Defining Recovery Potential in River Restoration: A Biological Data-Driven Approach
Abstract
:1. Introduction
2. Materials and Methods
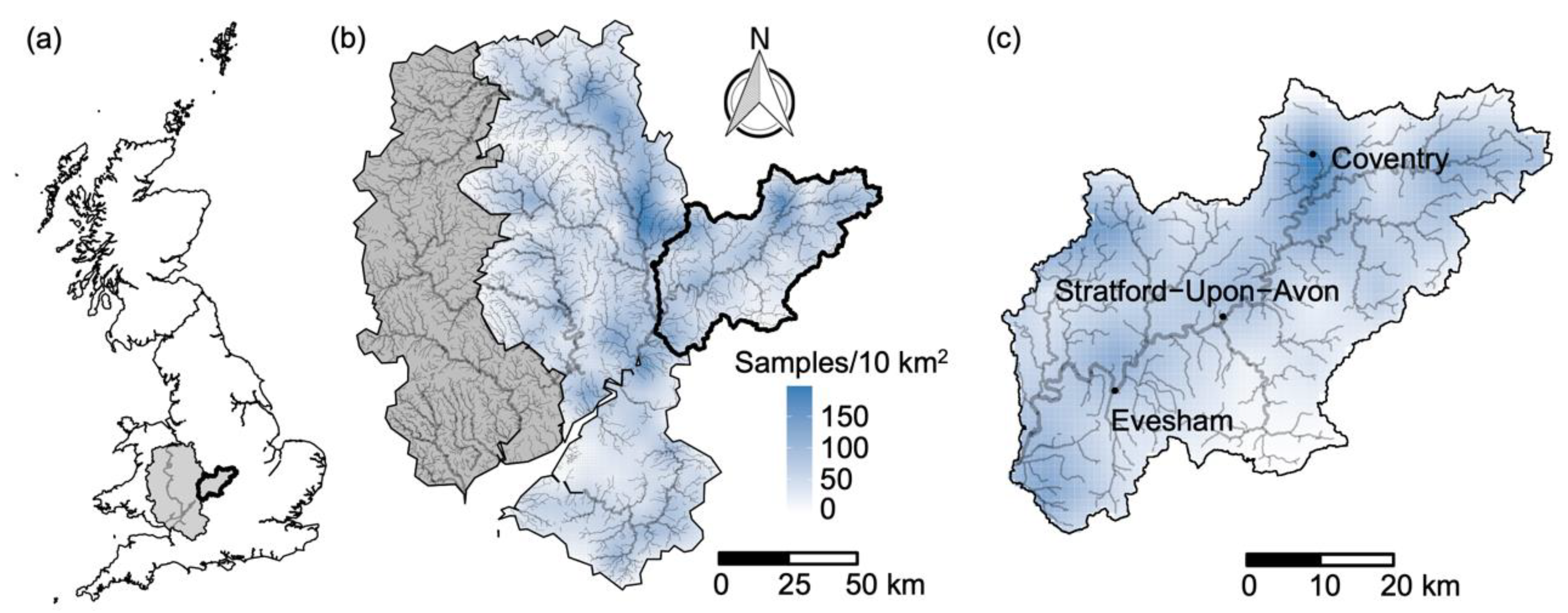
2.1. Study Area
2.2. Biological Data
2.3. Environmental Datasets
| Name | Short Name | Units | Source |
|---|---|---|---|
| River network | (none) | NA | OS Open Rivers [12]. Contains OS data © Crown copyright and database right 2021 |
| Channel resectioning index | CRI | Number of modifications | Modelled from UK River Habitat Survey database [13,15]. © Marc Naura 2016 |
| Log altitude | logalt | log(m) | River Invertebrate Classification Tool (RICT) [16]. © Centre for Ecology and Hydrology |
| Chalk cover | gcha | Proportion of upstream catchment | |
| Clay cover | gcla | ||
| Hard rock cover | groc | ||
| Limestone cover | glim | ||
| Discharge | qcat | Category (1–9) | |
| Longitudinal slope | slop | m km−1 | |
| P concentration (arable sources) | p_ara | mg L−1 | Source Apportionment-GIS (SAGIS) [17]. |
| P concentration (livestock sources) | p_liv | mg L−1 | |
| P concentration (sewage treatment sources) | p_stw | mg L−1 | |
| P concentration (urban sources) | p_urb | mg L−1 | |
| Water resource availability at Q30 | cams30 | Traffic light classification | Catchment Abstraction Management Strategy (CAMS) [18]. © Environment Agency copyright and/or database right 2015 |
| Water resource availability at Q95 | cams95 | ||
| Land cover (broadleaved woodland) | bwd | % cover | UK Centre for Ecology and Hydrology Land Cover Maps [19]: 2000 [20]; 2007 [21]; 2015 [22]. |
| Land cover (coniferous woodland) | cwd | ||
| Land cover (arable) | ara | ||
| Land cover (improved grassland) | igr | ||
| Land cover (semi-natural grassland) | sgr | ||
| Land cover (mountain, heath, bog) | hth | ||
| Land cover (urban) | urb |
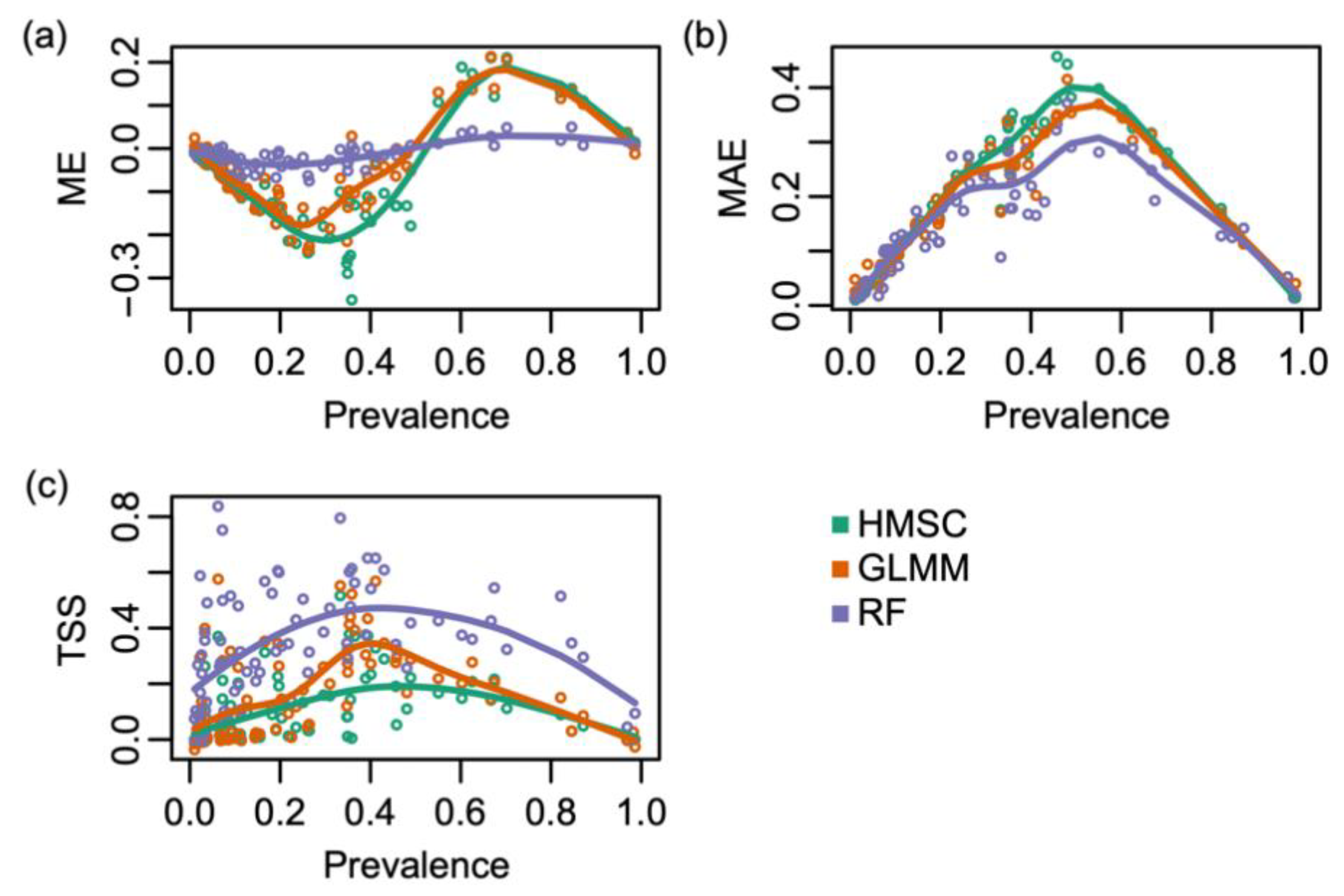
2.4. Species Distribution Modelling
2.5. Restoration Scenarios
3. Results
4. Discussion
4.1. Implications for River Restoration Planning
4.2. Limitations and Further Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brookes, A. Recovery and restoration of some engineered British river channels. In River Conservation and Management; Boon, P.J., Calow, P., Petts, G.E., Eds.; Wiley: Chichester, UK, 1992; pp. 337–352. [Google Scholar]
- Rosgen, D.L. Applied River Morphology; Wildland Hydrology: Pagosa Springs, CO, USA, 1996. [Google Scholar]
- Hering, D.; Aroviita, J.; Baattrup-Pedersen, A.; Brabec, K.; Buijse, T.; Ecke, F.; Friberg, N.; Gielczewski, M.; Januschke, K.; Köhler, J.; et al. Contrasting the roles of section length and instream habitat enhancement for river restoration success: A field study of 20 European restoration projects. J. Appl. Ecol. 2015, 52, 1518–1527. [Google Scholar] [CrossRef]
- Verdonschot, R.C.; Kail, J.; McKie, B.G.; Verdonschot, P.F. The role of benthic microhabitats in determining the effects of hydromorphological river restoration on macroinvertebrates. Hydrobiologia 2016, 769, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.F.; Moss, D.; Armitage, P.D.; Furse, M.T. A preliminary classification of running-water sites in Great Britain based on macroinvertebrate species and the prediction of community type using environmental data. Freshw. Biol. 1984, 14, 221–256. [Google Scholar] [CrossRef]
- Davies, P.E. Development of a national river bioassessment system (AUSRIVAS). In Assessing the Biological Quality of Freshwaters: RIVPACS and Other Techniques; Wright, J.F., Sutcliffe, D.W., Furse, M.T., Eds.; Freshwater Biological Association: Ambleside, UK, 2000; pp. 113–124. [Google Scholar]
- Metcalfe, R.A.; Mackereth, R.W.; Grantham, B.; Jones, N.; Pyrce, R.S.; Haxton, T.; Luce, J.J.; Stainton, R. Aquatic Ecosystem Assessments for Rivers; Ministry of Natural Resources: Peterborough, ON, Canada, 2013.
- Clarke, R.T.; Wright, J.F.; Furse, M.T. RIVPACS models for predicting the expected macroinvertebrate fauna and assessing the ecological quality of rivers. Ecol. Model. 2003, 160, 219–233. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Poff, N.L.; Bond, N.R.; Horne, A.; Merritt, D.M.; Reynolds, L.V.; Olden, J.D.; Ruhi, A.; Lytle, D.A. Prepare river ecosystems for an uncertain future. Nature 2019, 570, 301–303. [Google Scholar] [CrossRef]
- Environment Agency Ecology & Fish Data Explorer. Available online: https://environment.data.gov.uk/ecology/explorer/ (accessed on 19 October 2021).
- Water Framework Directive-United Kingdom Advisory Group. River Assessment Methods Benthic Invertebrate Fauna, River Invertebrate Classification Tool (RICT); SNIFFER: Edinburgh, UK, 2008. [Google Scholar]
- OS Open Rivers. Available online: https://www.ordnancesurvey.co.uk/business-government/products/open-map-rivers (accessed on 19 October 2021).
- Naura, M.; Clark, M.J.; Sear, D.A.; Atkinson, P.M.; Hornby, D.D.; Kemp, P.; England, J.; Peirson, G.; Bromley, C.; Carter, M.G. Mapping habitat indices across river networks using spatial statistical modelling of River Habitat Survey data. Ecol. Indic. 2016, 66, 20–29. [Google Scholar] [CrossRef] [Green Version]
- CEH Digital River Network of Great Britain (1:50,000). Available online: https://catalogue.ceh.ac.uk/id/7d5e42b6-7729-46c8-99e9-f9e4efddde1d (accessed on 19 October 2021).
- River Habitat Survey—Survey Details and Summary Results. Available online: https://data.gov.uk/dataset/4cb467c9-346e-44ac-85c6-6cd579111e2c/river-habitat-survey-survey-details-and-summary-results (accessed on 19 October 2021).
- River Invertebrate Classification Tool. Available online: https://www.sepa.org.uk/environment/water/aquatic-classification/river-invertebrate-classification-tool/ (accessed on 19 October 2021).
- UKWIR. Chemical Source Apportionment under the WFD (12/WW/02/3); UK Water Industry Research: London, UK, 2012. [Google Scholar]
- Abstraction Licensing Strategies (CAMS Process). Available online: https://www.gov.uk/government/collections/water-abstraction-licensing-strategies-cams-process (accessed on 19 October 2021).
- UKCEH Land Cover Maps. Available online: https://www.ceh.ac.uk/ukceh-land-cover-maps (accessed on 19 October 2021).
- Fuller, R.M.; Smith, G.M.; Sanderson, J.M.; Hill, R.A.; Thomson, A.G.; Cox, R.; Brown, N.J.; Clarke, R.T.; Rothery, P.; Gerard, F.F. Land Cover Map 2000 (1 km Dominant Aggregate Class, GB); NERC: Atlanta, GA, USA, 2002. [Google Scholar]
- Morton, R.D.; Rowland, C.S.; Wood, C.M.; Meek, L.; Marston, C.G.; Smith, G.M. Land Cover Map 2007 (1 km Dominant Aggregate Class, GB) v1.2; NERC: Atlanta, GA, USA, 2014. [Google Scholar]
- Rowland, C.S.; Morton, R.D.; Carrasco, L.; McShane, G.; O’Neil, A.W.; Wood, C.M. Land Cover Map 2015 (1 km Dominant Aggregate Class, GB); NERC: Atlanta, GA, USA, 2017. [Google Scholar]
- Norberg, A.; Abrego, N.; Blanchet, F.G.; Adler, F.R.; Anderson, B.J.; Anttila, J.; Araújo, M.B.; Dallas, T.; Dunson, D.; Elith, J.; et al. A comprehensive evaluation of predictive performance of 33 species distribution models at species and community levels. Ecol. Monogr. 2019, 89, e01370. [Google Scholar] [CrossRef]
- Hui, F.K.C.; Warton, D.I.; Foster, S.D.; Dunstan, P.K. To mix or not to mix: Comparing the predictive performance of mixture models vs. separate species distribution models. Ecology 2013, 94, 1913–1919. [Google Scholar] [CrossRef]
- Ovaskainen, O.; Tikhonov, G.; Norberg, A.; Guillaume Blanchet, F.; Duan, L.; Dunson, D.; Roslin, T.; Abrego, N. How to make more out of community data? A conceptual framework and its implementation as models and software. Ecol. Lett. 2017, 20, 561–576. [Google Scholar] [CrossRef]
- Daskalova, G.N.; Phillimore, A.B.; Myers-Smith, I.H. Accounting for year effects and sampling error in temporal analyses of invertebrate population and biodiversity change: A comment on Seibold et al. 2019. Insect Conserv. Divers. 2021, 14, 149–154. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Hmsc: Hierarchical Model of Species Communities. R Package Version 3.0-11. Available online: https://CRAN.R-project.org/package=Hmsc (accessed on 19 October 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wright, M.N.; Ziegler, A. ranger: A fast implementation of Random Forests for high dimensional data in C++ and R. Stat. Softw. 2017, 77, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés D’eau Douce: Systématique, Biologie, Écologie, 2nd ed.; Centre National de la Recherche Scientifique Press: Paris, France, 2010. [Google Scholar]
- Wilkes, M.A.; Edwards, F.; Jones, J.I.; Murphy, J.F.; England, J.; Friberg, N.; Hering, D.; Poff, N.L.; Usseglio-Polatera, P.; Verberk, W.C.; et al. Trait-based ecology at large scales: Assessing functional trait correlations, phylogenetic constraints and spatial variability using open data. Glob. Chang. Biol. 2020, 26, 7255–7267. [Google Scholar] [CrossRef]
- Van Looy, K.; Tonkin, J.D.; Floury, M.; Leigh, C.; Soininen, J.; Larsen, S.; Heino, J.; LeRoy Poff, N.; Delong, M.; Jähnig, S.C.; et al. The three Rs of river ecosystem resilience: Resources, recruitment, and refugia. River Res. Appl. 2019, 35, 107–120. [Google Scholar] [CrossRef] [Green Version]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. Available online: https://www.R-project.org/ (accessed on 19 October 2021).
- Wilkes, M.A.; Bennett, J.; Burbi, S.; Charlesworth, S.; Dehnen-Schmutz, K.; Rayns, F.; Schmutz, U.; Smith, B.; Tilzey, M.; Trenchard, L.; et al. Making way for trees? Changes in land-use, habitats and protected areas in Great Britain under “global tree restoration potential”. Sustainability 2020, 12, 5845. [Google Scholar] [CrossRef]
- England, J.; Wilkes, M.A. Does river restoration work? Taxonomic and functional trajectories at two restoration schemes. Sci. Total Environ. 2018, 618, 961–970. [Google Scholar] [CrossRef]
- Swan, C.M.; Brown, B.L. Metacommunity theory meets restoration: Isolation may mediate how ecological communities respond to stream restoration. Ecol. Appl. 2017, 27, 2209–2219. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]

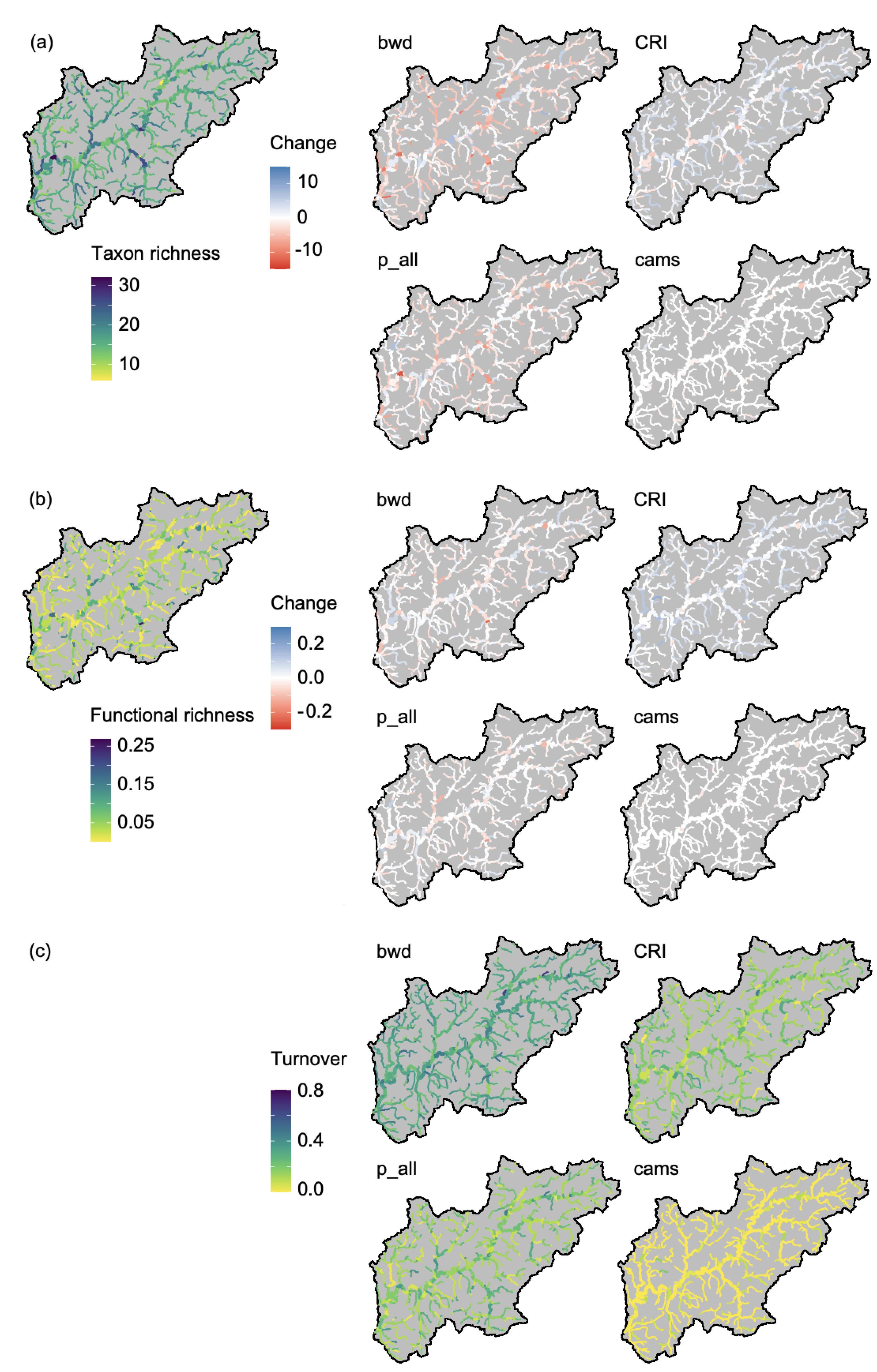
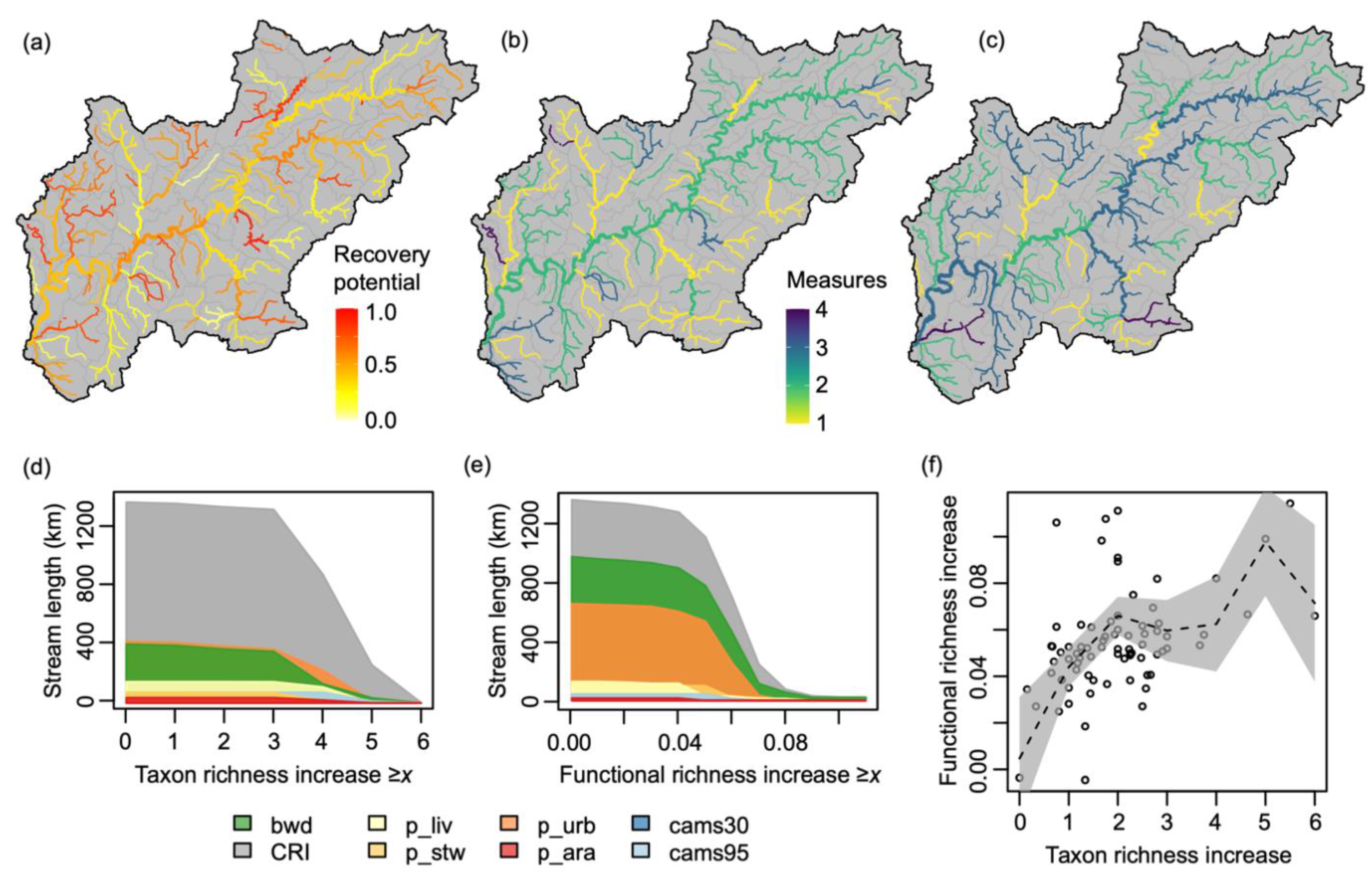
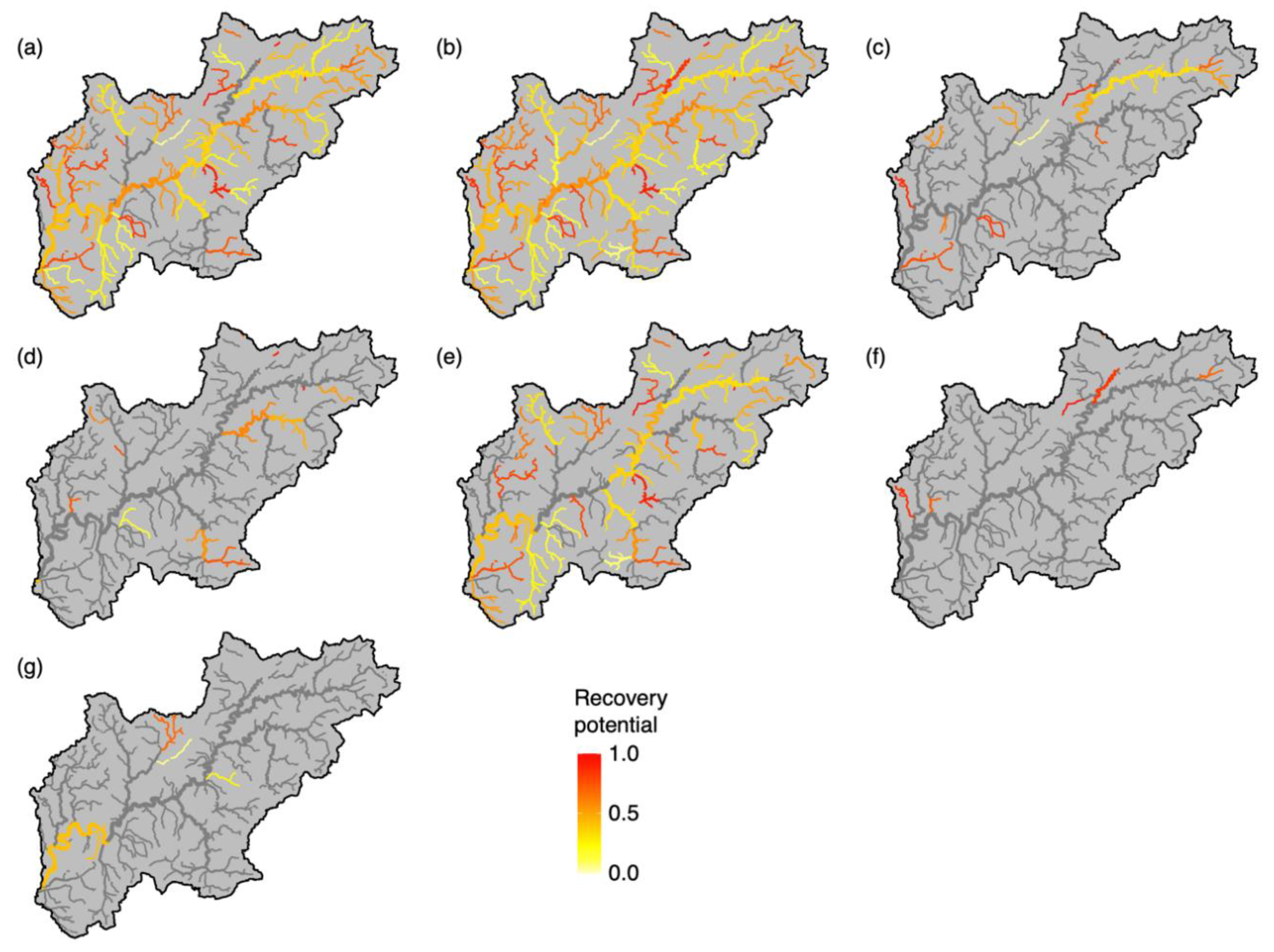
| Short Name | Description |
|---|---|
| bwd 1,2 | Increase broadleaved woodland cover within 1 km of river to 100% |
| CRI 1,2 | Remove all elements of channel resectioning (e.g., reinforced bed, banks) |
| p_all 1 | Reduce P concentrations from all sources by 50% |
| p_ara 2 | Reduce P concentrations from arable sources by 50% |
| p_liv 2 | Reduce P concentrations from livestock sources by 50% |
| p_stw 2 | Reduce P concentrations from sewage treatment sources by 50% |
| p_urb 2 | Reduce P concentrations from urban sources by 50% |
| cams 1 | Bring water resource availability classification at both Q30 and Q95 to “green” status |
| cams30 2 | Bring water resource availability classification at Q30 to “green” status |
| cams95 2 | Bring water resource availability classification at Q95 to “green” status |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkes, M.A.; Mckenzie, M.; Naura, M.; Allen, L.; Morris, M.; Van De Wiel, M.; Dumbrell, A.J.; Bani, A.; Lashford, C.; Lavers, T.; et al. Defining Recovery Potential in River Restoration: A Biological Data-Driven Approach. Water 2021, 13, 3339. https://doi.org/10.3390/w13233339
Wilkes MA, Mckenzie M, Naura M, Allen L, Morris M, Van De Wiel M, Dumbrell AJ, Bani A, Lashford C, Lavers T, et al. Defining Recovery Potential in River Restoration: A Biological Data-Driven Approach. Water. 2021; 13(23):3339. https://doi.org/10.3390/w13233339
Chicago/Turabian StyleWilkes, Martin A., Morwenna Mckenzie, Marc Naura, Laura Allen, Mike Morris, Marco Van De Wiel, Alex J. Dumbrell, Alessia Bani, Craig Lashford, Tom Lavers, and et al. 2021. "Defining Recovery Potential in River Restoration: A Biological Data-Driven Approach" Water 13, no. 23: 3339. https://doi.org/10.3390/w13233339
APA StyleWilkes, M. A., Mckenzie, M., Naura, M., Allen, L., Morris, M., Van De Wiel, M., Dumbrell, A. J., Bani, A., Lashford, C., Lavers, T., & England, J. (2021). Defining Recovery Potential in River Restoration: A Biological Data-Driven Approach. Water, 13(23), 3339. https://doi.org/10.3390/w13233339











