Abstract
Variations in the hydrological regime are among the anthropogenic pressures affecting biological assemblage structure in shallow freshwater lakes. We estimated the effects of the water level fluctuation range on the temporal dissimilarity of the macroinvertebrate community by sampling benthic macroinvertebrate assemblages monthly in 2017 and bimonthly in 2018. Then, we applied a boosted regression trees (BRT) model to quantitatively analyzing the relationship between macroinvertebrate abundance and microhabitat factors in different seasons. To distinguish differences in water level fluctuations at the sample site scale, we proposed a variable, namely, the percentage of water level fluctuation range (PWLFR). The results were as follows. (1) An increased water level fluctuation range would lead to more temporally heterogeneous macroinvertebrate communities. Temporal dissimilarity of macroinvertebrates increased linearly in response to increasing water level fluctuation range. (2) Species abundance presented seasonal characteristics, and the dominant factors affecting species abundance varied with the seasons. PWLFR was the dominant variable explaining macroinvertebrate abundance in summer. Macroinvertebrate abundance showed positive effects with increasing PWLFR. (3) The interaction between chlorophyll a and PWLFR in summer promoted an increase in macroinvertebrate abundance. These findings may provide a basis for the formulation of effective ecological water replenishment management decisions aimed at maintaining the stability of shallow lake ecosystems in arid and semi-arid regions.
1. Introduction
The regulation of water levels for flood control, water consumption, and irrigation is a major form of anthropogenic disturbance in shallow freshwater ecosystems, leading to declines in biodiversity and ecosystem services [1,2]. Implementing ecological water replenishment projects in shallow lakes with degraded ecological structure and function is currently an effective means to restore the health of their ecosystems [3]. However, variations in hydrological conditions will have a direct impact on variations in community structure in freshwater assemblages, such as spatial distributions of rheophilic and lentic taxa, and indirect effects, such as variations in food resource availability and substrate composition [4]. As a foundation of the stability of lake ecosystems, macroinvertebrates play an important role in nutrient circulation and energy flow in the freshwater food web [5]. Responses of the community structure and abundance of macroinvertebrates to variations in the hydrological regime are often used to evaluate the impact of human pressure on ecosystems [6,7,8,9]. Therefore, understanding the response of macroinvertebrate communities to variations in hydrological regimes in shallow lakes has become critical in the restoration and management of degraded shallow lake ecosystems.
Water level fluctuations are the main component of the variations in the hydrological regime [10]. Water level fluctuations can affect the species composition of macroinvertebrate communities at different temporal scales and spatial ranges [11,12]. At present, one of the main research directions on the ecological effects of water level fluctuation changes is to focus on the impacts of water level fluctuations on the spatial distribution and diversity of macroinvertebrate species [13,14,15], but little comprehensive consideration has been given to variations in dissimilarity in macroinvertebrate community structure caused by water level fluctuations across a variety of temporal scales. Many environmental factors (such as temperature, water chemistry, hydrology, and habitat availability) often exhibit seasonal oscillations [16], leading to seasonal pulses of resources [17]. Organisms have evolved corresponding life-history strategies to exploit seasonally generated niches, which will therefore affect macroinvertebrate community structure [16,18]. Considering the significant differences existing in the community structure of macroinvertebrates for different seasons, it is not sufficient to reveal the impacts of water level fluctuations on the community structure of macroinvertebrates based on the analysis at a certain time point or taking the entire study period as a whole. Because community variations usually involve a continuous time process, a temporal dissimilarity variation analysis in community structure is advantageous to understanding the succession of community structure over time compared with β-diversity across spatial contexts. If there are differences in the ability of species to adapt to changes in water level fluctuations, then this would cause temporal variation in community composition. Therefore, explicit studies on the temporal dissimilarity of community composition are needed to examine the effects of water level fluctuations on the temporal macroinvertebrate community process.
Most current research attempts to establish a relationship between water level fluctuations of the entire lake and variations in the overall macroinvertebrate community structure [19,20,21]. However, we have found a lack of studies that analyze this relationship at a microhabitat level. Elucidating the ecological impacts of water level fluctuations on shallow lakes is frequently hindered by heterogeneous distributions of organisms, a consequence of variability in microhabitat characteristics such as water chemistry, morphology, and habitat structure [16,22]. Macroinvertebrate community composition and total abundance show variations in the different microhabitats of Baiyangdian Lake [23]. Given the spatially heterogeneous nature within lakes, water level fluctuations analysis in microhabitats is of significance for detecting ecological impacts of water level regulations in shallow lakes.
Taking Baiyangdian Lake, a shallow lake in North China, as an example, we examined benthic communities characterized by water level fluctuations resulting from hydrological regime variations due to the implementation of a series of ecological water replenishment projects. The aim of this study was to provide an in-depth investigation on how and to what extent the water level fluctuation range affected the community structure of macroinvertebrates in a shallow lake. We tested the following hypotheses: (1) variety of temporal dissimilarity between macroinvertebrate community structure may reflect differences in the water level fluctuations of shallow lakes; (2) the range of water level fluctuations can be used to explain variations in macroinvertebrate total abundance at the microhabitat scale; and (3) taking into account the seasonal oscillations of microhabitat conditions (such as water chemistry, hydrology, and habitat availability), the explanatory power of the range of water level fluctuations in explaining variations in macroinvertebrate species abundance would vary seasonally. The results will contribute to evaluating the spatiotemporal ecological effects of variations in water level and formulating regulation rules for ecological water replenishment.
2. Materials and Methods
2.1. Study Area
A shallow lake, Baiyangdian Lake in northern China (Figure 1), was selected to investigate the responses of macroinvertebrate community dissimilarity and abundance to water level fluctuations. The slope of Baiyangdian Lake is relatively gentle, with a natural slope of 1/200–1/2000. The area of Baiyangdian Lake is approximately 306.73 km2, and the average water depth is 2–3 m. Known as the kidney of North China, Baiyangdian Lake plays an important role in maintaining the ecological balance of the Beijing–Tianjin–Hebei region. Affected by climate change and human activities, the inflow of Baiyangdian Lake has decreased, which has led directly to the degradation of the living environment of organisms and ecological problems such as the decrease in macroinvertebrate biomass and biodiversity [24]. In order to maintain a stable water level, the outlet of Baiyangdian Lake is often closed. In response to the ecological degradation of Baiyangdian Lake due to water resource shortages, ecological water replenishment has been implemented several times in recent years [15].
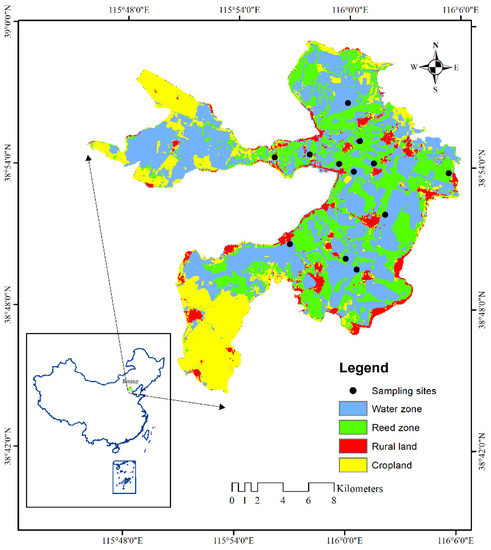
Figure 1.
Study area and map of sampling site locations in Baiyangdian Lake (this figure was produced from a Landsat 8 image at the high-water level in 2015).
2.2. Field Sampling
A total of 12 monitoring sites (Figure 1) in Baiyangdian Lake were selected to capture water depth gradient variations. We conducted 11 sampling events, sampling once a month from May to November 2017 and every other month from April to October 2018. At each sampling site, we collected a suite of environmental variables to characterize the microhabitat condition of each site. Microhabitat variables in the water body were measured in situ prior to macroinvertebrate sampling to avoid disturbing the water body when collecting sediments. Transparency (TR) in water was measured by a Secchi disk submerged below the surface, and the water temperature (WT), dissolved oxygen (DO), pH, total dissolved solids (TDS), and electrical conductivity of the sample sites were measured in situ with the multiparametric sonde YSI-6600 (YSI, Yellow Springs, OH, USA) at the same time. Then, the water depth (WD) was measured using a steel ruler. In addition, 1 L water samples were collected in plastic volumetric bottles from each sample site and brought back to the laboratory for water quality analyses. The analysis of total nitrogen (TN), total phosphorus (TP), chlorophyll a (Chl a) concentrations, and chemical oxygen demand (CODmn) in water was performed according to Chinese standards HJ 636—2012, GB 11893—1989, SL 88—2012, and GB 11892—1989, respectively (http://www.mee.gov.cn/ accessed on 28 November 2016).
Macroinvertebrates were collected by using the improved Peterson mud extractor (opening area of 1/16 m2). Two replicate samples were taken for macroinvertebrate community analysis at each site. At the sampling sites, the bottom mud samples were preliminarily sieved with 0.5 mm mesh, then large organic debris was removed and the retained materials in the screen were preserved in plastic containers with 75% ethanol. The samples were transported to the laboratory for subsequent sorting, counting, and identification. The taxonomy was identified at the lowest operational taxonomic unit (i.e., genus and species level) by the experimenters with experience in identifying species categories (Table S1).
2.3. Water Level Fluctuation Variables
The water level fluctuation range (WLFR) refers to the difference between the maximum and minimum water levels over some time period before sampling. This indicator can reflect the difference in the total volume of water bodies to a certain extent. The impact of water level fluctuation on macroinvertebrate species depends on the temporal scale under consideration [12]. In this study, the difference between the maximum value and the minimum value in one month before sampling was used to calculate the monthly water level fluctuation range. For each sampling point at the same sampling time, the range of the water level fluctuation was the same. To reflect the difference in water level fluctuations on the sample point scale, we proposed a new variable, namely, the percentage of water level fluctuation range (PWLFR), to quantify the difference in the degree of water level fluctuation between samples sites in a sampling. The expression of PWLFR in a sample site is as follows:
where WLFR is the monthly water level fluctuation range, and D is the water depth at the single sample site when sampling.
2.4. Dissimilarity Index
We examined differences in the temporal dissimilarity of the macroinvertebrate community in terms of species abundances among sampling events. The macroinvertebrate abundance datasets of each sampling event were prepared for the identification of the community composition in a single time-point. The temporal dissimilarity of the macroinvertebrate communities in two consecutive sampling events in this study was calculated using the meandist function in vegan package [25]. The meandist function is based on a dissimilarities matrix to find the mean within and between block dissimilarities. There are many indexes for estimating community dissimilarity, such as the Jaccard, Euclidean, and Bray–Curtis dissimilarity indexes. We used the Bray–Curtis distance, which was advantageous in revealing ecological gradients based on species abundance data [26], to calculate the Bray–Curtis dissimilarity matrix. To reduce the impact of rare species on the macroinvertebrate community, species that appeared only once in 11 sampling events were deleted (Table S1). The macroinvertebrate abundance data of all samples were transformed by log10(x + 1) to eliminate the influence of extreme values.
2.5. Data Analysis
We first determined whether the changing pattern of the dissimilarity index of the macroinvertebrate community in two consecutive sampling events was presented along the gradient of water level fluctuation ranges by directly regressing the dissimilarity index on the water level fluctuation range using the lm function. We used standardized residuals and Cook’s distance to identify outliers and influential observations. The studentized residual for the observation in August 2018 was −4.14 (larger than 3 in absolute value) and the Cook’s distance measure was 3.67 (greater than 1). Therefore, based on the studentized residuals and Cook’s distance measure, we classified the observation in August 2018 as an outlier and influential observation. It was omitted in the regression analysis of WLFR and dissimilarity index. Then, we explored the reasons for temporal dissimilarity changes of each sample site, considering the turnover and nestedness components. Means of these metrics for all sites indicate how much dissimilarity is due to replacements or losses of species between two consecutive sampling events. We used the beta.temp function in the betapart package [27] to separate dissimilarity into nestedness (βsne) and turnover (βsim) components.
The Kruskal–Wallis nonparametric test was used to compare the differences in the seasonal scales of the macroinvertebrate species abundance. Taking into account the significant seasonal differences of macroinvertebrate species, the abundance dataset of macroinvertebrates during the study period was split into two groups according to the significance of seasonal differences. The relationship between microhabitat factors and biocommunity indicators is very complex, and direct and simple relationships are not easy to obtain, which places high demands on statistical analysis methods. Some researchers have noted that the interaction between microhabitat factors can significantly affect biological responses [28]. Boosted regression trees (BRT) have the ability to fit complex nonlinear relationships and to identify the interaction between predictors without being affected by outliers [29,30].
We utilized BRT to examine the effect of the percentage of water level fluctuations and other habitat variables on macroinvertebrate community size (total species abundance at each site) in different seasonal groups. We also performed a log10(x + 1) transformation of the total species abundance data. When constructing the BRT model, three parameters (the bagging fraction, learning rate, and tree complexity) need to be optimized, and the details are as follows. (1) The bagging fraction that controls randomness is the proportion of randomly selected observation data used for modeling in the entire dataset; usually, the empirical value is 0.50–0.75 [30]. Due to the relatively small number of samples in this study, the bagging score was chosen to be 0.75. (2) The learning rate (lr) is used to reduce the contribution of each tree to the construction of the total model. The smaller the lr value, the more trees are needed. (3) Tree complexity (tc) is the number of nodes in a single tree. In fact, this value controls the maximum level of interaction between environmental factors that can be fitted in modeling. When the bagging score is determined, the values of lr and tc have a greater impact on the results of the model. In this study, we set the lr values as 0.05, 0.01, 0.005, 0.001, and 0.0005 and the tc values as 3, 4, and 5 and combined these two variables to fit the model. The optimal lr and tc values were determined based on the principle of reducing overfitting and minimizing the CV value. In addition, to ensure the reliability of the model, the constructed model should guarantee at least 1000 trees. The dismo package was used to implement the gbm.step function [31], and all calculations were conducted in R [32].
3. Results
3.1. Relationships between Macroinvertebrate Community Dissimilarity and Monthly Water Level Fluctuation Range
To verify hypothesis (1), stated in the Introduction section, we analyzed the response relationship between the macroinvertebrate community dissimilarity index and WLFR, as shown in Figure 2. The range of monthly water level fluctuation was 0.02–0.38 m. In 2017, the overall variation trend of the water level fluctuation range was relatively gentle, and extreme values of 0.29 m appeared in the spring. In the summer of 2018, the peak value of the water level fluctuation range was 0.38 m, while the water level fluctuation range of other months was smaller, with an average range value of 0.04 m. During the study period, the range of the community dissimilarity index between two consecutive sampling events, which illustrated the difference in the macroinvertebrate assemblages, was 0.43–0.66. Figure 2 shows a significant linear increasing trend for the community dissimilarity indexes of adjacent months in response to the range of water level fluctuations (p = 0.04, deviance explained = 47%). This trend indicated decreased homogenization of community composition with increasing monthly water level fluctuations. Further analysis results showed that as the gradient of the water level fluctuation range increases, species turnover accounts for a relatively high proportion of the community dissimilarity changes. This trend is more obvious in summer. When the water level fluctuation range exceeds 0.13 m, species turnover account is relatively high (beta.sim average value, 0.40 vs. beta.sne average value, 0.12); when the water level fluctuation range is less than 0.13 m, species nestedness accounts for a relatively high proportion (beta.sim average value, 0.07 vs. beta.sne average value, 0.36).
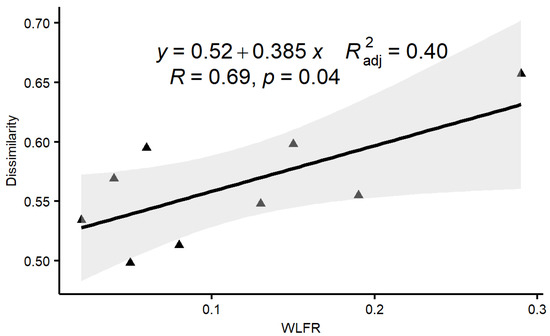
Figure 2.
Correlation between the dissimilarity of macroinvertebrate assemblages in two consecutive sampling events and the monthly water level fluctuation range (WLFR) (the solid line represents the estimated regression line for the dataset with outlier omitted).
3.2. Driving Factors of Variations in Community Abundance
A total of 33 species were collected during the study period (Table S1). Among them, Propsilocerus kamusi and Bithynia fuchsiana were the dominant emerging species in Baiyangdian Lake, and they appeared in each sampling. The average abundance of each sample site was 293 ± 378 ind/m2. The Kruskal–Wallis nonparametric test showed significant seasonal differences in species abundance and richness, among which summer was obviously different from the other two seasons (Figure 3). The species abundance and richness of macroinvertebrates presented a seasonal trend, being high in spring and autumn and low in summer (Figure 3). During the study period, there were no significant differences in species abundance between spring and autumn (Figure 3). Therefore, this study combined spring data and autumn data to explore the relationship between variations in macroinvertebrate species abundance and percentages of water level fluctuation ranges.
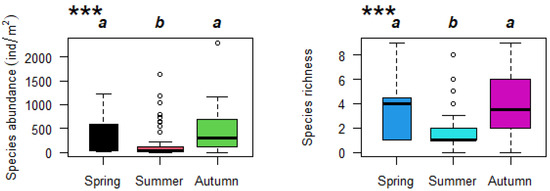
Figure 3.
Seasonal pattern of macroinvertebrate species abundance and richness in Baiyangdian Lake (the same letters indicate nonsignificant differences; *** represents significant differences existing among seasons (p < 0.001)).
Microhabitat factors influencing macroinvertebrate species abundance were different in summer from those in spring and autumn (Figure 4). The BRT model indicated that Chl a (45.2%) and PWLFR (18.1%) had the strongest influence on the species abundance of macroinvertebrates in summer (Figure 4a). Water depth and total nitrogen also had an obvious impact on species abundance in summer. The results of the BRT model indicated that high species abundance was most likely found in areas where the concentration of chlorophyll a was high, the range of water level fluctuations was large, and the water depth was relatively shallow in Baiyangdian Lake (Figure 4a). In summer, the impact of PWLFR on macroinvertebrate species abundance increased with an increase in PWLFR (Figure 4a). When PWLFR reached 20%, the increasing trend of this influence stopped and remained relatively stable. In contrast to the summer species abundance, in spring and autumn, the four dominant factors affecting the species abundance of macroinvertebrates were WT (30.4%), TDS (20.0%), Chl a (15.7%), and DO (10.7%) (Figure 4b). However, PWLFR did not show a powerful effect on species abundance in spring and autumn. The partial dependence results from the spring and autumn fitted model indicated that high species abundance occurred in the area with low WT and Chl a and high TDS and DO in the shallow lake. Species abundance declined with the increase in WT in spring and autumn.
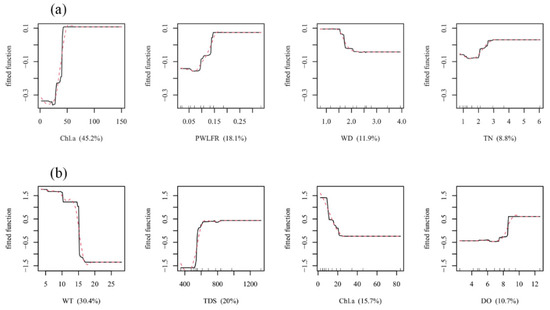
Figure 4.
Partial dependence plots for BRT analyses relating macroinvertebrate species abundance (log10(x + 1) transformation) to the top four most important habitat variables in (a) summer and (b) spring and autumn. The relative influences of each variable in percentage are shown on the x-axis. The y-axes are on the log10(x + 1) scale and are centered on zero as the over the data distribution. Chl a: Chlorophyll a; PWLFR: The percentage of water level fluctuation range; WD: Water depth; TN: Total nitrogen; WT: Water temperature; TDS: Total dissolved solids; DO: Dissolved oxygen.
The results showed interactions between the habitat factors in each season (Figure 5). The strongest interaction effects involved the PWLFR and Chl a in summer, and their interaction can increase macroinvertebrate species abundance. When PWLFR was less than 15%, as PWLFR increased, Chl a had a greater impact on species abundance (Figure 5a). When PWLFR exceeded 15%, the interaction between these two factors was not obvious. Compared with summer, the strongest interaction effects in spring and autumn were WT and TDS (Figure 5b). The species abundance in spring and autumn decreased rapidly with increasing WT. When considering TDS, the higher TDS is, the greater the decrease in species abundance with the increase in WT will be.
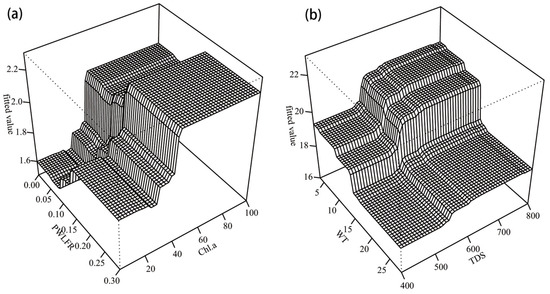
Figure 5.
Three-dimensional partial dependence plots for the strongest interaction in the BRT model for species abundance in (a) summer and (b) spring and autumn. Chl a: Chlorophyll a; PWLFR: The percentage of water level fluctuation range; WT: Water temperature; TDS: Total dissolved solids.
4. Discussion
4.1. Water Level Fluctuation Range Affecting the Temporal Dissimilarity of Macroinvertebrate Community Structure
Linear regression results of temporal dissimilarity and monthly water level fluctuation range matched our hypotheses that differences in the water level fluctuations would lead to temporal dissimilarity between macroinvertebrate community structures in shallow lakes. The results showed that as the extent of water level fluctuations increased, the community dissimilarity index showed an increasing trend. The effects of water level fluctuations on temporal dissimilarity are probably due to several reasons. Firstly, we explored the mechanism of temporal dissimilar change in terms of change in species composition. We found that the temporal dissimilar change reflects the combined effects of the species turnover and nestedness (βsim average value, 0.20 vs. βsne average value, 0.18) in Baiyangdian Lake during the study period. The size of WLFR may contribute to change the form of dissimilar change (dominated by species replacement or species nesting). Comparing the community structure changes in June each year with similar water level and temperature conditions, it was found that the difference in the range of water level fluctuations has changed the contribution of the turnover and nestedness of species to temporal dissimilarity changes. Species turnover (beta.sim, 0.40 vs. beta.sne, 0.12) with a large water level fluctuation range (0.29 m) had a great impact on temporal dissimilarity changes in 2017, while species nestedness (beta.sim, 0.07 vs. beta.sne, 0.36) with a small water level fluctuation range (0.06 m) played a leading role in 2018. This may indicate that the chance of species nestedness occurring is small in a relatively stable environment.
We then explored the mechanism of temporal dissimilarity change in terms of the changing trend of species abundance. Individual taxa responded differently to water level fluctuation changes [19,33,34]. Variations in the response differences of certain taxa abundance made the macroinvertebrate communities respond positively to water level fluctuations [2,20]. In our study, only Branchiura sowerbyi and Bithynia fuchsiana species abundance showed a decreasing trend with the increase of the water level fluctuation range, and other species abundance did not show a regular trend along the water level fluctuation range gradient. This indicates that, compared with other species, tolerant species (Branchiura sowerbyi) [35] and large niche overlapping species (Bithynia fuchsiana) [22,36] can adapt quickly to changes in habitat conditions caused by water level fluctuations. Trottier et al. [2] found that as winter water level drawdown amplitude increases, macroinvertebrate abundance decreases and community composition changes towards a higher-tolerance species. Macroinvertebrate populations may closely relate to seasonally induced signals that control the reproductive time of benthic species and the efficiency of resource assimilation [18]. For example, the dominant taxon Propsilocerus kamusi in Baiyangdian Lake had a significantly higher abundance in spring and autumn than in summer.
4.2. Dominant Microhabitat Factors Affecting Macroinvertebrate Abundance across Different Seasons
The community structure of macroinvertebrates in lake ecosystems is closely related to the habitat conditions in which they live [37,38]. Variations in abiotic conditions and intercorrelations over time further increased the difficulty of studying the community–habitat relationship [4]. Our findings indicated that macroinvertebrate abundance presented significant seasonal variability, and the specific manifestation was that the species abundance was high in spring and autumn and low in summer. This seasonal difference was evident in related research [39]. Seasonal variations in benthic community compositions and abundance were related to the availability of suitable microhabitats and the suitability of water physicochemical characteristics which result from water level fluctuations [4]. The research of Mwaijengo et al. [16] indicated that the macroinvertebrates in the wet season were mainly limited by chlorophyll a, dissolved oxygen, and phosphorus, while nitrogen and turbidity became important in the dry season. The BRT model showed that microhabitat factors in different seasons had different effects on macroinvertebrate abundance. The dominant local environmental factors that affected macroinvertebrate abundance in summer included Chl a, PWLFR, WD, and TN, while WT, TDS, Chl a, and DO dominated in spring and autumn. These results were in accordance with previous studies; that is, the relative importance of environmental factors influencing macroinvertebrates varied with the seasons [16]. The covariations and differences in many factors associated with water level fluctuation were probably other drivers of seasonal variations in macroinvertebrate abundance.
4.3. Ability of PWLFR to Explain Macroinvertebrate Abundance Varies across Different Seasons
Water level fluctuation disturbance can be considered the most important habitat factor explaining the seasonal variation pattern of macroinvertebrate abundance [40,41]. This study found that PWLFR, a microhabitat factor, had an important explanatory effect on benthic species in summer. However, macroinvertebrates in spring and autumn were not sensitive to the variations in PWLFR at the sample site scale. The summer water level of Baiyangdian Lake was generally low, but with the increase in precipitation, the water level was in an unstable period from a downward trend to an upward trend [15]. During this period, water level fluctuations had a dominant influence on macroinvertebrate abundance variations (Figure 4a). Due to the relatively stable hydrological fluctuation trends in spring and autumn, nutrients became the main factors influencing macroinvertebrate abundance. Factors such as the water level fluctuation range and water depth that characterize hydrological conditions only affected the macroinvertebrate abundance in summer, which was similar to previous findings [39]. Mesa [42] also reported that seasonal hydrological regimes play an important role during periods of high water-level instability, while biological interactions may play a dominant role during low-water periods.
4.4. Important Impact of PWLFR on Macroinvertebrate Abundance in Summer
The habitat conditions of shallow lakes are sensitive to short-term variations in hydrology, but the degree of influence is mediated by topographical conditions [11,43]. PWLFR, the variable proposed in this study, presented the difference of water level fluctuation range at the sample site scale. Across all sites, the BRT showed that PWLFR was a significant explanatory variable of macroinvertebrate abundance in summer (18.1%), which increased consistently with increasing PWLFR. This was consistent with previous findings from lakes in littoral areas. Research by Furey et al. [44] found that the macroinvertebrate density and biomass of adjusted lakes undergoing seasonal drawdown were higher than those of natural lakes with small seasonal water level variations. In a study of the relationship between aquatic insects and water level fluctuations in ponds in arid areas, ponds with fluctuating water levels had high species abundance [45]. These studies all emphasized that a certain degree of overall water level fluctuation was beneficial to the increase in the abundance of macroinvertebrate species. Our research further found that the difference in range of water level fluctuations among the sample sites also helped explain variations in macroinvertebrate species abundance. However, this contrasted with research by Trottier [2], who found that as the water level declined in winter, its species abundance decreased significantly. The difference between the two conclusions may be because the range of water level fluctuations in winter (0.3 to 7.2 m) significantly exceeded the range of water level fluctuations in summer in our study area (PWLF < 0.38). The range of water level fluctuations may have a threshold effect on macroinvertebrate abundance [19].
The BRT model captured the microhabitat factor interaction that affected macroinvertebrate abundance in the different seasons. Considering the interaction between habitat factors would improve the explanatory power of the model for variations in the species abundance. When studying the impact of environmental factors on the integrity of freshwater macroinvertebrates, Pilière et al. [28] noted that considering the interaction between environmental factors would increase the simulation ability of the model by 10%. Hydrology is often confounded by other microhabitat factors that influence macroinvertebrate abundance [46]. This study found that the interaction between PWLFR and Chl a in summer had the greatest impact on macroinvertebrate abundance. The external input of Chl a and the spatial distribution of Chl a in shallow lakes were closely related to water level fluctuations [47], and the interaction of the two may cause variations in macroinvertebrate abundance.
4.5. The Implications for Shallow Lake Management
Exploring variations in species abundance and their impacts on ecosystem structure and function under the influence of water level fluctuations is an imperative task in the management of shallow lake ecosystems. The current implementation of ecological water replenishment projects has increased the range of water level fluctuations, which may therefore have unanticipated effects on the whole aquatic ecosystem. This study provides empirical evidence that the response of the macroinvertebrate community in shallow lakes illustrates the potential of water level manipulation as a tool for managing the ecological integrity of shallow lakes. When we want to increase the abundance of macroinvertebrates while minimizing the impact of community turnovers in summer, we can do so by controlling the range of water level fluctuations. For example, when the PWLFR at the sample point scale is between 10% and 15% (Figure 4A), that is, when the WLFR is between 0.20 and 0.29 m, although the macroinvertebrate community is increasing temporal dissimilarly, the macroinvertebrate abundance shows an increasing trend. This will ensure that the stability of the entire macroinvertebrate community will not decrease due to species turnover or nestedness changes. Therefore, we may increase the minimum water level in summer by increasing the amount of ecological water replenishment supply in the early summer or reduce the water supply in autumn to regulate the maximum water level in spring and autumn to achieve the purpose of Baiyangdian water level management. From a management point of view, we suggest that the water level fluctuation range should be considered in ecological water replenishment to maintain ecological integrity in shallow lakes for future lake management.
5. Conclusions
To increase the understanding of the response of macroinvertebrate community structure to water level fluctuations, this study analyzed the impact of water level fluctuation range on the degree of variations in dissimilarity in the overall community structure at two consecutive sampling events, and then used the BRT model to quantitatively analyze the response of macroinvertebrate species abundance to PWLFR and other important microhabitat factors in different seasons at the microhabitat level.
Our study showed that there was a linear relationship between monthly WLFR and dissimilarity. This indicated that an increase in monthly WLFR may, to a certain extent, lead to a more heterogeneous macroinvertebrate community. Our data showed that the relative importance of microhabitat factors that affected macroinvertebrate abundance may differ among seasons. While in spring and autumn we did not find that PWLFR was the dominant variable, we reported that in summer, PWLFR could explain 18.1% of macroinvertebrate abundance changes. We also showed that when PWLFR was lower than 0.15, there was a clear positive interaction between Chl a and PWLFR that increased macroinvertebrate abundance at the sample site scale in summer. Shallow lake ecosystems management should consider the effects of water level fluctuations on macroinvertebrate community structure especially when the implementation of ecological water replenishment projects had a great impact on the hydrological regime.
This study focused on exploring the impacts of differences in water level fluctuations on macroinvertebrate abundance and did not involve the direct response mechanism of a single macroinvertebrate species. Future research will explore the driving mechanism of the influence of hydrological regime variations on the abundance or growth of a single population to further expand the current findings.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/w13233380/s1, Table S1: Macroinvertebrates species identified in Baiyangdian Lake during survey in this study.
Author Contributions
Data curation, S.Y., D.L., Y.Z.; Methodology, S.Y., X.W.; Project administration, Z.Y.; Supervision, T.S.; Validation, X.W.; Writing—original draft, S.Y.; Writing—review & editing, X.W. and T.S.; Funding acquisition, Z.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (Grant No. 52070024, 51679008, 51439001), and Beijing Advanced Innovation Program for Land Surface Science.
Data Availability Statement
The data presented in this study are available in Supplementary Material.
Acknowledgments
The author would like to thank all participants who took time to complete the survey. Their support was fundamental for this study. The author would also like to thank the editor and the anonymous referees for their critical feedback and valuable suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vorosmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Trottier, G.; Embke, H.; Turgeon, K.; Solomon, C.; Nozais, C.; Gregory-Eaves, I. Macroinvertebrate abundance is lower in temperate reservoirs with higher winter drawdown. Hydrobiologia 2019, 834, 199–211. [Google Scholar] [CrossRef]
- Chen, L.; Liu, S.; Wu, Y.; Xu, Y.J.; Chen, S.; Pang, S.; Gao, Z.; Zhang, G. Does Ecological Water Replenishment Help Prevent a Large Wetland from Further Deterioration? Results from the Zhalong Nature Reserve, China. Remote Sens. 2020, 12, 3449. [Google Scholar] [CrossRef]
- Alvarez-Cabria, M.; Barquin, J.; Juanes, J.A. Microdistribution patterns of macroinvertebrate communities upstream and downstream of organic effluents. Water Res. 2011, 45, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Hu, X.; He, Q.; Wu, Z.; Cheng, H.; Hu, Z.; Mazumder, A. Impacts of rapid urbanization on the water quality and macroinvertebrate communities of streams: A case study in Liangjiang New Area, China. Sci. Total Environ. 2018, 621, 1601–1614. [Google Scholar] [CrossRef]
- Serrano Balderas, E.C.; Grac, C.; Berti-Equille, L.; Armienta Hernandez, M.A. Potential application of macroinvertebrates indices in bioassessment of Mexican streams. Ecol. Indic. 2016, 61, 558–567. [Google Scholar] [CrossRef]
- Giorgio, A.; De Bonis, S.; Guida, M. Macroinvertebrate and diatom communities as indicators for the biological assessment of river Picentino (Campania, Italy). Ecol. Indic. 2016, 64, 85–91. [Google Scholar] [CrossRef]
- Sharifinia, M.; Mahmoudifard, A.; Imanpour Namin, J.; Ramezanpour, Z.; Yap, C.K. Pollution evaluation in the Shahrood River: Do physico-chemical and macroinvertebrate-based indices indicate same responses to anthropogenic activities? Chemosphere 2016, 159, 584–594. [Google Scholar] [CrossRef]
- Poikane, S.; Johnson, R.K.; Sandin, L.; Schartau, A.K.; Solimini, A.G.; Urbanic, G.; Arbaciauskas, K.; Aroviita, J.; Gabriels, W.; Miler, O.; et al. Benthic macroinvertebrates in lake ecological assessment: A review of methods, intercalibration and practical recommendations. Sci Total Environ. 2016, 543 Pt A, 123–134. [Google Scholar] [CrossRef]
- Batzer, D.P. The Seemingly Intractable Ecological Responses of Invertebrates in North American Wetlands: A Review. Wetlands 2012, 33, 1–15. [Google Scholar] [CrossRef]
- Leira, M.; Cantonati, M. Effects of water-level fluctuations on lakes: An annotated bibliography. Hydrobiologia 2008, 613, 171–184. [Google Scholar] [CrossRef]
- Hofmann, H.; Lorke, A.; Peeters, F. Temporal scales of water-level fluctuations in lakes and their ecological implications. Hydrobiologia 2008, 613, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Langer, T.A.; Cooper, M.J.; Reisinger, L.S.; Reisinger, A.J.; Uzarski, D.G. Water depth and lake-wide water level fluctuation influence on α- and β-diversity of coastal wetland fish communities. J. Great Lakes Res. 2018, 44, 70–76. [Google Scholar] [CrossRef]
- Pleskot, K.; Tóth, M.; Apolinarska, K. Distribution of subfossil chironomids (Diptera, Chironomidae) along a water depth gradient in the shallow Lake Spore, northern Poland. J. Limnol. 2019, 78, 3. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.J.; Wang, X.; Zhang, Y.L.; Liu, D.; Yi, Y.J.; Li, C.H.; Liu, Q.; Yang, Z.F. A hybrid PCA-GAM model for investigating the spatiotemporal impacts of water level fluctuations on the diversity of benthic macroinvertebrates in Baiyangdian Lake, North China. Ecol. Indic. 2020, 116, 11. [Google Scholar] [CrossRef]
- Mwaijengo, G.N.; Vanschoenwinkel, B.; Dube, T.; Njau, K.N.; Brendonck, L. Seasonal variation in benthic macroinvertebrate assemblages and water quality in an Afrotropical river catchment, northeastern Tanzania. Limnologica 2020, 82, 125780. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Bogan, M.T.; Bonada, N.; Rios-Touma, B.; Lytle, D.A. Seasonality and predictability shape temporal species diversity. Ecology 2017, 98, 1201–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, S.C.; Housley, L.; Back, J.A.; King, R.S. Freshwater eutrophication drives sharp reductions in temporal beta diversity. Ecology 2018, 99, 47–56. [Google Scholar] [CrossRef]
- Aroviita, J.; Hämäläinen, H. The impact of water-level regulation on littoral macroinvertebrate assemblages in boreal lakes. Hydrobiologia 2008, 613, 45–56. [Google Scholar] [CrossRef]
- Pander, J.; Knott, J.; Mueller, M.; Geist, J. Effects of environmental flows in a restored floodplain system on the community composition of fish, macroinvertebrates and macrophytes. Ecol. Eng. 2019, 132, 75–86. [Google Scholar] [CrossRef]
- Mathers, K.L.; White, J.C.; Fornaroli, R.; Chadd, R.; Vamosi, S. Flow regimes control the establishment of invasive crayfish and alter their effects on lotic macroinvertebrate communities. J. Appl. Ecol. 2020, 57, 886–902. [Google Scholar] [CrossRef]
- Evtimova, V.V.; Donohue, I. Water-level fluctuations regulate the structure and functioning of natural lakes. Freshw. Biol. 2016, 61, 251–264. [Google Scholar] [CrossRef]
- Yang, Y.F.; Yi, Y.J.; Zhou, Y.; Wang, X.; Zhang, S.H.; Yang, Z.F. Spatio-temporal variations of benthic macroinvertebrates and the driving environmental variables in a shallow lake. Ecol. Indic. 2020, 110, 11. [Google Scholar] [CrossRef]
- Yi, Y.; Lin, C.; Wang, W.; Song, J. Habitat and seasonal variations in bacterial community structure and diversity in sediments of a Shallow lake. Ecol. Indic. 2021, 120, 106959. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.B.; Simpson, G.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version. 2.0-10. CRAN 2013. Available online: https://CRAN.R-project.org/package=vegan (accessed on 28 November 2021).
- Baselga, A.; Freckleton, R. Separating the two components of abundance-based dissimilarity: Balanced changes in abundance vs. abundance gradients. Methods Ecol. Evol. 2013, 4, 552–557. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C. Betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Pilière, A.; Schipper, A.M.; Breure, T.M.; Posthuma, L.; de Zwart, D.; Dyer, S.D.; Huijbregts, M.A. Unraveling the relationships between freshwater invertebrate assemblages and interacting environmental factors. Freshw. Sci. 2014, 33, 1148–1158. [Google Scholar] [CrossRef]
- Theodoropoulos, C.; Vourka, A.; Stamou, A.; Rutschmann, P.; Skoulikidis, N. Response of freshwater macroinvertebrates to rainfall-induced high flows: A hydroecological approach. Ecol. Indic. 2017, 73, 432–442. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Hijmans, R.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. 2017. Available online: https://CRAN.R-project.org/package=dismo (accessed on 28 November 2021).
- R Core Team R. A Language and Environment for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 28 November 2021).
- Gathman, J.P.; Burton, T.M. A Great Lakes Coastal Wetland Invertebrate Community Gradient: Relative Influence of Flooding Regime and Vegetation Zonation. Wetlands 2011, 31, 329–341. [Google Scholar] [CrossRef]
- White, M.S.; Xenopoulos, M.A.; Metcalfe, R.A.; Somers, K.M.; Rosenfeld, J. Water level thresholds of benthic macroinvertebrate richness, structure, and function of boreal lake stony littoral habitats. Can. J. Fish. Aquat. Sci. 2011, 68, 1695–1704. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y. Effect of flow-sediment regime on benthic invertebrate communities: Long-term analysis in a regulated floodplain lake. Sci. Total Environ. 2019, 649, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pan, X.; Sun, C.; Shang, S.; Zhang, C.; Zhao, C.S.; Dong, B.; Zhang, Z. Analysing the ecological niche of water quality of key species in the aquatic ecosystem in Jinan City. Mar. Freshw. Res. 2019, 70, 656–669. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, H.; Vilmi, A.; Tolonen, K.T.; Tang, X.; Qin, B.; Gong, Z.; Heino, J. Relative roles of spatial processes, natural factors and anthropogenic stressors in structuring a lake macroinvertebrate metacommunity. Sci. Total Environ. 2017, 601–602, 1702–1711. [Google Scholar] [CrossRef]
- Donohue, I.; Jackson, A.L.; Pusch, M.T.; Irvine, K. Nutrient enrichment homogenizes lake benthic assemblages at local and regional scales. Ecology 2009, 90, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Scheibler, E.E.; Claps, M.C.; Roig-Juñent, S.A. Temporal and altitudinal variations in benthic macroinvertebrate assemblages in an Andean river basin of Argentina. J. Limnol. 2014, 73, 76–91. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Cabria, M.; Barquín, J.; Juanes, J.A. Macroinvertebrate community dynamics in a temperate European Atlantic river. Do they conform to general ecological theory? Hydrobiologia 2010, 658, 277–291. [Google Scholar] [CrossRef]
- Tupinambás, T.H.; Cortes, R.M.V.; Hughes, S.J.; Varandas, S.G.; Callisto, M. Macroinvertebrate responses to distinct hydrological patterns in a tropical regulated river. Ecohydrology 2016, 9, 460–471. [Google Scholar] [CrossRef]
- Mariana Mesa, L. Interannual and Seasonal Variability of Macroinvertebrates in Monsoonal Climate Streams. Braz. Arch. Biol. Technol. 2012, 55, 403–410. [Google Scholar] [CrossRef]
- Evtimova, V.V.; Donohue, I.; Frid, C. Quantifying ecological responses to amplified water level fluctuations in standing waters: An experimental approach. J. Appl. Ecol. 2014, 51, 1282–1291. [Google Scholar] [CrossRef]
- Furey, P.C.; Nordin, R.N.; Mazumder, A. Littoral benthic macroinvertebrates under contrasting drawdown in a reservoir and a natural lake. J. North. Am. Benthol. Soc. 2006, 25, 19–31. [Google Scholar] [CrossRef]
- Jooste, M.L.; Samways, M.J.; Deacon, C. Fluctuating pond water levels and aquatic insect persistence in a drought-prone Mediterranean-type climate. Hydrobiologia 2020, 847, 1315–1326. [Google Scholar] [CrossRef]
- Whiles, M.R.; Goldowitz, B.S. Macroinvertebrate communities in Central Platte River wetlands: Patterns across a hydrologic gradient. Wetlands 2005, 25, 462–472. [Google Scholar] [CrossRef]
- Queimalinos, C.; Reissig, M.; Dieguez Mdel, C.; Arcagni, M.; Ribeiro Guevara, S.; Campbell, L.; Cardenas, C.S.; Rapacioli, R.; Arribere, M. Influence of precipitation, landscape and hydrogeomorphic lake features on pelagic allochthonous indicators in two connected ultraoligotrophic lakes of North Patagonia. Sci Total Environ. 2012, 427–428, 219–228. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).