, 3230. https://doi.org/10.3390/w13223230
Stelmakh L, Kovrigina N. Phytoplankton Growth Rate and Microzooplankton Grazing under Conditions of Climatic Changes and Anthropogenic Pollution in the Coastal Waters of the Black Sea (Sevastopol Region). Water. 2021; 13(22):3230. https://doi.org/10.3390/w13223230
Chicago/Turabian StyleStelmakh, Lyudmyla, and Nelya Kovrigina. 2021. "Phytoplankton Growth Rate and Microzooplankton Grazing under Conditions of Climatic Changes and Anthropogenic Pollution in the Coastal Waters of the Black Sea (Sevastopol Region)" Water 13, no. 22: 3230. https://doi.org/10.3390/w13223230
APA StyleStelmakh, L., & Kovrigina, N. (2021). Phytoplankton Growth Rate and Microzooplankton Grazing under Conditions of Climatic Changes and Anthropogenic Pollution in the Coastal Waters of the Black Sea (Sevastopol Region). Water, 13(22), 3230. https://doi.org/10.3390/w13223230






